Intervenciones para la nefropatía crónica en pacientes con drepanocitosis
Appendices
Appendix 1. Glossary
Alloimmunisation
An immune response to foreign antigens as a result of exposure to donor blood transfusions
Enuresis
Inability to control urination and includes bedwetting by children
Epigenetic
Traits that are not determined by the DNA code itself but rather by modifications of the DNA bases or of proteins associated with DNA
Extravascular
Outside the blood vessel or vascular system
Glomerular
Network of filters in the kidney that filter waste from the blood
Glomerulosclerosis
Scarring or hardening of the glomeruli, the tiny blood vessels in the kidney
Haematopoiesis
The production of red blood cells, white blood cells, and platelets from stem cells within the bone marrow
Hypoxia
Lack of oxygen reaching the cells of the kidney
Intravascular
Within the blood vessel or vascular sytem
Ischaemia
Restriction of blood supply to tissues
Nephropathy
Damage to or disease of the kidney
Renal papillary necrosis
This is a disorder of the kidneys in which all or part of the kidney papillae die. The kidney papillae are the areas where the openings of the collecting ducts enter the kidney, and where the urine flows into the ureters
Renal tubules
Small tube‐shaped structures that remove salt, excess fluids, and waste products from the blood
Splenic sequestration
This happens when large pools of sickled red blood cells are trapped in the spleen resulting in damage to the spleen
Appendix 2. Search strategies
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Anemia, Sickle Cell] explode all trees
#2 MeSH descriptor: [Hemoglobin, Sickle] this term only
#3 ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbAS or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases")
#4 (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*)
#5 (sickle and SCD)
#6 ((Hb S or HbS or sickle) near/3 (disease* or thalass?emi*))
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Kidney Diseases] explode all trees
#9 MeSH descriptor: [Urologic Diseases] this term only
#10 ((kidney or renal) near/5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*))
#11 ("end‐stage renal" or "end‐stage kidney" or "endstage renal" or "endstage kidney" or "chronic kidney" or "chronic renal")
#12 (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD)
#13 MeSH descriptor: [Hematuria] this term only
#14 MeSH descriptor: [Proteinuria] explode all trees
#15 (proteinuria* or hematuria* or haematuria* or hemoglobinuria* or haemoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin near/2 creatine))
#16 MeSH descriptor: [Renal Replacement Therapy] explode all trees
#17 ((renal or kidney) near/3 (transplant* or replacement*))
#18 (predialysis or pre‐dialysis or dialysis or hemodialysis or haemodialysis or hemofiltration or haemofiltration or hemodiafiltration or haemodiafiltration)
#19 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 #7 and #19
#21 sickle cell nephropathy
#22 #20 or #21
MEDLINE (OvidSP)
1. exp Anemia, Sickle Cell/
2. Hemoglobin, Sickle/
3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw,kf.
4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw,kf.
5. (sickle and SCD).tw,kf.
6. ((Hb S or HbS or sickle) adj3 (disease* or thalass?emi*)).tw,kf.
7. or/1‐6
8. exp Kidney Diseases/
9. Urologic Diseases/
10. ((kidney or renal) adj5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)).tw,kf.
11. (end‐stage renal or end‐stage kidney or endstage renal or endstage kidney or chronic kidney or chronic renal).tw,kf.
12. (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD).tw,kf.
13. Hematuria/
14. exp Proteinuria/
15. (proteinuria* or h?ematuria* or h?emoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin adj2 creatine)).tw,kf.
16 exp Renal Replacement Therapy/
17. ((renal or kidney) adj3 (transplant* or replacement*)).tw,kf.
18. (predialysis or pre‐dialysis or dialysis or h?emodialysis or h?emofiltration or h?emodiafiltration).tw,kf.
19. or/8‐18
20. 7 and 19
21. sickle cell nephropathy.tw,kf.
22. 20 or 21
23. randomized controlled trial.pt.
24. controlled clinical trial.pt.
25. randomi*.tw.
26. placebo.ab.
27. clinical trials as topic.sh.
28. randomly.ab.
29. groups.ab.
30. trial.tw.
31. or/23‐30
32. exp animals/ not humans/
33. 31 not 32
34. 22 and 33
Embase (OvidSP)
1. exp Sickle Cell Anemia/
2. Hemoglobin S/
3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw,kw.
4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw,kw.
5. (sickle and SCD).tw,kw.
6. ((Hb S or HbS or sickle) adj3 (disease* or thalass?emi*)).tw,kw.
7. or/1‐6
8. exp Kidney Disease/
9. Urinary Tract Disease/
10. ((kidney or renal) adj5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)).tw,kw.
11. (end‐stage renal or end‐stage kidney or endstage renal or endstage kidney or chronic kidney or chronic renal).tw,kw.
12. (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD).tw,kw.
13. Hematuria/
14. exp Proteinuria/
15. (proteinuria* or h?ematuria* or h?emoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin adj2 creatine)).tw,kw.
16. exp Renal Replacement Therapy/
17. ((renal or kidney) adj3 (transplant* or replacement*)).tw,kw.
