Corticosteroides sistémicos para la otitis media aguda en niños
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012289.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 15 March 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Acute Respiratory Infections Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Respati W Ranakusuma (RR) drafted the protocol and contributed as a primary review author, selected studies for inclusion, extracted data, assessed the risk of bias, entered data into Review Manager 5, and carried out and interpreted the analysis.
Yupitri Pitoyo (YP) selected studies for inclusion, extracted data, and assessed the risk of bias.
Eka Dian Safitri (EDS) selected studies for inclusion, extracted data, and assessed the risk of bias.
Sarah Thorning (ST) developed and ran the search strategy, and obtained copies of studies.
Elaine M Beller (EMB) carried out and interpreted the analysis, contributed as the fourth review author for disagreements on methodological/statistical issues, and checked the correct use of grammar.
Sudigdo Sastroasmoro (SS) drafted the protocol, contributed to drafting the final review, and checked the correct use of grammar.
Chris B Del Mar (CDM) drafted the protocol and contributed as the fifth review author for disagreements on clinical issues (if needed), drafted the final review, and checked the correct use of grammar.
Declarations of interest
Respati W Ranakusuma: none known
Yupitri Pitoyo: none known
Eka Dian Safitri: none known
Sarah Thorning: none known
Elaine M Beller: her work on this review was supported by a grant from the National Health and Medical Research Council, Australia, to the Centre for Research in Evidence‐Based Practice, Bond University
Sudigdo Sastroasmoro: none known
Chris B Del Mar: none known
Acknowledgements
This review is part of a PhD project at the Centre for Research in Evidence‐Based Practice, Bond University (CREBP) Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Australia. It is supported by the CREBP, Bond University.
We would especially like to thank Clare Dooley and Liz Dooley for their assistance in the preparation of the protocol. We wish to thank the following people for commenting on the draft protocol: Jean Symes, Zaina AlBalawi, Brian Westerberg, Simona Nistor‐Grahl, Ravi Shankar, and Michelle Guppy. We thank the following people for commenting on the draft review: Shunjie Chua, Esther Martin Anna Granath, Mark Jones, and Michelle Guppy.
The methods section of this protocol is based on a standard template developed by Cochrane Airways and adapted by the Cochrane Acute Respiratory Infections Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 15 | Systemic corticosteroids for acute otitis media in children | Review | Respati W Ranakusuma, Yupitri Pitoyo, Eka D Safitri, Sarah Thorning, Elaine M Beller, Sudigdo Sastroasmoro, Chris B Del Mar | |
| 2016 Jul 20 | Systemic corticosteroids for acute otitis media in children | Protocol | Respati W Ranakusuma, Yupitri Pitoyo, Eka D Safitri, Sarah Thorning, Elaine M Beller, Sudigdo Sastroasmoro, Chris B Del Mar | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Child, Preschool; Humans; Infant;
PICOs
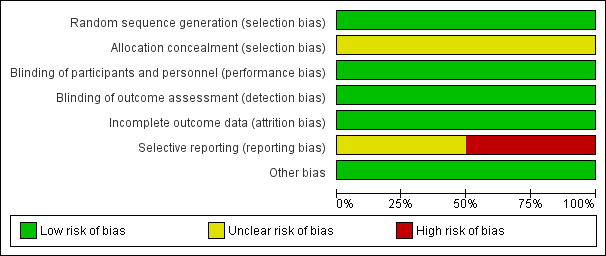
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Systemic corticosteroids versus placebo for children with acute otitis media, Outcome 1 Reduction of overall or specific symptoms at various time points.
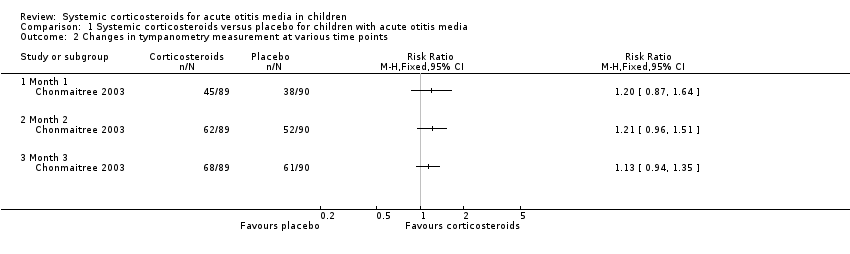
Comparison 1 Systemic corticosteroids versus placebo for children with acute otitis media, Outcome 2 Changes in tympanometry measurement at various time points.

Comparison 1 Systemic corticosteroids versus placebo for children with acute otitis media, Outcome 3 AOM recurrence at various time points.
| Systemic corticosteroids versus placebo for children with acute otitis media | ||||||
| Patient or population: children with acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with systemic corticosteroids | |||||
| Proportion of children with pain at various time points | ||||||
| Day 5 | Study population | Not estimable | (0 studies) | ‐ | Only pre‐treatment data were available. We could not retrieve post‐treatment data. | |
| 0 per 1000 | 0 per 1000 | |||||
| Day 14 | Study population | Not estimable | (0 studies) | ‐ | Only pre‐treatment data were available. We could not retrieve post‐treatment data. | |
| 0 per 1000 | 0 per 1000 | |||||
| Reduction of overall or specific symptoms at various time points | ||||||
| Day 5 | Study population | RR 1.06 | 179 | ⊕⊕⊝⊝ | This outcome was represented as the proportion of children for whom symptoms and inflamed eardrum(s) resolved and who did not require additional antibiotic treatment. | |
| 889 per 1000 | 942 per 1000 | |||||
| Day 14 | Study population | RR 1.05 | 179 | ⊕⊕⊝⊝ | This outcome was represented as the proportion of children for whom symptoms and inflamed eardrum(s) resolved and who did not require additional antibiotic treatment. | |
| 867 per 1000 | 910 per 1000 | |||||
| Reduction in overall or specific symptom duration | Study population | Not estimable | (0 studies) | ‐ | No study provided data for this outcome. | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects | Study population | Not estimable | (0 studies) | ‐ | The available data were reported as an overall result. We could not retrieve data from each individual group (overall side effects across all groups: drowsiness (22% to 34%), dry mouth (16% to 27%), increased urine amount (14% to 27%), nappy rash (7% to 32%), nervousness (7% to 20%), and decreased urine amount (0% to 11%)). | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to study limitations (unclear description of the allocation concealment and evidence of selective reporting) and one level due to indirectness (differences between the outcome of interest (i.e. in reporting, measurements) and reported outcomes). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reduction of overall or specific symptoms at various time points Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Day 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Changes in tympanometry measurement at various time points Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Month 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Month 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Month 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 AOM recurrence at various time points Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Month 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Month 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Month 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 During Month 4 to Month 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


