Corticosteroides intramusculares versus orales para reducir las recaídas después del alta del servicio de urgencias para el asma aguda
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | A prospective, randomised, non‐blinded clinical trial Comparison of IM dexamethasone to oral prednisolone Randomisation and allocation concealment methods were reported but methods were not described | |
| Participants | Patients with known history of asthma who presented to the ED with a moderate asthma exacerbation who did not require admission on presentation and had no evidence of varicella exposure or fever and did not take any systemic corticosteroids within 2 weeks of the start of the study A locally modified scoring system based on GINA guidelines for the management of asthma was used to evaluate the clinical response at presentation, and 5 days after treatment. Ages: enrolled patients 9 months to 14 years. Mean age of IM corticosteroid group: 79.7 months. Mean age of oral corticosteroid group: 45.4 months Reported enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Set in Jordan Sex: 20 men, 9 women | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Authors did not respond to requests for clarification. No registered protocol was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 16): "Patients were randomised to receive either a single Intramuscular (IM) dose of dexamethasone acetate (1.7 mg/kg) or prednisolone taken orally each day for 5 days (2 mg/kg//day)." |
| Allocation concealment (selection bias) | Unclear risk | Study did not comment on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Reported as a non‐blinded study. Quote (p. 16): "The study is prospective randomised and non‐blinded." |
| Blinding of outcome assessment (detection bias) | High risk | Reported as a non‐blinded study. Quote (p. 16): "The study is prospective randomised and non‐blinded." |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Source of funding not provided. Baseline imbalance detected, dexamethasone group significantly older than prednisolone group. Quote (p. 16): "Males out‐numbered females in both groups (13:4 in the dexamethasone group, and 7:5 in the second group). Patients in dexamethasone acetate group were older than those in prednisolone group (P = 0.007), which can risk of bias ably be explained by the small‐sized sample and the random assignment of patients to either group." |
| Methods | A prospective, randomised, double‐blinded, controlled clinical trial Comparison of IM betamethasone versus oral prednisone Randomisation was reported but method was not described. Allocation concealment: pharmacy provided randomised, sequentially numbered, sealed kits that contained an injection of betamethasone 2 mL and 7 placebo capsules, or an injection of saline 2 mL and 7 capsules of prednisone 50 mg each | |
| Participants | Patients who presented to the ED with an exacerbation of asthma and had no contraindication to prednisone or betamethasone and were likely to be discharged after treatment. The diagnosis of asthma was made clinically by the ED physician. Patients who received oral or parenteral corticosteroids within 24 hours of enrolment were not excluded from study. Ages: enrolled patients 18 years and above. Mean age of IM corticosteroid group: 31.6 years (SD: 13.1). Mean age of oral corticosteroids group: 21.1 years (SD: 10.7) Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Set in Canada Sex: 77 men, 94 women | |
| Interventions |
| |
| Outcomes |
| |
| Notes | No registered protocol was identified; however study authors were contacted and provided the study protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 148): "The hospital pharmacy provided sequentially numbered, sealed kits that were randomised to contain either ...." |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. Drug kits were sealed and sequentially numbered. Quote (p. 148): "The hospital pharmacy provided sequentially numbered, sealed kits that were randomised to contain either an injection of betamethasone 2 mL and seven placebo capsules, or an injection of saline 2 mL and seven capsules containing prednisone 50 mg each." |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded study. Drug kits opened by nurse not involved in study patient. Medication names were obscured so neither staff or patients knew medication name. Quote (p. 149): "After consent was obtained, a kit was opened by a nurse who was not involved with the study patient. This nurse drew up the 2 mL solution and placed a translucent sleeve over the syringe to obscure the medication but to allow the graduations to be seen. The primary nurse involved with the patient was given the covered syringe and capsules, and administered the intramuscular injection." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment completed by study coordinator blinded to treatment allocation. All instances of suspected relapses reviewed by investigators before un‐blinding. Quote (p. 149): "Patients were contacted by telephone on day 7 and 21 by a study coordinator blinded to the treatment allocation, and asked if they sought treatment for their asthma since the initial ED visit." "Before un‐blinding, all instances of suspected relapse were reviewed by two of the investigators." |
| Incomplete outcome data (attrition bias) | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 149). Excluded patients balanced between groups. Quote (p. 149‐50): "During the study, 176 patients with acute asthma agreed to participate in the trial. After random assignment, five patients were subsequently excluded: three patients required hospitalisation before ED discharge; one was discovered to be on chronic prednisone; and one refused the injection." "At day 7, three patients were lost to follow‐up ‐ two in the prednisone group and one in the betamethasone group. By day 21, 12 patients were lost to follow‐up ‐ four in the prednisone group and eight in the betamethasone group. At the completion of the study, follow‐up information was available for 159 patients (93%)." |
| Selective reporting (reporting bias) | Low risk | Protocol made available to authors by study investigators (protocol not registered). All pre‐selected outcomes reported. |
| Other bias | Low risk | Study appears to be free of other sources of bias. Source of funding stated. Quote (p. 152): "Supported by a grant by Research and Development of the University of Calgary, Calgary, Alberta." |
| Methods | A prospective, randomised, non‐blinded controlled clinical trial Comparison of a single dose of IM dexamethasone versus oral prednisolone Randomization was reported but method was not described. Allocation concealment method was accomplished using sequentially numbered opaque study packets containing group assignments. | |
