Ecografía del primer trimestre sola o en combinación con pruebas séricas del primer trimestre para la detección del síndrome de Down
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 230 participants Brazil ‐ private centres Dates not specified Pregnant women Mean age 35.8 years (21‐45 years) Singleton pregnancies Karyotyping performed at same time as NT 10‐14 weeks' gestation | |
| Study design | Diagnostic validation study to determine the best ROC cut‐off for NT Retrospective study of patient notes | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: chorionic villus biopsy, amniocentesis or blood or placenta used for fetal karyotyping | |
| Index and comparator tests | NT with cut‐off of 2.5 mm (found to be optimum cut‐off from ROC) (Sequoia, Aspen 128XP10‐Acuson and Toshiba SH140) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To define the best fixed cut‐off point for NT, and the accuracy of this cut‐off for all fetal aneuploidy screening and for trisomy of chromosome 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All patients received a reference standard |
| Differential verification avoided? | No | Women had different reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4130 participants France ‐ single centre May 1994 to December 1997 Pregnant women Mean maternal age 30.1 years (all under 38 years), 86% < 35, 14% ≥ 35 Singleton pregnancies 10‐14 weeks' gestation Crown‐rump length between 38 mm and 84 mm | |
| Study design | Prospective consecutive series study | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: prenatal karyotyping conducted (in 7.6% of patients) depending on presence of risk > 125, high maternal age, parental anxiety, history of chromosomal defects or parental translocation or abnormal second trimester scan age Cytogenetic testing of newborns with suspected abnormalities Postmortem on terminations of pregnancy or miscarriages Follow‐up to neonatal examination in newborn | |
| Index and comparator tests | Maternal age First trimester NT planned at 12‐13 weeks, 3 mm risk cut‐off Second trimester serum hCG between 14 and 17 weeks (Amerlite, Orthoclinical diagnostics machine), cut‐off 1:250 (Prenata software) Second trimester serum AFP between 14 and 17 weeks (Amerlite, Orthoclinical diagnostics machine), cut‐off 1:250 (Prenata software) Serum tests in 3790 women | |
| Follow‐up | Delivery and postnatal paediatric examination 35 lost to follow‐up and excluded from analysis Pregnancy loss in 37 women due to spontaneous abortion (n = 21) or intrauterine death (n = 16) 340 women had first trimester NT but not second trimester serum testing | |
| Aim of study | To compare first trimester NT and second trimester maternal serum measurements as alternative methods of antenatal screening in a low‐risk population and to evaluate the consequence of combining the results in the estimation of risk. | |
| Notes | Women lost to follow‐up are excluded in the final analysis. All antenatally detected cases were terminated. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT was not measured or not recorded in 219 women and these patients were excluded from the study |
| Withdrawals explained? | Yes | 340 women who did not want second trimester serum screening withdrew from that part of the study |
| Clinical features and settings | Women requesting screening (self‐paying service) and women attending on account of previous pregnancy history of fetal abnormality | |
| Participants | 3188 participants Cambridge, UK ‐ Maternity Hospital August 2001‐March 2004 Singleton pregnancies Pregnant women Median age 37 years (19‐46 years) 11‐14 weeks' gestation 45‐84 mm crown‐rump length Viable fetus | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: invasive testing offered to women with NT > 3 mm or risk > 1:250 as defined by combined NT and serum results (chorionic villus sampling from 11 weeks, amniocentesis from 15 weeks). Rapid in situ hybridisation test in patients with risk > 1:30. No details given of any follow‐up to birth | |
| Index and comparator tests | First trimester NT in all women (FMF methods) Second trimester serum biochemistry (AutoDELFIA(TM) time‐resolved fluorimmunoassay (Perkin Elmer)) at 14 weeks. Offered to patients with negative first trimester NT (2725 accepted, 85%) | |
| Follow‐up | Details of follow‐up to birth not given | |
| Aim of study | To determine the detection and false positive rates for trisomy 21 using 2‐stage combined nuchal translucency and triple testing whilst disclosing abnormal NT measurements at the scan | |
| Notes | Women with miscarriages excluded | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | 463 patients having NT did not go on to have serum testing |
| Clinical features and settings | Routine screening | |
| Participants | 10,273 participants with complete screening and outcome data Australia ‐ screening programme, independent ultrasound practices 24‐month period (dates not specified) Pregnant women Mean maternal age 34.9 years (screen positive) and 30.5 years (screen negative) Singleton pregnancies 11‐13 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | NT (FMF protocol) First trimester PAPP‐A and free ßhCG (90.2% by time resolved amplified cryptate emission technology, Kryptor random access immunoassay analyzer, Brahms, 9.8% by manual Ortho Clinical Diagnostic Immunometric I125 immunoassay for PAPP‐A, and Ortho Clinical Diagnostics Vitros ECi automated analyzer for ßhCG) Risk cut‐off 1:300 | |
| Follow‐up | Linkage to data collected by the Midwives Notification System and the Western Australia Birth Defects Registry and by searching laboratory records of all prenatal cytogenetics services in the state. 162 women lost to follow‐up were excluded Pregnancy loss in 54 women due to miscarriage (n = 35), stillbirth (n = 17) and neonatal death (n = 2) | |
| Aim of study | To investigate associations between combined first‐trimester screen result, pregnancy associated plasma protein level and adverse fetal outcomes in women | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 2032 participants with adequate imaging on ultrasound screening Budapest ‐ single centre January 2003 ‐ February 2010 Pregnant women Mean age 36.4 years (15‐46 years) (Down's syndrome) and 29.8 years (15‐49 years) (no Down's syndrome) 11‐20 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 52 cases Reference standards: amniocentesis or CVS (85% of women), or follow‐up to birth | |
| Index and comparator tests | First and second trimester fetal iliac angle (GE Medical System Kretztechnik GmbH & Co OHG, AC2‐5 transabdominal and IC5‐9 transvaginal curved array transducer and Medison Co., LTD EC4‐9ES transvaginal and C3‐7IM transabdominal curved array transducer) Measurement taken from a transverse section of the fetal pelvis Cut‐off angles of 75‐100o | |
| Follow‐up | Followed up to delivery (no cases were detected at birth) | |
| Aim of study | To present results of the sonographic measurement of the fetal iliac angle during the first and second trimesters of pregnancy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Different reference standards used |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | 95.2% had adequate imaging |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1656 participants France ‐ single centre January to December 1995 Singleton pregnancies Pregnant women Mean age 32 years (16‐46 years), 8.3% > 35 years Enrolled before 13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 5 cases Reference standards: amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on 1 or more index test. No details of reference standard for index test negative women | |
| Index and comparator tests | Maternal age NT at 12‐14 weeks (Toshiba SSA 270), risk cut‐point 1:250 First trimester (12‐14 weeks) serum AFP and free ßhCG (Elsa AFP and Elsa free ßhCG; Cis‐Bio International) Second trimester (15‐18 weeks) serum AFP and total hCG (AFP‐2T and hCG‐60; Ortho‐Clinical Diagnostics) All women had NT and serum testing | |
| Follow‐up | Details of follow‐up are not stated. Unclear whether women were followed up to birth. Of the 1656 women, 12 (0.7%) were lost to follow‐up, 2 had miscarriages, 2 had preterm premature ruptures of the membranes and 2 had intrauterine deaths. | |
| Aim of study | To evaluate the sequential combination of ultrasound screening for fetal aneoploidy at 11‐14 weeks with maternal biochemistry at 12‐14 and 15‐18 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 22,746 participants London ‐ 2 antenatal clinics January 2003 ‐ December 2008 Pregnant women Median age 39 years (Down's syndrome) and 34 years (non‐Down's syndrome) 11‐13 and 14‐22 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 106 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | First trimester NT, PAPP‐A and free ßhCG (details not reported) Second trimester AFP, uE3, free ßhCG and inhibin A (details not reported) Results in multiple publications | |
| Follow‐up | Data obtained from the Hospitals, the regional cytogenetic unit and the National Down Syndrome Cytogenetic Register | |
| Aim of study | To determine whether the standard deviation of NT measurements has decreased over time and, if so, to revise the estimate and assess the effect of revising the estimate of the standard deviation on the performance of antenatal screening for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 232 participants (all had NT and serum testing) 32 cases of Down's and 200 randomly selected controls (selected from series of 3731 women) Italy ‐ single centre July 1993 ‐ December 1996 Pregnant women 10 to 13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: CVS or amniocentesis | |
| Index and comparator tests | Maternal age First trimester NT (in longitudinal section of the fetus with caliper measurements to the nearest 0.1 mm) First trimester PAPP‐A (Amerlex‐M PAPP‐A IRMA, Ortho‐Clinical Diagnostics) First trimester free ßhCG (Elsa9free ßhCG CIS) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the potential effectiveness of maternal serum PAPP‐A and free ßhCG in combination with NT measurement in the first trimester of pregnancy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 516 participants London ‐ hospital birth centre Dates not reported Pregnant women Median maternal age 35 years (range 17‐49 years) 11‐13 weeks' gestation 16‐24 weeks' gestation in a sub‐sample of 183 women | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 51 cases Reference standard: CVS | |
| Index and comparator tests | First trimester fetal echocardiography (transabdominally with a 4‐8 MHz curvilinear transducer, Voluson 730 Expert, GE Medical Systems) in all women (425 successfully examined) and in the second trimester in 183 women | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To establish the feasibility of examining the subclavian artery at 11 + 0 to 13 + 6 weeks of gestation and to determine the prevalence of aberrant right subclavian artery (ARSA) in chromosomally normal and abnormal fetuses | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women received the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | 425/516 (82.4%) of women were successfully examined |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 3731 participants Spain October 1999 ‐ December 2002 Pregnant women 10 to 14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: CVS (high‐risk women) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester (10‐14 weeks) Ductus venous Doppler studies First trimester (10‐14 weeks) NT (FMF method) First trimester (10 weeks) serum PAPP‐A and free ßhCG (time‐resolved fluorescent assays, Perkin‐Elmer Life Sciences) Risk cutoffs 1:200, 1:250 or 1:300 DV ‐ Saggital view of quiescent fetus. When optimal record of DV obtained, measured only once. When reversed end diastolic flow present, 3 separated samples obtained. Maximum velocity manually drawn in 3 waveforms and PIV automatically obtained by software linked to equipment | |
| Follow‐up | Details given in Borrel 2004: follow‐up through phone enquiry, contact with attending obstetrician, births defects registry of Barcelona. Cases with missing follow‐up or unknown karyotype excluded from further analysis | |
| Aim of study | To estimate the improvement in screening efficiency when fetal ductus venosus Doppler studies are added to existing first trimester Down's syndrome screening protocols | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth (described in Borrel 2004) |
| Partial verification avoided? | Yes | All patients received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | 4 unaffected pregnancies could not be assessed with NT |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening and high‐risk referral | |
| Participants | 7250 participants: 6940 women undergoing routine screening (October 1999 ‐ December 2006) 310 women referred for CVS (October 1999 ‐ December 2007) Barcelona ‐ hospital clinic Pregnant women Mean maternal age 32 years 10‐13 and 15‐20 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 66 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT and ductus venosus pulsivity index (DVPI) (transabdominal ultrasound, Eccocee SSA and Power‐Vision 400, Toshiba Medical Systems, Voluson PRO, General Electrics Healthcare) First trimester PAPP‐A and free ßhCG (details not reported) Second trimester AFP, uE3, free ßhCG and inhibin A (details not reported) | |
| Follow‐up | From hospital clinic records, telephoning women or from the attending obstetrician. Obtained in 97.4% of pregnancies | |
| Aim of study | To assess the value of ductus venosus blood flow (expressed as pulsatility index, DVPI) in antenatal Down's syndrome screening when used with the combined and integrated tests | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population and selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Ductus venosus measurements were not obtained in 3.3% of pregnancies |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 22,280 participants with complete screening results and outcome data August 2001 ‐ October 2003 Australia ‐ State‐wide screening programme evaluation Pregnant women Median maternal age 31 years (range 14‐47 years), 20% ≥ 35 Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester PAPP‐A, free ßhCG and NT (details not reported) Risk cut‐point 1:300 | |
| Follow‐up | Data on outcome from the Western Australia Midwives data collection, Birth Defects Registry and hospital morbidity and mortality data | |
| Aim of study | To identify first trimester indicators of adverse pregnancy outcomes | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐ up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2996 participants Brazil ‐ University Hospital Estimated date of delivery pre December 1999 Pregnant women Median age 28 years (13‐46 years), 19.4% ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation (mean 12 weeks) | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: antenatal karyotyping (5.9% of pregnancies: 62% of high‐risk, 29% of medium‐risk and 3% of the low‐risk women) or follow‐up to birth (85.3% of women) | |
| Index and comparator tests | Maternal age First trimester (10‐14 weeks) NT Risk cut‐off 1:300 | |
| Follow‐up | 85.3% of women were followed up to birth. Of these, 65 were spontaneous miscarriages or intrauterine death with no karyotyping | |
| Aim of study | To assess the detection rate of chromosomal abnormalities using NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk patients undergoing routine screening | |
| Participants | 408 participants Italy Dates not reported Pregnant women Singleton pregnancies Aged ≥ 35 years (range 35‐44 years) 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 6 cases Reference standards: amniocentesis in women high risk on screening (16.2%) or follow‐up to birth in women who were low risk on screening | |
| Index and comparator tests | Maternal age NT with cut‐point 3 mm Serum free ßhCG (Schering RIA) and PAPP‐A (Chematil ELISA) Risk score cut‐point 1:250 | |
| Follow‐up | Follow‐up at birth in all by collaboration with mothers Women who miscarried were excluded from the study | |
| Aim of study | To evaluate the combined test of NT, serum markers and age in pregnant women 35 years of age and over to detect Down's syndrome | |
| Notes | No live births were Down's syndrome. All detected cases were terminated. 7 women were excluded due to miscarriages | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2131 women with 2339 fetuses New York ‐ single centre April 2000 to November 2002 Pregnant women Singleton or multifetal pregnancies Median age 33 years (interquartile range 31‐36), 36.2% ≥ 35 years | |
| Study design | Prospective consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth in 96.1% of patients | |
| Index and comparator tests | Maternal age NT (FMF methods) Combined risk score cut‐point 1:300 Each fetus with a separate chorion was considered individually when calculating the performance of NT but for monochorionic twins, only the fetus with the higher risk calculation was included | |
| Follow‐up | Attempted to obtain results for cytogenetic testing following miscarriage or termination or where Down's suspected at birth. Karyotype results or documented evidence of phenotypically normal baby was recorded in 96.1% of patients | |
| Aim of study | To examine the detection rate of chromosomal abnormalities using a combination of nuchal translucency and maternal age | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | No | Reference standard results were available for only 96% of patients |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | 19 patients could not be imaged |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 242 participants: 22 cases and 220 randomly selected controls China ‐ hospital screening programme August 2003 ‐ March 2007 Pregnant women Median maternal age, cases 30 years (20‐44 years) and controls 32 years (19‐40 years) 12‐14 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 22 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | First trimester frontomaxillary facial (FMF) angle (transabdominal ultrasound, ATL HDI 5000, Philips Medical Systems or Voluson 730 Pro, GE Medical systems, by clinicians accredited by the FMF) Measured with a protractor from printed and filed images Angle > 95th percentile taken as positive test result | |
| Follow‐up | Pregnancy outcome obtained from obstetric and neonatal files | |
| Aim of study | To evaluate the measurement of FMF angle at 11‐13 weeks, 6 days in a Chinese population and its applicability in screening for fetal trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Unclear | Only the most optimal images were included in the study and the proportion of images that were not included is not stated |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Screening programmes for syphilis and Down's syndrome | |
| Participants | 108 participants (27 cases of Down's syndrome, 81 controls) Denmark ‐ Statens Serum Institute Dates not specified Pregnant women 5‐11 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 27 affected cases (18 diagnosed in 2nd trimester, 9 at birth) Reference standard: karyotyping | |
| Index and comparator tests | Maternal age First trimester (week 11‐14) NT Frozen samples tested for: First trimester (week 5‐11) inhibin A (dimer assay kit MCA 950KZZ, Serotec) First trimester (week 5‐11) ßhCG (available for some samples) First trimester (week 5‐11) PAPP‐A (available for some samples) (combined PAPP‐A and ßhCG TrIFMA assay) Risk cutpoints of 1:100, 1:250 and 1:400 Performance assessed with SPlus algorithm | |
| Follow‐up | All diagnosis were verified by karyotyping | |
| Aim of study | To investigate whether inhibin A can be used in the first trimester for Down's syndrome screening | |
| Notes | Identified through the Danish central cytogenetic registry as part of quality assurance programme | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 335 participants: 74 cases and 261 controls matched for length of sample storage and maternal age Denmark ‐ screening programme Dates not reported Pregnant women Singleton pregnancies Median maternal age cases 37.5 years and controls 36.4 years 8‐13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 74 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (details not reported) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (AutoDelfia, PerkinElmer, Turku or Kryptor, Brahms) Frozen serum samples tested for: First trimester placental growth hormone (double monoclonal ELISA, DSL‐10‐19 200, Diagnostic Systems Laboratory Inc) Growth hormone binding protein (enzyme‐amplified ELISA, DSL‐10‐48 100, Diagnostic Systems Laboratory Inc) | |
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry | |
| Aim of study | To examine the potential of placental growth hormone and growth hormone binding protein as maternal serum screening markers for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 531 participants: 28 cases and 503 controls Denmark ‐ screening programme Dates not specified Pregnant women Singleton pregnancies Median age cases 36 years (range 25‐44 years) and controls 29 years (range 17‐45 years) 8‐14 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 28 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (details not reported) First trimester PAPP‐A and free ßhCG (details not reported) First trimester ADAM12s (AutoDELFIA/Delfia ADAM12 Research kit 4025‐0010, PerkinElmer Life and Analytical Sciences, on the 1235 AutoDELFIA automatic immunoassay system) | |
| Follow‐up | Cross‐referencing with the Danish Cytogenetic Central Registry | |
| Aim of study | To examine the efficiency of a second generation assay for ADAM12 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 970 fetuses (20 twin and 1 triplet pregnancy) UK Dates not specified Pregnant women Median age 37 years (16‐48 years) 11‐14 weeks' gestation (median 12 weeks) | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 88 cases Reference standard: CVS | |
| Index and comparator tests | Maxillary bone length Mid‐saggital view of fetal profile obtained for nasal bone. Transducer angled laterally so that the maxillary bone and mandible including the ramus and condylar process can be seen. Maxillary length measured with callipers. Magnified to 0.1 mm increment | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To determine the value of measuring maxillary length at 11‐14 weeks' gestation in screening for trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | CVS |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Study reports that measurements were made successfully in all cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 20, 418 participants UK ‐ Fetal Medicine Centre October 2001‐2004 Pregnant women Singleton pregnancies Median age 35 years (18‐50 years) 11‐13 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 140 cases Reference standards: CVS or amniocentesis in high‐risk women, or follow‐up to birth | |
| Index and comparator tests | Maternal age Presence of nasal Bone (FMF methods) First trimester NT (FMF methods) First trimester serum free ßhCG (Kryptor analyser, Brahms AG) First trimester serum PAPP‐A (Kryptor analyser, Brahms AG) | |
| Follow‐up | Data on pregnancy outcome from cytogenetics laboratory and by letters and telephone calls to patients, GPs and maternity units 656 patients excluded because karyotype was not known due to miscarriage (n = 185), termination of pregnancy (n = 85) or loss to follow‐up (n = 386) | |
| Aim of study | To investigate the impact of incorporating assessment of the nasal bone into first trimester combined screening by fetal nuchal translucency thickness and maternal serum biochemistry | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Reported that fetal NT and serum markers were successfully measured in all cases |
| Withdrawals explained? | Yes | Patients lost to follow‐up reported |
| Clinical features and settings | Routine screening | |
| Participants | 18,901 participants Australia ‐ South Australian Maternal Serum Antenatal Screening Program Dates not reported Pregnant women Median age 31.3 years Maternal and gestational age not reported | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 66 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT PAPP‐A and free ßhCG (details not reported) | |
| Follow‐up | Details not reported | |
| Aim of study | To compare different screening strategies for the detection of Down's syndrome and to consider the practical implications of using multiple screening protocols | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 57,057 participants June 1998 ‐ July 2007 UK ‐ 6 Hospitals Pregnant women Singleton pregnancies Mean age: Down's syndrome 38 years (range 16‐49 years) and healthy 29 years (range 13‐56 years) 10‐14 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 723 cases (307 from original cohort and 416 supplemented cases screened at the Fetal Medicine centre or Harris Birthright Research Centre for Fetal Medicine) Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Rick cut‐point 1:300 | |
| Follow‐up | Birth data collected at birth by the delivering hospital and stored in several databases which were merged. Only women with full records for screening and birth outcome included in the study | |
| Aim of study | To investigate if fetal sex has an impact on first trimester combined screening for aenuploidy | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 445 participants: 70 cases and 375 controls matched for storage time and gestational age January 2007 ‐ October 2008 UK Pregnant women Singleton pregnancies Mean maternal age cases 37.0 years (IQR 32.9 to 40.5 years) and controls 32.4 years (IQR 29.0 to 35.9 years) 11‐13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 70 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Frozen serum samples tested for: First trimester placental growth factor (Solid‐phase, 2‐site fluoroimmunometric research assay (4083‐0010) on 6000 DELFIA Xpress random access platform, PerkinElmer) | |
| Follow‐up | Karyotype and results for pregnancy outcome received from cytogenetics laboratories and maternity units where deliveries took place | |
| Aim of study | To examine placental growth factor levels in first trimester maternal serum in trisomy 21 pregnancies and to investigate the potential value of PIGF in a first trimester screening test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 17,229 participants UK ‐ 15 centres Dates not specified Pregnant women Median age 29.9 years, 15.4% ≥ 35 years 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 45 cases Reference standards: CVS (offered where women had high NT measurements), amniocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age NT (FMF method) in 73% of patients Clotted blood samples tested for: Free ßhCG and PAPP‐A (Kryptor analyser) in 98.4% of patients | |
| Follow‐up | Reported that the outcome of all pregnancies was followed up | |
| Aim of study | To evaluate the use of NT measurement in combination with biochemical markers as a first trimester test for Down's syndrome in routine antenatal setting | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Report average success rate of NT (72.9%) |
| Withdrawals explained? | Yes | Numbers of patients not undergoing NT and biochemical testing given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 292 participants (207 participants before 14 weeks' gestation) The Netherlands ‐ single centre 19 84‐1997 Pregnant women Cases: 37 with Down's syndrome Controls: 255 matched 5:1 with cases for maternal age (within 2 years), gestational age (within 2 weeks) and duration of sample storage (within 2 months) 9‐15 weeks' gestation (in a few cases, blood samples for serum testing taken at 15‐19 weeks) | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 37 cases (24 affected pregnancies in women with NT testing enrolled before 14 weeks' gestation) Reference standards: CVS and amniocentesis | |
| Index and comparator tests | Maternal age NT (FMF methods) with cut‐off > 3 mm Frozen serum samples tested for: First trimester free ßhCG and AFP (DELFIA dual labelled time resolved fluorescent assay) First trimester serum PAPP‐A (DELFIA research assay (CR61‐105)) First trimester serum AFP | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To determine the expected detection rate and false positive rate for Down's syndrome achievable by early pregnancy screening with combined measurements of serum PAPP‐A, free ßhCG and fetal nuchal translucency, with the addition of AFP | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | Yes | All women had karyotyping |
| Incorporation avoided? | Yes | Index test did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted without knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | In 11 controls, failed to measure NT |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 95,645 participants (40,815 in 2005 and 54,830 in 2006) Denmark ‐ 19 obstetrics and gynaecology departments January 2005 ‐ December 2006 Pregnant women Maternal and gestational age not reported First trimester | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 225 cases (121 in 2005 and 104 in 2006) Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (by nurses, midwives and doctors in accordance with FMF guidelines) First trimester PAPP‐A and free ßhCG (Brahms Kryptor, Brahms Immunodiagnostic Systems or Delfia Xpress, PerkinElmer) Risk cut‐point 1:300 | |
| Follow‐up | Information obtained from the Danish central cytogenetic registry. No details of follow‐up for women without pre or post‐natal chromosome analysis | |
| Aim of study | To evaluate the impact of a screening strategy in the first trimester, introduced in Denmark during 2004 to 2006, on the number of infants born with Down's syndrome and the number of CVS and amniocentesis, and to determine detection and false positive rates in the screened population in 2005 and 2006 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Information given on the proportion of women not undergoing screening |
| Clinical features and settings | Routine screening | |
| Participants | 21,959 participants Germany, Switzerland and Austria ‐ multicentre study June 1995‐May 2000 Pregnant women Median age 33 years (15‐49 years), 36.1% > 35 years Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 210 cases Reference standards: CVS, amniocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age NT (FMF methods) Risk cut‐points of 1:100 and 1:300 | |
| Follow‐up | Follow‐up in 92.2% of women. Loss to follow‐up was due to miscarriage (n = 258), termination of pregnancy (n = 125) or absence of antenatal karyotyping (n = 1463). Only those with follow‐up information included in the study | |
| Aim of study | To examine the effectiveness of screening for Down's syndrome using age and NT at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Reported that NT successfully measured in all cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4097 participants with complete data on pregnancy outcome Germany ‐ single examiner December 1997 ‐ November 2006 Pregnant women Singleton pregnancies Median age 35.1 years (range 13.2‐46.7 years) 11‐13 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 34 cases Reference standards: Karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Mixture model, Delta NT and multiple of the median methods | |
| Follow‐up | Patient history and ultrasound results were entered into a database and pregnancy outcome or chromosomal results added as they became available 74 (1.8%) of women were excluded from the study because of incomplete follow‐up information | |
| Aim of study | To validate the mixture model in a single operator dataset and to compare the detection rates for fetal chromosomal defects obtained from the mixture model with those obtained from either the delta NT or log multiple of the median approach | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1759 participants The Netherlands ‐ private practice (VU medical centre) May 2001‐October 2003 Pregnant women 49% ≤ 35 years, 51% ≥ 36 years 9‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: Invasive testing or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods using own medians) First trimester PAPP‐A and free ßhCG (ELIPS Perkin Elmer, Finland) | |
| Follow‐up | Follow‐up data from medical records and patient reports. Data from 242 patients (12%) were not available and these patients were excluded from the study. | |
| Aim of study | To determine the diagnostic value of the combination screening test for Down's syndrome in the first trimester of pregnancy | |
| Notes | Dutch language | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Incomplete investigation reported in 25 patients (1.2%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 13,267 participants (13,207 participant received both NT test and serum testing) Belgium ‐ multicentre study (35 centres) Data from January 2004‐April 2004 added to previous database from before 2003 Pregnant women First and second trimester testing | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standards: CVS, amniocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester PAPP‐A (ELISA 2397, DRG International Inc) and free ßhCG (IRMA K1P1001) Second trimester PAPP‐A and free ßhCG Risk cut‐points of 1:200 and 1:300 | |
| Follow‐up | Follow‐up to birth reported by mail by obstetricians. Non‐responding obstetricians contacted personally to obtain missing data. Results of follow‐up reported by mail by obstetricians. Non‐responding obstetricians contacted personally to obtain missing data Cases of miscarriages (n = 49) and other fetal chromosomal abnormalities excluded from the study. Unclear if other patients lost to follow‐up | |
| Aim of study | To evaluate the performance of a first trimester fetal aneuploidy screening programme | |
| Notes | Women with miscarriages or cases of other chromosomal defects were excluded from the study. 9 live births of babies with Down's syndrome | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Numbers of women excluded due to miscarriage or other chromosomal defects and numbers not undergoing NT and biochemical testing reported. |
| Clinical features and settings | Routine screening | |
| Participants | 1507 participants UK ‐ fetal medicine unit September 2007 ‐ December 2008 Pregnant women Median maternal age 35.4 years (range 18‐49 years) 9‐10, 11‐13 and > 14 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age Early first trimester PAPP‐A (9 weeks' gestation) (AutoDELFIA PAPP‐A kit, PerkinElmer LAS (UK) Ltd) First trimester NT (11‐13 weeks' gestation) (General Electric E8, Voluson 730 Pro, GE Healthcare) Second trimester AFP, free ßhCG and uE3 (at or after 14 weeks' gestation) (AutoDELFIA(TM) time‐resolved fluorimmunoassay, PerkinElmer Life Sciences) Second trimester tests given if first trimester risk low (< 1:100) or invasive testing declined Cut‐point for second‐stage risk 1:250 | |
| Follow‐up | Data recorded on a fetal medicine database and combined with data held on separate databases for pregnancy outcome and the regional cytogenetic laboratory. Cytogenetic test results available for all women delivering in the region | |
| Aim of study | To audit a model combining early PAPP‐A with NT and early triple test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 10,436 participants receiving both NT and serum testing and with complete follow‐up data Australia Data from 2‐year period (dates not specified) Pregnant women Mean age 30.7 years, 21.2% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: CVS, amniocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Clotted blood samples tested for: First trimester PAPP‐A (Kryptor random access immunoassay analyser or manual Ortho Clinical Diagnostics Immunometric I125 immunoassay) First trimester free ßhCG (Kryptor random access immunoassay analyser or Ortho Clinical Diagnostics Vitros ECi automated analyser) Risk cut‐point 1:300 | |
| Follow‐up | Data obtained from WA Midwives notification system and WA Birth defects registry. Missing information sought from referring doctor and ultrasound practice. Data linkage achieved in 10,436 (99.6%) of patients In index test negative patients, outcome for 160 women not known In index test positive patients, outcome in 2 women not known | |
| Aim of study | To audit the initial 2 years of conduct of the combined first trimester screening | |
| Notes | Women with miscarriages or multiple pregnancies were excluded from the study | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4233 participants Austria ‐ single hospital June 1993 to July 1996 Pregnant women Median age 28 years (15‐49 years), 6.9% ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 7 cases Reference standards: amniocentesis or CVS in patients with previous Down’s pregnancy, > 35 years or with a positive biochemical test result. Other women underwent scan at 22 weeks and, if NT > 2.5 mm special examination directed to examination of fetal heart. Follow‐up to birth | |
| Index and comparator tests | First trimester NT (cut‐off 2.5 mm) NT taken in saggital section. Distance between the end of the echogenic muscles of the c spine and the inner layer of echogenic skin with callipers on the line | |
| Follow‐up | No details given of methods of follow‐up. 138 women lost to follow‐up | |
| Aim of study | To determine the value of NT measurement for the detection of aneuploidies and other malformations in a low‐risk population | |
| Notes | It appears that Down’s syndrome was only picked up in cases where CVS or amniocentesis had been conducted and it s not clear if patients were followed up to birth | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Unclear | Amniocentesis or anomalies scan at 22 weeks. Unclear if women were also followed up to birth. |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT measurement was not possible in 2% of cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1807 participants with successful scans Turkey September 2003 ‐ December 2005 Pregnant women Singleton pregnancies Median maternal age 28.3 years (range 17‐45 years) 11‐14 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 9 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester fetal nasal bone (experienced maternal fetal specialists) First trimester PAPP‐A and free ßhCG (details not reported) Combined cut‐point 1:300 | |
| Follow‐up | Findings recorded in a computer database. Karyotype results obtained directly from the genetics department. Pregnancy outcomes obtained from hospital records or from parents via telephone interview. 110 women (5%) with terminations, miscarriages or malformations and unknown outcome were excluded from the study | |
| Aim of study | To evaluate the contribution of nasal bone assessment in first trimester Down's syndrome screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Evaluation of nasal bone was not possible in 9 (0.5%) cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 1306 women with 1317 fetuses (11 sets of twins) Australia ‐ 2 hospitals and 2 private practices September 1993 to September 1994 Pegnant women Singleton or multifetal pregnancies Median age 37 years (21‐48 years) 10 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 21 cases Reference standard: CVS | |
| Index and comparator tests | First trimester NT (ATL HDI ESP Diagnostic Ultrasound system), cut‐point 3 mm or more | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the accuracy of ultrasound measurement of nuchal thickness in first trimester fetuses for predicting fetal karyotype | |
| Notes | No measurement of NT was recorded in 126 cases (9.6%). All down’s syndrome fetuses terminated | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | CVS |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | No measurement of NT was recorded in 126 cases (9.6%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 6508 participants with known fetal outcome Germany ‐ 3 prenatal health centres August 1999 ‐ May 2007 Pregnant women Mean maternal age 31.1 years (16‐46 years), 22% ≥ 35 years First trimester | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 40 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF standards) First trimester PAPP‐A and free ßhCG (no details given) Different software programmes used with and without modification to exclude the role of maternal age | |
| Follow‐up | Methods of follow‐up not reported. Stated that only women with known fetal outcome were included in the study | |
| Aim of study | To analyse the impact in test performance of 3 widely used first trimester screening software programs if the maternal age was excluded from their calculation algorithm | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 7118 participants undergoing combined first trimester screening and a fetal abnormality scan Taiwan ‐ single hospital January 2004 ‐ December 2007 Pregnant women Median maternal age 30 years (range 15‐47 years) 8‐13 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 25 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (11‐13 weeks' gestation) (FMF accredited obstetricians) First trimester free ßhCG and PAPP‐A (8‐12 weeks' gestation) (time resolved amplified cryptate emission, automated Kryptor Analyser, Brahms) Combined cut‐point 1:300 Second trimester fetal abnormality scan (18‐22 weeks' gestation) for intracardiac echogenic focus (ICEF) (In accordance with the American Institute of Ultrasound in Medicine Practice Guideline) | |
| Follow‐up | All neonates examined postnatally and Hospital records reviewed | |
| Aim of study | To determine the relation between intracardiac echogenic focus and trisomy 21 in a population of fetuses previously evaluated by first trimester combined screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 16,153 participants Australia ‐ State screening programme February 2000 ‐ June 2002 Pregnant women Mean maternal age 33 years (range 16‐51 years), 18.5% ≥ 37 years 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 63 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF accredited ultrasonologists) First trimester PAPP‐A and free ßhCG (details not reported) First trimester AFP, inhibin A and uE3 added to first trimester results for women who were screened at 13 weeks' gestation (augmented screening, number not reported) | |
| Follow‐up | Probabilistic record linkage was used to link health records from the Genetic Health prenatal screening database, Perinatal Data Collection Unit and the Birth Defects Register. Written requests for pregnancy outcome were sent to referring health professionals. Pathology and cytogenetics reports were collected for confirmation of birth defects and/or karyotype 151 women were lost to follow‐up and these were excluded in the analysis Of the 16,003 women, pregnancy loss in 71 due to miscarriage (n = 68), stillbirth (n = 1) and neonatal death (n = 2) | |
| Aim of study | To follow up and evaluate the state‐wide first trimester combined screening programme for Down's syndrome and trisomy 18 at Genetic Health Services Victoria, Australia | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 38,584 participants Australia ‐ State screening programme 2003 ‐ 2004 Pregnant women Maternal age ≥ 37 years in 16.3% of women First and second trimester | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 110 cases Reference standards: karyotyping (CVS = 774, amniocentesis =1644) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT, PAPP‐A and free ßhCG (n = 38,584) (details not reported) | |
| Follow‐up | Probabilistic record linkage was used to link health records from the Prenatal Screening Database, prenatal diagnostic data from cytogenetic laboratories, the Victoria Birth Register (Perinatal Data collection Unit) and the Victoria Birth Defects Register | |
| Aim of study | To map prenatal screening and diagnostic testing pathways in Victorian pregnant women during 2003‐2004; measure the impact of prenatal diagnostic testing uptake on the effectiveness of prenatal screening for Down's syndrome; and assess factors influencing uptake of diagnostic testing following screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Invalid results obtained for 7.4% of first and 0.1% of second trimester screenings |
| Withdrawals explained? | Yes | 48% of pregnant women in the state did not undergo prenatal testing |
| Clinical features and settings | Routine screening | |
| Participants | 56,954 participants with available outcome data UK ‐ multicentre July 1999 ‐ April 2007 Pregnant women Singleton pregnancies Mean maternal age 35.4 years (range 14.1 to 52.2 years) 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 395 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT First trimester fetal heart rate (pulsed‐wave Doppler) First trimester nasal bone (FMF certified sonographers) First trimester ductus venous flow (FMF certified sonographers) First trimester flow across tricuspid valve (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Kryptor, Brahms AG or Delfia Express, Perkin Elmer) Multiple publications with different test evaluations | |