18. (predialysis or pre‐dialysis or dialysis or h?emodialysis or h?emofiltration or h?emodiafiltration).tw,kw.
19. or/8‐18
20. 7 and 19
21. sickle cell nephropathy.tw,kw.
22. 20 or 21
23. crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/
24. (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or doubl* blind* or singl* blind* or assign* or allocat* or volunteer*).mp.
25. 23 or 24
26. 22 and 25
27. limit 26 to embase
CINAHL (EBSCOHost)
S1 (MH "Anemia, Sickle Cell+")
S2 TX ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbAS or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases" OR sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*)
S3 TX ((Hb S or HbS or sickle) N3 (disease* or thalass?emi*))
S4 S1 OR S2 OR S3
S5 (MH "Kidney Diseases+")
S6 (MH "Urologic Diseases")
S7 ((kidney or renal) N5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*))
S8 ("end‐stage renal" or "end‐stage kidney" or "endstage renal" or "endstage kidney" or "chronic kidney" or "chronic renal")
S9 (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD)
S10 (MH "Hematuria")
S11 (MH "Proteinuria+")
S12 (proteinuria* or hematuria* or haematuria* or hemoglobinuria* or haemoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin N2 creatine))
S13 (MH "Renal Replacement Therapy+")
S14 ((renal or kidney) N3 (transplant* or replacement*))
S15 (predialysis or pre‐dialysis or dialysis or hemodialysis or haemodialysis or hemofiltration or haemofiltration or hemodiafiltration or haemodiafiltration)
S16 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
S17 S4 AND S16
S18 sickle cell nephropathy
S19 S17 OR S18
S20 (MH Clinical Trials+)
S21 PT Clinical Trial
S22 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*))
S23 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*))
S24 TI randomi* OR AB randomi*
S25 MH RANDOM ASSIGNMENT
S26 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three))
S27 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) )
S28 MH PLACEBOS
S29 TI placebo* OR AB placebo*
S30 MH QUANTITATIVE STUDIES
S31 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30
S32 S19 AND S31
PubMed
#1 ("hemoglobin S" OR "haemoglobin S" OR "hemoglobin SC" OR "haemoglobin SC" OR "hemoglobin SE" OR "haemoglobin SE" OR "hemoglobin SS" OR "haemoglobin SS" OR "hemoglobin C disease" OR "hemoglobin D disease" OR "hemoglobin E disease" OR "haemoglobin C disease" OR "haemoglobin D disease" OR "haemoglobin E disease" OR "Hb SC" OR HbSC OR HbAS OR HbSS OR HbAC OR "Hb SE" OR "Hb SS" OR "Hb C disease" OR "Hb D disease" OR "Hb E disease" OR "SC disease" OR "SC diseases" OR "sickle cell" OR "sickle cells" OR sicklemia OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR (sickle and SCD))
#2 ((Hb S OR HbS OR sickle) AND (disease* OR thalass?emi*))
#3 #1 OR #2
#4 ((kidney OR renal) AND (disease* OR injur* OR insufficienc* OR function* OR dysfunction* OR abnormal* OR damage* OR failure* OR complication* OR manifestation*))
#5 ("end‐stage renal" OR "end‐stage kidney" OR "endstage renal" OR "endstage kidney" OR "chronic kidney" OR "chronic renal" OR ESRF OR ESKF OR ESRD OR ESKD OR CKF OR CKD OR CRF OR CRD OR CAPD OR CCPD OR APD)
#6 (proteinuria* OR hematuria* OR haematuria* OR hemoglobinuria* OR haemoglobinuria* OR erythrocyturia* OR albuminuria* OR microalbuminuria* OR macroalbuminuria* OR (albumin AND creatine))
#7 ((renal OR kidney) AND (transplant OR transplants OR transplantation OR transplantations OR transplanted OR replacement*))
#8 (predialysis OR pre‐dialysis OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration)
#9 #4 OR #5 OR #6 OR #7 OR #8
#10 #3 AND #9
#11 sickle cell nephropathy
#12 #10 OR #11
#13 (random* OR blind* OR "control group" OR placebo* OR "controlled study" OR groups OR trial* OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR pubmed OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb])
#14 #12 AND #13
Transfusion Evidence Library
sickle AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy)
LILACS
tw:(sickle AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
IndMed
(sickle OR sicklemia OR sickled OR sickling OR SC disease) AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy) AND (randomized OR randomised OR randomly OR random OR blind OR blinded OR trial OR placebo OR control group OR groups)
KoreaMed & PakMediNet
"randomized controlled trial" [PT] AND sickle [ALL]
Web of Science CPCI‐S
TOPIC: (sickle OR sicklemia OR sickled OR sickling) AND TOPIC: (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy) AND TOPIC: (random* OR blind* OR control group OR placebo OR controlled study OR groups OR trial OR trials OR systematic review OR meta‐analysis OR metaanalysis OR medline OR pubmed OR cochrane OR embase)
ClinicalTrials.gov
Search Terms: kidney OR renal OR dialysis OR hemodialysis OR hemofiltration OR hemodiafiltration OR proteinuria OR hematuria OR hemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy
Condition: sickle cell anemia
Study Type: Interventional Studies
WHO ICTRP
Title: kidney OR renal OR dialysis OR hemodialysis OR hemofiltration OR hemodiafiltration OR proteinuria OR hematuria OR hemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy
Condition: sickle cell anemia
Recruitment Status: ALL