| Participants | Patients with known history of asthma who presented to the ED with moderate asthma exacerbation who did not require admission on presentation. Asthma was defined as at least 2 prior episodes of respiratory illness treated with bronchodilators. Clinical asthma score adapted from a previous pulmonary score was used to evaluate asthma children aged from 5 to 17 years measuring: respiratory rate, retractions and wheezing. Reported enrolling patients with moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Ages: enrolled patients 18 months to 7 years. Median age of IM corticosteroid group: 38 months (IQR: 29‐59). Median age of oral corticosteroid group: 42 months (IQR: 28‐60.5) Patients who received IV therapy (not due to vomiting but to severity of asthma) were excluded from study. Set in United States Sex: 110 men, 71 women | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Authors were not contacted No registered protocol was identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 521): "After written informed consent was obtained, we randomised patients to 1 of 2 treatment groups in blocks of 6, 8, or 10." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured via sequentially numbered opaque packets containing group assignments kept in ED and opened by physician immediately after enrolment. Quote (p. 522): "Allocation concealment was maintained by use of sequentially numbered opaque study packets containing group assignments, which were kept in the ED and opened by the treating physician immediately after enrolment." |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded study. Quote (p. 522): "Treating physicians were not masked to study group." "At the time of disposition from the ED or 3 hours after enrolment for those patients still in the ED, physicians not blinded to group assignment performed a standardized repeat physical examination including the assessment of a second asthma score." Quote (p. 527): "The only people masked to group assignment in this study were the investigators who assigned follow‐up scores at the 4‐day return visit." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessment completed via physician blinded to group assignment and not involved in patient's care on the initial visit. Quote (p. 523): "At the 4‐day follow‐up visit, a physician masked to group assignment and not involved in the patient's care on the initial visit performed a standard examination, including assignment of an asthma score. Physicians and guardians were instructed not to engage in any conversation before completion of the physical examination. After completing and recording this physical examination, the physician administered a structured interview regarding interim use of bronchodilators and ICS, guardian's perception of bronchodilators and ICS, guardian's perception of clinical improvement, need for further medical care since enrolment, and compliance with oral steroid regimen." |
| Incomplete outcome data (attrition bias) | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 523). Excluded patients balanced between groups. Quote (p. 523, 525‐6): "Of 194 randomised patients, 13 were subsequently excluded from the study (7 in the dexamethasone arm and 6 in the prednisolone arm) for the following reasons: 6 had initial asthma scores of only 2, 4 had previously participated, 1 had only one prior episode of wheezing, 1 had taken prednisolone within the previous month, and 1 patient was randomised to the dexamethasone group, but inappropriately given prednisolone in the ED. The guardians of 2 included patients randomised to dexamethasone withdrew their consent for further participation during the ED visit (one before drug administration, one afterward)." |
| Selective reporting (reporting bias) | Unclear risk | No available protocol |
| Other bias | Unclear risk | Source of funding was not stated. |
| Methods | A prospective, randomised, non‐blinded controlled clinical trial with blinded outcome assessors Comparison of single dose of IM dexamethasone versus oral prednisolone Randomization and allocation concealment were reported but methods were not discussed. | |
| Participants | Patients with known history of asthma who presented to the ED with mild to moderate asthma exacerbation who did not require admission on presentation Asthma was defined as recurrent coughing, wheezing, or shortness of breath responsive to corticosteroids or β₂‐agonists. Asthma exacerbation was defined as increased asthma signs and symptoms unresponsive to the patient’s routine asthma medications and additional β₂‐agonist therapy. Clinical asthma score was defined as a composite score of wheeze and cough scores ranging from 0 to 8. Reported to enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Ages: enrolled patients 6 months to 7 years. Mean age of IM corticosteroid group: 38 months (SD: 18). Mean age of oral corticosteroid group: 36 months (SD: 22) Patients were excluded from study if they received IV corticosteroids on the ED initial visit. Set in United States Sex: 23 men, 9 women | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Authors were not contacted. No registered protocol was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 299): "Once enrolled in the study, patients were randomised to receive either a single IM dose of dexamethasone acetate (Dalalone, Forest Pharmaceuticals, 16 mg/mL) or prednisone (either suspension 3 mg/mL or tablets—patient’s choice) taken orally each day for 5 days." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded study. Quote (p. 300‐1): "The study investigators were blinded to the patient’s treatment arm. Patients and parents were not blinded. The nurses in the paediatric clinic who administered the IM injections did not discuss the patients with the study investigators." "Investigator blinding was achieved in all but 4 patients. For 2 of these children, the nurses told an investigator that they had administered a shot, and the other 2 children disclosed what they had received (one IM Dex and one oral Pred). A second investigator, who was still blinded, completed the assessments." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to group assignment. Quote (p. 300): "The study investigators were blinded to the patient’s treatment arm" |
| Incomplete outcome data (attrition bias) | Low risk | Study attrition provided. Excluded patients balanced between groups. Quote: (p. 300): "From September 1996 through February 1997, we approached 35 children (age range, 8 months to 7 years) and their legal guardians concerning enrolment, and all but 2 agreed to participate in the study. Sixteen children received IM Dex, and 17 were randomised to receive oral Pred. One child, a 16‐month‐old (randomised to IM Dex), was withdrawn on her second study day for treatment of a persistent cough by an emergency department physician who noted no wheezing or respiratory distress but gave her an additional IM injection of dexamethasone." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | No source of funding provided. |
| Methods | A prospective, randomised, double‐blinded controlled clinical trial Comparison of IM methylprednisolone versus oral methylprednisolone Randomization and allocation concealment were reported but methods were not described. | |