| Follow‐up | Karyotype results and details of pregnancy outcome added to databases as they became available. Women without complete screening and outcome data (n = 3053, 5.1%) were excluded from the study | |
| Aim of study | To examine the performance of first‐trimester screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency thickness, fetal heart rate and maternal serum free ßhCG and PAPP‐A Other objectives in related publications | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2570 participants with available outcome data Korea ‐ hospital and womens healthcare centre January 2001 to December 2001 Pregnant women Mean age 29.9 years (SD 3.3 years) Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standard: amniocentesis or CVS in 419 patients considered high risk (NT > 2.5, aged > 35 years, positive biochemical test result, history of chromosomal abnormality, fetal structural abnormality at ultrasound or other reason). Follow‐up to birth | |
| Index and comparator tests | First trimester NT (FMF methods) (HDI 3000, ATL, Bothell, WA, USA) 3 measurements taken, largest one used for risk calculation Cut‐off 2.5 mm, 3.0 mm or 95th percentile of each CRL | |
| Follow‐up | Pregnancy outcomes ascertained from obstetric and neonatal medical records of live or stillborn babies Only patients with known pregnancy outcome included in the study 8 patients who terminated their pregnancies because of structural abnormalities on ultrasound with no karyotyping results were excluded. Karyotyping was performed in intrauterine fetal death (n = 4) cases | |
| Aim of study | To determine the value of NT with different cut‐offs for the detection of chromosomal aberrations | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 998 participants: 151 cases and 847 controls matched for gestational age, maternal weight, maternal age and storage time The Netherlands ‐ National institute for Public Health and the Environment 2004 ‐ 2006 Pregnant women Singleton pregnancies Median maternal age 37 years (interquartile range 36‐39 years) 8‐13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 151 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT Fresh serum samples tested for: First trimester free ßhCG and PAPP‐A (AutoDELFIA, PerkinElmer) Frozen serum samples tested for: First trimester ADAM 12s, total hCG, placental protein 13 (PP13) and placental growth factor (PlGF) (AutoDELFIA or DelfiaXpress, PerkinElmer) | |
| Follow‐up | Pregnancy outcome was recorded via questionnaires and self‐reporting by the participating women. Only samples for pregnancies with known outcome were selected as controls | |
| Aim of study | To evaluate the modelled predictive value of 3 current screening markers (PAPP‐A, free ßhCG and NT) and 4 potential screening markers (ADAM 12, total hCG, PP13 and PIGF) for Down's syndrome using different screening strategies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine referral | |
| Participants | 6906 participants with complete outcome data Germany ‐ gynaecologists practices January 2000 ‐ December 2003 Pregnant women Median maternal age 32 years (15‐48 years), 26.4% ≥ 35 years 11‐14 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases in gynaecologists practices Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified gynaecologists) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 | |
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (36%) were excluded from the study | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | 146 women (including 11 with down's syndrome) excluded as results could not be assigned to gynaecologists' or prenatal centre group |
| Clinical features and settings | Routine referral | |
| Participants | 3862 participants with complete outcome data Germany ‐ tertiary level prenatal centres January 2000 ‐ December 2003 Pregnant women Median maternal age 34 years (range 14‐46 years), 43.2% ≥ 35 years 11‐14 weeks' gestation | |
| Study design | Cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 | |
| Follow‐up | Data on pregnancy outcome were obtained by contacting the patient or their general gynaecologist. Women without complete outcome data (8%) were excluded from the study | |
| Aim of study | To evaluate and compare the screening performance for fetal trisomy 21 in the first trimester of pregnancy in general gynaecologists practices and specialised centres for prenatal care in Germany | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | 146 women (including 11 with down's syndrome) excluded as results could not be assigned to gynaecologists' or prenatal centre group |
| Clinical features and settings | Routine screening | |
| Participants | 10,251 participants USA September 1995 to June 1998 Pregnant women 34.7% ≥ 35 years Singleton pregnancies No diabetes 9‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 50 cases (33 had undergone biochemical testing) Reference standards: not reported | |
| Index and comparator tests | Maternal age First trimester NT in 5,809 (2018 ≥ 35 years).patients (FMF methods) Dried blood samples tested for: First trimester free ßhCG and PAPP‐A in 10,251 patients (enzyme‐linked immunosorbent asay procedures) | |
| Follow‐up | No details of follow‐up reported | |
| Aim of study | To assess the effectiveness of free ßhCG, PAPP‐A and NT for first trimester screening for Down's syndrome and trisomy 18 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Unclear | Unclear reference standard |
| Partial verification avoided? | Unclear | Unclear if all patients had a reference standard |
| Differential verification avoided? | Unclear | Unclear if choice of reference depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 3907 participants Sweden 2005 ‐ 2006 Pregnant women 51% of women aged ≥ 35 years 9‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 29 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF trained sonographers) First trimester free ßhCG and PAPP‐A (AutoDELFIA, PerkinElmer) | |
| Follow‐up | The dataset used contained outcomes for all pregnancies | |
| Aim of study | To provide the necessary mathematical formulae to construct a risk calculation package for Down's syndrome using maternal serum free ßhCG, PAPP‐A and NT measurements in the first trimester for use in a web‐based system | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 27,291 participants: 223 cases and 22,157 controls (not matched) The Netherlands ‐ The Dutch National Institute for Public Health and the Environment Dates not specified Pregnant women Maternal age not reported 8‐13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 223 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF trained sonographers) First trimester free ßhCG and PAPP‐A (automated dissociation‐enhanced lanthanide fluorescent immunoassay, AutoDELFIA, PerkinElmer) | |
| Follow‐up | Known outcomes for cases and controls | |
| Aim of study | To estimate the effect of timing of serum collection on screening performance | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 16,237 participants Hong Kong ‐ multicentre study 1997 to 2000 Pregnant women Mean age 30.5 years (19% ≥ 35 years) (unaffected pregnancies) 10‐14 weeks and 15‐18 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 35 cases Reference standards: women considered high risk offered CVS (0.7%) or amniocentesis (11.8%). Follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Second trimester free ßhCG and AFP (methods not stated) (All women underwent both NT and biochemical testing) | |
| Follow‐up | By review of hospital and laboratory records and by directly telephoning women. Participants who defaulted the second trimester serum tests (n = 1015) and those who miscarried after NT but before serum testing (n = 91) were excluded from the study. Outcome obtained in only 15,253 patients (93.9%) | |
| Aim of study | To report data on participants undergoing both first and second trimester methods of screening to assess the relative efficacy of different methods of screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | No | Not all women received a reference standard (6.1% had no ascertainment of pregnancy outcome) |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT successful in 99.8% of cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 10,185 participants (178 twin pregnancies; 10,363 fetuses) Hong Kong ‐ University Hospital June 2003 ‐ March 2007 Pregnant women Singleton or multifetal pregnancies Median maternal age 32 years (IQR 30‐35 years), 27.4% of women aged ≥ 35 years 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 34 cases Reference standards: amniocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF accredited doctors, HDI 5000 or HDI 3000, Philips Medical System) First trimester nasal bone assessment (7925 women) (FMF accredited doctors, HDI 5000 or HDI 3000, Philips Medical System) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms Diagnostica GmbH) Risk cut‐point 1:300 For twin pregnancies, a risk was calculated for each fetus based on the individual NT and maternal serum biochemistry corrected for twin pregnancies | |
| Follow‐up | Specific staff were allocated to contact all women for pregnancy and fetal outcome. Women were contacted by phone and mail. 5 screen positive and 50 screen negative cases had unknown outcome. | |
| Aim of study | To examine the effectiveness of first trimester fetal trisomy 21 screening using a combination of maternal age, NT and maternal serum free ßhCG and PAPP‐A levels in a predominantly Chinese population in Hong Kong | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Nasal bone status could not be determined in 176 women (2.2%) (2 with Down's syndrome) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 18,965 pregnancies UK ‐ University Hospital July 1998 ‐ January 2004 Maternal age not reported 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 37 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) Risk cut‐point 1:300 | |
| Follow‐up | Information on birth outcome from Harris birthright Research Centre database, the North East Regional Cytogenetic Laboratory, the National Down's syndrome register and the Basildon and Thurrock University Hospital database and, in some cases, maternal and paediatric records. For each case, screening results were linked to cytogenetic results/pregnancy outcome | |
| Aim of study | To evaluate NT scans with a view to comparing findings with other research centres | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 227 participants UK ‐ single centre Pregnant women Singleton pregnancies Median maternal age 35 years (17‐49 years) 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 20 cases Reference standard: CVS | |
| Index and comparator tests | First trimester presence of mitral gap (Doppler flow traces) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To investigate the possible association between a particular pulsed Doppler waveform pattern, mitral gap and trisomy 21 at 11 + 0 to 13 + 6 weeks | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | Choice of reference standard did not depend on index test results |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 19,614 participants with complete screening and outcome data UK ‐ multicentre January 2006 ‐ May 2007 Pregnant women Singleton pregnancies Median maternal age 34.5 years (14.1‐50.1 years) 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 122 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT and fetal heart rate First trimester ductus venous blood flow velocity waveforms (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (Delfia Xpress, PerkinElmer) | |
| Follow‐up | Karyotype results and details on pregnancy outcome were added to the database as soon as they became available. Women without complete outcome data (5.3%) were excluded from the study | |
| Aim of study | To investigate the performance of first trimester screening for aneuploidies by including assessment of ductus venosus flow in the combined test of maternal age, fetal NT thickness, fetal heart rate and serum free ßhCG and PAPP‐A | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 6324 participants USA ‐ multicentre study (15 centres) May 2002 to December 2002 Pregnant women Mean age 30.1 years (16‐47 years), 22.1% ≥ 35 years Singleton pregnancies 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases Reference standards: karyotyping in 587 (amniocentesis n = 510; neonatal cord blood n = 41; products of conception and autopsy material n = 31), or follow‐up to birth | |
| Index and comparator tests | Nasal bone imaging Fetal image in a perfect saggital plane with fetal spine down. Angle of insonation of ultrasound beam with fetal profile close to 45 degrees. Image magnified significantly until 2 echogenic lines are visible in region of fetal nose. Transducer tilted from side to side to distinguish fetal skin from nasal bone. Deeper echogenic line noted to become more echolucent at its distal end | |
| Follow‐up | A tracking programme with up to 10 contact options for each patient used for follow‐up Follow‐up to birth in 6228 patients (98.5%) and adequate nasal bone imaging in 4801 (75.9%) | |
| Aim of study | To evaluate first trimester nasal bone imaging as a screening tool for aneuploidy | |
| Notes | Only 17% of patients who had miscarriage or termination of pregnancy had karyotype information available | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | No | Not all women received a reference standard (1.5% had no ascertainment of pregnancy outcome) |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Nasal bone screening successful in 4801 cases (75.9%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 38,033 participants USA ‐ multicentre study (15 centres) October 1999 to December 2002 Pregnant women Mean maternal age 30.1 years (SD 5.8 years.); 8199 (21.6%) aged ≥ 35 years Singleton pregnancies Live fetuses 10‐13 and 15‐18 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 92 cases Reference standards: amniocentesis offered to women with positive results from any screening test or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT in 36,306 patients (92.9%) First trimester PAPP‐A and free ßhCG in 37,843 patients (99.5%) Second trimester AFP, total hCG, uE3 and inhibin A in 35,236 patients (92.6%) All data in 33,546 patients (88.2%) | |
| Follow‐up | Follow‐up with computerised tracking system. Medical records were reviewed in cases of 1) possible medical problem suspected 2) positive screening test results with no karyotype data, 3) 10% random sample of all enrolled patients Follow‐up to birth in 36,378 patients (97%) | |
| Aim of study | To evaluate first trimester and/or second trimester screening tool for Down's syndrome | |
| Notes | Unclear which types of patients did not have follow‐up data. Appears that aborted/miscarried fetuses did not have follow‐up | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | No | Not all women received a reference standard (3% had no ascertainment of pregnancy outcome, patients not excluded from study) |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT failed or rejected at review in only 7.1% |
| Withdrawals explained? | Yes | Details given for patients who did not undergo different index tests |
| Clinical features and settings | Routine screening | |
| Participants | 1521 participants (18 twin and 2 triplet pregnancies; 1543 fetuses) Italy Pregnant women Singleton or multifetal pregnancies Median maternal age 31.3 years (range 18‐45 years), 19.7% ≥ 35 years 11‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF accredited sonographers) First trimester serum free ßhCG and PAPP‐A (Kryptor analyser, Brahms) Risk cut‐point 1:300 | |
| Follow‐up | Follow‐up obtained by analysis of fetal karyotype, from patient notes and by telephoning patients | |
| Aim of study | To evaluate the performance of the combined test compared to the NT measurement alone, in fetal aneuploidy screening in the general population and in pregnant women aged 35 years and over | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Screening of patients ≥ 35 years of age | |
| Participants | 262 participants Indonesia ‐ 4 hospitals January 2001 to January 2003 Pregnant women Mean age 37.7 years (35‐43 years) Singleton pregnancies 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: amniocentesis (unclear in which patients this was conducted) or follow‐up to birth | |
| Index and comparator tests | First trimester NT (all patients) with > 3.0 mm cut‐off (FMF methods, Apoge 800‐ATL, SSD 680‐Aloka, Logic alpha 200 GE, Veluson 730 Pro GE) First trimester nasal bone assessment (97 (55%) patients who also had NT) | |
| Follow‐up | Follow‐up to birth in patients with no nasal bone and NT > 3 mm. Unclear if screen‐negative patients had follow‐up to birth | |
| Aim of study | Evaluation of a non‐invasive method to screen for Down's syndrome at a maternal age of 35 years or more | |
| Notes | No cases of Down’s detected that were not picked up in screening tests | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 139 participants: 31 cases and 108 controls (3:1 with cases, matched for time of study, geographic location and to be within 5‐year age interval) Sweden ‐ data from Swedish Nuchal Translucency Trial Dates not reported Pregnant women Mean age cases 38.5 years (SD 4.0 years) and controls 35.5 years (SD 4.0 years) Singleton pregnancies 8‐14 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standards: not reported | |
| Index and comparator tests | Maternal age First trimester NT (12‐14 weeks) (method not specified) Frozen serum samples PAPP‐A and free ßhCG in sample taken at 8‐14 weeks (Auto Delfia Instrument) Risk cut‐points of 1:250 and 1:350 (Lifecycle software used to calculate risk) | |
| Follow‐up | No details of methods used to follow women‐up | |
| Aim of study | To determine to what extent adding first trimester serum screening to NT would change the detection rate and test positive rate for Down's syndrome | |
| Notes | Part of NUPP trial | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Details given for women who did not agree to take part |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 486 participants UK and Portugal Dates not reported Pregnant women Singleton pregnancies Median age 35 years (17‐46 years) 10‐14 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 38 cases Reference standard: fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks | |
| Index and comparator tests | Maternal age First trimester NT (SSD, Aloka) First trimester ductus venosus flow velocity: measured transabdominally (5‐MHz curvilinear probe, Ecocee, Toshiba) or transvaginally (SSD 2000, Aloka) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To assess the possible role of Doppler ultrasound assessment of ductus venous blood flow in screening for chromosomal abnormalities at 11 to 14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Reported that measurements made successfully in all cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 515 participants Portugal Dates not reported Pregnant women Median age 35 years (17‐46 years) Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 43 cases Reference standards: fetal karyotyping. In cases where NT above 95th percentile, follow‐up scan conducted at 14‐16 weeks | |
| Index and comparator tests | Maternal age First trimester NT (SSD, Aloka) First trimester ductus venous Doppler evaluation ‐ ductus venosus flow velocity ‐ abnormal flow is defined as absent or reversed flow of blood in the ductus venosus, normal flow defined as presence. Measurement made by obtaining the right ventral midsaggital plane of the fetal trunk in fetal quiescence. Pulsed Doppler gate placed in distal portion of umbilical sinus. 5 consecutive high‐quality waveforms used to measure peak velocity during ventricular systole and diastole, the lowest forward velocity during atrial contraction in late diastole and the pulsatility index. Up to 10 minutes allowed for measurements | |
| Follow‐up | All women received karyotyping. Unclear if patients followed up to birth | |
| Aim of study | To review the role of Doppler ultrasound in screening for chromosomal abnormalities at 11 to 14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Reported that Doppler measurements made successfully in all cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 256 participants who were referred to unit for fetal karyotyping and had NT and Doppler studies UK ‐ tertiary referral fetal medicine unit Conducted over 18 months, dates not reported Pregnant women Median age 35 years (15‐42 years) 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 30 cases Reference standard: CVS or follow‐up | |
| Index and comparator tests | Maternal age First trimester NT First trimester ductus venous Doppler studies (ATI HDL 5000 US machine with curvilinear TV probe) | |
| Follow‐up | Follow‐up based on ultrasounds findings, examination at birth, postmortem examination in cases of intrauterine death or termination of pregnancy and by telephone interviews with parents | |
| Aim of study | To assess the role of first trimester Doppler assessment of the ductus venosus in screening for fetal aneuploidy in pregnancies at 11‐14 weeks of gestation | |
| Notes | 2 live births with Down’s syndrome. Appears to be a high‐risk invasive testing study but some people did not appear to get karyotyping but were followed up. Probably the majority got karyotyping | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All participants had a reference standard |
| Differential verification avoided? | Yes | All participants had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Doppler studies failed in 4 cases (1.5%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 32,478 participants with available outcome data Australia ‐ screening programme 2005 ‐ 2006 Pregnant women Singleton pregnancies Median maternal age 31 years (14‐48 years), 24.3% of women aged ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 94 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT PAPP‐A and free ßhCG (details not reported) Risk cut‐point 1:300 | |
| Follow‐up | Diagnostic data collected from cytogenetic laboratories. Screening data linked to Western Australia diagnostic data, hospital morbidity and mortality data, midwives notification data and the Birth Defects Registry data through the Department of Health Western Australias Data Linkage Branch. Outcome data available for 92.3% of screened women | |
| Aim of study | To investigate socio‐demographic characteristics in the uptake of prenatal aneuploidy screening in Western Australia and to identify potential barriers to screening access | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 595 participants Israel January 1999 ‐ January 2004 Pregnant women Mean age, healthy 30.3 years (SD 4.5), Down's syndrome 33.7 years (SD 4.9) Singleton pregnancies 11‐14 weeks' gestation and second trimester screening | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 24 cases Reference standards: amniocentesis (recommended for women with higher risk on first or second trimester testing) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (11‐14 weeks) First trimester PAPP‐A and free ßhCG (methods detailed in Maymon 2001) (some analysed retrospectively from banked samples) Second trimester PAPP‐A and free ßhCG (methods detailed in Maymon 2001) | |
| Follow‐up | Delivery outcome obtained by telephone interview or medical records. Information was available for all uneventful pregnancies and delivery outcomes. It is unclear whether information on terminations of pregnancy or miscarriages was available. | |
| Aim of study | To evaluate the cross‐trimester multiple marker correlation and the minimum marker combination needed for detecting various chromosomal aneuploides | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 243 participants: 19 cases and 224 consecutive controls USA ‐ antenatal sonographic unit October 2005 ‐ May 2007 Pregnant women Singleton pregnancies 11‐13 and 14‐28 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (according to FMF criteria) Second trimester nuchal skin‐fold (according to published criteria) First trimester free ßhCG and PAPP‐A (details not reported) | |
| Follow‐up | Cases detected through karyotyping. Stated that controls had normal pregnancies | |
| Aim of study | To assess whether there is a correlation between nuchal translucency and nuchal skin‐fold measurements in Down's syndrome and in normal pregnancies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Different reference standards used |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine referral | |
| Participants | 124,205 participants Germany Dates not reported Pregnant women Maternal age not reported Singleton pregnancies First trimester | |
| Study design | Retrospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 500 cases Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (only data obtained by level II or III certified sonographers included) First trimester free ßhCG and PAPP‐A (Brahms Kryptor system) FMF Germany risk calculation Risk cut‐point 1:150 | |
| Follow‐up | Details not reported | |
| Aim of study | To demonstrate that the variability of the FPR can be reduced through adjusting the concentrations of free ßhCG and PAPP‐A measured in the maternal serum by meaning of a nonlinear regression function modelling the dependence of these variables on maternal weight | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 7447 participants UK ‐ hospital maternity unit January 1995 to January 2000 Pregnant women Mean age 30.1 years (13‐50 years), 21.1% ≥ 35 years, 11.9% ≥ 37 years 10‐14 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 23 cases Reference standards: karyotyping in women considered at risk due to index test results, age or family history or those with considerable anxiety (632 women, 8.5%). Follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT in all patients (fetus in mid‐sagittal section. Maximum thickness of subcutaneous translucency between skin and soft tissue overlying the C‐spine with the fetus in the ventral position) Second trimester AFP, free ßhCG in 65% of patients with NT (radio‐immunoassay and immunoradiometric assays) | |
| Follow‐up | Outcome at birth assess from hospital database, labour ward records or directly from patients. Follow‐up data in 7447 patients (87% of initial patient cohort). Patients without follow‐up excluded | |
| Aim of study | To asses the effectiveness of antenatal screening for trisomy 21 by first trimester sonography followed by second trimester biochemical screening | |
| Notes | 2nd trimester data not analysed 4 live births: 1 diagnosed before birth and chose not to abort. 3 diagnosed after birth (no invasive testing was conducted) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 333 participants Spain ‐ fetal medicine unit February 2007 ‐ January 2009 Pregnant women Singleton pregnancies Mean maternal age 32.7 years (range 16.7‐47.5 years) 11‐14 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 20 cases Reference standard: CVS | |
| Index and comparator tests | First trimester nasal bone (FMF certified sonographer) First trimester ductus venosus (FMF certified sonographer) First trimester tricuspid regurgitation (FMF certified sonographer) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate detection and false positive rates of the ultrasound markers ‐ nasal bone, ductus venosus flow and tricuspid regurgitation, during the first trimester in a population at high‐genetic risk and to study the influence of a 2‐stage screening policy after previous combined screening on the rate of invasive procedures | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | Choice of reference standard did not depend on index test results |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | 5 (1.5%) women did not have measurements obtained for nasal bone and tricuspid regurgitation and 10 (3%) did not have measurements obtained for ductus venosus |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 6831 participants Spain ‐ fetal medicine unit February 2007 ‐ January 2009 Pregnant women Maternal age not reported 9‐11 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographer) First trimester PAPP‐A and free ßhCG (DELFIA Xpress random access platform, PerkinElmer) | |
| Follow‐up | Details not reported | |
| Aim of study | To evaluate detection and false positive rates of the ultrasound markers ‐ nasal bone, ductus venosus flow and tricuspid regurgitation, during the first trimester in a population at high‐genetic risk and to study the influence of a 2‐stage screening policy after previous combined screening on the rate of invasive procedures | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 16,654 participants Italy ‐ single centre 2001‐2004 Pregnant women Median age 32 years (14‐49 years) Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 96 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, No information given regarding machines used) First trimester nasal bone examination (transabdominal ultrasound in mid‐sagittal view) Annual audit of screening performance (medians) | |
| Follow‐up | Outcome at birth as recorded in hospital database (provided by outcome sheets or telephone interviews). Of 32,000 cases in the database, 16,654 (52%) patients had NT, nasal bone assessment and follow‐up data available. Patients without follow‐up data were excluded from the study | |
| Aim of study | To evaluate the feasibility and diagnostic accuracy of fetal NT and nasal bone assessment at 11‐14 weeks for screening of trisomy 21 | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | In 13 cases (1.3%) not possible to ascertain if nasal bone was visible |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4538 participants who had follow‐up data available Spain ‐ tertiary hospital July 1999 ‐ October 2004 Pregnant women Mean age 31.1 years (14‐49 years), 25.9% of patients ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: invasive testing offered to women considered high risk from screening results or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (Methods described by Nicholaides) First trimester PAPP‐A and free ßhCG (Kryptor Trace system, CIS Bio International) Risk cut‐point 1:270 | |
| Follow‐up | Only patients with postnatal results available are included in the study | |
| Aim of study | To report the experience of using of use of the combined first trimester screening test | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 6471 fetuses with available outcome data Korea July 2004 ‐ March 2006 Pregnant women Singleton or multifetal pregnancies Mean maternal age: Down's syndrome 35.5 years (SD 4.8 years), non‐Down's syndrome 31.7 years (SD 3.4 years) 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standard: karyotyping or follow‐up to birth | |
| Index and comparator tests | First trimester fetal nasal bone assessment (Voluson 730, LOGIQ 400 or 5, GE Medical Systems or HDI 500, Philips Medical systems) (American Registry of Diagnostic Medical Sonographers certified Sonographers) | |
| Follow‐up | Obstetric and neonatal outcome obtained from medical records, karyotyping reports and, when needed, telephone conversations with parents or physicians. A total of 7834 fetuses were included in the study but 1047 fetuses (13.4%) without available outcome data were excluded. The remaining 6787 fetuses included 154 twin pregnancies. Assessment of fetal nasal bone was possible in 6490 (95.6%) of the 6787 fetuses. Comparison of nasal assessments between the control population and Down's cases was performed in 6471 fetuses | |
| Aim of study | To evaluate the role of nasal bone assessment in first‐trimester screening for Down's syndrome in the Korean population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Assessment of fetal nasal bone was not possible in 297 women (4.4%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 5694 participants who had first trimester NT and biochemical testing France ‐ 9 centres serving 12 maternity units January 1998 ‐ June 2001 Pregnant women Singleton pregnancies Maternal age not reported 11‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 26 cases Reference standards: invasive testing (offered to women with high NT measurement) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester nuchal translucency in 98% of patients (methods not specified. 60 sonographers ‐ 2 trained by Fetal Medicine Foundation, who trained 30 in turn. 8 received specific training in France, and 20 were self‐taught. Machines not specified) Frozen serum tested for: First trimester PAPP‐A (99% of patients), free ßhCG 99% of patients and AFP (93% of patients) (time‐resolved fluorescent assay, Perkin‐Elmer Life sciences) Risk cut‐point 1:250 | |
| Follow‐up | Data from the French national screening programme used for follow‐up at birth. 211 women (3.7%) who did not return after NT or were found to be > 14 weeks were excluded. It is unclear how many patients had follow‐up to birth | |
| Aim of study | Prospective study of NT and retrospective evaluation of serum (in same patient population) to evaluate whether or not to move the national French Down's screening programme to a first trimester programme | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Women with NT too small to measure assumed to have NT of < 0.5 mm. |
| Withdrawals explained? | Yes | Women failing to return or who more than 14 weeks pregnant were excluded (214). |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 827 participants UK ‐ research centre for fetal medicine January 1990 ‐ October 1991 Pregnant women Median age 38 years (22‐47 years) 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: fetal karyotyping by amniocentesis (52%) or CVS (48%) | |
| Index and comparator tests | Maternal age First trimester NT (curvilinear 5MHz transducer, Aloka 650 CO Limited) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To examine the significance of fetal NT at 10‐14 weeks' gestation in the prediction of abnormal fetal karyotype | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Amniocentesis or CVS |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | No | Women had different reference standards |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 75,821 participants with available information on outcome UK ‐ Various hospitals and a fetal medicine centre June 1998 ‐ December 2003 Pregnant women Median age 31 years (13‐49 years) Singleton pregnancies 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 325 cases Reference standards: amniocentesis or CVS (patients considered high risk based on screening). First trimester presence/absence of nasal bone, presence/absence of tricuspid regurgitation or normal/abnormal Doppler studies (patients of intermediate risk on first trimester screening and did not undergo CVS or amniocentesis. With the addition of information from these tests, if adjusted risk was high, CVS was performed). Follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (Kryptor analyser, Brahms AG) Risk cut‐point 1:300 | |
| Follow‐up | Follow‐up data from cytogenetics laboratories, patients, GPs or maternity units where they delivered. Patients without follow‐up information due to miscarriage or termination (n = 490) or loss to follow‐up (n = 2117) were excluded from the study. | |
| Aim of study | To evaluate the performance of first trimester screening for trisomy 21 by a combination of maternal age, fetal NT and maternal serum free ßhCG and PAPP‐A. In addition, the impact of a new individual risk orientated 2‐stage approach to first trimester screening was examined | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Exclusions due to loss to follow‐up and missing information for women with miscarriages or terminations of pregnancy explained |
| Clinical features and settings | Routine screening | |
| Participants | 2515 participants Finland ‐ primary care centres and maternity clinics of hospitals During 1999 Pregnant women 17.5% aged ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: invasive testing (patients considered high risk based on NT screening) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (≥ 3 mm) (64% of women) (method not described) Fresh serum tested for: First trimester free ßhCG and PAPP‐A (Wallac analytes and 1st trimester risk calculation programme maternal weight correction) Risk cut‐point 1:250 | |
| Follow‐up | Follow‐up data from maternity clinics and the National Research and Development Centre for Welfare and Health. Test negative patients followed up by contacting all maternity clinics and the National Research and Development Centre for Welfare and Health. Unclear if follow‐up information was obtained in all cases | |
| Aim of study | To evaluate efficacy of combining first trimester maternal serum and fetal NT measurement in screening for Down's syndrome in Finland | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening in a high‐risk population | |
| Participants | 2529 participants UK October 1994 to April 1995 Pregnant women Singleton pregnancies Median age 34 years (15‐47 years), 47% ≥ 35 years 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases Reference standards: karyotyping performed (27% of women) due to increased NT (14%), advanced maternal age (10%), previous chromosomally abnormal child (0.5%) or parental anxiety (2%). Ultrasound examination at 20 weeks (65% of patients). Follow‐up to birth (9% of women) | |
| Index and comparator tests | Maternal age First trimester NT (methods not stated) Fresh serum (or serum frozen over a weekend) tested for: First trimester free ßhCG (immunoradiometric assay, CIS) | |
| Follow‐up | Pregnancy outcome obtained from maternity units or the patients themselves. Follow‐up information only appears to have been obtained in 9% of cases (second trimester ultrasound used as reference standard for other women) | |
| Aim of study | To measure the contribution of maternal serum free beta hCG in a screening programme for fetal trisomy 21 based on fetal NT in the first trimester of pregnancy | |
| Notes | No proper results data are presented for this study | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | No | Invasive testing, ultrasound at 20 weeks or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1000 participants Australia ‐ public and private sector venues September 1997 to September 1999 Pregnant women Singleton or multifetal pregnancies (2000 fetuses including 25 sets of dichorionic twins, 7 sets of monochorionic twins and 4 sets of triplets but the numbers amongst the 1000 fetuses reported in the paper were not stated) Median age 32 years 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 8 cases Reference standards: CVS, amniocentesis, neonatal karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age NT (FMF methods) | |
| Follow‐up | Follow‐up from cytogenetics laboratory records but the completeness of follow‐up is not reported | |
| Aim of study | To evaluate a risk assessment tool based on first trimester NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women had a reference standard |
| Differential verification avoided? | Unclear | Unclear if the choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 22,340 participants Australia ‐ 13 ultrasound practices August 2001 to October 2003 Singleton pregnancies Pregnant women Median age 31 years (14‐47 years), 20% ≥ 35 years 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 60 cases Reference standards: CVS or amniocentesis (women assessed to be high risk on screening) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (machine not stated) All study participants underwent all tests Risk cut‐point 1:300 | |
| Follow‐up | Follow‐up data obtained by review of the Midwives Notification System and the Birth Defects Registry. 415 patients (1.8%) excluded due to no follow‐up data. Patients with multiple pregnancies or incomplete screens (n = 3946) were also excluded from the study | |
| Aim of study | To assess fetal outcomes for pregnancies identified at increase risk for Down's syndrome by first trimester combined ultrasound examination and maternal serum biochemistry | |
| Notes | Appears likely that patients with miscarriages and terminations excluded | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Details given of patients excluded due to incomplete screening data or loss to follow‐up |
| Clinical features and settings | Routine screening | |
| Participants | 14,487 participants undergoing first trimester screening (a separate cohort of 30,792 pregnancies were evaluated for integrated screening) November 2002 ‐ December 2005 Canada ‐ 2 hospitals Pregnant women Singleton pregnancies Mean maternal age 34 years 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 62 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (most sonographers had FMF certification) First trimester free ßhCG and PAPP‐A (DSX Four Plate Automated ELISA Processing system, Dynex Technologies and DPC Immulite 2000 automated immunoassay analyser, Siemens Medical Solutions Diagnostics) Risk cut‐point 1:200 or NT ≥ 3.5 mm Results presented with and without adjustment for bias due to miscarriages (viability bias) | |
| Follow‐up | From cytogenetics databases in both Hospitals, the Canadian Institute for Health Information, labour and delivery databases, written and phone follow‐up with care providers and phone follow‐up with women after birth | |
| Aim of study | To evaluate the performance of integrated prenatal screening and first trimester combined screening for trisomy 21 in a large Canadian urban centre | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening of general and high‐risk women | |
| Participants | 2010 participants (744 in subgroup undergoing NT testing) Italy Dates not reported Recruited through private physician or genetic counselling program for women of advanced maternal age Pregnant women Aged 15‐46 years, 35% ≥ 35 years Singleton pregnancies 9‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 11 cases (7 in subgroup with NT testing) Reference standards: not reported | |
| Index and comparator tests | Maternal age First trimester NT (37% of patients) (FMF methods, Toshiba SSA 250A or Acuson XP 10) First trimester free ßhCG and PAPP‐A (all patients) (dried blood samples, enzyme‐linked immunosorbent assays) Risk cut‐point 1:380 | |
| Follow‐up | Not reported | |
| Aim of study | To evaluate first trimester combined screening for Down's syndrome | |
| Notes | Unclear as to what reference standard (if any) was used. All cases of Down's syndrome identified had been picked up by screening | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Unclear | Reference standard not reported |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | Unclear | Unclear if the choice of reference standard depended on screening results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Details given of women undergoing NT but not biochemical testing |
| Clinical features and settings | Routine screening (2 centres) or in referred patients (1 centre) | |
| Participants | 1089 participants undergoing fetal nasal bone assessment Italy/The Netherlands ‐ 3 centres February 2002 to April 2002 Pregnant women Singleton pregnancies Median age 31.7 years (SD 4.0) in unaffected cases and 36.5 years (SD 4.1) in affected cases 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standards: CVS or amniocentesis (women considered high risk on screening on the basis of NT and biochemical results, but not on nasal bone screening, or if requested due to age or anxiety), or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester nasal bone assessment First trimester NT First trimester free ßhCG First trimester PAPP‐A | |
| Follow‐up | Reported that karyotyping was performed postnatally. It is unclear in which cases this was conducted | |
| Aim of study | To assess the feasibility of measuring nasal bone length in first trimester pregnancy and to confirm if the absence of fetal nasal bone is a marker for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Nasal bone assessment was successfully conducted in 94.3% of women |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2411 participants Italy Dates not reported Pregnant women Median age 30.5 years (SD 8.2) First trimester (gestational weeks not reported) | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standard: not reported | |
| Index and comparator tests | Maternal age First trimester nasal bone assessment (FMF methods) First trimester NT, free ßhCG and PAPP‐A Data from other studies used to generate statistical parameters to estimate performance of first trimester screening with and without nasal bone evaluation) Risk cut‐point 1:250 | |
| Follow‐up | No details reported for any follow‐up to birth | |
