Intervenciones para el tratamiento de la osteonecrosis del maxilar inferior relacionada con la medicación
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012432.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 06 October 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Oral Health Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Drafted the protocol: NB, OZ
Wrote the protocol: NB, OZ
Developed the search strategy: NB, OZ, and Anne Littlewood (Trials Search Co‐ordinator from the Cochrane Oral Health Group)
Searched for trials: NB, HH, OZ
Extracted data: NB, HH, OZ
Assessed trial for risk of bias: NB, HH, OZ
Assessed quality of the evidence: NB, OZ
Contacted authors of ongoing RCTs: OZ
Performed statistical analysis: NB, BM, OZ
Wrote the review: NB, OZ
Produced ’Summary of findings’ table: NB, OZ
Sources of support
Internal sources
-
Institute of Pharmacology of Natural Products & Clinical Pharmacology, and Institute of Epidemiology and Medical Biometry, Ulm University, Ulm, Germany.
-
Oral Medicine, Diagnosis, and Periodontology Department, Faculty of Dentistry, Cairo University, Egypt.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships‐alliances). Contributors over the past year have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; Swiss Society for Endondontology, Switzerland
Declarations of interest
There are no financial conflicts of interest and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review.
-
Natalie H Beth‐Tasdogan: no interests to declare
-
Benjamin Mayer: no interests to declare
-
Heba Hussein: no interests to declare
-
Oliver Zolk: no interests to declare
Acknowledgements
We thank the editorial team at Cochrane Oral Health, especially Martin McCabe, Anne Littlewood, Laura CI MacDonald, Helen Wakeford, Tanya Walsh, Helen Worthington, and Jo Weldon. We would like acknowledge the external referees Professor Juliet Compston, Professor Thomas B Dodson, and Dr Athanassios Kyrgidis for their helpful feedback, and Jason Elliot‐Smith for final copy editing of the protocol for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Jul 12 | Interventions for managing medication‐related osteonecrosis of the jaw | Review | Natalie H Beth-Tasdogan, Benjamin Mayer, Heba Hussein, Oliver Zolk, Jens-Uwe Peter | |
| 2017 Oct 06 | Interventions for managing medication‐related osteonecrosis of the jaw | Review | Natalie H Beth‐Tasdogan, Benjamin Mayer, Heba Hussein, Oliver Zolk | |
| 2016 Nov 09 | Interventions for managing medication‐related osteonecrosis of the jaw (MRONJ) | Protocol | Natalie H Beth‐Tasdogan, Benjamin Mayer, Heba Hussein, Oliver Zolk | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Angiogenesis Inhibitors [adverse effects];
- Anti-Bacterial Agents [therapeutic use];
- Bisphosphonate-Associated Osteonecrosis of the Jaw [prevention & control, therapy];
- Bone Density Conservation Agents [adverse effects, therapeutic use];
- Denosumab [adverse effects, therapeutic use];
- Dental Care;
- Diphosphonates [adverse effects, therapeutic use];
- Hyperbaric Oxygenation;
- Imidazoles [adverse effects, therapeutic use];
- Intercellular Signaling Peptides and Proteins [therapeutic use];
- Jaw Diseases [*chemically induced, prevention & control, *therapy];
- Oral Health;
- Osteonecrosis [*chemically induced, prevention & control, *therapy];
- Postoperative Complications [prevention & control, therapy];
- Prostatic Neoplasms [drug therapy];
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Time Factors;
- Tooth Extraction [adverse effects];
- Zoledronic Acid;
Medical Subject Headings Check Words
Female; Humans; Male;
PICOs

Study flow diagram. Results of the search strategy for inclusion of studies in this review
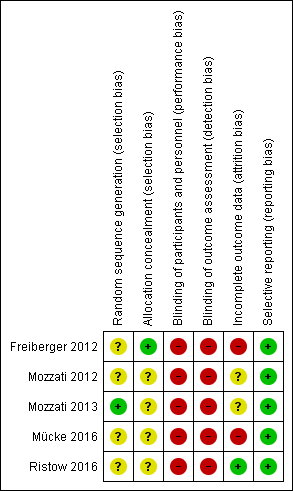
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 1 MRONJ (incidence proportion).
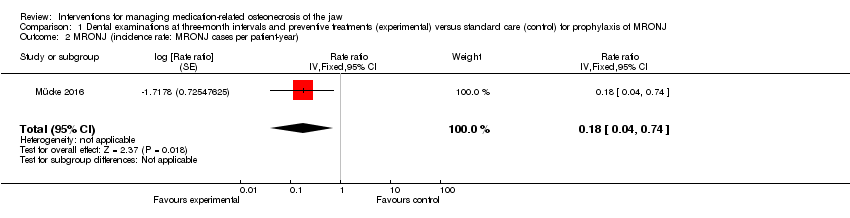
Comparison 1 Dental examinations at three‐month intervals and preventive treatments (experimental) versus standard care (control) for prophylaxis of MRONJ, Outcome 2 MRONJ (incidence rate: MRONJ cases per patient‐year).