| Participants | Patients who presented to the ED with an acute asthma and were discharged after ED treatment Inclusion criteria required eligible patients to fulfil the American Thoracic Society for the diagnosis of asthma. Ages: enrolled patients 15 to 55 years. Mean age of IM corticosteroid group: 42 years (SEM: 4). Mean age of oral corticosteroid group: 37 years (SEM:4) Exacerbation severity not discussed. Exacerbation severity estimated as severe based on baseline PEF Patients who received corticosteroids at the time of presentation were excluded from study. Patients who needed admission or remained in ED for more than 6 hours were excluded from study. Set in United States Sex: 11 men, 7 women | |
| Interventions |
| |
| Outcomes | Follow‐up between days 5 and 7
| |
| Notes | Authors did not respond to requests for clarification. No registered protocol was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Quote (p. 11): "All subjects were given an IV injection of 4 mg/kg of methylprednisolone sodium succinate, then randomised in double‐blind fashion to receive either an IM injection of methylprednisolone sodium acetate..." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind study. Oral placebo and oral methylprednisolone was indistinguishable from each other but unsure if IM placebo and IM methylprednisolone was indistinguishable. Quote (p. 11): "All subjects were given an IV injection of 4 mg/kg of methylprednisolone sodium succinate, then randomised in double‐blind fashion to receive either an IM injection of methylprednisolone sodium acetate..." "Oral placebo and oral methylprednisolone were indistinguishable, and the dosage and tapering schedule were the same as in our previous study." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Missing patients balanced between groups. Quote (p. 12): "Follow‐up was obtained by return visit in seven of eight patients in group 1, and in nine of ten patients in group 2." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No source of funding provided |
| Methods | A prospective, randomised, non‐blinded clinical trial with blinded outcome assessors Comparison of IM dexamethasone versus oral prednisone Randomization was stated but method was not described. Allocation concealment was accomplished using sealed opaque packets. | |
| Participants | Patients, who had 2 prior episodes of wheezing and were treated with beta‐2‐adrenergic agents, presented to ED with mild to moderate wheezing and did not require admission to hospital Enrolment of patients was based on the pulmonary index (0 to 3), assessment of a clinical score for asthma, and pulse oximetry on arrival to ED. Ages: enrolled patients 3 to 16 years. Mean age of IM corticosteroid group: 82 months (SD: 46). Mean age of oral corticosteroid group: 63 months (SD: 36) Reported enrolling patients with mild‐moderate exacerbations. Unable to assess exacerbation severity based on baseline PEF Patients were excluded from study if they received corticosteroids within the last month or were hospitalised for asthma treatment within the last 2 months prior to the study. Set in United States Sex: 24 men, 18 women | |
| Interventions |
| |
| Outcomes | Followed up on day 5 either by assessment in out‐patient clinic or via telephone interview
| |
| Notes | Author was contacted and provided clarification on allocation concealment and source of funding No registered protocol was identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how patients were randomised. Study authors confirmed via personal communication that randomisation was in blocks. Quote (p. 421): "Patients were randomly assigned by sealed packets to receive either oral prednisone tablets 2 mg/kg or IM dexamethasone 0.3 mg/kg." Personal communication: "Randomization was in blocks." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured via sealed packets. Study authors confirmed via personal communication that the sealed packets were opaque. Quote (p. 421): "Patients were randomly assigned by sealed packets to receive either oral prednisone tablets 2 mg/kg or IM dexamethasone 0.3 mg/kg." Personal communication: "Sealed packets were opaque" |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded study. Quote (p. 421): "The assigned corticosteroid was administered in an unblinded manner immediately after enrolment in the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded. Stated that Investigators (Physicians) were blinded to patient's treatment. Quote (p. 422): "Patient follow‐up was conducted on the fifth day after discharge from the PED by a physician blinded to group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Patient exclusions balanced between groups. Quote (p. 422): "Forty‐four patients participated in the pilot study of whom 23 received a single dose of IM dexamethasone and 21 received 3 days of oral prednisone. Two patients from the IM dexamethasone group were admitted to the hospital shortly after enrolment and therefore were removed from the study. The remaining 42 patients were discharged to home, resulting in an even distribution between the IM dexamethasone and oral prednisone groups." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Study authors confirmed via personnel communication that the study was not funded. Personal communication: "Study was funded via supplies only (decadron from Merck Pharmaceuticals)." |
| Methods | A prospective, randomised, double blinded, placebo‐controlled clinical trial Comparison of single dose of IM methylprednisolone versus oral methylprednisolone Randomisation was accomplished using computer‐generated random set of numbers (20 blocks). Allocation concealment was maintained by research pharmacist using computer generated set of numbers to package medications in balanced blocks (20). Intramuscular injection (methylprednisolone and placebo) with similar appearances and covered content were prepared and administered by a nurse who was not involved in the study. Oral methylprednisolone and placebo were identical in appearance and were given in identical containers. | |
| Participants | Patients diagnosed with asthma (based on the American Thoracic Society Guidelines (1962), PEF ≤ 70% predicted during the ED visit with a minimum PEF ≥ 40% predicted, and included both clinical symptoms and physical examination findings), who presented with an asthma exacerbation and were expected to be discharged after the ED treatment Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Ages: enrolled patients 18 to 45 years. Mean age of IM corticosteroid group: 33 years (SD: 8). Mean age of oral corticosteroid group: 33 years (SD: 8) Patients who received systemic corticosteroids (within 1 month prior to the study), theophylline or inhaled anticholinergic agents were excluded from study. Set in United States Sex: 56 men, 131 women | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Authors were contacted, but were unable to provide protocol No registered protocol was identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation completed via block randomised computer generated numbers. Quote (p. 363): "The medication was prepared and