| Aim of study | To determine the benefit of including nasal bone assessment in addition to standard first trimester markers as a screening test for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | Unclear | Unclear if the choice of reference standard depended on screening results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 194 participants Argentina October 2001 ‐ January 2002 Pregnant women Median age 36 years (19‐44 years) Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 5 cases Reference standard: CVS | |
| Index and comparator tests | First trimester nasal bone assessment (frontal saggital section of the fetal face. Angle of insonation of fetal nose close to 90 degree angle) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To evaluate the association of nasal bone on ultrasound and Down's syndrome fetuses at 11‐14 weeks' gestation | |
| Notes | States in text that there were 6 cases of trisomy 21 | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | CVS |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Unsuccessful nasal bone assessment in 6% |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1473 participants The Netherlands tertiary maternity unit June 1994 to March 1997 Pregnant women Mean age 31.4 years (SD 5.7), 24% ≥ 35 years Singleton pregnancies 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 9 cases Reference standards: prenatal karyotyping offered to patients considered high risk or maternal anxiety (conducted in 24%) or follow‐up to birth | |
| Index and comparator tests | Maternal age NT (FMF method, Hitachi machines, 6 sonographers instructed to take 'sufficient time') Risk cut‐point ≥ 3 mm | |
| Follow‐up | Follow‐up to outcome assessment in the delivery room. 68 women (4.4%) were excluded from the study due to loss to follow‐up | |
| Aim of study | To evaluate the effectiveness of NT measurement in the detection of trisomy 21 in a low‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | Unclear | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Unsuccessful NT measurement in 4.3% |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 2247 participants undergoing NT and fetal karyotyping The Netherlands ‐ prenatal diagnostic centre February 1994 to July 1997 Singleton pregnancies Pregnant women Mean age 37.6 years (22‐46 years) 10‐14 weeks' gestation | |
| Study design | Consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 36 cases Reference standard: prenatal karyotyping | |
| Index and comparator tests | Maternal age FT NT (maximal saggital thickness of NT, corrected for gestational age) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To examine the discriminatory capacity of NT measurement in the detection of trisomy 21 and other chromosomal anomalies | |
| Notes | No follow‐up information on 12 miscarriages | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Unsuccessful NT measurement in 2.4% |
| Withdrawals explained? | Yes | Patients excluded due to sonographically detected fetal abnormalities at NT measurement, no karyotyping or miscarriages |
| Clinical features and settings | Routine screening | |
| Participants | 10,775 participants Canada ‐ General Hospital October 2003 ‐ November 2004 Pregnant women Mean maternal age 32.3 years (SD 4.6 years) 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age FT NT (encouraged to only accept measurements from sonographers with FMF certification) FT PAPP‐A (AutoDELFIA, PerkinElmer) FT hyperglycosylated‐hCG (Nichols Advantage Specialty system, Nochols Institute Diagnosics) | |
| Follow‐up | From electronic record searches of local patient and cytogenetic records and case finding of local and regional birth records | |
| Aim of study | To validate Down's syndrome screening protocols that include hyperglycosylated‐hCG measurements | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4615 participants USA ‐ single institution January 2003 to September 2004 Pregnant women Singleton pregnancies Mean age 33.0 years (IQR 31.0‐36.0) 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 22 cases Reference standards: CVS or amniocentesis. Cytogenetic testing in cases of miscarriage. Follow‐up to birth. | |
| Index and comparator tests | Maternal First trimester NT (FMF methods) First trimester PAPP‐A and free ßhCG (dried blood spots, methodology described elsewhere) | |
| Follow‐up | Outcome information from computerised medical record review. Numbers of patients lost to follow‐up not reported | |
| Aim of study | To evaluate the performance of maternal age, fetal NT, PAPP‐A and free ßhCG for aneuploidy screening | |
| Notes | Appears that all cases of Down’s were diagnosed prenatally by karyotyping | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | High‐risk referral for invasive testing | |
| Participants | 544 participants UK ‐ tertiary referral fetal medicine unit December 2001 to November 2003 Pregnant women Median age 37 years (19‐46 years) Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 47 cases Reference standard: CVS | |
| Index and comparator tests | Maternal age First trimester NT (methods not reported), risk cut‐point 1:300 First trimester nasal bone examination (mid‐sagittal view with beam of the ultrasound transducer being parallel to the nasal bones, previously described) First trimester ductus venous flow (abnormal defined as absent or reversed flow. Angle of insonation < 30 degrees. 3 minutes allotted time. NB previously described) | |
| Follow‐up | 100% karyotyping | |
| Aim of study | To assess the role of fetal ductus venous and nasal bone evaluation in first trimester screening for Down’s syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | CVS |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | Yes | All women had the same reference standard |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Not possible to satisfactorily assess ductus venous flow in 4 cases (0.6%) and nasal bones in 52 cases (8.3%) |
| Withdrawals explained? | Yes | 158 patients not included in the study due to time restrictions or due to the patient declining taking part |
| Clinical features and settings | Routine screening | |
| Participants | 7116 participants UK ‐ single institution December 2001 to November 2003 Pregnant women Singleton pregnancies Mean age 31.4 years (14.5‐50.2 years) 10‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (all patients) (mid‐sagittal view) First trimester nasal bone assessment | |
| Follow‐up | Outcome information from computerised hospital records. Results cross‐matched with the registry of the Regional Genetics Service. No report of how many patients lost to follow‐up | |
| Aim of study | To assess the role of fetal nasal bone evaluation in first trimester screening for trisomy 21 in selected and unselected pregnancies | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Nasal bones could not be satisfactorily assessed in 9.9% of fetuses |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1800 participants Spain ‐ hospital fetal medicine department June 2003 to April 2004 Pregnant women Singleton pregnancies Mean age 30.1 years (15‐46 years) (SD 5.37), 18% ≥ 35 years First trimester (before week 14) | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 7 cases Reference standards: invasive testing offered to patients considered high risk at screening (> 1:300) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF method (Accuson XP10, Mountain View, California) Maximum allotted time of 20 minutes) First trimester nasal bone assessment (in 93.4% of patients) Risk cut‐point 1:300 PAPP‐A and free ßhCG (Delfia Xpress 6000 immunoanalyzer, Perkin Elmer) ‐ not used in study Published population parameters used (Wald 2003) | |
| Follow‐up | Follow‐up in all patients without invasive testing by 1) monitoring all births and miscarriages at the hospital, 2) continued contact with the genetics departments and 3) telephone follow‐up. States in abstract that only fetuses with complete follow‐up results included in the study | |
| Aim of study | To evaluate the utility of determining the presence or absence of nasal bone in a low‐risk fetal population | |
| Notes | 5 cases diagnosed by invasive testing, 2 by follow‐up | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Nasal bones could not be satisfactorily assessed in 6.6% of fetuses |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4776 participants undergoing NT and/or biochemical screening Finland ‐ hospitals or health care centres 1999 ‐ 2000 Pregnant women Singleton pregnancies Mean maternal age 29.5 years, 17.7% ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (Trained personnel) First trimester PAPP‐A and free ßhCG (AutoDelfia kits, PerkinElmer) Risk cut‐point 1:250 | |
| Follow‐up | Outcomes obtained from all maternity clinics, the Finnish Register of Congenital Malformations and the National Research and Development Centre for Welfare and Health. Follow‐up was complete in 99% of live‐born infants. Data on miscarriages (n = 68) received from the National Research and Development Centre for Welfare and Health | |
| Aim of study | To evaluate whether first trimester screening markers are altered in pregnancies affected both by other chromosomal defects than trisomy 21 and structural anomalies and whether it is possible to detect these pregnancies by combined ultrasound and biochemical screening | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 9118 participants France ‐ 2 tertiary and 4 primary referral centres March 1994 to December 1997 Pregnant women Median age 30.5 years (18‐37 years) Singleton pregnancies 12‐14 and 14‐17 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 21 cases Reference standards: amniocentesis offered to patients with NT > 3 mm or serum marker risk was > 1:250, or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT in 98.6% of women (FMF methods) Second trimester free ßhCG (beta hCG ELISA immunoradiometric assay) and AFP (AFP ELISA immunoradiometric assay) in 91.1% of women Both NT and biochemical testing in 60.4% of women | |
| Follow‐up | Details of the method of follow‐up not given. 3.4% of patients were lost to follow‐up and were excluded from the study. This included 113 women (1.2%) with miscarriages | |
| Aim of study | To assess the performance of combined first trimester sonographic screening and second trimester serum screening | |
| Notes | Includes cost‐effectiveness analysis | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women had a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT was not able to be measured in 93 women (1.5%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 14,934 participants Canada ‐ multicentre study Pregnant women Singleton pregnancies Mean maternal age 30.9 (SD 4.5) years 11‐13 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down's syndrome: 51 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (trained assessors following protocol) First trimester PAPP‐A and free ßhCG (PerkinElmer Life Sciences) Second trimester ultrasound and/or serum markers (free ßhCG and AFP or total hCG, AFP and uE3) performed in some cases Risk cut‐point 1:250 | |
| Follow‐up | Notebooks in maternity hospitals used to record information on patient characteristics, screening and outcome at birth. Data obtained from cytogenetic laboratories and DASDY database (contains results of birth examinations). Letters sent to women with missing outcome information and, after 3 months, if there was no response, they were contacted by telephone | |
| Aim of study | To evaluate the performance, acceptability and cost‐effectiveness ratio of a pragmatic approach to screening for Down's syndrome based on the combined first trimester test supplemented by routine ultrasound at 20‐22 weeks in the general population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | 554 women (3.7%) did not undergo screening |
| Clinical features and settings | Routine screening | |
| Participants | 10,854 pregnancies with complete outcome data China ‐ University Hospital January 2005 ‐ May 2008 Pregnant women Singleton pregnancies Median maternal age 33.1 years, 30.1% of women aged ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 32 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF accredited sonographers, HDI 5000, Philips Medical System) First trimester PAPP‐A and free ßhCG (kryptor analyser, Brahms Diagnostica GmbH) Contingent screening strategies
Intermediate risk cut‐points 1:50 to 1:1000 | |
| Follow‐up | Fetal karyotypes entered into database when available. Data on pregnancy outcomes obtained either from local maternity database for those who delivered in the unit or via telephone calls to patients | |
| Aim of study | To assess the relative performance of a multi‐stage first trimester screening protocol for fetal Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 21,492 participants France ‐ Single Health Authority district January 2001 ‐ December 2003 Pregnant women Median maternal age 30.7 years (18.0‐46.3 years) 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 80 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (sonographers trained to FMF standards) First trimester PAPP‐A and free ßhCG (time resolved fluorescent assay, PerkinElmer Life Sciences) Routine abnormality scan for structural malformations (20‐24 weeks) Femur length at routine abnormality scan | |
| Follow‐up | Case report forms completed by attending obstetrician or midwife throughout pregnancy and delivery. Databases of certified laboratories cross‐checked with delivery and outcome data in all maternity units, the databases of all cytogenetic laboratories, the database of the health authority (DASDY), contact with women by mail 3 months after expected delivery and direct telephone with women. | |
| Aim of study | To evaluate the performance of the contingent use of femur length at routine mid‐trimester scan in screening for Down's syndrome in women having previously undergone first trimester screening with disclosure of risk estimates | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4248 participants Spain ‐ Screening database managed by the Fetaltest project To December 2005 Pregnant women Singleton pregnancies Mean maternal age 30.6 years (14‐46 years) 11‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) First trimester PAPP‐A and free ßhCG (details not reported) Cut‐point 1:300 and detection rate at 5% FPR | |
| Follow‐up | Follow‐up by supervising the live births and miscarriages at the Hospital together with continuous contact with the genetics department. 24 pregnancies ended in miscarriage and were lost to follow‐up. In 269 women not giving birth at that Hospital, only those karyotyped were followed up. In total, 287 women (6.8%) were lost to follow‐up | |
| Aim of study | To determine whether delta‐NT could be extrapolated successfully from 1 centre‐specific NT reference curve to another and thus to empirically calculate the likelihood ratios of delta‐NT | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 3185 participants UK ‐ single hospital November 1996 to November 1998 Pregnant women Mean age 28 years (SD 5) 11‐14 and 6‐20 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 8 cases Reference standards: invasive testing (women with high risk on screening) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, transabdominal route) in 84% of women. NT risk cut‐point of 1:100 or if NT measurement > 95th centile for that particular CRL considered screen positive. Confirmatory NT test conducted in all women positive on first NT screening Second trimester AFP, ßhCG and uE3 in 49% of women. Serum risk cut‐point 1:250 | |
| Follow‐up | Follow‐up from computerised maternity records, the neonatal database and the hospital termination of pregnancy and miscarriage record books | |
| Aim of study | To present data on the performance of biochemical screening in a population with a prior low‐risk screening result | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | In 122 (4.3%) of women, a second NT scan was needed since the first 1 failed to obtain a measurement |
| Withdrawals explained? | Yes | Of 3704 women booked for hospital delivery, 3185 had at least 1 screening test and were included in the study |
| Clinical features and settings | Routine screening | |
| Participants | 10,668 participants with complete outcome data Germany ‐ private centre November 2000 ‐ December 2006 Pregnant women Singleton pregnancies Maternal age ≥ 35 years in 31.0% of women 11‐13 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 59 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified physicians) First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms GmbH) Cut‐point 1:300 | |
| Follow‐up | Information provided by either obstetric departments or obstetricians. Results from CVS and amniocentesis, as well as karyotypes from aborted fetal tissue or from postnatal investigations were used. 3.9% of women were lost to follow‐up and were excluded from the study | |
| Aim of study | To assess the performance of a combined first trimester screening concept for trisomies 21, 18 and 13 applied to a low‐ and high‐risk patient sample in a specialised private centre for prenatal medicine | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4033 participants The Netherlands ‐ multicentre (44 centres) study July 2002 to May 2004 Singleton pregnancies Pregnant women aged 18‐47 years (median 36.5 years) 10‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: invasive testing or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) First trimester free ßhCG and PAPP‐A (AutoDELFIA analyser) | |
| Follow‐up | Women were asked to fill in a questionnaire about outcome of pregnancy. A second request was sent by mail if necessary 784 patients were lost to follow‐up (16.2%) and were excluded from the study | |
| Aim of study | To report the results of a first trimester combined‐test screening programme in a multicentre routine clinical setting | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 9342 participants Austria ‐ single institution January 1994 to December 1998 Pregnant women Singleton pregnancies Mean age 28 years (15‐46 years), 10.7% ≥ 35 years 10‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 19 cases Reference standards: CVS (offered to patients with first trimester NT > 3.5 mm), amniocentesis (offered to patients with first trimester NT 2.5‐3.4 mm, high risk on second trimester serum testing (> 1:250) and those > 35 years) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (5‐MHz transducer, Acuson Corp) Second trimester AFP, uE3 and hGC (triple test) offered to patients not undergoing first trimester invasive testing (99.7% of women) (AMERLEX‐M 2nd Trimester kits, Ortho Clinical Diagnostics) | |
| Follow‐up | Patients included in study if they were delivered in the same hospital where they were screened. All newborns were examined for malformations by a paediatrician after delivery. | |
| Aim of study | To evaluate screening for trisomy 21 in a low‐risk population utilising a combination of NT measurement in the first trimester and the triple test in the second trimester | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4802 participants Austria ‐ single institution December 1997 to April 2000 Singleton pregnancies Pregnant women 13.0% > 35 years 10‐12 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 14 cases Reference standards: CVS and amniocentesis (offered to patients with increased risk (> 1:400) at first trimester screening. CVS recommended when NT > 3.5 or when women did not want to wait until the 15th week for amniocentesis), or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (transabdominal transducer, 5‐MHz curvilinear Transducer, Acuson, Mountain View), cut‐point 2.5 mm First trimester PAPP‐A and free ßhCG (done radioimmunologically, kits by Ortho Clinical Diagnostics) Combined risk cut‐point 1:250 | |
| Follow‐up | Patients without follow‐up information (n = 92, 2%) were excluded from the study. 27 women with spontaneous abortions were also excluded from the study | |
| Aim of study | To determine the detection rate of the combined test, NT alone and maternal age alone in a non‐selected population at a false positive rate of about 5% | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All patients received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Women not attending visits were excluded from the study |
| Clinical features and settings | Routine screening | |
| Participants | 4523 participants UK ‐ single institution July 1996 to November 1997 Pregnant women Mean age 29.4 years (16‐47 years) 10‐14 weeks' gestation | |
| Study design | Prospective consecutive cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: invasive testing (women considered high risk on screening) or follow‐up to birth | |
| Index and comparator tests | Maternal age NT (Sagittal plane by transabdominal (92.7%) and transvaginal (7.3%) sonography) Adjusted risk cut‐point 1:270 | |
| Follow‐up | Pregnancy outcome obtained via questionnaires, examination by neonatologist and outcome cross‐referenced with regional cytogenetics registry. 26 test‐negative patients lost to follow‐up and excluded from the study | |
| Aim of study | To evaluate first trimester pregnancy screening for fetal aneuploidy and congenital heart defects by maternal age and NT measurement in an unselected population | |
| Notes | 3 live births, 9 termination of pregnancy | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2053 participants Australia ‐ private practice (Sydney Ultrasound for Women) July 2000 to May 2002 Pregnant women Median age 32 years (15‐44 years), 29% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort study | |
| Target condition and reference standard(s) | Down’s syndrome: 5 affected cases Reference standards: invasive testing or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, sagittal plane, ATL 5000; Philips) First trimester free ßhCG and PAPP‐A (kryptor analyser, Brahms Diagnostics) All participants had all tests Risk cut‐point 1:300 | |
| Follow‐up | Data obtained from referring doctors or patients via letter, phone or completed feedback form given at the time of consultation. Only cases of known outcome included in the study. 68 (1.3%) lost to follow‐up, largely due to miscarriage (n = 20) and loss to follow‐up (n = 40). | |
| Aim of study | To report the sensitivity of combined first trimester biochemistry and ultrasound screening for Down's syndrome in an Australian private practice specialising in obstetric ultrasound | |
| Notes | Only women having biochemical testing before NT were included in the study. This was done to avoid bias from women declining biochemical testing following negative NT. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1287 participants Chile ‐ fetal medicine centre January 2003 ‐ January 2006 Pregnant women Median maternal age 33 years (range 14‐47 years), 35.4% ≥ 35 years Singleton pregnancies 11‐14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 31 cases Reference standards: CVS, amniocentesis, cordocentesis or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT and nasal bone assessment (Accuvix XQ, Medison or Voluson 730, GE Healthcare) (only included in study if scanned by 1 of 2 fetal medicine specialists following FMF guidelines) | |
| Follow‐up | Cases of chromosomal abnormality were identified from the cytogenetics laboratory logbook, which recorded all the cytogenetic studies performed prenatally, after a spontaneous abortion or fetal death, or in neonates with physical abnormalities. Information from the remaining cases was obtained from the delivery records and neonatal discharge summaries, which recorded the condition of the neonate at birth and the physical examination performed by a neonatologist | |
| Aim of study | To report their experience with first trimester screening for trisomy 21 by using the combination of NT thickness and nasal bone assessment | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 96,127 participants UK ‐ multicentre study (22 centres) Women due to deliver before June 1997 Pregnant women Median age 31 years (14‐49 years) Singleton pregnancies 10 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 326 cases Reference standards: CVS and amniocentesis (9.6% of women) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (sagittal section) Risk cut‐point 1:300 | |
| Follow‐up | Each women given a request form to complete about the outcome of pregnancy. 4184 women (4.2%) were excluded due to loss to follow‐up or due to miscarriages that were not karyotyped | |
| Aim of study | To investigate the assessment of risk by a combination of maternal age and fetal NT thickness, measured by ultrasonography at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 19,694 participants Denmark ‐ 2 centres July 2005 ‐ June 2007 Pregnant women Singleton pregnancies Maternal age: healthy mean age 30.4 years (16‐45 years), 16.5% ≥ 35 years, Down's syndrome median age 34 years (23‐44 years) 8‐13 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 100 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (TRACE technology, Kryptor instrument, Brahms AG) | |
| Follow‐up | Details not reported. It was stated that, for non‐Down's syndrome pregnancies, only those with known healthy fetus were included | |
| Aim of study | To develop 2 alternative risk calculation programmes to assess whether the screening efficacies for T13, T18 and T21 could be improved by using our locally estimated medians | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Women referred for invasive testing or self‐referred for screening | |
| Participants | 1156 participants: 210 cases and 946 controls matched for gestational and maternal age UK ‐ fetal medicine research centre Dates not specified Pregnant women Median maternal age 38 years (19‐46 years) (cases) and 36 years (15‐47 years) (controls) 10‐14 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 210 cases Reference standards: invasive testing (high‐risk women) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (methods not reported) Frozen serum samples tested for: First trimester free ßhCG and PAPP‐A (Kryptor analyser, time resolved amplified cryptate emission (TRACE)) | |
| Follow‐up | Stated that pregnancy outcome was ascertained in all women | |
| Aim of study | To examine the potential impact of combining maternal age with fetal NT thickness and maternal serum free ßhCG and PAPP‐A in screening for trisomy 21 at 10‐14 weeks of gestation | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 278 participants: 54 cases and 224 controls (no details of how selected) UK ‐ OSCAR screening program Samples collected since 1998 Pregnant women Median maternal age 36 years (20‐44 years) (cases) and 30 years (16‐41 years) (controls) 11‐13 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 54 cases Reference standards: details not reported | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Frozen serum samples tested for: First trimester free ßhCG, PAPP‐A and ThCG (Kryptor Analyser (TRACE) and automated immunofluorescent assays) | |
| Follow‐up | Methods for follow‐up to birth not reported | |
| Aim of study | To assess serum hyperglycosylated hCG for use in the first trimester of pregnancy as a marker of Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth (Nicolaides 2005(OSCAR screening program)) |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear of all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 622 participants: 55 cases and 567 controls matched for gestational age Denmark ‐ screening programme Dates not reported Pregnant women Median maternal age cases 35.8 years, controls 29.3 years 8‐13 weeks' gestation (results modelled on only cases where testing conducted before 10 weeks' gestation) | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 55 cases (31 tested before 10 weeks' gestation) Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (details not reported) Fresh serum samples tested for: First trimester PAPP‐A and free ßhCG (Kryptor analyser, Brahms) Frozen samples tested for: First trimester ADAM 12 (measured blind to clinical outcome) (manual DELFIA assay, PerkinElmer Life & Analytical Sciences) | |
| Follow‐up | Details not reported | |
| Aim of study | To establish the effectiveness or otherwise of ADAM 12 as an early screening marker | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 5000 participants UK ‐ maternity clinic Over a 3 year period ‐ dates not specified Pregnant women Singleton pregnancies Median age 32 years (14‐45 years), 27% ≥ 35 years 11 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 15 cases Reference standards: invasive testing offered to women with screening risk of > 1:250 or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, ATL HDI 3500, ATL HDI 3000, Toshiba SSA‐340A and Kretz Voluson) First trimester free ßhCG and PAPP‐A (Clotted venous blood samples, AutoDELFIA immunoassy, Perkin Elmer) | |
| Follow‐up | Details not reported | |
| Aim of study | To assess the effectiveness of combined ultrasound and biochemical screening for chromosomal abnormalities in singleton pregnancies in a routine antenatal clinic and laboratory setting | |
| Notes | Fetal loss rate for invasive testing was 1.4% (3/212) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT not successfully measured in 25 patients (0.5%) |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 7096 participants with information available on pregnancy outcome Slovenia ‐ 2 outpatient clinics November 1999 ‐ May 2006 Pregnant women Singleton pregnancies Median maternal age 28.6 years (range 15‐42 years), 2.5% ≥ 36 years 11‐14 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (2 FMF certified sonographers) (3.5‐5 MHz and 8‐4 MHz transducers Toshiba Corevision Pro and 2‐5 MHz and 9.3‐3.7 MHz transducers GE Healthcare Voluson 730 Pro) Cut‐off 1/300 | |
| Follow‐up | Pregnancy outcomes were obtained from participating women, referring gynaecologists, paediatricians and maternity units and were missing in 3% (n = 225) of cases. Karyotype results were reported from the cytogenetics laboratory. Only women with known outcome were included in the study analysis | |
| Aim of study | To evaluate screening for trisomy 21 by maternal age and NT in a low‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 4611 women due to deliver before July 1996 Greece ‐ 4 medical centres Dates not specified Singleton pregnancies Median maternal age 29 years (16‐48 years), 7.8% ≥ 37 years 10 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: CVS or amniocentesis or follow‐up to birth. Unclear reference standard in cases of intrauterine death, miscarriages and terminations | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, transabdominally with 5 or 3.5 MHz curvilinear translucer or transvaginally with 5 MHz transducer) Pandya's risk criteria | |
| Follow‐up | Results of fetal karyotyping and pregnancy outcome were entered into the database when they became available | |
| Aim of study | To evaluate first trimester screening for chromosomal defects by fetal NT thickness at 10‐14 weeks of gestation in 4 medical centres in Greece | |
| Notes | 1 set of parents continued with diagnosed Down’s pregnancy to birth, 9 terminated. 1 case of Down’s syndrome only detected at birth. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 9802 participants UK ‐ district general hospital November 1994 to November 1998 Pregnant women Singleton pregnancies Mean age 29 years (15‐45 years) 10 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases Reference standards: CVS (offered to patients considered high risk on screening) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (transabdominally, Toshiba SSA‐250, Accuson 128XP/4 or Aloka 650CL with 3.5‐7.5 curvilinear transducers) | |
| Follow‐up | Pregnancy outcomes from hospital records and general practitioners. Karyotype results or postnatal tests were provided by the local Regional Cytoenetics laboratory. The proportion of patients who were followed up is not reported (49 patients had not given birth at the time of analysis of outcomes) | |
| Aim of study | To evaluate the effectiveness of 10‐14 week NT measurement in routine ultrasounds screening for Down's syndrome | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Unsuccessful NT in 10.1% of patients |
| Withdrawals explained? | Yes | Patients not included due to ineligibility described |
| Clinical features and settings | High‐risk referral | |
| Participants | 445 fetuses with increased risk based on NT or biochemical testing and information available on pregnancy outcome The Netherlands ‐ fetal medicine unit September 1996 ‐ March 2008 Mean maternal age 34.5 years (19‐45 years) First trimester | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 72 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | First trimester ductus venosus pulsatility index and Ductus venosus a‐wave (methods reported elsewhere) | |
| Follow‐up | Pregnancy outcome was obtained from standard follow‐up forms filled in and returned by patients, maternity wards or midwife practices and by reviewing neonatal, pathology and clinical paediatric notes. When the baby was born without structural defects or dysmorphic features, the chromosomes were assumed to be normal. In all cases of enlarged NT or antenatal suspicion of abnormal development, the infant was investigated by a neonatologist, paediatric cardiologist or geneticist. | |
| Aim of study | To investigate if ductus venosus pulsatility index for veins and a‐wave measurements can increase the accuracy of first trimester Down's syndrome screening in a high‐risk population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Satisfactory waveform measurements made in 98% of cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 691 participants: 46 cases and 645 controls Denmark ‐ nationwide screening programme Dates not reported Pregnant women Singleton pregnancies Mean maternal age cases 35 years, controls 31 years 8‐11 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 46 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (11‐13 weeks' gestation) (FMF certified sonographers) First trimester PAPP‐A and free ßhCG (fresh serum, 8‐11 weeks' gestation) (Kryptor analyser, Brahms) First trimester ADAM 12 (frozen serum, 8‐11 weeks' gestation) (Kyptor analyser, assay by Cezanne SAS, TRACE technology) | |
| Follow‐up | Not reported | |
| Aim of study | To determine whether ADAM 12 is a useful serum marker for fetal trisomy 21 using the mixture model | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of some index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 10,189 participants UK ‐ screening programme July 2000 ‐ October 2005 Pregnant women Median maternal age 33.1 years, 36.9% ≥ 35 years 9‐14 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 44 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (trained sonographers) First trimester PAPP‐A and free ßhCG (DELFIA fluoroimmunoassay, PerkinElmer LAS) Contingent: biochemistry high risk cut‐off 1:42, low risk cut‐off 1:1000. If biochemical/maternal age risk between 1:42 and 1:1000, NT results added and combined risk calculated. Final cut‐point 1:250 | |
| Follow‐up | Not reported | |
| Aim of study | To assess the performance of a 2‐stage screening protocol for Down's syndrome based on initial serum marker analysis for all women and NT measurement only in women with intermediate risks | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 7534 participants Finland ‐ screening programme 2002‐2004 Pregnant women Singleton pregnancies Mean maternal age 29.6 years, 18.6% ≥ 35 years 10‐12 weeks' gestation | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 30 cases (24 underwent NT as well as biochemical testing) Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (trained nurses, midwives and doctors) (4765 women) First trimester PAPP‐A and free ßhCG (details not reported) (all women) Cut‐point 1:250 | |
| Follow‐up | Contacted chromosome laboratory at the department of clinical genetics in the Oulu university clinic and the Finish Register of Congenital Malformation and the National Research and Development Centre for Welfare and Health | |
| Aim of study | To compare the efficacy of both separate and combined maternal serum testing and fetal NT measurement in the first trimester screening for Down's syndrome in northern Finland | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 1752 participants Italy ‐ ultrasound and prenatal diagnosis unit December 2001 to June 2002 Pregnant women Median age 32 years (18‐47 years) 11 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases Reference standards: CVS or follow‐up to birth | |
| Index and comparator tests | Maternal age Nasal bone assessment (ultrasound examinations with Aloka SSD‐1700 or ATL‐Philips 5000 HCD) | |
| Follow‐up | Follow‐up to birth in all cases of abnormalities. Not reported if there was follow‐up in screen‐negative patients | |
| Aim of study | To evaluate the significance of nasal bone ossification as a marker fir trisomy 21 at 11 to 14 weeks' gestation in an unselected population | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | In 154 cases (8.1%) fetal profile was not obtained |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 39,983 participants UK and Austria ‐ multicentre trial September 1996 to April 2000 Pregnant women 9‐13 and 14‐20 weeks' gestation | |
| Study design | Case‐control study | |
| Target condition and reference standard(s) | Down's syndrome: 85 cases Reference standards: invasive testing (following second trimester screening) or follow‐up to birth | |
| Index and comparator tests | First trimester NT (midsaggital section, optimal magnification of thickness of translucent space between inner skin surface and fascia covering cervical spine (white black interface (oute) ‐ black white interface (inner), 41 models of ultrasound machine, 20 minutes allotted scanning time) First and second trimester serum AFP, hCG, uE3, PAPP‐A, free ßhCG (time resolved fluoroimmunoassay, AutoDELFIA) First and second trimester inhibin A (Sandwich enzyme linked immunosorbent assay, Oxford bioinnovation) First and second trimester urinary beta core fragment, total hCG, ITA and free ßhCG (ITA and beta core fragment, Quest diagnostics USA) | |
| Follow‐up | Follow‐up by: 1) staff at local hospitals completed a study outcome form at, or just after delivery, 2) study records of CVS, amniocentesis or karyotype at birth linked to information from cytogenic laboratories, 3) study records linked to records of cases of Down's syndrome from the National Down's Syndrome Cytogenetic Register, 4) information obtained from local obstetrical outcome records, 5) forms sent to all women with a request to return details of the outcome of their pregnancy, 6) individual searches in respect of women whose outcomes of pregnancy had not been obtained by any of the previous methods. 4% of total patient cohort did not have a documented outcome of pregnancy. Unclear if any of these were included in the nested case‐control study | |
| Aim of study | To identify the most effective, safe and cost‐effective strategy for antenatal screening for Down's syndrome using NT, maternal serum and urine markers in the first and second trimesters of pregnancy and maternal age in various combinations | |
| Notes | Performance of screening assessed at 17 weeks' gestation. Study tried to be non‐interventional in the first trimester ‐ second trimester testing was aimed to be used as the basis for any referral for invasive testing | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Unclear | Unclear if all index tests interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | Rates of NT failure on average 9%. Pre‐10 weeks' gestation, > 33% failure rate, declined to 7% at 12 weeks |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 8216 participants USA multicentre study (12 prenatal diagnostic centres) Dates not specified Singleton pregnancies Pregnant women Mean age 35 years (SD 4.6), 50% ≥ 35 years 11 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 61 cases Reference standards: invasive testing. Miscarriage with cytogenetic testing. Follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Dried blood samples tested for: First trimester free ßhCG and PAPP‐A (dried blood samples, enzyme‐linked immunoadsorbent assay as previously described) Risk cut‐point 1:270 | |
| Follow‐up | Follow‐up to birth by directly following up women and reviewing delivery records. An effort was also made to obtain information on terminated or miscarried pregnancies. 196 (2.3%) of patients without follow‐up information were excluded and women with a previous trisomy 18 or 21 pregnancy were also excluded | |
| Aim of study | To evaluate the use of combined first trimester markers for aneuploidy in clinical practice | |
| Notes | 16 live Down’s syndrome births | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 2231 participants USA January 2005 ‐ January 2008 Pregnant women Singleton pregnancies Mean maternal age 36.7 years (SD 3.2 years) First and second trimester | |
| Study design | Retrospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (Sonographers credited by FMF or Nuchal Translucency Quality Review Program) First trimester PAPP‐A and free ßhCG (details not reported) Second trimester ultrasound (in 884 women) Cut‐point for combined test 1:220 | |
| Follow‐up | Down's syndrome cases ascertained from pre‐natal genetic database, including prenatal and newborn testing or physical examination at birth | |
| Aim of study | To evaluate the trisomy 21 screening performance of the first trimester combined test followed by second trimester genetic sonography | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Referrals for screening | |
| Participants | 8622 participants Denmark ‐ 3 obstetrics departments March 1998 to June 2001 Pregnant women Mean age 29 years, 10.8% ≥ 35 years Singleton pregnancies 11 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases Reference standards: invasive testing (in cases of increased risk) or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods, Logic 700 MR machine) (all women) First trimester free ßhCCG (AFP/ßhCG Auto Delfia kit) and PAPP‐A (In‐house ELISA (Sandwich)) in 6,441 women (75%) Risk cut‐point 1:250 | |
| Follow‐up | Cross‐checking with all the chromosome laboratories in Denmark. Follow‐up in 96.2% of pregnancies through patients records | |
| Aim of study | To determine the performance of screening for Down's syndrome and other major chromosomal abnormalities using NT, free ßhCG and PAPP‐A in a prospective study of a non‐selected population | |
| Notes | Uptake of screening was 73% (9,941 accepted out of 13,621 offered screening) Women with miscarriages excluded from the study 3 live Down’s syndrome births | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT could not be measured in 2.5% of cases |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 20,293 participants with complete outcome data The Netherlands ‐ nationwide screening programme May 2004 ‐ July 2006 Pregnant women Singleton pregnancies Median maternal age 34.9 years (15‐48 years) 8‐14 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 87 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF protocols) First trimester PAPP‐A and free ßhCG (AutoDELFIA analyser, PerkinElmer, Turku) Cut‐point for combined test 1:250 | |
| Follow‐up | Pregnancy outcome was evaluated by questionnaire and collected through self‐reporting of the participating women. Due to strict privacy rules of the Dutch Personal Data Protection Act, the researchers were allowed to send a reminder letter to collect missing data only once. Women without complete information on outcome were excluded from the study | |
| Aim of study | To study the performance of the first‐trimester combined test between 2004 and 2006 compared to a previous period to investigate changes in time and identify reasons for sub‐optimal performance | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | Yes | Only 65% of biochemistry screened women (n = 41,782) had NT results |
| Clinical features and settings | Routine screening | |
| Participants | 37,488 participants with complete outcome data UK ‐ single centre July 1999 ‐ July 2005 Pregnant women Singleton pregnancies Median maternal age 35.2 years (16‐52 years) 11‐13 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down's syndrome: 264 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT First trimester PAPP‐A and free ßhCG (Kryptor system, Brahms AG) | |
| Follow‐up | Maternal characteristics and test results were recorded in a computer database and karyotype results and details on pregnancy outcomes added as they became available. Women without complete outcome data (n = 1231, 3.2%) were excluded from the study | |
| Aim of study | To examine the validity of methods used to derive patient‐specific risks form NT measurements | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Yes | All women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given |
