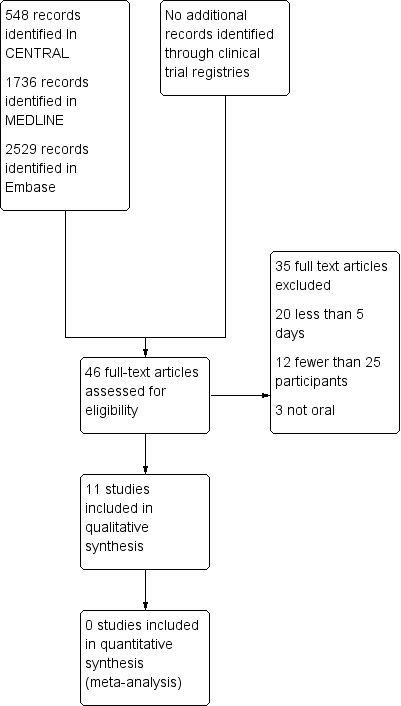
Fármacos antiinflamatorios no esteroideos orales (AINE) para el dolor por cáncer en adultos
Appendices
Appendix 1. Search strategy for CENTRAL (via CRSO)
-
MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES (15201)
-
(NSAID* or "non‐steroidal anti‐inflammatory drug*"):TI,AB,KY (3754)
-
((aceclofenac or acemetacin or azapropazone or celecoxib or ketoprofen or dexketoprofen or diclofenac or dipyrone or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or ibuprofen or Indomet?acin or “mefenamic acid” or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid)):TI,AB,KY (14109)
-
(Apazone or Ketoprofen or Diclofenac or Etodolac or Fenoprofen or Flurbiprofen or Ibuprofen or Indomethacin or Mefenamic Acid or Naproxen or Piroxicam or Sulindac):MH (5867)
-
#1 OR #2 OR #3 OR #4 (23883)
-
MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES (48784)
-
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*):TI,AB,KY (112035)
-
#6 OR #7 (117073)
-
MESH DESCRIPTOR Pain EXPLODE ALL TREES (34252)
-
(pain* or nocicept* or neuropath*):TI,AB,KY (99145)
-
#9 OR #10 (104045)
-
#5 AND #8 AND #11 (548)
Appendix 2. Search strategy for MEDLINE (via Ovid)
-
exp Anti‐Inflammatory Agents, Non‐Steroidal/ (181078)
-
(NSAID* or "non‐steroidal anti‐inflammatory drug*".tw. (24689)
-
Apazone/ or Ketoprofen/ or Diclofenac/ or Etodolac/ or Fenoprofen/ or Flurbiprofen/ or Ibuprofen/ or Indomethacin/ or Mefenamic Acid/ or Naproxen/ or Piroxicam/ or Sulindac/ (51468)
-
(aceclofenac or acemetacin or azapropazone or celecoxib or ketoprofen or dexketoprofen or diclofenac or dipyrone or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or ibuprofen or Indomet?acin or “mefenamic acid” or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid).tw (66755)
-
1 or 2 or 3 or 4 (202029)
-
exp Neoplasms/ (2963191)
-
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*).mp. (3245993)
-
5 or 6 (3579379)
-
exp Pain/ (349136)
-
(pain* or nocicept* or neuropath*).mp. (682211)
-
8 or 9 (756162)
-
randomized controlled trial.pt. (456758)
-
controlled clinical trial.pt. (93340)
-
randomized.ab. (348554)
-
placebo.ab. (171262)
-
drug therapy.fs. (1967607)
-
randomly.ab. (239695)
-
trial.ab (363855)
-
12 or 13 or 14 or 15 or 16 or 17 or 18 (2663271)
-
5 and 8 and 11 and 19 (1736)
Appendix 3. Search strategy for Embase (via Ovid)
-
exp Anti‐Inflammatory Agents, Non‐Steroidal/ (533081)
-
(NSAID* or "non‐steroidal anti‐inflammatory drug*").tw. (42811)
-
(aceclofenac or acemetacin or azapropazone or celecoxib or ketoprofen or dexketoprofen or diclofenac or dipyrone or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or ibuprofen or Indomet?acin or mefenamic or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid).tw. (94288)
-
Apazone/ or Ketoprofen/ or Diclofenac/ or Etodolac/ or Fenoprofen/ or Flurbiprofen/ or Ibuprofen/ or Indomethacin/ or Mefenamic Acid/ or Naproxen/ or Piroxicam/ or Sulindac/
-
1 or 2 or 4 (545302)
-
exp Neoplasms/ (4021384)
-