block‐randomised by a research pharmacist who used a computer‐generated set of random numbers to package the medications in balanced blocks of 20 (i.e., each block of 20 medication packets contained 10 packets of oral methylprednisolone plus an IM placebo and 10 packets of IM methylprednisolone plus oral placebo." |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled central allocation. Quote (p. 363): "The randomisation code was held by the pharmacist and was not broken during the course of the study." "To the best of our knowledge, allocation concealment was maintained." |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded study. Corticosteroids administered to patients via a study nurse not involved with the patient. Intramuscular injection was administered in a private setting with no one else present. Nurse instructed not to provide patients or staff information on contents of the syringe. Nurse covered the syringe so that other staff or patients could not see what was in the syringe. Oral placebo and corticosteroids was identical in appearance. Quote (p. 363): "The injection was reconstituted and administered by an ED nurse who was not blinded to the treatment but who had no involvement in any aspect of the study. This individual was instructed not to provide the patient, physician, or study personnel with any information about the contents of the syringe. Although the placebo and methylprednisolone injections were similar in appearance, the nurse also was instructed not to allow anyone to see the contents of the syringe. The injection was administered in a private setting with no one else present. The oral methylprednisolone and oral placebo were identical in appearance and were given to patients in identical containers. To the best of our knowledge, allocation concealment was maintained." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 364). Excluded patients balanced between groups. Quote (p. 364): "One hundred ninety patients were entered into the study over a 60‐month period from November 1997 to November 2002. As shown in the diagram of the Consolidated Standards of Reporting trials, three patients (all in the oral methylprednisolone/IM placebo group) were removed after study entry due to protocol violations. One patient’s asthma was too severe to be discharged safely from the ED, a second patient did not receive a ‐agonist prescription at ED discharge, and a third patient was instructed by the primary physician to discontinue the study medication at day 5. Seven patients (IM administration group, three patients; oral administration group, four patients) were lost to follow‐up and were excluded from the primary efficacy analysis. The remaining 180 patients, 92 of whom received IM methylprednisolone plus oral placebo, and 88 of whom received oral methylprednisolone plus IM placebo, completed the protocol and were available for follow‐up at 10 days. All patients reached for follow‐up at 10 days were successfully contacted again for follow‐up at 21 days. No patients who were lost to follow‐up at 10 days were available for follow‐up at 21 days." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Study funded by pharmaceutical company. Authors state that pharmaceutical company just provided financial support, and was not involved in the design, execution, data analysis, or manuscript preparation. Quote (p. 362): "This research was funded in part by an unrestricted grant from Pharmacia & Upjohn (currently, Pfizer), who, other than providing financial support, were not involved in any way with the design, execution, data analysis, or manuscript preparation." |
| Methods | A prospective, randomised, double blinded, placebo‐controlled clinical trial Comparison of single dose of IM dexamethasone versus oral dexamethasone Randomization was accomplished using computer‐generated random set of numbers (9 blocks). Allocation concealment was reported but method was not described. | |
| Participants | Patients diagnosed with asthma (based on the American Thoracic Society criteria, 1962), presented to ED with acute asthma and did not require hospital admission Exacerbation severity not discussed. Exacerbation severity estimated as mild/moderate based on baseline PEF Ages: enrolled patients 16 to 60 years. Mean age of IM corticosteroid group: 37 years (SD: 4). Mean age of oral corticosteroid group: 40 years (SD: 4) Patients who received systemic corticosteroids prior to enrolment in the study were excluded. Set in Taiwan Sex: 20 men, 16 women | |
| Interventions | Patients were divided into 3 groups (A, B, and C)
Tapering of dexamethasone was as follows: 3.0 mg day 1 and 2, 2.0 mg day 3, 1.5 mg day 4, 1.0 mg day 5, 0.75 mg day 6 and 0.5 mg day 7)
| |
| Outcomes | Primary and secondary outcomes were not defined.
| |
| Notes | Attempts to contact the authors failed No registered protocol was identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised via block randomised computer generated numbers. Quote (p. 26): "The subjects were randomly assigned into one of three groups using a double blind model. Randomisation was by means of a set of computer‐generated set of random‐numbers in blocks of nine." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blinded study. Oral corticosteroids and oral placebo were indistinguishable from each other but no information provided as to whether IM corticosteroids were indistinguishable from IM placebo. Quote (p. 26): "The subjects were randomly assigned into one of three groups using a double blind model." "The oral dexamethasone and oral placebo were indistinguishable." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided on outcome assessment blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Study did not report attrition/exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | Source of funded not provided. |
| Methods | A prospective, randomised, double‐blinded, placebo‐controlled clinical trial Comparison of IM triamcinolone versus oral prednisone Randomization was accomplished using computer‐generated random numbers. Allocation concealment was reported and discussed as pharmacy controlled by using computerized generated set of numbers to package medications and placebo into sequentially numbered kits. | |
| Participants | Patients presented to the ED with an asthma exacerbation that had an initial PEF < 350 L/min and did not require admission to hospital on ED presentation The diagnosis of asthma was according to the American Thoracic Society criteria (1987) Patients were excluded from study if had received corticosteroids within 2 weeks prior to ED presentation or were unable or unwilling to attend follow‐up evaluation. Ages: Enrolled patients 18 to 50 years. Mean age of IM corticosteroid group: 31 years (SD: 9). Mean age of oral corticosteroid group: 32 years (SD: 9) Exacerbation severity not reported. Exacerbation severity estimated as mild/moderate based on baseline PEF Set in United States Sex: 47 mean, 107 women | |
| Interventions |
| |
| Outcomes | Patients were followed up 7 to 10 days after ED enrolment.