| Clinical features and settings | Routine screening | |
| Participants | 223,361 pregnant women UK, Denmark and Cyprus – multicentre Some data from UK and Denmark in previous publications Dates not reported Singleton pregnancies Median maternal age 31.9 years (IQR 27.7‐35.8 years) 7‐14 weeks' gestation | |
| Study design | Cohort | |
| Target condition and reference standard(s) | Down’s syndrome: 886 cases Reference standards: karyotyping or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (details not reported) First trimester PAPP‐A and free βhCG (Kryptor system, Brahms AG or Delfia Express sustem, PerkinElmer, Waltham) | |
| Follow‐up | Karyotype results and details on pregnancy outcomes were added to databases as soon as they became available | |
| Aim of study | To establish an algorithm for first trimester combined screening for trisomy 21 with biochemical testing from 7 to 14 weeks’ gestation and ultrasound testing at 11‐13 weeks | |
| Notes | Taken results modelled for PAPP‐A and free ßhcg at 12 weeks as that was most common time for testing (44% of women) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | No | |
| Withdrawals explained? | No | |
| Clinical features and settings | Routine screening | |
| Participants | 10,001 participants Italy ‐ genetic diagnosis centre May 1996 to unspecified date Pregnant women Median age 33 years (14‐48 years) Singleton pregnancies 10 to 14 weeks' gestation | |
| Study design | Prospective cohort | |
| Target condition and reference standard(s) | Down's syndrome: 64 cases Reference standards: amniocentesis, CVS or follow‐up to birth | |
| Index and comparator tests | Maternal age First trimester NT (FMF methods) Risk cut‐points of 1:100, 1:200 and 1:300 | |
| Follow‐up | Outcome obtained from women themselves. 1422 patients (11%) with no data on follow‐up outcome and 202 patients with miscarriages were excluded from the study | |
| Aim of study | To examine the distribution of fetal NT thickness in normal and abnormal fetuses in Sardinia and to determine its effectiveness as a screening tool | |
| Notes | Study design unclear (maybe a case‐control study) | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard |
| Differential verification avoided? | No | Choice of reference standard depended on index test results |
| Incorporation avoided? | Yes | Reference standard was independent of the index test |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice |
| Uninterpretable results reported? | Yes | NT could not be measured in 25 (0.2%) of cases |
| Withdrawals explained? | No | No details of withdrawals given |
AFP:alpha‐fetoprotein
ßhCG: beta human chorionic gonadotrophin
CVS: chorionic villus sampling
DELFIA: dual labelled time resolved fluorescent assay
DVPI: ductus venosus pulsivity index
FMF: frontomaxillary facial
hCG: human chorionic gonadotrophin
NT: nuchal translucency
PAPP‐A: pregnancy‐associated plasma protein A
PIGF: placental growth factor
uE3: unconjugated oestriol
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Unable to extract useful data | |
| No Down's syndrome pregnancies | |
| Unable to extract useful data | |
| Not Down’s syndrome specific | |
| Study only includes cases of Down’s syndrome | |
| Unable to extract useful information | |
| Results presented in another study | |
| Unable to extract useful data | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS | |
| No diagnostic data | |
| Less than 5 Down's syndrome pregnancies | |
| Study only includes test‐positives | |
| Quantitative fluorescent polymerase chain reaction study | |
| Discussion article | |
| Likely fewer than 80% of pregnancies dated by USS | |
| Women screened at greater than 24 weeks' gestation | |
| Second trimester ultrasound | |
| Unable to extract useful data | |
| Review article | |
| Unable to ascertain whether part of screening population in Rozenberg et al. No response from authors, therefore excluded to reduce risk of data replication. | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to obtain paper | |
| USS markers greater than 14 weeks' gestation | |
| USS markers greater than 14 weeks' gestation | |
| USS markers greater than 14 weeks' gestation | |
| USS markers greater than 14 weeks' gestation | |
| Review article | |
| Data from the FASTER trial | |
| No Down's pregnancies in study population | |
| No Down's pregnancies in study population | |
| No Down's pregnancies in study population | |
| Unable to extract useful data | |
| No Down's pregnancies in study population | |
| Second trimester ultrasound study | |
| No diagnostic data | |
| Unclear method of confirmation of gestational age | |
| Male versus female fetuses | |
| Results are not specific to Down’s syndrome | |
| No Down's syndrome pregnancies in study | |
| Less than 80% follow‐up | |
| Less than 80% follow‐up | |
| No Down's pregnancies in study population | |
| Less than 80% follow‐up | |
| Statistical modelling (computer simulation) | |
| Modelled data | |
| Less than 80% of pregnancies dated by USS | |
| Editorial | |
| No Down's pregnancies included | |
| Mathematical model | |
| No follow‐up information | |
| Less than 80% of pregnancies USS dated | |
| Less than 80% of pregnancies USS dated | |
| Gestational age not USS estimated | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in study population | |
| All healthy pregnancies | |
| Cost‐effectiveness analysis | |
| Not a proper sample ‐ most had elevated NT | |
| Review article | |
| Unable to extract useful data | |
| Study of testing on amniocentesis samples | |
| Population risk factor calculations | |
| No diagnostic data | |
| No diagnostic data | |
| No diagnostic data | |
| No diagnostic data | |
| No follow‐up data | |
| Based on SURUSS data ‐ second trimester serum parameters not actually measured | |
| Unable to extract useful data | |
| Second trimester ultrasound | |
| Review article | |
| Screen‐negative population gestations not confirmed by ultrasound | |
| Review article | |
| USS screening inclusive of women greater than 14 weeks' gestation | |
| Review article | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Second trimester ultrasound | |
| Unable to extract useful data | |
| No data for false positive rates | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| Second trimester ultrasound | |
| Second trimester ultrasound | |
| No diagnostic data | |
| No diagnostic data | |
| No follow‐up for false negatives | |
| Review article | |
| No Down's syndrome pregnancies in study population | |
| Less than 5 Down's cases in study population | |
| Unable to extract useful data | |
| No diagnostic data | |
| Likely that fewer than 80% of gestational age confirmed by USS | |
| Case series No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in study population | |
| Less than 5 Down's cases in study population | |
| Study of maternal DNA testing | |
| Study of testing amniotic fluid | |
| Not possible to calculate specificity | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| No diagnostic data | |
| Less than 5 Down's syndrome pregnancies in study population | |
| Unable to obtain translation | |
| Unable to extract useful data ‐ attempted to contact author | |
| Review article | |
| USS at greater than 14 weeks | |
| USS at greater than 14 weeks | |
| USS at greater than 14 weeks | |
| Unable to extract useful data | |
| Review article | |
| No diagnostic data | |
| Less than 80% of pregnancies had gestational age confirmation by ultrasound | |
| Less than 80% of pregnancies had gestational age confirmation by ultrasound | |
| No Down's syndrome pregnancies in study population | |
| Adjustment factors for smokers | |
| Gestational age not confirmed by USS | |
| Gestational age not confirmed by USS | |
| No gestational age limits given | |
| Paper presenting adjustment factors | |
| Data modelled on 4 meta‐analysed studies | |
| Unable to extract useful data | |
| Review article | |
| Abnormal scans only in study population | |
| Less than 5 Down's syndrome pregnancies in study population | |
| ST ultrasound | |
| No Down's syndrome pregnancies in study population | |
| Not specific to Down’s syndrome | |
| Unable to extract useful information | |
| Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response | |
| Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response | |
| Unable to extract useful data | |
| No follow‐up information | |
| Second trimester ultrasound | |
| Unable to obtain translation | |
| Unable to obtain translation | |
| DNA testing of blood samples from parents | |
| Comment | |
| Gestational age by USS only in screen‐positive population | |
| Ultrasound confirmation of gestational age performed in screen‐positive women only | |
| No information for specificity | |
| Second trimester ultrasound | |
| Unable to extract useful data | |
| Fewer than 5 Down's syndrome pregnancies in population | |
| Not specific to Down’s syndrome | |
| Review article | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Data previously presented in another study | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Audit | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Study of karyotyping | |
| Study only examines screen‐positives | |
| Study only examines abnormal fetuses | |
| ST ultrasound | |
| Diagnostic data from other studies | |
| Diagnostic data from other studies | |
| Diagnostic parameters from other studies | |
| No diagnostic data | |
| Comparison of male versus female fetuses | |
| Second trimester ultrasound | |
| Fewer than 80% of study population had gestational age confirmed by USS | |
| Only 1 case of Down’s syndrome | |
| Greater than 14 weeks USS screening | |
| Likely that fewer than 80% of pregnancies had gestational age estimated by USS | |
| Study of FISH technique | |
| ST ultrasound | |
| No diagnostic data | |
| ST ultrasound | |
| Unable to obtain translation | |
| Unable to obtain translation | |
| Systematic review and guidelines | |
| Unable to extract useful data | |
| Study of fetal samples | |
| Less than 80% follow‐up | |
| Less than 80% follow‐up | |
| Unaffected pregnancies only | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Less than 80% of pregnancies had gestational age confirmed by ultrasound scan | |
| Study of FISH technique | |
| Less than 5 Down's pregnancies in study population | |
| Gestational age greater than 24 weeks | |
| Study of findings on amniocentesis | |
| Second trimester ultrasound | |
| Less than 80% of pregnancies had gestational age confirmed by ultrasound scan | |
| Editorial | |
| Unable to extract useful data | |
| Less than 5 Down's pregnancies in study population | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS | |
| No Down’s syndrome data | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in study population | |
| Correlation between markers, not evaluation of screening tests | |
| Unable to extract useful data | |
| Gestation unclear | |
| Gestation based on LMP | |
| Unable to extract useful data | |
| Study of testing on CVS and amniocentesis samples | |
| Study of FISH diagnosis | |
| Unclear method of determination of gestational age Unable to extract useful data | |
| CME | |
| Diagnostic data from other studies used | |
| Second trimester ultrasound scans | |
| Unable to obtain translation | |
| No Down's syndrome pregnancies in study population | |
| Adjustment factors | |
| No Down's syndrome pregnancies in study population | |
| No Down's pregnancies | |
| Same data as Liu 2010 | |
| No Down's syndrome pregnancies in study population | |
| Not possible to obtain detection rate | |
| No diagnostic data | |
| Study of cardiac function in pregnancies with normal and abnormal NT results | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Editorial/commentary | |
| Modelling | |
| Second trimester ultrasound | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in population | |
| Less than 5 Down's pregnancies in population | |
| Review | |
| Unable to extract useful data | |
| No diagnostic data | |
| Not Down’s syndrome specific | |
| Review article | |
| Gestational age estimated by USS in fewer than 80% of cases | |
| Normal pregnancies only | |
| Gestation greater than 14 weeks for USS | |
| Study of DNA testing on maternal blood | |
| No Down's syndrome pregnancies in study population | |
| Less than 5 Down's syndrome pregnancies in study population | |
| Unable to obtain translation | |
| ST ultrasound | |
| Screen‐positive pregnancies only | |
| No diagnostic data | |
| Not Down’s syndrome detection | |
| ST ultrasound | |
| Fewer than 80% pregnancies had gestational age estimated by USS | |
| Not possible to obtain complete diagnostic data | |
| Summary article | |
| Less than 5 Down's syndrome pregnancies in population | |
| Less than 5 Down's syndrome pregnancies in population | |
| Less than 80% follow‐up Unable to ascertain proportion of population with gestational age confirmed by USS | |
| Assumption of normal karyotype without reference standard in significant proportion of control pregnancies | |
| FPR only calculated for subset of the cohort | |
| Unable to obtain translation | |
| Review article | |
| Validation of a specific assay | |
| Less than 80% of pregnancies had gestational age confirmed by ultrasound scan | |
| Review article | |
| Less than 5 Down's syndrome pregnancies in population | |
| Unable to extract useful information | |
| No new data | |
| No Down's syndrome pregnancies in study population | |
| Modelled data | |
| Adjustment factor | |
| Uses data from other published studies | |
| No Down's cases in population | |
| Modelled population | |
| No Down's syndrome pregnancies in study population | |
| Unable to extract useful data | |
| Simulation | |
| Unable to extract useful data | |
| Fewer than 80% pregnancies had gestational age estimated by USS | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Study of women's decisions about screening | |
| Male versus female fetuses | |
| Fewer than 80% of pregnancies USS dated | |
| Unable to extract useful data | |
| No diagnostic data | |
| Down's syndrome pregnancies only | |
| Unable to separate twins from singletons therefore unable to extract useful data | |
| Appears to be a review article (French) | |
| Unable to obtain translation | |
| Unable to obtain translation | |
| No diagnostic data | |
| Unable to obtain translation | |
| Unable to extract useful data | |
| Second trimester ultrasound | |
| Editorial | |
| Not possible to separate out data for cases of Down’s syndrome | |
| Pooled test results | |
| Gestational age by LMP only | |
| FPR and DR obtained from different cohorts | |
| Fewer than 80% of gestational ages estimated by USS | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Pooled test results | |
| Likely fewer than 80% evaluated for gestational age by ultrasound examination | |
| Likely fewer than 80% evaluated for gestational age by ultrasound examination | |
| Review article | |
| Review article | |
| No diagnostic data | |
| Abnormal screening results only | |
| Unable to obtain paper | |
| No Down's syndrome pregnancies in study population | |
| No normal test results included therefore unable to extract meaningful data | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in study population | |
| Modelled data | |
| USS dating on screen positive women only | |
| Observed versus expected cases of Down's syndrome in a population | |
| Gestational age not confirmed by ultrasound scan | |
| Editorial | |
| Part of Merz 2011 cohort | |
| Normal pregnancies only | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| Unable to extract useful data | |
| Fewer than 80% gestational age estimated by USS | |
| Fewer than 80% gestational age estimated by USS | |
| Gestational age greater than specified limits | |
| No diagnostic data | |
| No diagnostic data | |
| No diagnostic data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Less than 5 Down's syndrome pregnancies | |
| Review article | |
| Greater than 24 weeks' gestation | |
| No diagnostic data | |
| No Down's syndrome pregnancies in study population | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Gestational age greater than 24 weeks | |
| Unable to extract meaningful data ‐ unable to separate double and triple test data | |
| No Down's syndrome pregnancies in study population | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Fewer than 80% USS dated | |
| Unable to extract useful data | |
| Not specific Down’s syndrome results | |
| No diagnostic data | |
| No diagnostic data | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Study of outcomes of abnormal NT results | |
| Review article | |
| Review article | |
| Unable to obtain translation ‐ appears to be a review article | |
| Unable to obtain translation ‐ appears to be a review article | |
| Unable to obtain translation ‐ appears to be a review article | |
| Unable to obtain translation ‐ appears to be a review article | |
| Unable to obtain translation ‐ appears to be a review article | |
| Review article | |
| No Down's pregnancies in study population | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Less than 80% of gestational ages confirmed by USS | |
| Unable to extract useful data | |
| Out of FT screening time frame | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Gestational age of greater than 14 weeks in USS population | |
| ST ultrasound | |
| ST ultrasound | |
| No results presented | |
| ST ultrasound | |
| Unable to extract useful data | |
| No diagnostic data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Less than 80% follow‐up | |
| No Down's syndrome pregnancies in study population | |
| Second trimester USS | |
| Only healthy pregnancies | |
| No diagnostic data | |
| Twin data used in calculation of the median | |
| Fewer than 80% USS dated | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| Meta‐analysis | |
| Unable to extract meaningful data | |
| Less than 5 Down's syndrome pregnancies in population | |
| Study of outcomes of abnormal NT results | |
| Review article | |
| No diagnostic data | |
| ST ultrasound | |
| Editorial, no new data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in population | |
| Smokers versus non smokers | |
| ST ultrasound | |
| Unable to extract useful data | |
| Likely fewer than 80% USS dated | |
| Gestational age confirmed by USS in less than 80% of population | |
| Gestational age confirmed by USS in less than 80% of population | |
| Only 2 cases of Down's syndrome | |
| Women screened prior to recruitment | |
| Unable to extract useful data | |
| Abnormal results only | |
| No diagnostic data | |
| Comparison of prevalence and predicition | |
| Comparison of a marker in women of different ethnic origins | |
| Unable to extract useful data | |
| Unable to obtain translation | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Review article | |
| Method of ascertainment of gestational age unclear Twin gestations included in general population | |
| Second trimester USS | |
| Fewer than 5 Down's cases | |
| Explanation of mathematical techniques | |
| Unable to extract useful data | |
| Not full diagnostic data | |
| No Down's syndrome pregnancies in study population | |
| Down's syndrome pregnancies excluded from study | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| Editorial | |
| No Down's pregnancies | |
| Editorial ‐ summary of FASTER results | |
| Review article | |
| Review article | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| USS greater than 14 weeks' gestation | |
| No Down's syndrome pregnancies in population | |
| Unable to determine method of confirmation of gestational age | |
| High‐risk results only included (i.e. no screen‐negative group for comparison) | |
| No Down's pregnancies in population | |
| Unable to ascertain how numbers calculated and from which populations | |
| Unable to extract useful data | |
| No diagnostic data | |
| Included in Sahota 2010 | |
| Unable to obtain paper | |
| Only 1 case of Down’s syndrome | |
| Down's syndrome secondary to Robertsonian translocation only. No controls | |
| No Down's syndrome pregnancies in population | |
| Fewer than 80% had gestational age estimated by USS | |
| Gestation greater than 14 weeks for nuchal scanning | |
| Down's syndrome and Edward's syndrome affected pregnancies only | |
| Unable to extract useful data | |
| Full study information not given | |
| Unable to extract useful data | |
| Not specific to Down’s syndrome | |
| No separate Down’s syndrome data | |
| No diagnostic data | |
| Not specific to Down’s syndrome | |
| Not specific to Down’s syndrome | |
| Not specific to Down’s syndrome | |
| No follow‐up data for test negatives | |
| No Down's pregnancies in study population | |
| Less than 5 Down's syndrome pregnancies in study population | |
| Review article | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in population | |
| No Down’s syndrome specific information for specificity | |
| Review article | |
| Gestational age confirmed by USS in less than 80% of population | |
| Analysis of screen‐positive results | |
| Review/meta‐analysis | |
| Unable to extract useful data | |
| Meta‐analysis of second trimester ultrasound markers | |
| Population study, not examining DTA | |
| Study of prevalence, not screening | |
| Study of prevalence, not screening | |
| Less than 80% follow‐up | |
| Observation of Down's prevalence stratified by age | |
| Editorial | |
| ST ultrasound | |
| No diagnostic data | |
| ST ultrasound | |
| Fewer than 80% USS dated | |
| Likely fewer than 80% USS dated | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Fewer than 80% USS dated | |
| No Down's pregnancies in study population | |
| Unable to extract useful data | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS | |
| Unable to extract useful data | |
| No Down's pregnancies in population | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS | |
| Statistical modelling, aneuploid pregnancies only in study population | |
| No Down's pregnancies in population | |
| Unable to extract useful data | |
| Review | |
| Statistical methods paper | |
| Examination of median shifts rather than an evaluation of screening | |
| No Down's syndrome pregnancies in population | |
| No Down's syndrome pregnancies in population | |
| No Down's cases | |
| Male versus female fetuses | |
| No Down's cases in population | |
| No Down's pregnancies in population | |
| No Down's pregnancies in population | |
| Comparsison of fetal sex | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in population | |
| No Down's pregnancies | |
| Risk validation study | |
| No Down's syndrome pregnancies in population | |
| Demonstration of median changes with time, rather than evaluation of screening | |
| No Down's pregnancies in population | |
| No Down's pregnancies in population | |
| Calculation of weight correction factor | |
| Fewer than 5 Down's syndrome pregnancies | |
| Calculation of smoking correction factor | |
| No Down's pregnancies | |
| No Down's pregnancies | |
| Comparison of 2 different assays ‐ not actual screening evaluation | |
| Unable to extract appropriate data for unaffected pregnancies | |
| Comparison of male and female fetuses | |
| No diagnostic data | |
| Literature review | |
| Review article | |
| ST ultrasound | |
| Unable to extract useful data | |
| Review article | |
| Unable to ascertain method of confirmation of gestational age | |
| Only 55% gestational ages estimated by USS | |
| No Down's syndrome pregnancies in study population | |
| Examination of inter‐observer variation in NT scanning | |
| No diagnostic data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Gestational age not confirmed by USS | |
| Information on screen‐positive pregnancies only | |
| No Down's syndrome pregnancies in study population | |
| Editorial | |
| No Down's syndrome pregnancies in population | |
| Unable to extract useful data | |
| Not possible to obtain full diagnostic data | |
| Unable to obtain translation | |
| Less than 5 Down's syndrome pregnancies in study population | |
| Fewer than 80% pregnancies had gestational age estimated by USS | |
| Unable to extract useful data | |
| Not possible to obtain full diagnostic data | |
| No Down's syndrome pregnancies in study population Software comparison study | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| ST ultrasound | |
| Second trimester USS | |
| Less than 80% had gestational age confirmed by ultrasound | |
| Gestational age not confirmed by USS | |
| No Down's pregnancies in study | |
| Less than 80% had gestational age confirmed by ultrasound | |
| No Down's pregnancies in study | |
| No Down's pregnancies in study | |
| No USS dating | |
| No Down's syndrome pregnancies in population | |
| Review article | |
| No Down's pregnancies | |
| Dated by LMP | |
| No Down's syndrome pregnancies in population | |
| Gestational age greater than 24 weeks | |
| Data modelled on 3 separate populations of women | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Gestational age not confirmed by USS | |
| No Down's syndrome pregnancies | |
| Modelled on several studies, some of which have no USS dating | |
| No cases | |
| Less than 80% had gestational age confirmed by USS | |
| Modelled on SURRUS data | |
| Unable to extract useful data | |
| No Down's syndrome pregnancies in study population | |
| ST ultrasound | |
| Review article | |
| No Down's syndrome pregnancies in study population | |
| No Down's syndrome pregnancies in study population | |
| No diagnostic data | |
| Unable to extract useful data | |
| Study of women's views on screening | |
| Cohort split into people having different tests and non‐representative samples of women assessed for each test | |
| Abnormal results only (cystic hygroma) | |
| Less than 80% of pregnancies had gestational age confirmed by USS | |
| Adjustment factors | |
| Less than 80% of pregnancies had gestational age confirmed by USS | |
| No diagnostic data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Unable to extract useful data | |
| Likely fewer than 80% USS dated | |
| Review | |
| No Down's syndrome pregnancies in study population | |
| Less than 5 Down's syndrome pregnancies in population | |
| Mathematical model | |
| Simulation study, no new data | |
| Only cases of false negatives and true negatives included | |
| Second trimester USS | |
| Unable to extract useful data | |
| Method of determination of gestational age unclear | |
| Unable to extract useful data | |
| Male versus female fetuses | |
| Unable to obtain translation | |
| Fewer than 80% pregnancies had gestational age estimated by USS | |
| No diagnostic data | |
| Only aneuploid pregnancies included in study | |
| No Down's cases in population | |
| No diagnostic data | |
| Unable to obtain translation | |
| Second trimester ultrasound | |
| Inappropriate study design |
CVS: chorionic villus sampling
DR: detection rate
FPR: false positive rate
LMP: last menstrual period
NT: nuchal translucency
USS: ultrasound scan
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Aberrant right subclavian artery Show forest plot | 1 | 425 |
| Test 1  Aberrant right subclavian artery. | ||
| 2 Frontomaxillary facial angle >95 percentile Show forest plot | 1 | 242 |
| Test 2 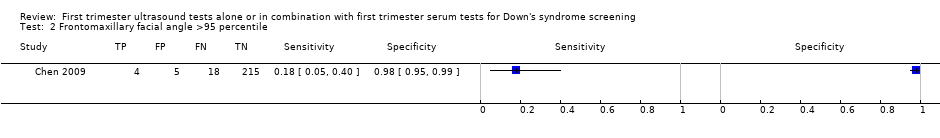 Frontomaxillary facial angle >95 percentile. | ||
| 3 Presence of mitral gap Show forest plot | 1 | 217 |
| Test 3  Presence of mitral gap. | ||
| 4 Maxillary bone length, 5% percentile Show forest plot | 1 | 927 |
| Test 4 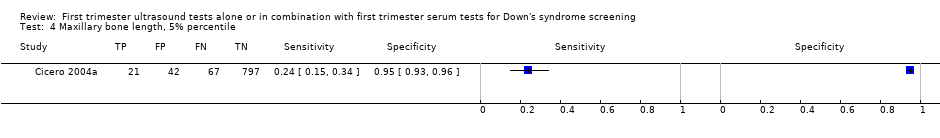 Maxillary bone length, 5% percentile. | ||
| 5 Tricuspid regurgitation Show forest plot | 1 | 312 |
| Test 5  Tricuspid regurgitation. | ||
| 6 Iliac angle 90 degrees Show forest plot | 1 | 2032 |
| Test 6  Iliac angle 90 degrees. | ||
| 7 Ductus venosus a‐wave reversed Show forest plot | 1 | 378 |
| Test 7  Ductus venosus a‐wave reversed. | ||
| 8 Ductus venosus pulsivity index > 95 percentile Show forest plot | 1 | 378 |
| Test 8  Ductus venosus pulsivity index > 95 percentile. | ||
| 9 Nasal bone, mixed cut‐points Show forest plot | 11 | 48279 |
| Test 9 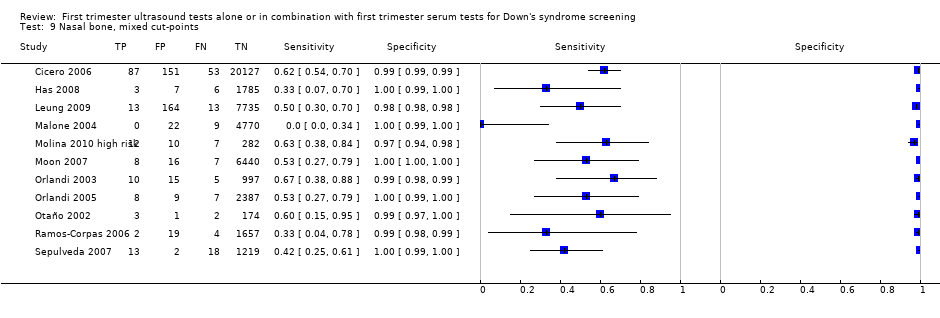 Nasal bone, mixed cut‐points. | ||
| 10 NT, 2.5 mm Show forest plot | 4 | 11835 |
| Test 10 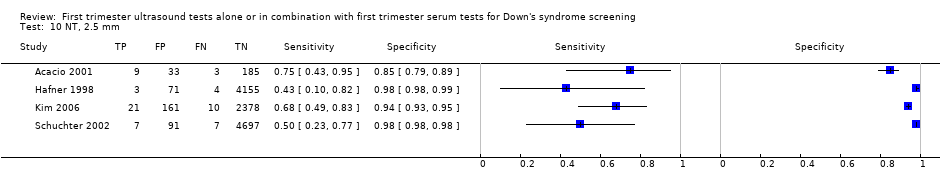 NT, 2.5 mm. | ||
| 11 NT, 3 mm Show forest plot | 6 | 10381 |
| Test 11  NT, 3 mm. | ||
| 12 NT, 5FPR Show forest plot | 3 | 63885 |
| Test 12 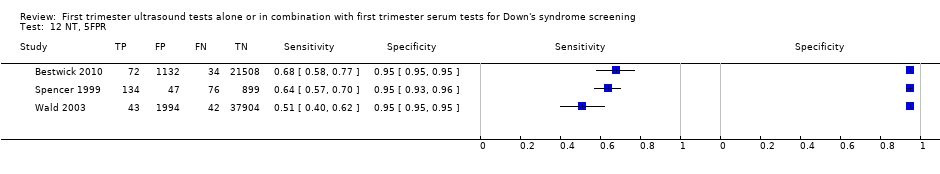 NT, 5FPR. | ||
| 13 NT, mixed cut‐points Show forest plot | 13 | 90978 |
| Test 13  NT, mixed cut‐points. | ||
| 14 NT and age, risk 1:100 Show forest plot | 1 | 10668 |
| Test 14  NT and age, risk 1:100. | ||
| 15 NT and age, risk 1:250 Show forest plot | 10 | 79412 |
| Test 15 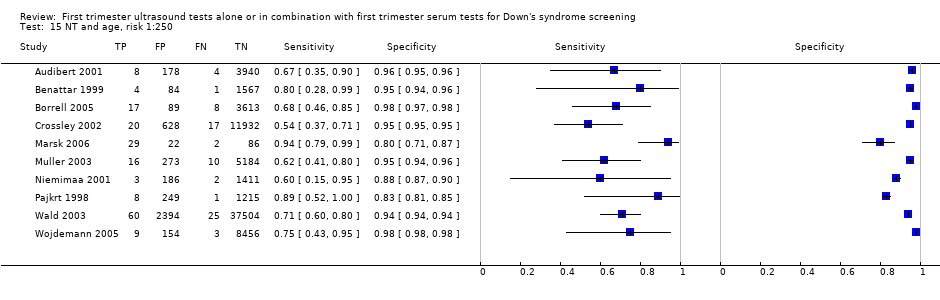 NT and age, risk 1:250. | ||
| 16 NT and age, risk 1:300 Show forest plot | 23 | 252811 |
| Test 16 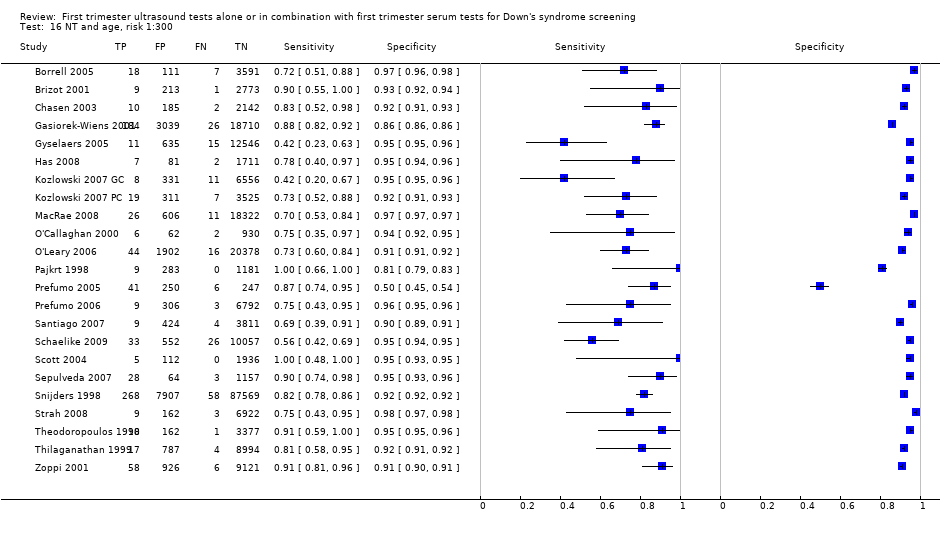 NT and age, risk 1:300. | ||
| 17 NT and age, 1FPR Show forest plot | 4 | 98453 |
| Test 17  NT and age, 1FPR. | ||
| 18 NT and age, 3FPR Show forest plot | 4 | 98453 |
| Test 18 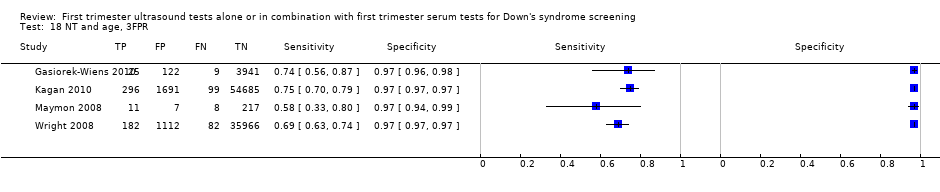 NT and age, 3FPR. | ||
| 19 NT and age, 5FPR Show forest plot | 22 | 288853 |
| Test 19 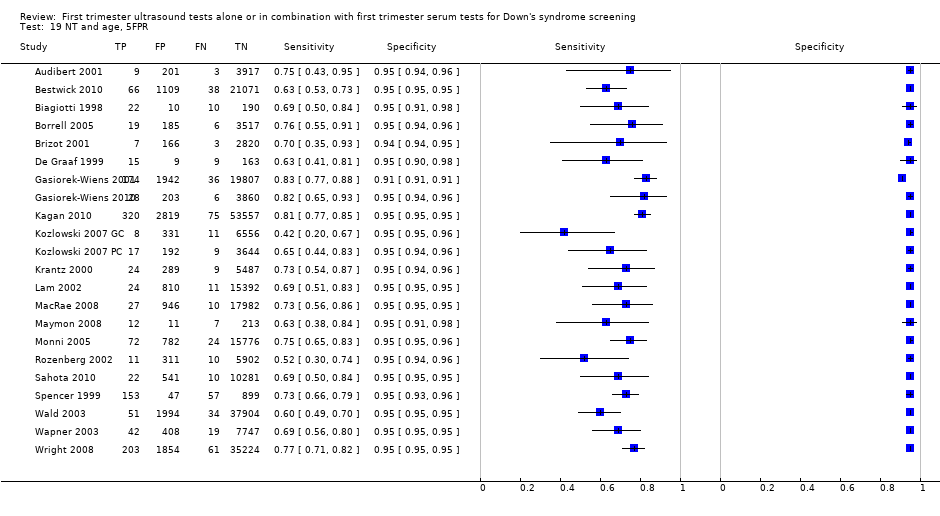 NT and age, 5FPR. | ||
| 20 NT and age, mixed cut‐points Show forest plot | 50 | 530874 |
| Test 20 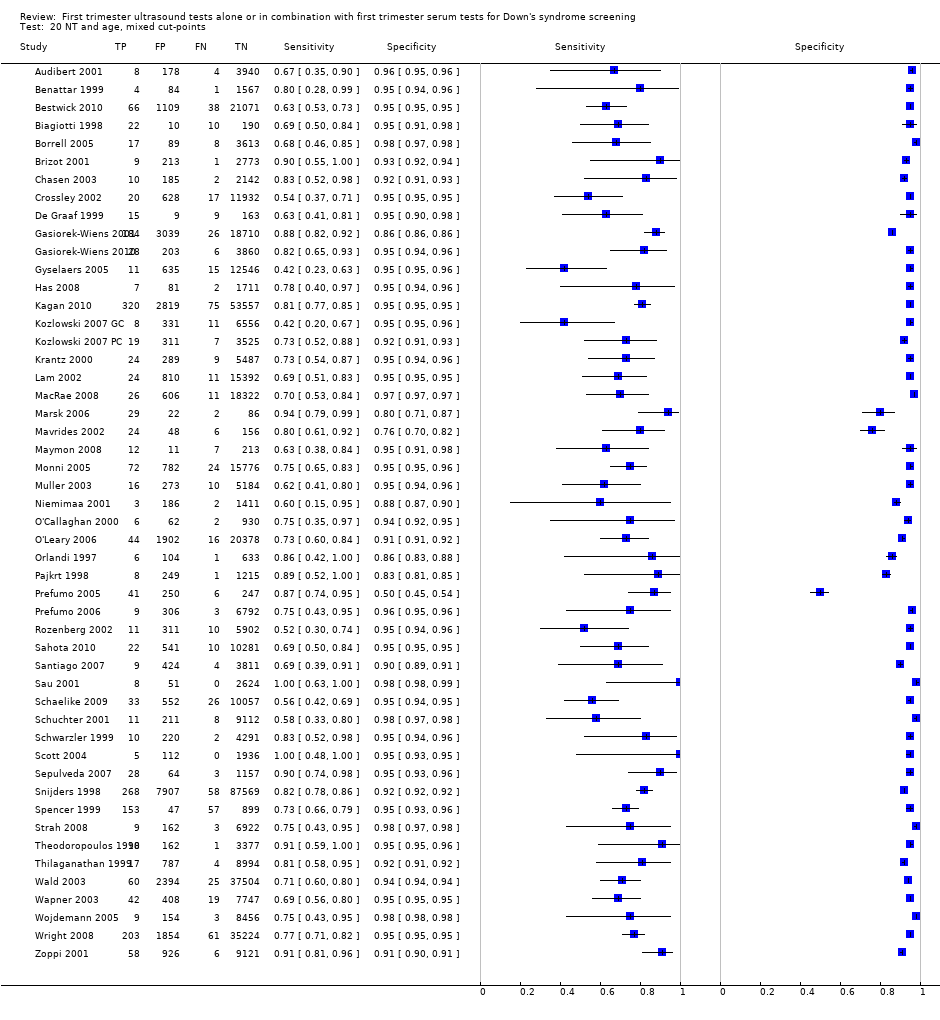 NT and age, mixed cut‐points. | ||
| 21 NT and nasal bone, Absent NB + NT ≥ 95th centile Show forest plot | 1 | 486 |
| Test 21  NT and nasal bone, Absent NB + NT ≥ 95th centile. | ||
| 22 Ductus and age, risk 1:250 Show forest plot | 1 | 3731 |
| Test 22  Ductus and age, risk 1:250. | ||
| 23 Ductus and age, 5FPR Show forest plot | 2 | 3965 |
| Test 23  Ductus and age, 5FPR. | ||
| 24 Ductus and age, mixed cut‐points Show forest plot | 5 | 5331 |
| Test 24  Ductus and age, mixed cut‐points. | ||
| 25 Ductus, NT and age, risk 1:100 Show forest plot | 1 | 19736 |
| Test 25  Ductus, NT and age, risk 1:100. | ||
| 26 Ductus, NT and age, risk 1:250 Show forest plot | 1 | 3727 |
| Test 26  Ductus, NT and age, risk 1:250. | ||
| 27 Ductus, NT and age, 5FPR Show forest plot | 2 | 3961 |
| Test 27  Ductus, NT and age, 5FPR. | ||
| 28 Ductus, NT and age, mixed cut‐points Show forest plot | 3 | 23697 |
| Test 28  Ductus, NT and age, mixed cut‐points. | ||
| 29 Age and nasal bone, mixed cut‐points Show forest plot | 4 | 25303 |
| Test 29  Age and nasal bone, mixed cut‐points. | ||
| 30 Age, NT and tricuspid blood flow, risk 1:100 Show forest plot | 1 | 19736 |
| Test 30  Age, NT and tricuspid blood flow, risk 1:100. | ||
| 31 Age, NT and nasal bone, risk 1:100 Show forest plot | 1 | 19736 |
| Test 31  Age, NT and nasal bone, risk 1:100. | ||
| 32 Age, NT and nasal bone, risk 1:300 Show forest plot | 4 | 9963 |
| Test 32  Age, NT and nasal bone, risk 1:300. | ||
| 33 Age, NT and nasal bone, mixed cut‐points Show forest plot | 5 | 29699 |
| Test 33  Age, NT and nasal bone, mixed cut‐points. | ||
| 34 Age, NT, nasal bone and ductus, risk NT>1:300 AND abnormal DV flow AND absent NB Show forest plot | 1 | 544 |
| Test 34  Age, NT, nasal bone and ductus, risk NT>1:300 AND abnormal DV flow AND absent NB. | ||
| 35 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 20305 |
| Test 35  Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 36 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 41842 |
| Test 36  Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points. | ||
| 37 Age, NT and free ßhCG, 1st trimester, 5FPR Show forest plot | 4 | 4986 |
| Test 37 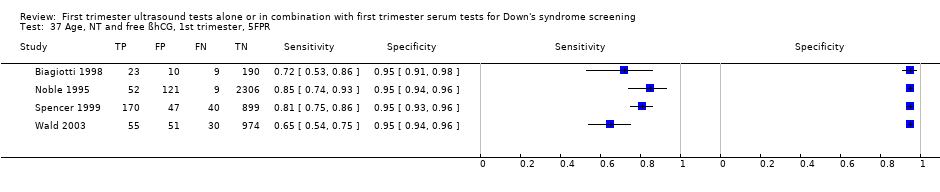 Age, NT and free ßhCG, 1st trimester, 5FPR. | ||
| 38 Age, NT and free ßhCG, 1st trimester, risk 1:240 Show forest plot | 1 | 5809 |
| Test 38  Age, NT and free ßhCG, 1st trimester, risk 1:240. | ||
| 39 Age, NT and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 5 | 10795 |
| Test 39  Age, NT and free ßhCG, 1st trimester, mixed cut‐points. | ||
| 40 Age, NT and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 1507 |
| Test 40  Age, NT and PAPP‐A, 1st trimester, risk 1:100. | ||
| 41 Age, NT and PAPP‐A, 1st trimester, risk 1:185 Show forest plot | 1 | 5809 |
| Test 41  Age, NT and PAPP‐A, 1st trimester, risk 1:185. | ||
| 42 Age, NT and PAPP‐A, 1st trimester, 5FPR Show forest plot | 3 | 2498 |
| Test 42  Age, NT and PAPP‐A, 1st trimester, 5FPR. | ||
| 43 Age, NT and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 5 | 9814 |
| Test 43  Age, NT and PAPP‐A, 1st trimester, mixed cut‐points. | ||
| 44 Age, NT and total hCG, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 44  Age, NT and total hCG, 1st trimester, 5FPR. | ||
| 45 Age, NT and AFP, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 45  Age, NT and AFP, 1st trimester, 5FPR. | ||
| 46 Age, NT and ITA, 1st trimester, 5FPR Show forest plot | 1 | 278 |
| Test 46  Age, NT and ITA, 1st trimester, 5FPR. | ||
| 47 Age, NT and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| Test 47  Age, NT and inhibin, 1st trimester, risk 1:100. | ||
| 48 Age, NT and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| Test 48  Age, NT and inhibin, 1st trimester, risk 1:250. | ||
| 49 Age, NT and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| Test 49  Age, NT and inhibin, 1st trimester, risk 1:400. | ||
| 50 Age, NT and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 50  Age, NT and inhibin, 1st trimester, 5FPR. | ||
| 51 Age, NT and inhibin, 1st trimester, mixed cut‐points Show forest plot | 2 | 1150 |
| Test 51 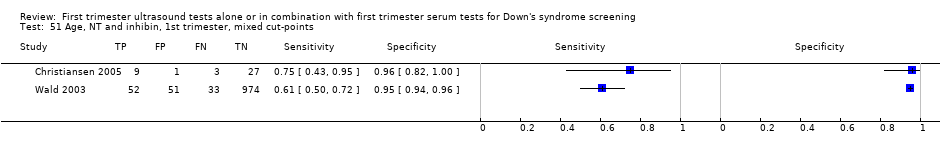 Age, NT and inhibin, 1st trimester, mixed cut‐points. | ||
| 52 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:100 Show forest plot | 10 | 102332 |
| Test 52  Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:100. | ||
| 53 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:150 Show forest plot | 5 | 177643 |
| Test 53 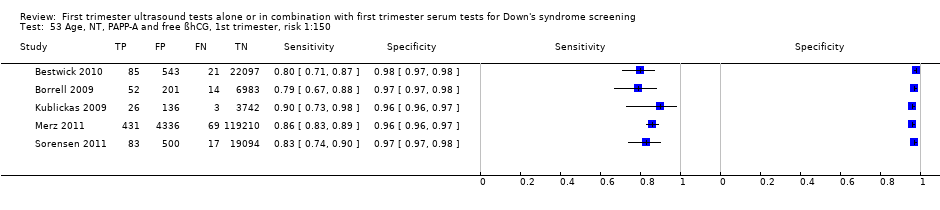 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:150. | ||
| 54 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:200 Show forest plot | 8 | 135768 |
| Test 54  Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:200. | ||
| 55 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:220 Show forest plot | 1 | 2231 |
| Test 55  Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:220. | ||
| 56 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:250 Show forest plot | 25 | 174712 |
| Test 56  Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:250. | ||
| 57 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:300 Show forest plot | 29 | 544681 |
| Test 57  Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:300. | ||
| 58 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 1FPR Show forest plot | 7 | 88874 |
| Test 58 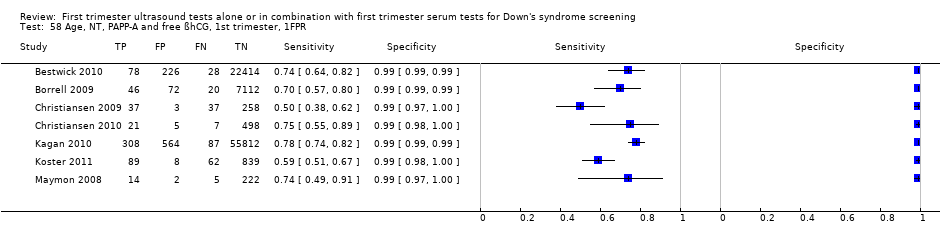 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 1FPR. | ||
| 59 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 3FPR Show forest plot | 9 | 312680 |
| Test 59  Age, NT, PAPP‐A and free ßhCG, 1st trimester, 3FPR. | ||
| 60 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 5FPR Show forest plot | 24 | 391874 |
| Test 60 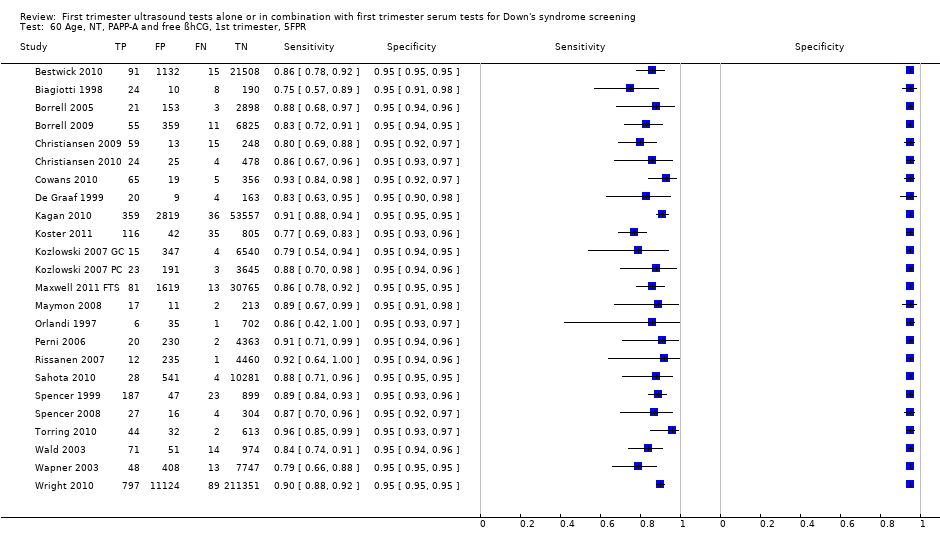 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 5FPR. | ||
| 61 Age, NT, PAPP‐A and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 69 | 1173853 |
| Test 61  Age, NT, PAPP‐A and free ßhCG, 1st trimester, mixed cut‐points. | ||
| 62 Age, NT, PAPP‐A and uE3, 1st trimester, 5FPR Show forest plot | 1 | 576 |
| Test 62  Age, NT, PAPP‐A and uE3, 1st trimester, 5FPR. | ||
| 63 Age, NT, PAPP‐A and ITA, 1st trimester, 5FPR Show forest plot | 2 | 11053 |
| Test 63 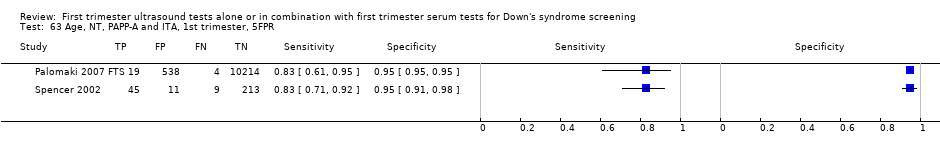 Age, NT, PAPP‐A and ITA, 1st trimester, 5FPR. | ||
| 64 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| Test 64  Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:100. | ||
| 65 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| Test 65  Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:250. | ||
| 66 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| Test 66  Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:400. | ||
| 67 Age, NT, PAPP‐A and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 67  Age, NT, PAPP‐A and inhibin, 1st trimester, 5FPR. | ||
| 68 Age, NT, PAPP‐A and inhibin, 1st trimester, mixed cut‐points Show forest plot | 2 | 1150 |
| Test 68  Age, NT, PAPP‐A and inhibin, 1st trimester, mixed cut‐points. | ||
| 69 Age, NT, PAPP‐A and ADAM12, 1st trimester, 5FPR Show forest plot | 2 | 1042 |
| Test 69 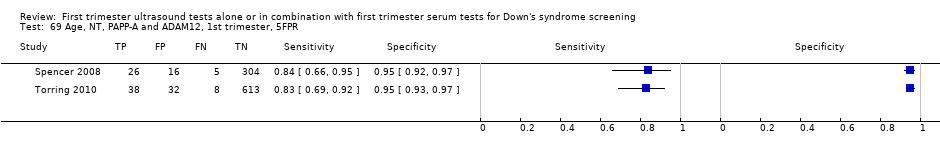 Age, NT, PAPP‐A and ADAM12, 1st trimester, 5FPR. | ||
| 70 Age, NT, PAPP‐A and ADAM12, 1st trimester, risk 1:250 Show forest plot | 1 | 691 |
| Test 70  Age, NT, PAPP‐A and ADAM12, 1st trimester, risk 1:250. | ||
| 71 Age, NT, free ßhCG and ADAM12, 1st trimester, 5FPR Show forest plot | 1 | 351 |
| Test 71  Age, NT, free ßhCG and ADAM12, 1st trimester, 5FPR. | ||
| 72 Age, NT, AFP and free ßhCG, 1st trimester, risk 1:250 Show forest plot | 1 | 1656 |
| Test 72  Age, NT, AFP and free ßhCG, 1st trimester, risk 1:250. | ||
| 73 Age, NT, AFP and free ßhCG, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 73  Age, NT, AFP and free ßhCG, 1st trimester, 5FPR. | ||
| 74 Age, NT, AFP and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 2 | 2766 |
| Test 74  Age, NT, AFP and free ßhCG, 1st trimester, mixed cut‐points. | ||
| 75 Age, NT, AFP and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 75  Age, NT, AFP and PAPP‐A, 1st trimester, 5FPR. | ||
| 76 Age, NT, total hCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 76  Age, NT, total hCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 77 Age, NT, total hCG and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 77  Age, NT, total hCG and inhibin, 1st trimester, 5FPR. | ||
| 78 Age, NT, free ßhCG and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 78  Age, NT, free ßhCG and inhibin, 1st trimester, 5FPR. | ||
| 79 Age, NT, PAPP‐A, free ßhCG, 1st trimester serum, ductus venosus pulsivity index, 5FPR Show forest plot | 1 | 7250 |
| Test 79  Age, NT, PAPP‐A, free ßhCG, 1st trimester serum, ductus venosus pulsivity index, 5FPR. | ||
| 80 Age, free ßhCG and PAPP‐A, if risk 1:42‐1:1000, NT, final 1:250 risk Show forest plot | 1 | 10189 |
| Test 80  Age, free ßhCG and PAPP‐A, if risk 1:42‐1:1000, NT, final 1:250 risk. | ||
| 81 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 2 | 26986 |
| Test 81  Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:100. | ||
| 82 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:250 Show forest plot | 2 | 10325 |
| Test 82  Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:250. | ||
| 83 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 10325 |
| Test 83  Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 84 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 30061 |
| Test 84  Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points. | ||
| 85 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 19736 |
| Test 85  Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:100. | ||
| 86 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:300 Show forest plot | 1 | 1801 |
| Test 86  Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:300. | ||
| 87 Age, NT, tricuspid blood flow, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 19736 |
| Test 87  Age, NT, tricuspid blood flow, free ßhCG and PAPP‐A, 1st trimester, risk 1:100. | ||
| 88 Age, NT, fetal heart rate, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 76385 |
| Test 88  Age, NT, fetal heart rate, free ßhCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 89 Age, NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:200 Show forest plot | 1 | 19736 |
| Test 89  Age, NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:200. | ||
| 90 age, NT, fetal heart rate, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 19614 |
| Test 90  age, NT, fetal heart rate, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 91 Age, NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A,1st trimester, 5FPR Show forest plot | 1 | 19736 |
| Test 91  Age, NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A,1st trimester, 5FPR. | ||
| 92 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, risk 1:250 Show forest plot | 1 | 5483 |
| Test 92  Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, risk 1:250. | ||
| 93 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 1306 |
| Test 93 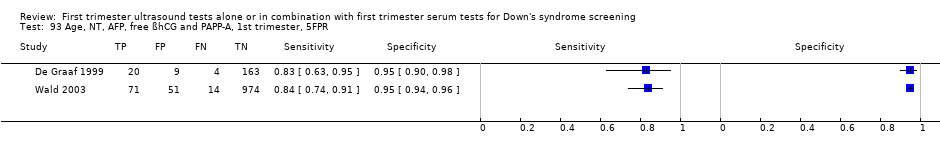 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, 5FPR. | ||
| 94 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 6789 |
| Test 94  Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points. | ||
| 95 Age, NT, total hCG, inhibin and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 95  Age, NT, total hCG, inhibin and PAPP‐A, 1st trimester, 5FPR. | ||
| 96 Age, NT, PAPP‐A, free ßhCG and PGH, 1st trimester, 5FPR Show forest plot | 1 | 335 |
| Test 96  Age, NT, PAPP‐A, free ßhCG and PGH, 1st trimester, 5FPR. | ||
| 97 Age, NT, PAPP‐A, free ßhCG and GHBP, 1st trimester, 5FPR Show forest plot | 1 | 335 |
| Test 97  Age, NT, PAPP‐A, free ßhCG and GHBP, 1st trimester, 5FPR. | ||
| 98 Age, NT, PAPP‐A, free ßhCG and PIGF, 1st trimester, 5FPR Show forest plot | 2 | 1443 |
| Test 98  Age, NT, PAPP‐A, free ßhCG and PIGF, 1st trimester, 5FPR. | ||
| 99 Age, NT, PAPP‐A, free ßhCG and total hCG, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| Test 99  Age, NT, PAPP‐A, free ßhCG and total hCG, 1st trimester, 5FPR. | ||
| 100 Age, NT, PAPP‐A, free ßhCG and PP13, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| Test 100  Age, NT, PAPP‐A, free ßhCG and PP13, 1st trimester, 5FPR. | ||
| 101 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, 5FPR Show forest plot | 4 | 2571 |
| Test 101  Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, 5FPR. | ||
| 102 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, risk 1:250 Show forest plot | 2 | 1222 |
| Test 102 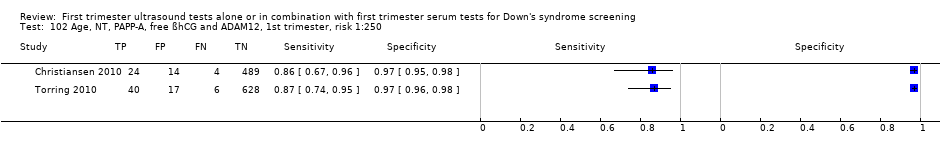 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, risk 1:250. | ||
| 103 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, mixed cut‐points Show forest plot | 4 | 2571 |
| Test 103 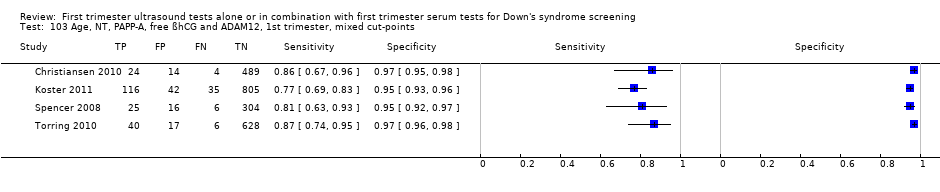 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, mixed cut‐points. | ||
| 104 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| Test 104  Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:100. | ||
| 105 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| Test 105  Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:250. | ||
| 106 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| Test 106  Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:400. | ||
| 107 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 107  Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, 5FPR. | ||
| 108 Age, NT, PAPP‐A, free ßhCG, ADAM12 and PlGH, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| Test 108  Age, NT, PAPP‐A, free ßhCG, ADAM12 and PlGH, 1st trimester, 5FPR. | ||
| 109 Age, NT, total hCG, inhibin, PAPP‐A, AFP and uE3, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 109  Age, NT, total hCG, inhibin, PAPP‐A, AFP and uE3, 1st trimester, 5FPR. | ||
| 110 Age, NT, free ßhCG, inhibin, PAPP‐A, AFP and uE3,1st trimester, 5FPR Show forest plot | 1 | 1110 |
| Test 110  Age, NT, free ßhCG, inhibin, PAPP‐A, AFP and uE3,1st trimester, 5FPR. | ||
| 111 Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG and PlGF, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| Test 111  Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG and PlGF, 1st trimester, 5FPR. | ||
| 112 Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG, PlGF and PP13, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| Test 112  Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG, PlGF and PP13, 1st trimester, 5FPR. | ||
| 113 NT, free ßhCG and PAPP‐A, 1st trimester incidence rate 63.3% Show forest plot | 1 | 6508 |
| Test 113  NT, free ßhCG and PAPP‐A, 1st trimester incidence rate 63.3%. | ||
| 114 NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age < 35 years Show forest plot | 5 | 19057 |
| Test 114 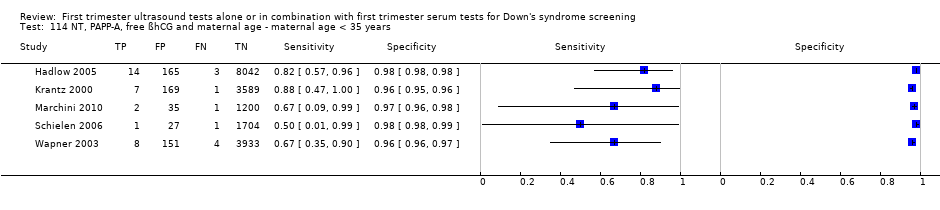 NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age < 35 years. | ||
| 115 NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age ≥ 35 years Show forest plot | 5 | 10980 |
| Test 115  NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age ≥ 35 years. | ||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
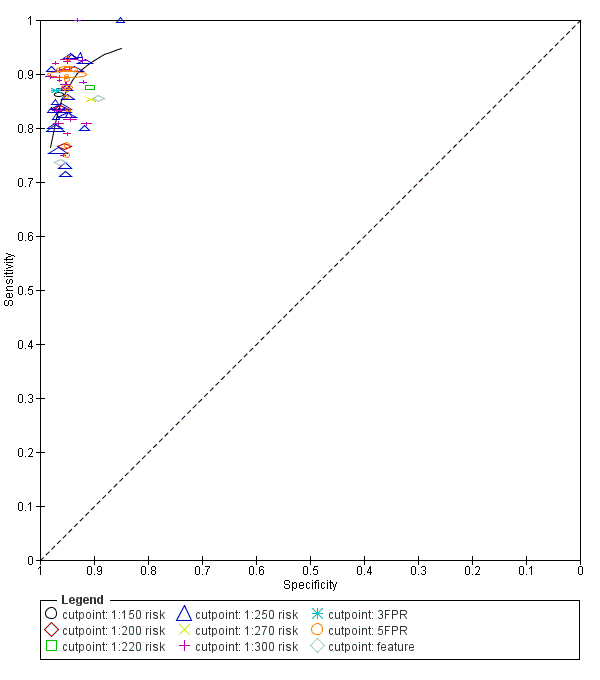
Study estimates of sensitivity and specificity with a summary ROC curve for the NT, PAPP‐A, free ßhCG and maternal age test combination at different cut‐points. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.