(neoplasm* or malignan* or tumour* or tumor* or cancer* or carcinoma*).mp. (4471451)
-
6 or 7 (4961554)
-
exp Pain/ (1138211)
-
(pain* or nocicept* or neuropath*).mp. (1310694)
-
9 or 10 (1578116)
-
(random* or factorial* or crossover or "cross‐over" or cross‐over).tw. (1253752)
-
(placebo* or (doubl* adj blind*) or (singl* adj blind*)).tw. (331489)
-
(assign* or allocat*).tw. (420484)
-
crossover procedure/ (55966)
-
double‐blind procedure/ (142603)
-
Randomized Controlled Trial/ (488123)
-
12 or 13 or 14 or 15 or 16 or 17 (1675367)
-
5 and 8 and 11 and 18 (2529)
Appendix 4. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
-
High: randomised trials; or double‐upgraded observational studies.
-
Moderate: downgraded randomised trials; or upgraded observational studies.
-
Low: double‐downgraded randomised trials; or observational studies.
-
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
-
limitations in the design and implementation of available studies suggesting high likelihood of bias;
-
indirectness of evidence (indirect population, intervention, control, outcomes);
-
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
-
imprecision of results (wide confidence intervals);
-
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
-
large magnitude of effect;
-
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
-
dose‐response gradient.
Appendix 5. Summary of results in individual studies: efficacy
| Study | Intervention | Participants with at least 50% and at least 30% reduction in pain | Participants with pain no worse than mild | Participants with PGIC of much improved or very much improved | Other measures of pain intensity or pain relief |
| NSAID versus NSAID | |||||
| Nimesulide 2 x 200 mg daily, n = 34 | No data | No data | No data | In participants completing 2 weeks (43) | |
| Diclofenac Na (Voltaren) 4 x 50 mg daily, n = 33 | Responder defined as ≥ 50% PI reduction or PI ≤ 40/100 But individual data given only for pain ≥40/100 mm on day 2 | No data | Mean PR: | ||
| Celecoxib 2 x 200 mg daily, n = 28 (26) | No data | No data | No data | No difference between groups at baseline or end of study Mean PI reduced from about 60/100 to about 45/100 | |
| Ketorolac 3 x 10 mg daily Cross‐over, n = 137 | No data | No data | PGE "good or complete": | 15 participants had relief with ketorolac but not diclofenac; 14 participants had relief with diclofenac but not ketorolac | |
| Dexketoprofen trometamol 4 x 25 mg daily, n = 57 | No data | PI < 30/100 at end of study: | Overall efficacy of medication quite or very effective: | PID ≥ 20/100 at end of study: | |
| Ketorolac 4 x 10 mg daily, n = 50 | No data | No data | Pain Integrated Score halved in first week (mostly by 1 day) from about 40 to 20 Patients remaining on treatment > 14 days: | ||
| Naproxen 2 x 500 mg daily, n = 28 | No data | No data | No data | PI reduced by about 15/100 in both groups, with large SDs | |