| |
| Notes | Author was contacted and provided clarification on the methodology of blinding and outcome data. Was unable to provide copy of the study protocol. No registered protocol was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation completed via computer generated set of random numbers. Quote (p. 334): "A computer‐generated set of random numbers was used by the hospital pharmacy to package the active medications and placebos into sequentially numbered kits." |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled allocation concealment. Medications and placebos packaged in sequentially numbered kits. Quote (p. 334): "A computer‐generated set of random numbers was used by the hospital pharmacy to package the active medications and placebos into sequentially numbered kits." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded study. Study authors confirmed via personnel communication that the study medications were indistinguishable from each other. Personal communication: "Syringes were prefilled and tablets looked similar for placebo and prednisone. Each patient received injection and pill bottle. They brought their bottle to follow‐up." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors (physicians) were blinded to the patient's group assignment. Quote (p. 335): "Physicians evaluating patients at follow‐up remained blinded to drug group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Detailed information on study attrition provided in flow diagram provided (p. 337). Excluded patients balanced between groups. Quote (p. 335‐6): "We approached 186 patients who were potentially eligible; 15 (9%) refused to participate, and 3 (2%) resided out of town and could not return for follow‐up. A total of 168 patients were enrolled, 82 in the triamcinolone group and 86 in the prednisone group. Fourteen patients were withdrawn from analysis: 6 (3 in each group) for protocol violations because the patient was older than 50 years of age, and 8 (1 in the triamcinolone group and 7 in the prednisone group) because they were lost to follow‐up. The final study population was 154 patients, 78 in the triamcinolone group and 76 in the prednisone group." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No other potential source of bias found. Source of funding provided. Quote (p. 333): "Research supported by the Summa Health System Foundation." |
Abbreviations
ED: emergency department
FEV1: forced expiratory volume
FVC: forced vital capacity
ICS: inhaled corticosteroids
IM: intramuscular
MDI: metered dose inhaler
PEF: peak expiratory flow
IV: intravenous
SD: standard deviation
SEM: standard error of mean
IQR: interquartile range
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Did not compare IM versus oral corticosteroids | |
| Not an RCT/CCT | |
| Not an RCT/CCT | |
| Did not provide a single dose of intramuscular corticosteroids | |
| Did not compare IM versus oral corticosteroids | |
| Not an RCT/CCT | |
| Patients not discharged from ED | |
| Did not provide a single dose of intramuscular corticosteroids | |
| Not an RCT/CCT | |
| Not an RCT/CCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | No abstract for full publication available. Unable to assess |
| Participants | No abstract for full publication available. Unable to assess |
| Interventions | No abstract for full publication available. Unable to assess |
| Outcomes | No abstract for full publication available. Unable to assess |
| Notes | No abstract for full publication available. Unable to make assessment on study eligibility |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| Analysis 1.1  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 1 Relapse. | ||||
| 2 Relapse intention to treat Show forest plot | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| Analysis 1.2  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 2 Relapse intention to treat. | ||||
| 3 Subgroup analysis: children versus adults Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| Analysis 1.3  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 3 Subgroup analysis: children versus adults. | ||||
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge. | ||||
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations Show forest plot | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| Analysis 1.5 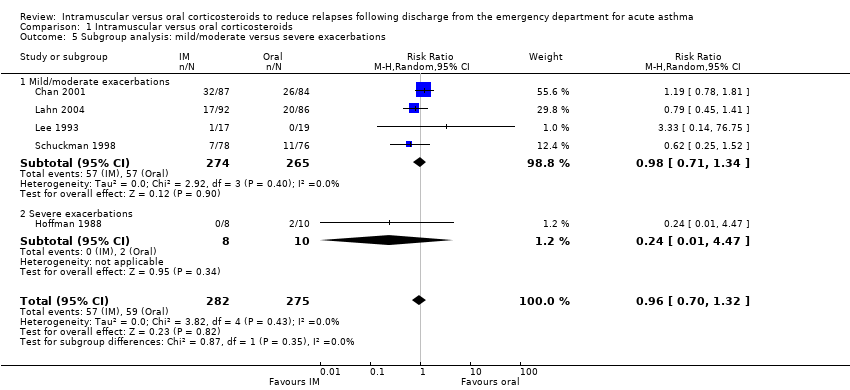 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 5 Subgroup analysis: mild/moderate versus severe exacerbations. | ||||
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias Show forest plot | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| Analysis 1.6 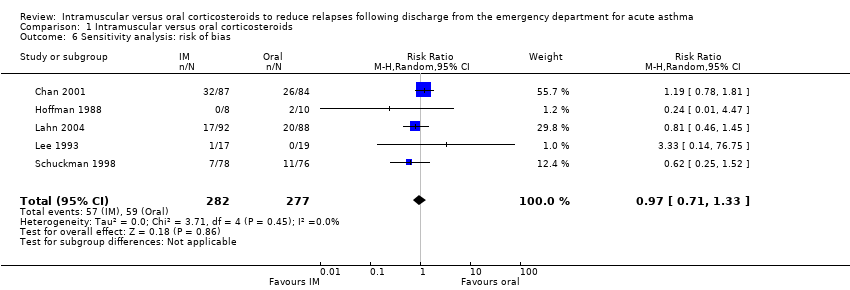 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 6 Sensitivity analysis: risk of bias. | ||||
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days Show forest plot | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| Analysis 1.7  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days. | ||||