Study estimates of sensitivity and specificity with a summary ROC curve for NT and maternal age across different cut‐points. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.
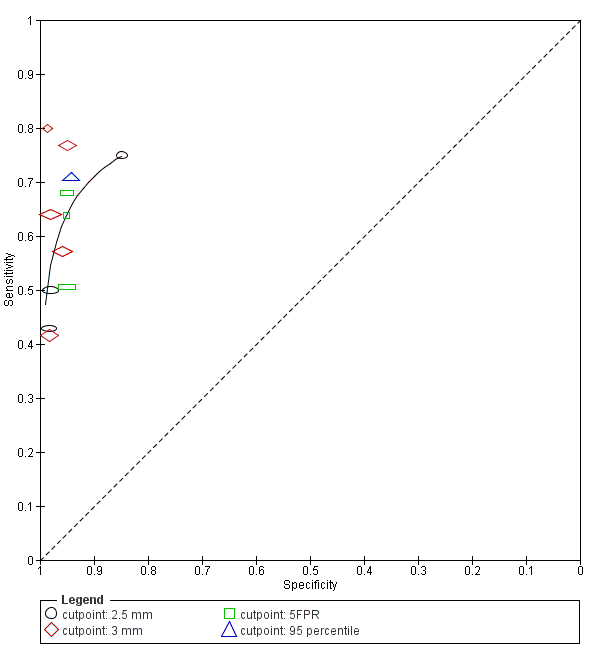
Study estimates of sensitivity and specificity with a summary ROC curve for NT. Each symbol represents a pair of sensitivity and specificity at one cut‐point from each study.