| Naproxen Na 2 x 550 mg daily, n = 50 | No data | No data | No data | Mean Integrated Pain Score decreased by ˜25 points in both groups (> halved) to 15.7 with naproxen and 17 with diclofenac [judged equivalent to < 35/100 on VAS] In participants who switched to step II | |
| Diflunisal 2 x 500 mg daily Cross‐over, n = 50 | No data | No data | No data | Mean reduction in PI (scale 0 ‐ 10): | |
| NSAID versus opioid | |||||
| Ketorolac tromethamine 4 x 10 mg daily, n = 34 | No data | No data | No data | Mean daily PR greater for combination than ketorolac over 7 days; statistically significant for days 2 and 4 | |
| See above | |||||
| Dipyrone 3 x 1000 mg daily, n = 41 | ≥ 50% pain improvement: | No data | No data | Decrease in mean PI on day 7: | |
| n: number of participants in treatment arm; Na: sodium; PGE: Patient Global Evaluation; PGIC: Patient Global Impression of Change; PI: pain intensity; PID: pain intensity difference; PR: pain relief; SD: standard deviation | |||||
Appendix 6. Summary of results in individual studies: rescue medication, adverse events, withdrawals
| Study | Intervention | Use of rescue or breakthrough medication | Participants with any adverse event and serious adverse events | Participants with specific adverse events | Withdrawals |
| NSAID versus NSAID | |||||
| Nimesulide 2 x 200 mg daily, n = 34 | No data | Any AE: no data SAE: none specifically reported Deaths: | Somnolence: Nimesulide 6/34 Naproxen 6/34 Dyspepsia: Nimesulide 18/34 Naproxen 12/34 | AE: Participant refusal or lost to follow‐up: | |
| Diclofenac Na (Voltaren) 4 x 50 mg daily, n = 33 | No data | Any AE: no data SAE: none reported | Somnolence: Diclofenac 0/33 Nefopam 2/33 Aspirin + codeine 4/33 Dyspepsia: Diclofenac 0/33 Nefopam 3/33 Aspirin + codeine 1/33 | All cause: AE: Diclofenac 1/33 LoE: | |
| Celecoxib 2 x 200 mg daily, n = 28 (26) | No data | Any AE: no data SAE: none | Dyspepsia: Celecoxib 2/28 Diclofenac 5/28 | AE: none 2 participants in each group withdrew before 3 weeks because depression worsened from moderate to severe | |
| Ketorolac 3 x 10 mg daily Cross‐over, n = 137 | No data | Any AE: SAE: none reported | Dyspepsia: Ketorolac 6/128 Diclofenac 5/129 | All cause: Ketorolac 10/128 (due to intolerance [AE] 2, LoE 23 ‐ group not given) | |
| Dexketoprofen trometamol 4 x 25 mg daily, n = 57 | Dexketoprofen 71% | Any AE: [Note, these data from text. Data in table 6 indicated dexketoprofen 12 and ketorolac 19 ‐ but 'treatment‐related'. Unable to reconcile] SAE: | Dyspepsia: Dexketoprofen 3/57 Ketorolac 3/58 | 1 participant in each group had missing post‐baseline efficacy data AE: | |
| Ketorolac 4 x 10 mg daily, n = 50 | No data | Any "symptoms" of high intensity 1st week: Ketorolac 20/50 Diclofenac 26/50 2nd week: Ketorolac 15/50 Diclofenac 21/50 | Somnolence: Ketorolac 2/50 Diclofenac 4/50 Anorexia: Ketorolac 1/50 Diclofenac 6/50 Dry mouth: Ketorolac 4/50 Diclofenac 5/50 Dyspepsia: Ketorolac 2/50 Diclofenac 2/50 | AE: "none due to drug treatment" Compliance issues: | |