Sickle cell nephropathy pathophysiology in sickle cell disease: Adapted fromOkafor 2013andNath 2015
RBC: red blood cells; FSGS: focal segmental glomerulosclerosis; ESRD: end‐stage renal disease

Structure of the kidney. From: Wikispaces. Human Physiology. 12. Urology.https://humanphysiology2011.wikispaces.com/12.+Urology

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Hydroxyurea vs placebo, Outcome 1 Slower progression or improvement in GFR (mL per min per 1·73 m²).

Comparison 1 Hydroxyurea vs placebo, Outcome 2 Improvement in ability to concentrate urine (mOsm/kg).

Comparison 1 Hydroxyurea vs placebo, Outcome 3 SAEs assessed with acute chest syndrome.
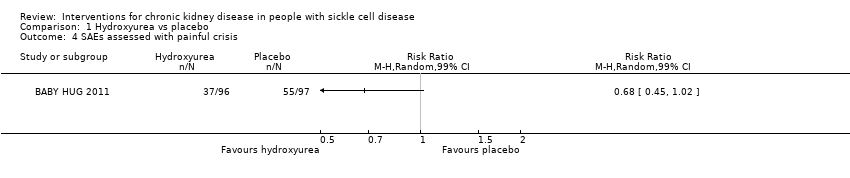
Comparison 1 Hydroxyurea vs placebo, Outcome 4 SAEs assessed with painful crisis.
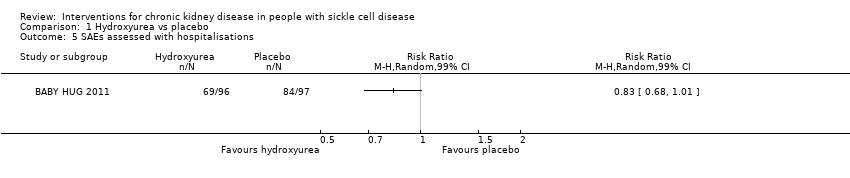
Comparison 1 Hydroxyurea vs placebo, Outcome 5 SAEs assessed with hospitalisations.