| 8 Sensitivity analysis: fixed effects Show forest plot | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| Analysis 1.8  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 8 Sensitivity analysis: fixed effects. | ||||
| 9 Sensitivity analysis: corticosteroids in ED Show forest plot | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
| Analysis 1.9  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 9 Sensitivity analysis: corticosteroids in ED. | ||||
| 10 Serious adverse events; hospitalization following discharge Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.10 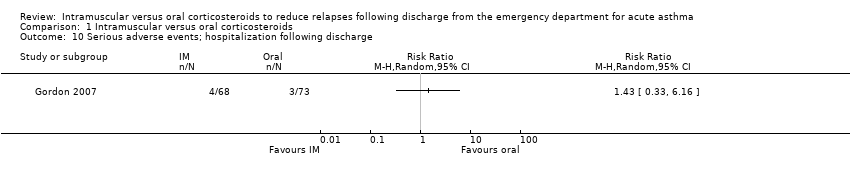 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 10 Serious adverse events; hospitalization following discharge. | ||||
| 11 Adverse events Show forest plot | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| Analysis 1.11  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 11 Adverse events. | ||||
| 12 Adverse events: nausea/vomiting/GI distress Show forest plot | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
| Analysis 1.12 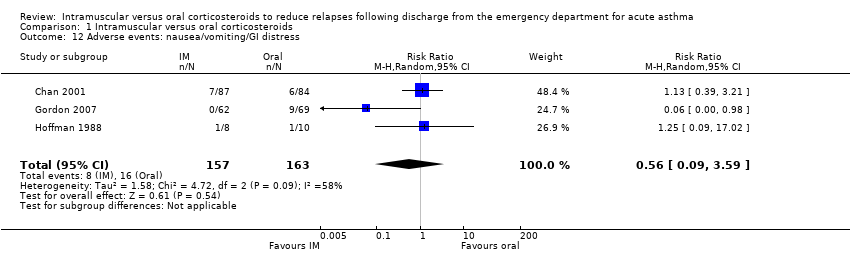 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 12 Adverse events: nausea/vomiting/GI distress. | ||||
| 13 Adverse events: insomnia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 13 Adverse events: insomnia. | ||||
| 14 Adverse events: personality changes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.14 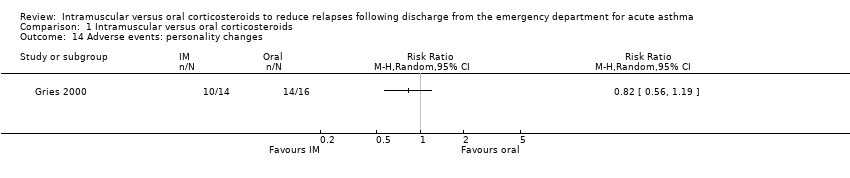 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 14 Adverse events: personality changes. | ||||
| 15 Adverse events: pain Show forest plot | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
| Analysis 1.15  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 15 Adverse events: pain. | ||||
| 16 Adverse events: swelling Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
| Analysis 1.16 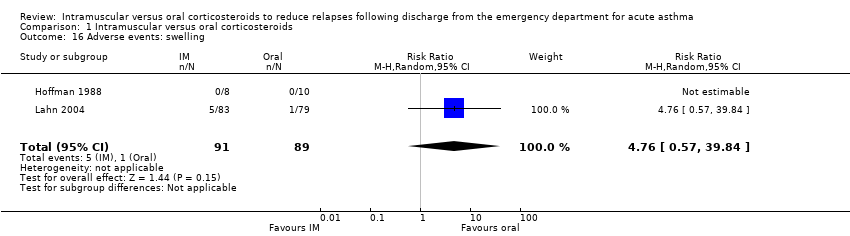 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 16 Adverse events: swelling. | ||||
| 17 Adverse events: redness Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
| Analysis 1.17  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 17 Adverse events: redness. | ||||
| 18 Pulmonary function: peak expiratory flow (L/min) Show forest plot | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
| Analysis 1.18 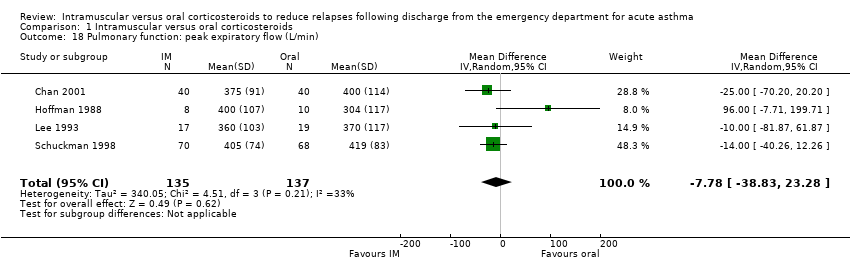 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 18 Pulmonary function: peak expiratory flow (L/min). | ||||
| 19 Pulmonary function: FEV₁/FVC (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.19 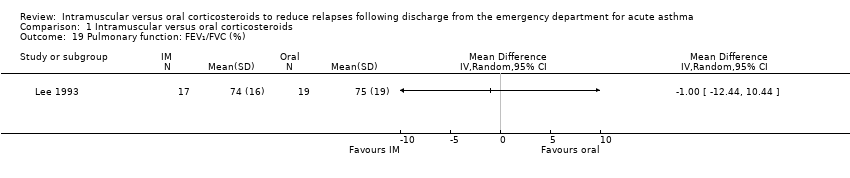 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 19 Pulmonary function: FEV₁/FVC (%). | ||||
| 20 Symptom persistence Show forest plot | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| Analysis 1.20  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 20 Symptom persistence. | ||||
| 21 Symptom persistence: cough Show forest plot | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
| Analysis 1.21  Comparison 1 Intramuscular versus oral corticosteroids, Outcome 21 Symptom persistence: cough. | ||||
| 22 Symptom persistence: wheezing Show forest plot | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
| Analysis 1.22 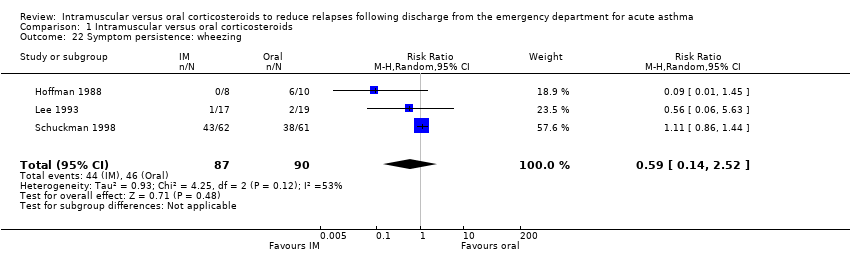 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 22 Symptom persistence: wheezing. | ||||
| 23 24‐hour beta agonist use Show forest plot | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |
| Analysis 1.23 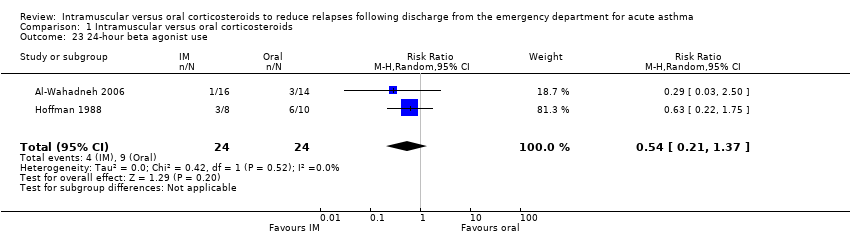 Comparison 1 Intramuscular versus oral corticosteroids, Outcome 23 24‐hour beta agonist use. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 1 Relapse.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 2 Relapse intention to treat.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 3 Subgroup analysis: children versus adults.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 5 Subgroup analysis: mild/moderate versus severe exacerbations.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 6 Sensitivity analysis: risk of bias.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 8 Sensitivity analysis: fixed effects.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 9 Sensitivity analysis: corticosteroids in ED.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 10 Serious adverse events; hospitalization following discharge.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 11 Adverse events.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 12 Adverse events: nausea/vomiting/GI distress.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 13 Adverse events: insomnia.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 14 Adverse events: personality changes.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 15 Adverse events: pain.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 16 Adverse events: swelling.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 17 Adverse events: redness.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 18 Pulmonary function: peak expiratory flow (L/min).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 19 Pulmonary function: FEV₁/FVC (%).