| Naproxen 2 x 500 mg daily, n = 28 | No data | "No side effects were reported and haematological and biochemical parameters did not significantly alter during the study" SAE: 2 deaths not related to study drugs | None | All cause: 5/28 | |
| Naproxen Na 2 x 550 mg daily, n = 50 | No data | Any AE: no data Deaths: Naproxen 2/50 | Somnolence: Naproxen 4/50 Diclofenac 10/50 Dry mouth: Naproxen 6/50 Diclofenac 8/50 Dyspepsia: Naproxen 6/50 Diclofenac 8/50 | All cause: AE: Pain resolved: | |
| Diflunisal 2 x 500 mg daily Cross‐over, n = 50 | No rescue medication allowed | Any AE: no data SAE: 1 death due to disseminated malignancy (treatment at time not given) | Somnolence: Diflunisal 0/50 Dipyrone 2/50 Anorexia: Diflunisal 2/50 Dipyrone 1/50 Dyspepsia: Diflunisal 2/50 Dipyrone 1/50 | AE: none 2 participants lost to follow‐up | |
| NSAID versus opioid | |||||
| Ketorolac tromethamine 4 x 10 mg daily, n = 34 | No data | Any AE: SAE: none reported | Somnolence: Ketorolac 1/34 Paracetamol + codeine 3/40 Anorexia: Ketorolac 1/34 Paracetamol + codeine 1/40 Dry mouth: Ketorolac 0/34 Paracetamol + codeine 2/40 Dyspepsia: Ketorolac 5/34 Paracetamol + codeine 4/40 | All cause: AE: | |
| See above | |||||
| Dipyrone 3 x 1000 mg daily, n = 41 | Dipyrone 3000 mg 17/41 | Any AE: SAE: none reported | Somnolence: Dipyrone 3000 mg 4/41 Dipyrone 600 mg 9/38 Morphine 18/42 Anorexia: Dipyrone 3000 mg 0/41 Dipyrone 600 mg 0/38 Morphine 1/42 Dyspepsia: Dipyrone 3000 mg 0/41 Dipyrone 600 mg 1/38 Morphine 1/42 | No AE withdrawals | |
| AE: adverse event; GIH: gastrointestinal haemorrhage; LoE: lack of effect; Na: sodium; SAE: serious adverse event | |||||
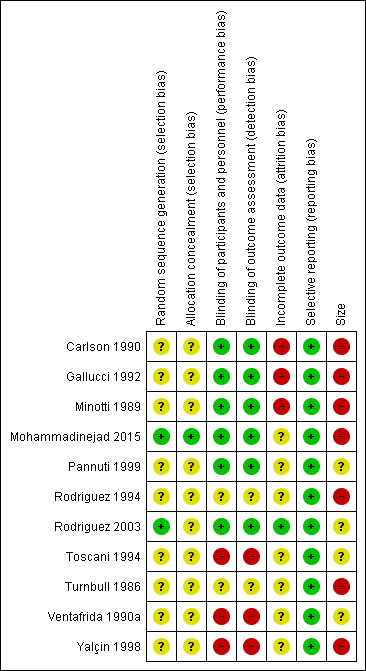
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| NSAID for cancer pain ‐ non‐controlled data | ||||
| Patient or population: people with cancer pain Settings: inpatient or outpatient Intervention: any NSAID, and dose Comparison: no control ‐ cohort of treated participants | ||||
| Outcomes | Probable outcome with NSAID | No of Participants | Quality of the evidence | Comments |
| Participants with at least 30% or at least 50% reduction in pain | No data | No data | Very low | Limited data, several risks of bias |
| PGIC much or very much improved | No data | No data | Very low | Limited data, several risks of bias |
| Pain no worse than mild at one or two weeks (or equivalent) | Range of estimates from 260 in 1000 to 510 in 1000 | 4 studies 415 participants randomised | Very low | Limited data, several risks of bias |
| Serious adverse events | 2 serious adverse events reported | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Adverse events | Dry mouth 10% Loss of appetite 4% Somnolence 9% Dyspepsia 9% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Withdrawals | All cause 23% Lack of efficacy 24% Adverse event 5% | Variously reported in studies | Very low | Limited data, several risks of bias |
| Death | 22 deaths, not clearly related to treatment | 11 studies 949 participants | Very low | Limited data, several risks of bias |
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||