Detection rates (% sensitivity) at a 5% false positive rate for nine of the most evaluated first trimester ultrasound markers alone or in combination with first trimester serum tests. A = NT, PAPP‐A, free ßhCG and maternal age; B = NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age; C = NT, PAPP‐A and maternal age; D = NT, nasal bone and maternal age; E= NT, free ßhCG and maternal age; F= NT and maternal age; G = NT; H = Nasal bone and maternal age; and I = Ductus and maternal age.
Each square represents the summary sensitivity for a test strategy at a 5% false positive rate. The size of each square is proportional to the number of Down's cases. The estimates are shown with 95% confidence intervals. The test strategies are ordered on the plot according to decreasing detection rate. For each test strategy, the number of included studies, Down's syndrome cases and pregnancies are shown on the horizontal axis.

Forest plot of the NT, PAPP‐A, free ßhCG and maternal age test strategy by maternal age group (< 35 years versus ≥ 35 years).
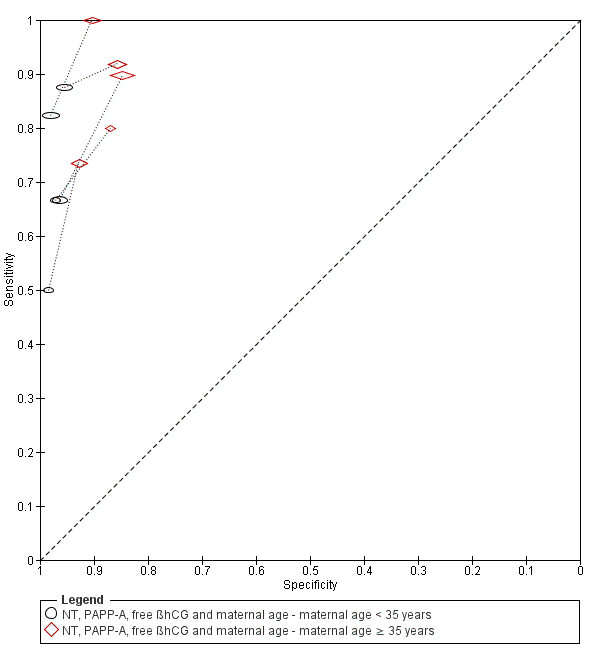
Summary ROC plot of the NT, PAPP‐A, free ßhCG and maternal age test strategy by maternal age group (< 35 years versus ≥ 35 years).

Aberrant right subclavian artery.

Frontomaxillary facial angle >95 percentile.

Maxillary bone length, 5% percentile.

Ductus venosus a‐wave reversed.

Ductus venosus pulsivity index > 95 percentile.

Nasal bone, mixed cut‐points.

NT and age, mixed cut‐points.

NT and nasal bone, Absent NB + NT ≥ 95th centile.

Ductus and age, risk 1:250.

Ductus and age, mixed cut‐points.

Ductus, NT and age, risk 1:100.

Ductus, NT and age, risk 1:250.

Ductus, NT and age, mixed cut‐points.

Age and nasal bone, mixed cut‐points.

Age, NT and tricuspid blood flow, risk 1:100.

Age, NT and nasal bone, risk 1:100.

Age, NT and nasal bone, risk 1:300.

Age, NT and nasal bone, mixed cut‐points.

Age, NT, nasal bone and ductus, risk NT>1:300 AND abnormal DV flow AND absent NB.

Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.

Age, NT and free ßhCG, 1st trimester, 5FPR.

Age, NT and free ßhCG, 1st trimester, risk 1:240.

Age, NT and free ßhCG, 1st trimester, mixed cut‐points.

Age, NT and PAPP‐A, 1st trimester, risk 1:100.

Age, NT and PAPP‐A, 1st trimester, risk 1:185.

Age, NT and PAPP‐A, 1st trimester, 5FPR.

Age, NT and PAPP‐A, 1st trimester, mixed cut‐points.

Age, NT and total hCG, 1st trimester, 5FPR.

Age, NT and AFP, 1st trimester, 5FPR.

Age, NT and ITA, 1st trimester, 5FPR.

Age, NT and inhibin, 1st trimester, risk 1:100.

Age, NT and inhibin, 1st trimester, risk 1:250.

Age, NT and inhibin, 1st trimester, risk 1:400.

Age, NT and inhibin, 1st trimester, 5FPR.

Age, NT and inhibin, 1st trimester, mixed cut‐points.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:100.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:150.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:200.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:220.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:250.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:300.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, 1FPR.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, 3FPR.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, 5FPR.

Age, NT, PAPP‐A and free ßhCG, 1st trimester, mixed cut‐points.

Age, NT, PAPP‐A and uE3, 1st trimester, 5FPR.

Age, NT, PAPP‐A and ITA, 1st trimester, 5FPR.

Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:100.

Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:250.

Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:400.

Age, NT, PAPP‐A and inhibin, 1st trimester, 5FPR.

Age, NT, PAPP‐A and inhibin, 1st trimester, mixed cut‐points.

Age, NT, PAPP‐A and ADAM12, 1st trimester, 5FPR.

Age, NT, PAPP‐A and ADAM12, 1st trimester, risk 1:250.

Age, NT, free ßhCG and ADAM12, 1st trimester, 5FPR.

Age, NT, AFP and free ßhCG, 1st trimester, risk 1:250.

Age, NT, AFP and free ßhCG, 1st trimester, 5FPR.

Age, NT, AFP and free ßhCG, 1st trimester, mixed cut‐points.

Age, NT, AFP and PAPP‐A, 1st trimester, 5FPR.

Age, NT, total hCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, total hCG and inhibin, 1st trimester, 5FPR.

Age, NT, free ßhCG and inhibin, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG, 1st trimester serum, ductus venosus pulsivity index, 5FPR.

Age, free ßhCG and PAPP‐A, if risk 1:42‐1:1000, NT, final 1:250 risk.

Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.

Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:250.

Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.

Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.

Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:300.

Age, NT, tricuspid blood flow, free ßhCG and PAPP‐A, 1st trimester, risk 1:100.

Age, NT, fetal heart rate, free ßhCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:200.

age, NT, fetal heart rate, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A,1st trimester, 5FPR.

Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, risk 1:250.

Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, 5FPR.

Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points.

Age, NT, total hCG, inhibin and PAPP‐A, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and PGH, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and GHBP, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and PIGF, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and total hCG, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and PP13, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, risk 1:250.

Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, mixed cut‐points.

Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:100.

Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:250.

Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:400.

Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG, ADAM12 and PlGH, 1st trimester, 5FPR.

Age, NT, total hCG, inhibin, PAPP‐A, AFP and uE3, 1st trimester, 5FPR.

Age, NT, free ßhCG, inhibin, PAPP‐A, AFP and uE3,1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG and PlGF, 1st trimester, 5FPR.

Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG, PlGF and PP13, 1st trimester, 5FPR.

NT, free ßhCG and PAPP‐A, 1st trimester incidence rate 63.3%.

NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age < 35 years.

NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age ≥ 35 years.
| Review question | What is the accuracy of ultrasound based markers alone and in combination with maternal age and/or first trimester serum markers for screening for Down's syndrome? | |||||
| Population | Pregnant women at less than 14 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome. Some studies were undertaken in women identified to be at high risk based on maternal age. | |||||
| Settings | All settings. | |||||
| Numbers of studies, pregnancies and Down's syndrome cases | 126 studies (reported in 152 publications) involving 1,604,040 fetuses of which 8454 were Down's syndrome cases | |||||
| Index tests | Risk scores computed using maternal age and first trimester ultrasound and serum markers for ultrasound markers ‐ NT, nasal bone, ductus venosus Doppler, maxillary bone length, fetal heart rate, aberrant right subclavian artery, frontomaxillary facial angle, presence of mitral gap, tricuspid regurgitation, tricuspid blood flow and iliac angle 90 degrees ‐ and serum markers ‐ inhibin A, AFP, free ßhCG, total hCG, PAPP‐A, uE3, ADAM 12, PlGF, PGH, ITA (h‐hCG), GHBP and PP13. | |||||
| Reference standards | Chromosomal verification (amniocentesis and CVS undertaken during pregnancy, and postnatal karyotyping) and postnatal macroscopic inspection. | |||||
| Study limitations | 116 studies only used selective chromosomal verification during pregnancy, and were at risk of under‐ascertainment of Down's syndrome cases due to pregnancy loss between administering the serum test and the reference standard. | |||||
| Test strategy | Studies | Women (Down's cases) | Sensitivity (95% CI) | Specificity (95% CI)* | Consequences in a hypothetical cohort of 10,000 pregnant women assuming Down’s syndrome affects approximately one in 800 live‐born babies | |
| Missed cases | False positives | |||||
| Nasal bone | 11 | 48,279 (290) | 49 (34, 64) | 99 (99, 100) | 7 | 100 |
| NT | 13 | 90,978 (593) | 70 (61, 78) | 95 | 4 | 500 |
| NT and maternal age | 50 | 530,874 (2701) | 71 (66, 75) | 95 | 4 | 500 |
| Nasal bone and maternal age | 4 | 25,303 (165) | 68 (28, 92) | 95 | 4 | 500 |
| Ductus and maternal age | 5 | 5331 (165) | 68 (49, 83) | 95 | 4 | 500 |
| NT, nasal bone and maternal age | 5 | 29,699 (221) | 78 (55, 91) | 95 | 3 | 500 |
| NT, free ßhCG and maternal age | 5 | 10,795 (421) | 77 (72, 82) | 95 | 3 | 500 |
| NT, PAPP‐A and maternal age | 5 | 9814 (372) | 81 (75, 86) | 95 | 3 | 500 |
| NT, PAPP‐A, free ßhCG and maternal age | 69 | 1,173,853 (6010) | 87 (86, 89) | 95 | 2 | 500 |
| NT, PAPP‐A, free ßhCG, ADAM 12 and maternal age | 4 | 2571 (256) | 82 (75, 87) | 95 | 3 | 500 |
| *We estimated sensitivity (with a 95% confidence interval) at a 5% false positive rate from the summary ROC curve obtained for each test except nasal bone. For nasal bone, the pooled specificity is reported because the cut‐point was absence or presence of nasal bone, and all studies reported false positive rates below 5% so estimation of sensitivity at a fixed 5% FPR was not appropriate. | ||||||
| Test strategy | Studies | Women (Down's cases) | Sensitivity* (95% CI) | Specificity* (95% CI) | Threshold |
| Without maternal age | |||||
| Ultrasound markers alone | |||||
| Aberrant right subclavian artery | 1 | 425 (51) | 8 (2, 19) | 99 (98, 100) | Feature |
| Frontomaxillary facial angle | 1 | 242 (22) | 18 (5, 40) | 98 (95, 99) | > 95th percentile |
| Presence of mitral gap | 1 | 217 (20) | 20 (6, 44) | 87 (81, 91) | Feature |
| Maxillary bone length | 1 | 927 (88) | 24 (15, 34) | 95 (93, 96) | 5th centile |
| Tricuspid regurgitation | 1 | 312 (20) | 50 (27, 73) | 98 (96, 99) | Feature |
| Iliac angle 90 degrees | 1 | 2032 (52) | 60 (45, 73) | 98 (97, 98) | Feature |
| Ductus venosus a‐wave reversed | 1 | 378 (72) | 68 (56, 79) | 70 (64, 75) | Feature |
| Ductus venosus pulsivity index | 1 | 378 (72) | 81 (70, 89) | 58 (52, 63) | > 95th percentile |
| NT and nasal bone | 1 | 486 (38) | 89 (75, 97) | 93 (91, 95) | Absent nasal bone and NT ≥ 95th centile |
| Ultrasound and double serum markers | |||||
| NT, free ßhCG and PAPP‐A | 1 | 6508 (40) | 90 (76, 97) | 95 (95, 96) | First trimester incidence rate 63.3% |
| With maternal age | |||||
| Ultrasound markers alone | |||||
| NT‐adjusted risk > 1:300 and abnormal ductus venosus flow and absent nasal bones | 1 | 544 (47) | 21 (11, 36) | 100 (99, 100) | 1:300 risk |
| NT and ductus | 3 | 23,697 (177) | 76 to 93 | 73 to 99 | 5% FPR, 1:250 risk, feature |
| NT and tricuspid blood flow | 1 | 19,736 (122) | 85 (78, 91) | 97 (97, 98) | 1:100 risk |
| Ultrasound and single serum markers | |||||
| NT and inhibin A | 2 | 1150 (97) | 61 to 75 | 95 to 96 | 5% FPR, 1:250 risk |
| NT and AFP | 1 | 1110 (85) | 61 (50, 72) | 95 (94, 96) | 5% FPR |
| NT and total hCG | 1 | 1110 (85) | 61 (50, 72) | 95 (94, 96) | 5% FPR |
| NT and ITA | 1 | 278 (54) | 80 (66, 89) | 95 (91, 98) | 5% FPR |
| Ultrasound and double serum markers | |||||
| NT, AFP and free ßhCG | 2 | 2766 (90) | 66 to 100 | 93 to 95 | 5% FPR, 1:250 risk |
| NT, PAPP‐A and inhibin A | 2 | 1150 (97) | 80 to 83 | 95 to 96 | 5% FPR, 1:250 risk |
| NT, total hCG and inhibin A | 1 | 1110 (85) | 62 (51, 73) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG and inhibin A | 1 | 1110 (85) | 66 (55, 76) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG and ADAM 12 | 1 | 351 (31) | 68 (49, 83) | 95 (92, 97) | 5% FPR |
| NT, PAPP‐A and uE3 | 1 | 576 (24) | 79 (58, 93) | 95 (93, 97) | 5% FPR |
| NT, total hCG and PAPP‐A | 1 | 1110 (85) | 80 (70, 88) | 95 (94, 96) | 5% FPR |
| NT, AFP and PAPP‐A | 1 | 1110 (85) | 80 (70, 88) | 95 (94, 96) | 5% FPR |
| NT, PAPP‐A and ITA | 2 | 11,053 (77) | 83 (73, 90) | 95 | 5% FPR |
| NT, PAPP‐A and ADAM 12 | 2 | 1042 (77) | 83 (73, 90) | 95 | 5% FPR |
| Free ßhCG and PAPP‐A, if risk between 1:42 and 1:1000 (intermediate risk), NToffered, final composite risk !:250 | 1 | 10,189 (44) | 89 (75, 96) | 94 (94, 95) | 1:250 risk |
| NT, ductus, free ßhCG and PAPP‐A | 3 | 30,061 (212) | 83 to 96 | 97 to 99 | 1:100 risk, 1:250 risk |
| NT, nasal bone, free ßhCG and PAPP‐A | 3 | 41,842 (271) | 89 to 94 | 95 to 98 | 5% FPR, 1:100 risk, 1:300 risk |
| NT, PAPP‐A, free ßhCG and ductus venosus pulsivity index | 1 | 7,250 (66) | 89 (79, 96) | 95 (94, 95) | 5% FPR |
| NT, tricuspid blood flow, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 91 (84, 95) | 97 (97, 98) | 1:100 risk |
| NT, fetal heart rate, free ßhCG and PAPP‐A | 2 | 76,385 (517) | 92 (89, 94) | 95 | 5% FPR |
| NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 95 (90, 98) | 96 (95, 96) | 1:200 risk |
| NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A | 1 | 19,736 (122) | 96 (91, 99) | 95 (95, 95) | 5% FPR |
| NT, fetal heart rate, ductus, free ßhCG and PAPP‐A | 1 | 19,614 (122) | 97 (92, 99) | 95 (95, 95) | 5% FPR |
| Ultrasound and triple serum markers | |||||
| NT, AFP, free ßhCG and PAPP‐A | 3 | 6789 (135) | 73 to 84 | 95 | 5% FPR, 1:250 risk |
| NT, PAPP‐A, free ßhCG and PP13 | 1 | 998 (151) | 77 (69, 83) | 95 (93, 96) | 5% FPR |
| NT, PAPP‐A, free ßhCG and total hCG | 1 | 998 (151) | 77 (69, 83) | 95 (93, 96) | 5% FPR |
| NT, total hCG, inhibin A and PAPP‐A | 1 | 1110 (85) | 81 (71, 89) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG, inhibin A and PAPP‐A | 1 | 1110 (85) | 84 (74, 91) | 95 (94, 96) | 5% FPR |
| NT, PAPP‐A, free ßhCG and PGH | 1 | 335 (74) | 86 (77, 93) | 95 (92, 97) | 5% FPR |
| NT, PAPP‐A, free ßhCG and PIGF | 2 | 1443 (221) | 88 (70, 95) | 95 | 5% FPR |
| NT, PAPP‐A, free ßhCG and GHBP | 1 | 335 (74) | 91 (81, 96) | 95 (92, 97) | 5% FPR |
| Ultrasound and quadruple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12 and PlGF | 1 | 998 (151) | 79 (72, 86) | 95 (93, 96) | 5% FPR |
| Ultrasound and quintuple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12, total hCG and PlGF | 1 | 998 (151) | 79 (72, 86) | 95 (93, 96) | 5% FPR |
| NT, total hCG, inhibin A, PAPP‐A, AFP and uE3 | 1 | 1110 (85) | 84 (74, 91) | 95 (94, 96) | 5% FPR |
| NT, free ßhCG, inhibin A, PAPP‐A, AFP and uE3 | 1 | 1110 (85) | 86 (77, 92) | 95 (94, 96) | 5% FPR |
| Ultrasound and sextuple serum markers | |||||
| NT, PAPP‐A, free ßhCG, ADAM 12, total hCG, PlGF and PP13 | 1 | 998 (151) | 80 (73, 86) | 95 (93, 96) | 5% FPR |
| *Tests evaluated by at least one study are presented in the table. Where there were two studies at the same threshold, estimates of summary sensitivity and summary specificity were obtained by using univariate fixed‐effect logistic regression models to pool sensitivities and specificities separately. If the threshold used was a 5% FPR, then only the sensitivities were pooled. The range of sensitivities and specificities are presented where meta‐analysis was not performed because there were only two or three studies and no common threshold. | |||||
| Ratio of DORs (95% CI); P value (Studies) | Nasal bone | NT | Nasal bone and age | Ductus and age | NT and age | NT, nasal bone and age | NT, free ßhCG and age | NT, PAPP‐A and age | NT, PAPP‐A, free ßhCG and age |
| NT | – | ||||||||
| Nasal bone and age | – | – | |||||||
| Ductus and age | 1.19 (0.12, 11.4); P = 0.84 (K = 1) | – | 0.85 (0.21, 3.41); P = 0.76 (K = 1) | ||||||
| NT and age | 0.62 (0.13, 2.93); P = 0.50 (K = 2) | 1.25 (0.90, 1.74); P = 0.17 (K = 3) | 0.84 (0.48, 1.49); P = 0.52 (K = 3) | 1.07 (0.51, 2.23); P = 0.85 (K = 3) | |||||
| NT, nasal bone and age | 0.61 (0.12, 3.10); P = 0.50 (K = 2) | – | 4.01 (1.51, 10.6); P = 0.01 (K = 2) | 0.95 (0.23, 3.97); P = 0.93 (K = 1) | 1.05 (0.70, 1.56); P = 0.82 (K = 5) | ||||
| NT, free ßhCG and age | – | 2.15 (1.33, 3.50); P = 0.007 (K = 2) | – | – | 1.47 (1.00, 2.15); P = 0.05 (K = 4) | – | |||
| NT, PAPP‐A and age | – | 2.86 (1.73, 4.73); P = 0.001 (K = 2) | – | – | 1.88 (1.27, 2.78); P = 0.004 (K = 4) | – | 1.28 (0.84, 1.93); P = 0.23 (K = 4) | ||
| NT, PAPP‐A, free ßhCG and age | 3.83 (0.89, 16.4); P = 0.07 (K = 2) | 4.35 (2.00, 9.46); P = 0.015 (K = 4) | – | 3.00 (0.42, 21.2); P = 0.19 (K = 1) | 3.19 (2.19, 4.66); P < 0.0001 (K = 25) | 1.23 (0.63, 2.40); P = 0.50 (K = 2) | 2.06 (1.31, 3.22); P = 0.004 (K = 4) | 1.61 (1.02, 2.55); P = 0.043 (K = 4) | |
| NT, PAPP‐A, free ßhCG, ADAM 12 and age | – | – | – | – | – | – | – | – | 0.87 (0.49, 1.52); P = 0.60 (K = 4) |
| – Indicates pairs of tests where there were no head‐to head comparisons of the two tests in a study. Direct comparisons were made using only data from studies that compared each pair of tests in the same population. Ratio of diagnostic odds ratios (DORs) were computed by division of the DOR for the test in the row by the DOR for the test in the column. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the row is higher than that of the test in the column; if the ratio is less than one, the diagnostic accuracy of the test in the column is higher than that of the test in the row. | |||||||||
| Ratio of DORs (95% CI); P value | Nasal bone | NT | Nasal bone and age | Ductus and age | NT and age | NT, nasal bone and age | NT, free ßhCG and age | NT, PAPP‐A and age | NT, PAPP‐A, free ßhCG and age | |
| DOR (95% CI) Studies | 132 (71, 245) K = 11 | 45 (31, 67) K = 13 | 40 (7, 224) K = 4 | 41 (18, 92) K = 5 | 46 (37, 57) K = 50 | 66 (24, 180) K = 5 | 65 (51, 84) K = 5 | 80 (59, 109) K = 5 | 133 (114, 155) K = 69 | |
| NT | 45 (31, 67) K = 13 | 0.34 (0.16, 0.71); P = 0.006 | ||||||||
| Nasal bone and age | 40 (7, 224) K = 4 | 0.31 (0.05, 1.90); P = 0.18 | 0.90 (0.16, 5.05); P = 0.89 | |||||||
| Ductus and age | 41 (18, 92) K = 5 | 0.31 (0.11, 0.87); P = 0.03 | 0.90 (0.37, 2.20); P = 0.80 | 1.00 (0.11, 9.34); P = 1.00 | ||||||
| NT and age | 46 (37, 57) K = 50 | 0.35 (0.19, 0.66); P = 0.002 | 1.02 (0.66, 1.58); P = 0.92 | 1.14 (0.23, 5.61); P = 0.87 | 1.14 (0.52, 2.49); P = 0.74 | |||||
| NT, nasal bone and age | 66 (24, 180) K = 5 | 0.50 (0.14, 1.81); P = 0.26 | 1.47 (0.47, 4.58); P = 0.48 | 1.64 (0.12, 21.5); P = 0.62 | 1.64 (0.33, 8.08); P = 0.46 | 1.43 (0.52, 3.98); P = 0.48 | ||||
| NT, free ßhCG and age | 65 (51, 84) K = 5 | 0.49 (0.25, 0.98); P = 0.04 | 1.44 (0.89, 2.34); P = 0.12 | 1.61 (0.26, 10.1); P = 0.56 | 1.61 (0.65, 3.99); P = 0.26 | 1.41 (1.02, 1.96); P = 0.04 | 0.98 (0.30, 3.19); P = 0.98 | |||
| NT, PAPP‐A and age | 80 (59, 109) K = 5 | 0.61 (0.29, 1.25); P = 0.16 | 1.77 (1.05, 3.00); P = 0.04 | 1.98 (0.30, 13.1); P = 0.42 | 1.98 (0.76, 5.15); P = 0.14 | 1.73 (1.19, 2.53); P = 0.005 | 1.21 (0.35, 4.13); P = 0.73 | 1.23 (0.74, 2.05); P = 0.35 | ||
| NT, PAPP‐A, free ßhCG and age | 133 (114, 155) K = 69 | 1.00 (0.55, 1.84); P = 1.00 | 2.93 (1.96, 4.40); P < 0.0001 | 3.27 (0.68, 15.8); P = 0.14 | 3.27 (1.53, 7.00); P = 0.003 | 2.87 (2.21, 3.72); P < 0.0001 | 2.00 (0.73, 5.45); P = 0.17 | 2.03 (1.52, 2.72) P < 0.0001 | 1.65 (1.17, 2.34) P = 0.005 | |
| NT, PAPP‐A, free ßhCG, ADAM 12 and age | 85 (58, 124) K = 4 | 0.64 (0.30, 1.37); P = 0.23 | 1.88 (1.07, 3.32); P = 0.03 | 2.10 (0.31, 14.1); P = 0.39 | 2.10 (0.78, 5.63); P = 0.12 | 1.84 (1.19, 2.84); P = 0.007 | 1.28 (0.37, 4.47); P = 0.65 | 1.30 (0.81, 2.09) P = 0.26 | 1.06 (0.61, 1.86) P = 0.81 | 0.64 (0.43, 0.96) P = 0.03 |
| Indirect comparisons were made using all available data for each pair of tests. Ratios of diagnostic odds ratios (DORs) were computed by division of the DOR for the test in the row by the DOR for the test in the column. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the row is higher than that of the test in the column; if the ratio is less than one, the diagnostic accuracy of the test in the column is higher than that of the test in the row. | ||||||||||
| Study | NT, PAPP‐A, free ßhCG and age | Nasal bone | NT and age | NT | Maternal age (range) in years | Reference standard | Population | Study design | Study location |
| X | Mean 35.8 (21‐45) | CVS biopsy, amniocentesis or blood or placenta used for fetal karyotyping | High‐risk referral for invasive testing | Retrospective study of patient notes | South America | ||||
| X | Mean 30.1, all < 38, 86% < 35, 14% ≥ 35 | Prenatal karyotype conducted (in 7.6% of patients) depending on presence of risk > 125, high maternal age, parental anxiety, history of chromosomal defects or parental translocation or abnormal second trimester scan age | Routine screening | Prospective consecutive series | France | ||||
| X | Median 37 (19‐46) | Invasive testing offered to women with NT > 3 mm or risk > 1:250 as defined by combined NT and serum results (CVS from 11 weeks, amniocentesis from 15 weeks). Rapid in situ hybridisation test in patients with risk > 1:30. No details given of any follow‐up to birth | Women requesting screening (self‐paying service) and women attending on account of previous pregnancy history of fetal abnormality | Prospective cohort | UK | ||||
| X | Mean 34.9 for screen positives, 30.5 for screen negatives | Karyotyping or follow‐up to birth | Routine screening | Cohort | Australia | ||||
| Mean 36.4 (15‐46) for Down's cases, 29.8 (15‐49) for unaffected pregnancies | Amniocentesis or CVS (85% of women) or follow‐up to birth | High‐risk referral for invasive testing | Cohort | Budapest | |||||
| X | Mean 32 (16‐46), 8.3% > 35 | Amniocentesis due to maternal age > 38 years (6.1% or women). Karyotyping encouraged for women with positive result on one or more index test. No details of reference standard for index test negative women | Routine screening | Prospective cohort | France | ||||
| X | X | X | Median 39 for Down's cases, 34 for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | ||
| X | X | Unclear (maybe all ≥ 38) | Amniocentesis or CVS | High‐risk referral for invasive testing | Case control | Italy | |||
| Median 35 (17‐49) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | |||||
| X | X | Not reported | CVS (high‐risk women) or follow‐up to birth | Routine screening | Retrospective cohort | Spain | |||
| X | Mean 32 | Karyotyping or follow‐up to birth | Routine screening and high‐risk referral | Prospective cohort | Spain | ||||
| X | Median 31 (14‐47), 20% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | ||||
| X | Median 28 (13‐46), 19.4% ≥ 35 | Antenatal karyotyping (5.9% of pregnancies: 62% of high‐risk, 29% of medium‐risk and 3% of the low‐risk women). Follow‐up to birth (85.3% of women) | Routine screening | Prospective cohort | Brazil | ||||
| X | ≥ 35 (35‐44) | Amniocentesis in women high risk on screening (16.2%). Follow‐up at birth in women who were low risk on screening | High‐risk patients undergoing routine screening | Retrospective cohort | Italy | ||||
| X | Median 33 (IQR 31‐36), 36.2% ≥ 35 | Karyotyping or follow‐up to birth in 96.1% of patients | Routine screening | Prospective consecutive cohort | USA | ||||
| Median 30 (20‐44) for Down's cases, 32 (19‐40) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | China | |||||
| X | Not reported | Karyotyping | Screening programmes for syphilis and Down's syndrome | Case control | Denmark | ||||
| X | Median 37.5 for Down's cases, 36.4 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | ||||
| X | Median 36 (25‐44) for Down's cases, 29 (17‐45) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | ||||
| Median 37 (16‐48) | CVS | High‐risk referral for invasive testing | Prospective cohort | USA | |||||
| X | Median 35 (18‐50) | CVS or amniocentesis (in high risk women) or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| Cocciolone 2008 (first trimester screening cohort) | X | Median 31.3 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Australia | |||
| X | Mean 38 (16‐49) for Down's cases, 29 (13‐56) for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | ||||
| X | Mean 37.0 (IQR 32.9‐40.5) for Down's cases, 32.4 (IQR 29.0‐35.9) for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | UK | ||||
| X | X | Median 29.9, 15.4% ≥ 35 | CVS (offered where women had high NT measurements), amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| X | X | Not reported | CVS and amniocentesis | High‐risk referral for invasive testing | Case control | Netherlands | |||
| X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Cohort | Denmark | ||||
| X | Median 33 (15‐49), 36.1% > 35 | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Germany, Switzerland and Austria | ||||
| X | Median 35.1 (13.2‐46.7) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | ||||
| X | 49% ≤ 35, 51% ≥ 36 | Invasive testing or follow‐up to birth | Routine screening | Retrospective cohort | Netherlands | ||||
| X | X | Not reported | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Belgium | |||
| Median 35.4 (18‐49) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | |||||
| X | Mean 30.7, 21.2% ≥ 35 | CVS, amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | Australia | ||||
| X | Median 28 (15‐49) 6.9% ≥ 35 | Amniocentesis or CVS in patients with previous Down’s pregnancy, > 35 years or with a positive biochemical test result. Other women underwent scan at 22 weeks and, if NT >2.5 mm special examination directed to examination of fetal heart. Follow‐up to birth | Routine screening | Prospective cohort | Austria | ||||
| X | X | X | Median 28.3 (17‐45) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Turkey | ||
| X | Median 37 (21‐48) | CVS | High‐risk referral for invasive testing | Prospective cohort | Australia | ||||
| X | Mean 31.1 (16‐46), 22% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Germany | ||||
| X | Median 30 (15‐47), mean 29.8 (SD 3.3) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Taiwan | ||||
| X | Mean 33 (16‐51), 18.5% ≥ 37 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | ||||
| Jaques 2010 FTS (first trimester screening) | X | Mean 16.3% ≥ 37 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Australia | |||
| X | X | Mean 35.4 (14.1‐52.2) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | |||
| X | Mean 29.9 (SD 3.3) | Amniocentesis or CVS in patients considered high risk (NT > 2.5, aged > 35 years, positive biochemical test result, history or chromosomal abnormality, fetal structural abnormality at ultrasound or other reason). Follow‐up to birth | Routine screening | Retrospective cohort | South Korea | ||||
| X | Median 37 (IQR 36‐39) | Karyotyping or follow‐up to birth | Routine screening | Case control | Netherlands | ||||
| Kozlowski 2007 GC (Gynaecologists' practices) | X | X | Median 32 (15‐48), 26.4% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | ||
| Kozlowski 2007 PC (Prenatal centre) | X | X | Median 34 (14‐46), 43.2% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Cohort | Germany | ||
| X | X | 34.7% ≥ 35 | Not reported | Routine screening | Prospective cohort | USA | |||
| X | 51% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Sweden | ||||
| X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Case control | Netherlands | ||||
| X | Mean 30.5 (19% ≥ 35) for unaffected pregnancies | Women considered high risk offered CVS (0.7%) or amniocentesis (11.8%). | Routine screening | Prospective cohort | Hong Kong | ||||
| X | X | Median 32 (IQR 30‐35), 27.4% ≥ 35 | Amniocentesis or follow‐up to birth | Routine screening | Prospective cohort | China | |||
| X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | ||||
| Median 35 (17‐49) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | |||||
| Median 34.5 (14.1‐50.1) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | |||||
| X | Mean 30.1 (16‐47), 22.1% ≥ 35 | Amniocentesis (in women considered high risk, n = 510) or follow‐up to birth | Routine screening | Prospective cohort | USA | ||||
| X | 21.6% ≥ 35 | Amniocentesis offered to women with positive results from any screening test. Follow‐up to birth | Routine screening | Prospective cohort | USA | ||||
| X | Median 31.3 (18‐45), 19.7% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Italy | ||||
| X | Mean 37.8 (35‐43) | Amniocentesis (unclear in which patients this was conducted) or follow‐up to birth | Screening of patients ≥ 35 years of age | Prospective cohort | Indonesia | ||||
| X | X | Mean 38.5 (SD 4.0) for Down's cases, 35.5 (SD 4.0) for controls | Not reported | Routine screening | Case control | Sweden | |||
| Median 35 (17‐46) | Fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks | High‐risk referral for invasive testing | Prospective cohort | UK and Portugal | |||||
| Median 35 (17‐46) | Fetal karyotyping. In cases where NT above 95th percentile or abnormal ductus venousus flow, follow‐up scan conducted at 14‐16 weeks | High‐risk referral for invasive testing | Prospective cohort | Portugal | |||||
| X | Median 35 (15‐42) | CVS or follow‐up | High‐risk referral for invasive testing | Prospective cohort | UK | ||||
| Maxwell 2011 FTS (first trimester screening cohort) | X | Median 31 (14‐48), 24.3% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| Mean 33.7 (SD 4.9) for Down's cases, 30.3 (SD 4.5) for controls | Amniocentesis (recommended for women with higher risk on first or second trimester testing) or follow‐up to birth | Routine screening | Case control | Israel | |||||
| X | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Case control | USA | |||
| X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Germany | ||||
| X | Mean 30.1 (13‐50), 21.1% ≥ 35, 11.9% ≥ 37 | Karyotyping in women considered at risk due to index test results, age or family history or those with considerable anxiety (632 women, 8.5%) or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| Molina 2010 high risk (High‐risk cohort) | X | Mean 32.7 (16.7‐47.5) | CVS | High‐risk referral for invasive testing | Cohort | Spain | |||
| Molina 2010 screening (Screening cohort) | X | Not reported | Karyotyping or follow‐up to birth | Routine screening | Cohort | Spain | |||
| X | Median 32 (14‐49) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Italy | ||||
| X | Mean 31.1 (14‐49), 25.9% ≥35 | Invasive testing offered to women considered high risk from screening results or follow‐up to birth | Routine screening | Prospective cohort | Spain | ||||
| X | Mean 35.5 (SD 4.8) for Down's cases, 31.7 (SD 3.4) for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Korea | ||||
| X | X | Not reported | Invasive testing (offered to women with high NT measurement) or follow‐up to birth | Routine screening | Retrospective cohort | France | |||
| X | Median 38 (22‐47) | Fetal karyotyping by amniocentesis (52%) or CVS (48%) | High‐risk referral for invasive testing | Prospective cohort | UK | ||||
| X | Median 31 (13‐49) | Amniocentesis or CVS (patients considered high risk based on screening). First trimester presence/absence of nasal bone, presence/absence of tricuspid regurgitation or normal/abnormal Doppler studies (patients of intermediate risk on first trimester screening and did not undergo CVS or amniocentesis. With the addition of information from these tests, if adjusted risk was high, CVS was performed). Follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| X | X | 17.5% ≥ 35 | Invasive testing (patients considered high risk based on NT screening) or follow‐up to birth. | Routine screening | Prospective cohort | Finland | |||
| Median 34 (15‐47), 47% ≥ 35 | Karyotyping performed (27% of women) due to increased NT (14%), advanced maternal age (10%), previous chromosomally abnormal child (0.5%) or parental anxiety (2%). | Routine screening in a high risk population | Prospective cohort | UK | |||||
| X | Median 32 | CVS, amniocentesis or neonatal karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Australia | ||||
| X | X | Median 31 (14‐47), 20% ≥ 35 | CVS or amniocentesis (women assessed to be high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| Okun 2008 FTS (first trimester screening cohort) | X | Mean 34 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | |||
| X | X | Range 15 to 46, 35% ≥ 35 | Not reported | Routine screening | Prospective cohort | Italy | |||
| X | Median 31.7 (SD 4.0) for Down's cases, 36.5 (SD 4.1) for unaffected pregnancies | CVS or amniocentesis (women considered high risk on screening on the basis of NT and biochemical results, but not on nasal bone screening, or if requested due to age or anxiety) or follow‐up to birth | Routine screening (2 centres) or in referred patients (1 centre) | Prospective cohort | Italy and Netherlands | ||||
| X | Median 30.5 (SD 8.2) | Not reported | Routine screening | Retrospective cohort | Italy | ||||
| X | Median 36 (19‐44) | CVS | High‐risk referral for invasive testing | Prospective cohort | Argentina | ||||
| X | Mean 31.4 (SD 5.7), 24% ≥ 35 | Prenatal karyotyping offered to patients considered high risk or maternal anxiety (conducted in 24%) or follow‐up to birth | Routine screening | Prospective cohort | Netherlands | ||||
| X | Mean 37.6 (22‐46) | Prenatal karyotyping | High‐risk referral for invasive testing | Consecutive cohort | Netherlands | ||||
| Palomaki 2007 FTS (first trimester screening cohort) | Mean 32.3 (SD 4.6) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | ||||
| X | Median 33.0 (IQR 31.0‐36.0) | CVS or amniocentesis. Cytogenetic testing in cases of miscarriage. Follow‐up to birth. | Routine screening | Retrospective cohort | USA | ||||
| X | Median 37 (19‐46) | CVS | High‐risk referral for invasive testing | Prospective cohort | UK | ||||
| X | Mean 31.4 (14.5‐50.2) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| X | Mean 30.1 (15‐46) (SD 5.37), 18% ≥ 35 | Invasive testing offered to patients considered high risk at screening (> 1:300) or follow‐up to birth | Routine screening | Prospective cohort | Spain | ||||
| X | 29.5, 17.7% ≥35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Finland | ||||
| X | Median 30.5 (18‐37) | Amniocentesis offered to patients with NT >3mm or serum marker risk was > 1:250, or follow‐up to birth | Routine screening | Prospective cohort | France | ||||
| X | Mean 30.9 (SD 4.5) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Canada | ||||
| X | X | Median 33.1, 30.1% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | China | |||
| X | Median 30.7 (18.0‐46.3) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | France | ||||
| X | X | Mean 30.6 (14‐46) | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Spain | |||
| X | Mean 28 (SD 5) | Invasive testing (women with high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| X | X | 31.0% ≥35 | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Germany | |||
| X | Median 36.5 (18‐47) | Invasive testing or follow‐up to birth | Routine screening | Retrospective cohort | Netherlands | ||||
| X | Mean 28 (15‐46), 10.7% ≥ 35 | CVS (offered to patients with first trimester NT > 3.5 mm), amniocentesis (offered to patients with first trimester NT 2.5‐3.4 mm, high risk on second trimester serum testing (> 1:250) and those > 35 years) or follow‐up to birth | Routine screening | Retrospective cohort | Austria | ||||
| X | X | 13% > 35 | CVS and amniocentesis (offered to patients with increased risk (> 1:400) at first trimester screening. CVS recommended when NT > 3.5 or when women did not want to wait until the 15th week for amniocentesis), or follow‐up to birth | Routine screening | Prospective cohort | Austria | |||
| X | Mean 29.4 (16‐47) | Invasive testing (women considered high risk on screening) or follow‐up to birth | Routine screening | Prospective consecutive cohort | UK | ||||
| X | X | Median 32 (15‐44), 29% ≥ 35 | Invasive testing or follow‐up to birth | Routine screening | Prospective cohort | Australia | |||
| X | X | Median 33 (14‐47), 35.4% ≥ 35 | CVS, amniocentesis, cordocentesis or follow‐up to birth | Routine screening | Prospective cohort | Chile | |||
| X | Median 31 (14‐49) | CVS and amniocentesis (9.6% of women) or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| X | Median 34 (23‐44) for Down's cases; mean 30.4 (16‐45), 16.5% ≥ 35 for unaffected pregnancies | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Denmark | ||||
| X | X | X | Median 38 (19‐46) for Down's cases, 36 (15‐47) for controls | Invasive testing (high‐risk women) or follow‐up to birth | Routine screening | Case control | UK | ||
| Median 36 (20‐44) for Down's cases, 30 (16‐41) for controls | Not reported | Routine screening | Case control | UK | |||||
| X | Median 35.8 for Down's cases, 29.3 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | ||||
| X | Median 32 (14‐45), 27% ≥ 35 | Invasive testing offered to women with screening risk of > 1:250 or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| X | Median 28.6 (15‐42) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Slovenia | ||||
| X | Median 29 (16‐48), 7.8% ≥ 37 | CVS or amniocentesis or follow‐up to birth. Unclear reference standard in cases of intrauterine death, miscarriages and terminations. | Routine screening | Prospective cohort | Greece | ||||
| X | Mean 29 (15‐45) | CVS (offered to patients considered high risk on screening) or follow‐up to birth | Routine screening | Prospective cohort | UK | ||||
| Mean 34.5 (19‐45) | Karyotyping or follow‐up to birth | Routine screening | Prospective cohort | Netherlands | |||||
| X | Mean 35 for Down's cases, 31 for controls | Karyotyping or follow‐up to birth | Routine screening | Case control | Denmark | ||||
| X | Median 33.1, 36.9% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | UK | ||||
| X | Mean 29.6, 18.6% ≥ 35 | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | Finland | ||||
| Median 32 (18‐47) | CVS or follow‐up to birth | Routine screening | Prospective cohort | Italy | |||||
| X | X | X | Not reported | Invasive testing (following second trimester screening) or follow‐up to birth | Routine screening | Case control | UK and Austria | ||
| X | X | Mean 35 (SD 4.6), 50% ≥ 35 | Invasive testing. Miscarriage with cytogenetic testing. Follow‐up to birth | Routine screening | Prospective cohort | USA | |||
| X | Mean 36.7 (SD 3.2) | Karyotyping or follow‐up to birth | Routine screening | Retrospective cohort | USA | ||||
| X | X | Mean 29, 10.8% ≥ 35 | Invasive testing (in cases of increased risk) or follow‐up to birth | Referrals for screening | Prospective cohort | Denmark | |||
| X | Median 34.9 (15‐48) | Karyotyping or follow‐up to birth | Routine screening | Cohort | Netherlands | ||||
| X | Median 35.2 (16‐52) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK | ||||
| X | Median 31.9 (IQR 27.7‐35.8) | Karyotyping or follow‐up to birth | Routine screening | Cohort | UK, Denmark and Cyprus | ||||
| X | Median 33 (14‐48) | Amniocentesis, CVS or follow‐up to birth | Routine screening | Prospective cohort | Italy | ||||
| *The study provided data for the subset of women with maternal age of 35 or more. X indicates that the test was evaluated in the study. CVS = chorionic villus sampling; IQR = interquartile range; SD = standard deviation. | |||||||||
| Correction made for missing false negatives in studies with delayed verification of test negatives | NT | NT and maternal age | NT, PAPP‐A, free ßhCG and maternal age | ||||||
| Ratio of DORs (95% CI); P value | Sensitivity at 5% FPR (95% CI) (studies) | Ratio of DORs (95% CI); P value | Sensitivity at 5% FPR (95% CI) (studies) | Ratio of DORs (95% CI); P value | Sensitivity at 5% FPR (95% CI) (studies) | ||||
| Screening (n = 9) | High risk (n = 4) | Screening (n = 46) | High risk (n = 4) | Screening (n = 66) | High risk (n =3) | ||||
| No FN correction | 0.68 (0.26, 1.77); P = 0.40 | 73 (62, 81) | 64 (45, 80) | 0.34 (0.17, 0.69); P = 0.003 | 72 (68, 76) | 47 (31, 63) | 0.41 (0.16, 1.00); P = 0.05 | 88 (86, 89) | 74 (54, 88) |
| FN increased +10% | 0.69 (0.27, 1.78); P = 0.40 | 70 (59, 79) | 62 (42, 78) | 0.40 (0.20, 0.82); P = 0.01 | 69 (64, 73) | 47 (31, 64) | 0.48 (0.19, 1.20); P = 0.11 | 86 (84, 87) | 74 (53, 88) |
| FN increased +20% | 0.74 (0.29, 1.92); P = 0.50 | 69 (57, 78) | 62 (42, 78) | 0.43 (0.21, 0.89); P = 0.02 | 67 (63, 71) | 47 (31, 64) | 0.51 (0.20, 1.28); P = 0.15 | 85 (83, 87) | 74 (54, 88) |
| FN increased +30% | 0.81 (0.31, 2.09); P = 0.63 | 67 (55, 76) | 62 (42, 78) | 0.46 (0.22, 0.97); P = 0.04 | 66 (61, 70) | 47 (30, 64) | 0.55 (0.22, 1.38); P = 0.20 | 84 (82, 86) | 74 (54, 88) |
| FN increased +40% | 0.76 (0.29, 2.02); P = 0.55 | 66 (53, 76) | 59 (39, 77) | 0.50 (0.24, 1.02); P = 0.06 | 64 (60, 68) | 47 (31, 64) | 0.59 (0.24, 1.48); P = 0.26 | 83 (81, 85) | 74 (54, 88) |
| FN increased +50% | 0.81 (0.30, 2.15): P = 0.65 | 64 (52, 75) | 59 (39, 77) | 0.52 (0.25, 1.08); P = 0.08 | 63 (58, 67) | 47 (30, 64) | 0.62 (0.25, 1.56); P = 0.31 | 82 (80, 84) | 74 (54, 88) |
| DOR = diagnostic odds ratio | |||||||||
| Test | No. of studies | No. of participants |
| 1 Aberrant right subclavian artery Show forest plot | 1 | 425 |
| 2 Frontomaxillary facial angle >95 percentile Show forest plot | 1 | 242 |
| 3 Presence of mitral gap Show forest plot | 1 | 217 |
| 4 Maxillary bone length, 5% percentile Show forest plot | 1 | 927 |
| 5 Tricuspid regurgitation Show forest plot | 1 | 312 |
| 6 Iliac angle 90 degrees Show forest plot | 1 | 2032 |
| 7 Ductus venosus a‐wave reversed Show forest plot | 1 | 378 |
| 8 Ductus venosus pulsivity index > 95 percentile Show forest plot | 1 | 378 |
| 9 Nasal bone, mixed cut‐points Show forest plot | 11 | 48279 |
| 10 NT, 2.5 mm Show forest plot | 4 | 11835 |
| 11 NT, 3 mm Show forest plot | 6 | 10381 |
| 12 NT, 5FPR Show forest plot | 3 | 63885 |
| 13 NT, mixed cut‐points Show forest plot | 13 | 90978 |
| 14 NT and age, risk 1:100 Show forest plot | 1 | 10668 |
| 15 NT and age, risk 1:250 Show forest plot | 10 | 79412 |
| 16 NT and age, risk 1:300 Show forest plot | 23 | 252811 |
| 17 NT and age, 1FPR Show forest plot | 4 | 98453 |
| 18 NT and age, 3FPR Show forest plot | 4 | 98453 |
| 19 NT and age, 5FPR Show forest plot | 22 | 288853 |
| 20 NT and age, mixed cut‐points Show forest plot | 50 | 530874 |
| 21 NT and nasal bone, Absent NB + NT ≥ 95th centile Show forest plot | 1 | 486 |
| 22 Ductus and age, risk 1:250 Show forest plot | 1 | 3731 |
| 23 Ductus and age, 5FPR Show forest plot | 2 | 3965 |
| 24 Ductus and age, mixed cut‐points Show forest plot | 5 | 5331 |
| 25 Ductus, NT and age, risk 1:100 Show forest plot | 1 | 19736 |
| 26 Ductus, NT and age, risk 1:250 Show forest plot | 1 | 3727 |
| 27 Ductus, NT and age, 5FPR Show forest plot | 2 | 3961 |
| 28 Ductus, NT and age, mixed cut‐points Show forest plot | 3 | 23697 |
| 29 Age and nasal bone, mixed cut‐points Show forest plot | 4 | 25303 |
| 30 Age, NT and tricuspid blood flow, risk 1:100 Show forest plot | 1 | 19736 |
| 31 Age, NT and nasal bone, risk 1:100 Show forest plot | 1 | 19736 |
| 32 Age, NT and nasal bone, risk 1:300 Show forest plot | 4 | 9963 |
| 33 Age, NT and nasal bone, mixed cut‐points Show forest plot | 5 | 29699 |
| 34 Age, NT, nasal bone and ductus, risk NT>1:300 AND abnormal DV flow AND absent NB Show forest plot | 1 | 544 |
| 35 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 20305 |
| 36 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 41842 |
| 37 Age, NT and free ßhCG, 1st trimester, 5FPR Show forest plot | 4 | 4986 |
| 38 Age, NT and free ßhCG, 1st trimester, risk 1:240 Show forest plot | 1 | 5809 |
| 39 Age, NT and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 5 | 10795 |
| 40 Age, NT and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 1507 |
| 41 Age, NT and PAPP‐A, 1st trimester, risk 1:185 Show forest plot | 1 | 5809 |
| 42 Age, NT and PAPP‐A, 1st trimester, 5FPR Show forest plot | 3 | 2498 |
| 43 Age, NT and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 5 | 9814 |
| 44 Age, NT and total hCG, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 45 Age, NT and AFP, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 46 Age, NT and ITA, 1st trimester, 5FPR Show forest plot | 1 | 278 |
| 47 Age, NT and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| 48 Age, NT and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| 49 Age, NT and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| 50 Age, NT and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 51 Age, NT and inhibin, 1st trimester, mixed cut‐points Show forest plot | 2 | 1150 |
| 52 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:100 Show forest plot | 10 | 102332 |
| 53 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:150 Show forest plot | 5 | 177643 |
| 54 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:200 Show forest plot | 8 | 135768 |
| 55 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:220 Show forest plot | 1 | 2231 |
| 56 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:250 Show forest plot | 25 | 174712 |
| 57 Age, NT, PAPP‐A and free ßhCG, 1st trimester, risk 1:300 Show forest plot | 29 | 544681 |
| 58 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 1FPR Show forest plot | 7 | 88874 |
| 59 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 3FPR Show forest plot | 9 | 312680 |
| 60 Age, NT, PAPP‐A and free ßhCG, 1st trimester, 5FPR Show forest plot | 24 | 391874 |
| 61 Age, NT, PAPP‐A and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 69 | 1173853 |
| 62 Age, NT, PAPP‐A and uE3, 1st trimester, 5FPR Show forest plot | 1 | 576 |
| 63 Age, NT, PAPP‐A and ITA, 1st trimester, 5FPR Show forest plot | 2 | 11053 |
| 64 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| 65 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| 66 Age, NT, PAPP‐A and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| 67 Age, NT, PAPP‐A and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 68 Age, NT, PAPP‐A and inhibin, 1st trimester, mixed cut‐points Show forest plot | 2 | 1150 |
| 69 Age, NT, PAPP‐A and ADAM12, 1st trimester, 5FPR Show forest plot | 2 | 1042 |
| 70 Age, NT, PAPP‐A and ADAM12, 1st trimester, risk 1:250 Show forest plot | 1 | 691 |
| 71 Age, NT, free ßhCG and ADAM12, 1st trimester, 5FPR Show forest plot | 1 | 351 |
| 72 Age, NT, AFP and free ßhCG, 1st trimester, risk 1:250 Show forest plot | 1 | 1656 |
| 73 Age, NT, AFP and free ßhCG, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 74 Age, NT, AFP and free ßhCG, 1st trimester, mixed cut‐points Show forest plot | 2 | 2766 |
| 75 Age, NT, AFP and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 76 Age, NT, total hCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 77 Age, NT, total hCG and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 78 Age, NT, free ßhCG and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 79 Age, NT, PAPP‐A, free ßhCG, 1st trimester serum, ductus venosus pulsivity index, 5FPR Show forest plot | 1 | 7250 |
| 80 Age, free ßhCG and PAPP‐A, if risk 1:42‐1:1000, NT, final 1:250 risk Show forest plot | 1 | 10189 |
| 81 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 2 | 26986 |
| 82 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, risk 1:250 Show forest plot | 2 | 10325 |
| 83 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 10325 |
| 84 Age, NT, ductus, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 30061 |
| 85 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 19736 |
| 86 Age, NT, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:300 Show forest plot | 1 | 1801 |
| 87 Age, NT, tricuspid blood flow, free ßhCG and PAPP‐A, 1st trimester, risk 1:100 Show forest plot | 1 | 19736 |
| 88 Age, NT, fetal heart rate, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 76385 |
| 89 Age, NT, fetal heart rate, nasal bone, free ßhCG and PAPP‐A, 1st trimester, risk 1:200 Show forest plot | 1 | 19736 |
| 90 age, NT, fetal heart rate, ductus, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 19614 |
| 91 Age, NT, fetal heart rate, tricuspid blood flow, free ßhCG and PAPP‐A,1st trimester, 5FPR Show forest plot | 1 | 19736 |
| 92 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, risk 1:250 Show forest plot | 1 | 5483 |
| 93 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, 5FPR Show forest plot | 2 | 1306 |
| 94 Age, NT, AFP, free ßhCG and PAPP‐A, 1st trimester, mixed cut‐points Show forest plot | 3 | 6789 |
| 95 Age, NT, total hCG, inhibin and PAPP‐A, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 96 Age, NT, PAPP‐A, free ßhCG and PGH, 1st trimester, 5FPR Show forest plot | 1 | 335 |
| 97 Age, NT, PAPP‐A, free ßhCG and GHBP, 1st trimester, 5FPR Show forest plot | 1 | 335 |
| 98 Age, NT, PAPP‐A, free ßhCG and PIGF, 1st trimester, 5FPR Show forest plot | 2 | 1443 |
| 99 Age, NT, PAPP‐A, free ßhCG and total hCG, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| 100 Age, NT, PAPP‐A, free ßhCG and PP13, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| 101 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, 5FPR Show forest plot | 4 | 2571 |
| 102 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, risk 1:250 Show forest plot | 2 | 1222 |
| 103 Age, NT, PAPP‐A, free ßhCG and ADAM12, 1st trimester, mixed cut‐points Show forest plot | 4 | 2571 |
| 104 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:100 Show forest plot | 1 | 40 |
| 105 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:250 Show forest plot | 1 | 40 |
| 106 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, risk 1:400 Show forest plot | 1 | 40 |
| 107 Age, NT, free ßhCG, PAPP‐A and inhibin, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 108 Age, NT, PAPP‐A, free ßhCG, ADAM12 and PlGH, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| 109 Age, NT, total hCG, inhibin, PAPP‐A, AFP and uE3, 1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 110 Age, NT, free ßhCG, inhibin, PAPP‐A, AFP and uE3,1st trimester, 5FPR Show forest plot | 1 | 1110 |
| 111 Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG and PlGF, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| 112 Age, NT, PAPP‐A, free ßhCG, ADAM12, total hCG, PlGF and PP13, 1st trimester, 5FPR Show forest plot | 1 | 998 |
| 113 NT, free ßhCG and PAPP‐A, 1st trimester incidence rate 63.3% Show forest plot | 1 | 6508 |
| 114 NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age < 35 years Show forest plot | 5 | 19057 |
| 115 NT, PAPP‐A, free ßhCG and maternal age ‐ maternal age ≥ 35 years Show forest plot | 5 | 10980 |