Comparison 1 Hydroxyurea vs placebo, Outcome 6 SAEs assessed with stroke.
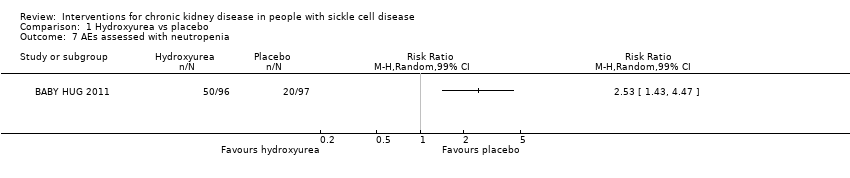
Comparison 1 Hydroxyurea vs placebo, Outcome 7 AEs assessed with neutropenia.

Comparison 1 Hydroxyurea vs placebo, Outcome 8 AEs assessed with thrombocytopenia.

Comparison 1 Hydroxyurea vs placebo, Outcome 9 Number of participants transfused.

Comparison 2 ACEI (captopril) vs placebo, Outcome 1 Slower progression or reduction in proteinuria (mg/day).

Comparison 2 ACEI (captopril) vs placebo, Outcome 2 Other drug‐related adverse events (dry cough).
| Hydroxyurea compared to placebo for preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with hydroxyurea | |||||
| Slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) | The mean slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) was 146.64 (43.7) | MD 0.58 higher | ‐ | 142 | ⊕⊝⊝⊝ | |
| Improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) | The mean improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) was 494.57 (110.07) | MD 42.23 higher | ‐ | 178 | ⊕⊕⊝⊝ | |
| SAEs assessed with acute chest syndrome | Study population | RR 0.39 | 193 | ⊕⊕⊝⊝ | ||
| 186 per 1000 | 72 per 1000 | |||||
| SAEs assessed with painful crisis | Study population | RR 0.68 | 193 | ⊕⊕⊝⊝ | ||
| 567 per 1000 | 386 per 1000 | |||||
| SAEs assessed with hospitalisations | Study population | RR 0.83 | 193 | ⊕⊕⊝⊝ | ||
| 866 per 1000 | 719 per 1000 | |||||
| Mortality due to any cause | No deaths reported in either group | not estimable | 193 | ⊕⊕⊝⊝ | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by one due to unclear risk of attrition bias. | ||||||
| ACEI compared to placebo in preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with ACEI | |||||
| Slower progression or reduction in proteinuria (mg/day 6 months follow‐up) | The mean slower progression or reduction in proteinuria (mg/day 6 months follow‐up) was 76 (45) | MD 49.00 lower | ‐ | 22 | ⊕⊝⊝⊝ | |
| Improvement in ability to concentrate urine mOsm/kg | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with acute chest syndrome | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with painful crisis | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with hospitalisations | Not reported | ‐ | ‐ | ‐ | ||
| Mortality due to any cause | Not reported | ‐ | ‐ | ‐ | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ||
| ACEI: angiotensin converting enzyme inhibitor; CI: confidence interval; MD: mean difference; SAEs: serious adverse events | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the quality of evidence by two due to unclear or high risk of bias in all domains. | ||||||
| Unadjusted HRs reported in BABY HUG 2011 | ||
| Outcome | HR | 95% CI |
| Acute chest syndrome | 0.36 | 0.15 to 0.87 |
| Painful crisis | 0.54 | 0.36 to 0.83 |
| Hospitalisations | 0.73 | 0.53 to 1.00 |
| Neutropenia | 3.0 | 1.7 to 5.1 |
| Thrombocytopenia | 1.6 | 0.6 to 4.1 |
| Transfusions | 0.55 | 0.32 to 0.96 |
| CI: confidence interval | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or improvement in GFR (mL per min per 1·73 m²) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement in ability to concentrate urine (mOsm/kg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SAEs assessed with acute chest syndrome Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 4 SAEs assessed with painful crisis Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 5 SAEs assessed with hospitalisations Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 6 SAEs assessed with stroke Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 99% CI) | Totals not selected | |
| 7 AEs assessed with neutropenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 8 AEs assessed with thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 9 Number of participants transfused Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Slower progression or reduction in proteinuria (mg/day) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 6 months follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Other drug‐related adverse events (dry cough) Show forest plot | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |