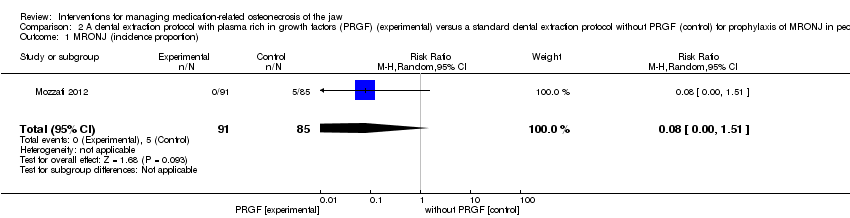
Comparison 2 A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) versus a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions, Outcome 1 MRONJ (incidence proportion).
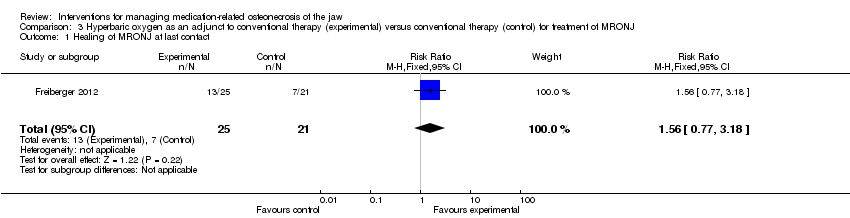
Comparison 3 Hyperbaric oxygen as an adjunct to conventional therapy (experimental) versus conventional therapy (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ at last contact.

Comparison 4 Autofluorescence‐guided bone surgery (experimental) versus tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ, Outcome 1 Healing of MRONJ (defined as mucosal integrity) at 1 year.
| Dental examinations at three‐month intervals and preventive treatments (experimental) compared to standard care (control) for prophylaxis of MRONJ | ||||||
| Population: prophylaxis of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard care (control) | Risk with dental examinations at three‐month intervals and preventive treatments (experimental) | |||||
| MRONJ (incidence proportion) (follow‐up: mean 32 months) | 233 per 1000 | 23 per 1000 | RR 0.10 | 253 | ⊕⊕⊝⊝ | Participants: high‐risk ( i.e. individuals with cancer exposed to intravenous zoledronic acid The outcome MRONJ was also reported as number of cases per patient‐year (incidence rate) rate ratio 0.18 (95% CI 0.04 to 0.74) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by two levels due to very serious risk of bias (high and unbalanced rate of crossovers after randomisation, high drop‐out rates due to high mortality, failure to adhere to the intention‐to‐treat principle, the mean follow‐up differed between experimental and control group). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| A dental extraction protocol with plasma rich in growth factors (PRGF) (experimental) compared to a standard dental extraction protocol without PRGF (control) for prophylaxis of MRONJ in people treated with IV bisphosphonates who need dental extractions | ||||||
| Population: people treated with IV bisphosphonates who need dental extractions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with a standard dental extraction protocol without PRGF (control) | Risk with a dental extraction protocol with PRGF (experimental) | |||||
| MRONJ (incidence proportion) | 59 per 1000 | 5 per 1000 | RR 0.08 | 176 | ⊕⊝⊝⊝ | Participants: high risk, i.e. individuals with cancer exposed to IV zoledronic acid |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (high or unclear risk of selection bias, performance bias, detection bias, and attrition bias). IV = intravenous MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) compared to conventional therapy (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with conventional therapy (control) | Risk with hyperbaric oxygen therapy as an adjunct to conventional therapy (experimental) | |||||
| Healing of MRONJ (follow‐up: up to 24 months (outcome was measured at last follow‐up)) | 333 per 1000 | 520 per 1000 | RR 1.56 | 46 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, detection bias, and attrition bias; failure to adhere to the intention‐to‐treat principle). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| Autofluorescence‐guided bone surgery (experimental) compared to tetracycline fluorescence‐guided bone surgery (control) for treatment of MRONJ | ||||||
| Population: treatment of MRONJ | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with tetracycline fluorescence‐guided bone surgery (control) | Risk with autofluorescence‐guided bone surgery (experimental) | |||||
| Healing of MRONJ (follow‐up: 1 year) | 889 per 1000 | 933 per 1000 | RR 1.05 | 34 participants included in the analysis | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. We downgraded the quality of the evidence by three levels due to imprecision and very serious risk of bias (unclear and high risk of selection bias, performance bias, and detection bias). MRONJ = medication‐related osteonecrosis of the jaw RCT = randomised controlled trial | ||||||
| MRONJ stage | Description |
| AT RISK | No apparent necrotic bone in patients who have been treated with oral or intravenous bisphosphonates |
| STAGE 0 | No clinical evidence of necrotic bone but nonspecific clinical findings, radiographic changes, and symptoms |
| STAGE 1 | Exposed and necrotic bone or fistulas that probes to bone in patients who are asymptomatic and have no evidence of infection |
| STAGE 2 | Exposed and necrotic bone or fistulas that probes to bone associated with infection as evidenced by pain and erythema in the region of exposed bone with or without purulent drainage |
| STAGE 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and ≥ 1 of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e. inferior border and ramus in mandible, maxillary sinus, and zygoma in maxilla) resulting in pathologic fracture, extraoral fistula, oral antral, or oral nasal communication, or osteolysis extending to inferior border of the mandible or sinus floor |
| From the American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw‐‐2014 update (Ruggiero 2014) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| 2 MRONJ (incidence rate: MRONJ cases per patient‐year) Show forest plot | 1 | Rate ratio (Fixed, 95% CI) | 0.18 [0.04, 0.74] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 MRONJ (incidence proportion) Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ at last contact Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healing of MRONJ (defined as mucosal integrity) at 1 year Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.30] |