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 20 Symptom persistence.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 21 Symptom persistence: cough.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 22 Symptom persistence: wheezing.

Comparison 1 Intramuscular versus oral corticosteroids, Outcome 23 24‐hour beta agonist use.
| Intramuscular corticosteroids compared to Oral corticosteroids for acute asthma | |||||
| Patient or population: patients with acute asthma | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Oral corticosteroids | Intramuscular corticosteroids | ||||
| Relapse | 201 per 1000 | 12 fewer per 1000 | RR 0.94 | 804 | ⊕⊕⊝⊝ |
| Relapse within 10 days post‐discharge | 154 per 1000 | 40 fewer per 1000 (from 75 fewer to 11 more) | RR 0.74 | 742 | ⊕⊕⊕⊝ |
| Relapse occurring after 10 days post‐discharge | 245 per 1000 | 2 fewer per 1000 | RR 0.99 | 556 | ⊕⊕⊝⊝ |
| Adverse events | 294 per 1000 | 50 fewer per 1000 | RR 0.83 | 404 | ⊕⊕⊝⊝ |
| Pulmonary function: Peak expiratory flow | The mean pulmonary function: peak expiratory flow ranged across control groups from | The mean pulmonary function: peak expiratory flow in the intervention groups was | 272 | ⊕⊕⊕⊝ | |
| Symptom persistence | 537 per 1000 | 317 fewer per 1000 | RR 0.41 | 80 | ⊕⊕⊝⊝ |
| 24‐hour beta agonist use | 375 per 1000 | 172 fewer per 1000 | RR 0.54 | 48 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded 1 level for risk of bias. Majority of studies received an unclear risk of bias for random sequence generation and selective outcome reporting | |||||
| Studies | Pulmonary function: Eligibility criteria | Exacerbation severity |
| Severity estimated using modified scoring system based on GINA guidelines. Reported to enrolling patients with mild‐moderate exacerbations, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported mean baseline PEF greater than 200 L/min: IM group: 270 L/min (SD: 103); oral group: 261 L/min (SD: 104). | Mild/moderate | |
| Reported to enrolling patients identified as moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Applied adapted exacerbation severity score (unspecified). Reported to enrolling patients rated as mild/moderate, however baseline pulmonary function of the groups was not reported. | Unable to assess | |
| Reported baseline mean PEF of enrolled patients of less than 150 L/min: IM group: 129 L/min (SD:14); oral group: 141 L/min (SD: 14). | Severe | |
| Exacerbation severity estimated via pulmonary index score. Study reported to enrolling patients with mild/moderate exacerbations, however baseline pulmonary function was not reported. | Unable to assess | |
| Eligibility criteria required patients to have a PEFR of ≤ 70% predicted with a minimum PEFR of ≥ 40%. Reported PEF of enrolled patients was ≥ 200 L/min: IM group: 205 L/min (SD: 70); oral group: 209 L/min (SD: 72). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 210 L/min (SD: 30); oral group: 208 L/min (SD: 26). | Mild/moderate | |
| Reported mean baseline PEF of ≥ 200 L/min: IM group: 243.6 L/min (SD: 64); oral group: 244.7 L/min (SD: 83). | Mild/moderate | |
| Abbreviations: GINA = Global Initiative for Asthma; PEF = Peak expiratory flow; PEFR = Peak expiratory flow rate; IM = intramuscular; SD = standard deviation | ||
| Studies | Location/setting | Co‐interventions | Corticosteroid doses and durations | Methyprednisolone equivalency | Relapse outcome |
| Jordan, ED | Provided in ED: not stated Provided at discharge: SABA | Dexamethasone (IM) 1.7 mg/kg Mean dose: 24 mg Single dose Prednisolone (oral) 2 mg/kg/day for 5 days Mean dose: 19.2 mg per day Total dose: 96 mg | IM Methylprednisone equivalency: 120 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 76.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/16 Day 21 Oral group 3/14 Day 21 | |
| Canada, ED | Provided in ED: SABA, methylxanthines, supplemental oral/IV corticosteroids Provided at discharge: Methylxanthines, unspecified inhaled beta₂‐agonists, and ICS | Betamethasone (IM) 12 mg Single dose. Received placebo capsules over 7 days. Prednisone (oral) 50 mg a day for 7 days. Received a single placebo injection Total dose: 350 mg | IM Methylprednisone equivalency: 72 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 280 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 12/86 Day 7 Oral group 19/82 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA, ipratropium bromide. IV corticosteroids for patients who vomited oral corticosteroids. Provided at discharge: Inhaled beta₂‐agonists and ICS | Dexamethasone (IM) 0.6 mg/kg (max 16 mg) Single dose Prednisolone (oral) 2 mg/kg (max 50 mg) daily for 5 days Total: 250 mg | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12‐36 hours) Oral Methylprednisone equivalency: 200 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 8/69 Day 4 Oral group 11/73 Day 4 IM group 15/68 Day 14 Oral group 16/73 Day 14 | |
| United States, Tertiary medical center | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) Patients 6 to 12 months old received 16 mg. Patients 13 to 35 months old received 24 mg. Children ≥ 36 months received 36 mg. Single dose Prednisone (oral) 2 mg/kg a day for 5 days Total dose: unclear | IM Methylprednisone equivalency: Unable to assess Oral Methylprednisone equivalency: Unable to assess | IM group 1/15 Day 28 Oral group 3/17 Day 28 | |
| United States, ED | Provided in ED: SABA, epinephrine, methylxanthines, IV corticosteroids Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Methylprednisonlone (IM) 80 mg Single dose. Received placebo capsules for 7 days Methylprednisolone (oral) Tapering dose over 7 days. Total dose: 216 mg Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 216 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/8 day Day 7 Oral group 2/10 Day 7 | |
| United States, Pediatric ED | Provided in ED: SABA Provided at discharge: SABA | Dexamethasone (IM) 0.3 mg/kg Total dose: 15 mg Single dose Prednisone (oral) 2 mg/kg a day for 3 days Total dose: 100 mg | IM Methylprednisone equivalency: 75 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 0/21 Day 5 Oral group 0/21 Day 5 | |
| United States, ED | Provided in ED: inhaled beta₂‐agonists, IV corticosteroids Provided at discharge: SABA | Methylprednisolone (IM) 160 mg Single dose. Received placebo capsules for 8 days Methylprednisolone (oral) Tapering dose over 8 days. Total dose: 160 mg (tapering dose 32 mg day 1). Received placebo injection | IM Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 80 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 13/92 Day 10 Oral group 12/88 Day 10 IM group 17/92 Day 21 Oral group 20/88 Day 21 | |
| Taiwan, ED | Provided in ED: SABA, methylxanthines Provided at discharge: Methylxanthine and inhaled beta₂‐agonists | Dexamethasone (IM) 10 mg Single dose. Received placebo capsules for 7 days Dexamethasone (oral) Tapering dose over 7 days. 11.75 mg total. Received placebo injection | IM Methylprednisone equivalency: 50 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 58.8 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 1/17 Day 7 Oral group 0/19 Day 7 | |
| United States, ED | Provided in ED: SABA, oral/IV corticosteroids Provided at discharge: SABA, antibiotics, ICS, cromolyn sodium, ipratropium bromide | Triamcinolone (IM) 40 mg Single dose. Received placebo capsules for 5 days Prednisone (oral) 40 mg a day for 5 days. Total dose: 160 mg Received placebo injection | IM Methylprednisone equivalency: 40 mg Duration: intermediate half‐life (12 to 36 hours) Oral Methylprednisone equivalency: 160 mg Duration: intermediate half‐life (12 to 36 hours) | IM group 7/78 Day 7 Oral group 11/76 Day 7 | |
| ED = emergency department; SABA = short‐acting beta₂‐agonists; LABA = long‐acting beta₂‐agonists; IV = intravenous; IM = intramuscular; ICS = inhaled corticosteroids | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 2 Relapse intention to treat Show forest plot | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 3 Subgroup analysis: children versus adults Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations Show forest plot | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias Show forest plot | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days Show forest plot | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
| 8 Sensitivity analysis: fixed effects Show forest plot | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| 9 Sensitivity analysis: corticosteroids in ED Show forest plot | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
| 10 Serious adverse events; hospitalization following discharge Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events Show forest plot | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 12 Adverse events: nausea/vomiting/GI distress Show forest plot | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
| 13 Adverse events: insomnia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14 Adverse events: personality changes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15 Adverse events: pain Show forest plot | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
| 16 Adverse events: swelling Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
| 17 Adverse events: redness Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
| 18 Pulmonary function: peak expiratory flow (L/min) Show forest plot | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
| 19 Pulmonary function: FEV₁/FVC (%) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Symptom persistence Show forest plot | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
| 21 Symptom persistence: cough Show forest plot | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
| 22 Symptom persistence: wheezing Show forest plot | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
| 23 24‐hour beta agonist use Show forest plot | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |


