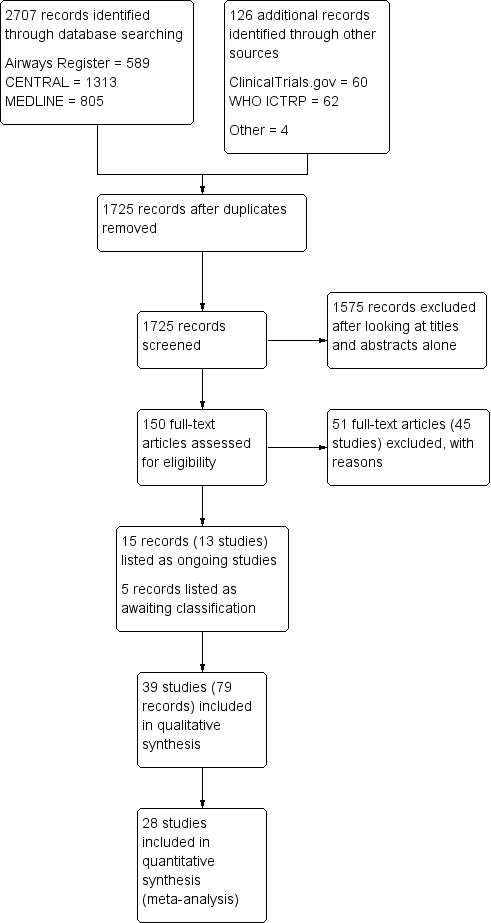
Intervenciones para mejorar el cumplimiento con los corticosteroides inhalados para el asma
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 24 weeks Setting: Medical Research Institute of New Zealand and the P3 Research Clinical Trials Unit at Bowen Hospital, Wellington, New Zealand Trial registration: ACTRN12606000508572 | |
| Participants | Population: 111 adolescents and adults with asthma randomised to intervention (combination inhaler) (n = 57) or control (separate inhaler) (n = 54) Age: 16 to 65 years; mean (SD) age in the adherence group 45.5 (13.8) years and in the control group 49.2 (11.2) years Baseline asthma severity: Those with a significant exacerbation in the last month were excluded Inclusion criteria: adults in the Wellington region 16 to 65 years of age; diagnosis of asthma; and currently taking ICS at a stable dose with or without a separate LABA inhaler Exclusion criteria: diagnosis of chronic obstructive pulmonary disease, current use of a combination ICS/LABA inhaler, pregnant or lactating women, history of other clinically significant disease, significant exacerbation of asthma in the previous month requiring clinic or hospital attendance Percentage withdrawn: 5.3% from the adherence group and 9.3% from the control group Other allowed medication: not reported | |
| Interventions | Intervention summary: 125 mg FP and 25 mg salmeterol in a combination Smartinhaler, 2 actuations twice daily. The Smartinhaler casing recorded the date and time of each actuation. Participants were not told that adherence would be monitored Control summary: 125 mg FP and 25 mg salmeterol in separate Smartinhalers, 2 actuations twice daily. The Smartinhaler casing recorded the date and time of each actuation. Participants were not told that adherence would be monitored Complex intervention: no | |
| Outcomes | Outcomes measured: FEV1, ACQ, Asthma Exacerbation Questionnaire, need for oral steroids or doctor visits over previous 6 weeks. Primary adherence measure was percentage of doses taken over last 6 weeks of the study; secondary adherence measures were adherence during the other 6‐week periods of the study, percentage of fully adherent days, proportion who were > 50%, > 80% or > 90% adherent over each 6‐week period, overuse defined as > 2 doses taken within a 6‐hour period or > 4 doses within a 24‐hour period (% of days when this occurred) Adherence calculation: electronic Smartinhaler data ‐ number of doses taken as a percentage of those prescribed. All calculations were made after exclusion of dose dumping, defined as 6 or more actuations within a 5‐minute period | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: GlaxoSmithKline (GSK) GSK ID number: SAM106689 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by computer‐generated random code supplied by a statistician. The sequence was imbedded in a Microsoft Access Database (Microsoft Corp, Redmond, Wash) by a third party and concealed from the researchers until the time the subject was enrolled and entered into the database" |
| Allocation concealment (selection bias) | Low risk | "The sequence was imbedded in a Microsoft Access Database (Microsoft Corp, Redmond, Wash) by a third party and concealed from the researchers until the time the subject was enrolled and entered into the database" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Although participants were aware that they were taking combined or separate inhalers, adherence was measured covertly with a SmartInhaler; this was the main outcome measured. However, ACQ may be at risk of performance bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Although blinding of outcome assessors was not described, adherence was measured objectively with a SmartInhaler; this was the only outcome measured. However, ACQ is participant reported and may be at risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Only 8 participants withdrew (3 from the combined inhaler group and 5 from the separate inhaler group). All are accounted for in the flow diagram, and drop‐out occurred for similar reasons |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (ACTRN12606000508572). All outcomes listed in trial registration have been clearly reported, but the study mentions the Asthma Exacerbation Questionnaire and the need for oral steroids and doctor visits over the previous 6 weeks, which are not reported in the paper |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 4 months Setting: 1 paediatric asthma clinic within an outer metropolitan general hospital in Australia Trial registration: not reported | |
| Participants | Population: 26 children with asthma randomised to receive adherence feedback (n = 14) or usual care (n = 12) Age: 6 to 14 years; mean age in the adherence feedback group 9.1 years and in the control group 9.3 years Baseline asthma severity: intervention group: FEV1 % predicted = 72.9, mean fluticasone dose (mcg/d) 300; number with symptoms or reliever use 3 or more times per week = 10. Control group: FEV1 % predicted = 77.5, mean fluticasone dose (mcg/d) 250, number with symptoms or reliever use 3 or more times per week = 8 Inclusion criteria: Children given a diagnosis of asthma at between 6 and 14 years of age (inclusive) were eligible for enrolment if their asthma was not well controlled despite prescribed preventive medication. Suboptimal control was based on reported history of asthma symptoms (wheeze or limitation of activity) occurring more than twice a week and requiring reliever medication and/or reduced lung function (reproducible FEV1 < 80% predicted) Exclusion criteria: not reported Percentage withdrawn: no withdrawal from trial Other allowed medication: not reported | |
| Interventions | Intervention summary: Adherence data collected via Smartinhaler were shared with the child, parent and physician during consultation for those allocated to the intervention group. These data were incorporated in the management plan for the coming month. Reviews were performed monthly with the child's usual physician Control summary: Children in the control group had their Smartinhaler collected and were given a new device. Their adherence remained unknown to parent, child and respiratory physician. Reviews were performed monthly with the child's usual physician Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence, symptoms (via questionnaire), lung function Adherence calculation: Adherence was calculated as a percentage of prescribed doses registered by the Smartinhaler, between midnight and midday or between midday and midnight for morning and evening doses, respectively, or at any time during the day for once‐daily dosing | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After providing informed written consent, children were randomly allocated to either the intervention or control group through the use of sealed opaque envelopes" Not clear how the order of sealed envelopes was generated |
| Allocation concealment (selection bias) | Low risk | "After providing informed written consent, children were randomly allocated to either the intervention or control group through the use of sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Although primary outcome ‐ adherence ‐ was measured by an electronic counter, other outcomes (such as SABA use) may be subject to performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Main outcome ‐ adherence ‐ objectively measured, but other outcomes (such as reported SABA use) subject to detection bias as the unblinded parent is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified; symptoms measured but not reported so could not be included in meta‐analysis. No measure of variance is given for the adherence outcome, nor for the secondary outcomes of FEV1 and controller medication use. P values are not exact (1 decimal place). Other outcomes reported appropriately |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 90 weeks Setting: 1 site. Brazil Trial registration: ADERE PEDIATRIC 1 (GSK trial register) | |
| Participants | Population: 298 children with asthma randomised to a telephone follow‐up intervention (n = 149) or to usual care (n = 149) Age: 6 to 14 years; mean age (SD) in the intervention group 8.9 (2.4) years and in the control group 9.0 (2.5) years Baseline asthma severity: Of those who completed the trial in the intervention group, 67 had moderate and 41 severe asthma, and in the control group, 74 had moderate and 37 severe asthma Inclusion criteria: moderate or severe asthma defined by SPT II Brazillian Consensus on Asthma Management Exclusion criteria: comorbidities that may interfere with study evaluation, systemic steroids required for more than 7 days; patients treated with allergen immunotherapy Percentage withdrawn: 28% from the intervention group and 27% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: medical guidance and follow‐up telephone call from a healthcare professional every 15 days Control summary: medical guidance; no telephone follow‐up Complex intervention: no | |
| Outcomes | Outcomes measured: level of compliance, disease control evaluated by 5‐point questionnaire, quality of life (SF‐36) Adherence calculation: percentage of actual number of doses of salmeterol/fluticasone propionate divided by number of expected doses | |
| Notes | Type of publication: pharmaceutical company report Funding: GlaxoSmithKline NB: participants from non‐intervention group not followed up, no conclusions drawn from protocol. No peer‐reviewed publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized to intervention or non‐intervention" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel; described as open‐label |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors; described as open‐label |
| Incomplete outcome data (attrition bias) | High risk | > 25% drop‐out in both groups. Control group not followed up as planned, so missing data for entire outcomes for this group. Study protocol was violated |
| Selective reporting (reporting bias) | High risk | Multiple planned outcomes, including primary outcome (adherence 'not available'), or available only for the intervention group |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: 10 weeks Setting: single site; participants recruited through newspaper adverts; in association with community allergy practices. USA Trial registration: not reported | |
| Participants | Population: 50 adults with asthma randomised to an interactive voice response (IVR) intervention (n = 25) or usual care (UC) (n = 25) Age: 18 to 65 years; mean age (SD) in IVR group 39.6 (12.8) years and in UC group 43.5 (14.3) years Baseline asthma severity: physician‐diagnosed asthma for which they were prescribed daily inhaled corticosteroid treatment; no other severity information given Inclusion criteria: adults 18 to 65 years old who had physician‐diagnosed asthma for which they were prescribed daily inhaled corticosteroid treatment. Participants were recruited through newspaper advertising and in co‐operation with community allergy practices and received $25 for each completed study visit Exclusion criteria: significant disease or disorder that, in the opinion of the investigator, might influence results of the study or the patient’s ability to participate in the study (this included other chronic health disorders, current substance abuse or dependence, mental retardation or psychiatric disorder); current participation in another asthma‐related research or clinical trial Percentage withdrawn: no withdrawal Other allowed medication: not specifically reported | |
| Interventions | Intervention summary: 2 automated IVR telephone calls separated by 1 month, with 1 additional call if recently reported symptoms of poorly controlled disease or failure to fill a prescription. Calls were completed in less than 5 minutes and included content designed to inquire about asthma symptoms, deliver core educational messages, encourage refilling of inhaled corticosteroid prescriptions and increase communication with providers Control summary: usual care Complex intervention: no | |
| Outcomes | Outcomes measured: AQLQ, ACT, BMQ, adherence with use of an electronic monitor Adherence calculation: electronic adherence device or canister weight to give a mean % adherence (exact details of calculation not provided) | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: supported by the Investigator‐Sponsored Study Program of AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization table generated before study initiation determined group assignment by order of entry into the study |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants described. Main outcome ‐ adherence ‐ objectively measured, but other outcomes such as ACQ and AQLQ subject to performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Investigators remained blind to treatment until final data set was completed. However, for participant‐reported outcomes such a AQLQ and ACQ, the participant is the outcome assessor; therefore these outcomes are at high risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Although attrition not specifically reported, end of study data given for all 50 randomised participants |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, but all outcomes stated in methods clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: parallel‐group randomised controlled trial; blinding not stated Duration: 2 months Setting: set in New Zealand; no other details reported Trial registration: not reported | |
| Participants | Population: 40 children with asthma randomised to an inhaler alarm intervention (n = 20) or usual care (n = 20) Age: 7 to 17 years; no further details reported Baseline asthma severity: 'symptomatic asthma despite being on inhaled corticosteroids' Inclusion criteria: children aged 7 to 17 years with symptomatic asthma despite taking inhaled corticosteroids Exclusion criteria: not reported Percentage withdrawn: withdrawal not reported Other allowed medication: not reported | |
| Interventions | Intervention summary: inhaler alarm with 14 different tones, 1 for each morning and evening of the week Control summary: usual care (inhaler alarm turned off) Complex intervention: no | |
| Outcomes | Outcomes measured: AQLQ, prebronchodilator FEV1, use of salbutamol, adherence to inhaled steroid Adherence calculation: Adherence was expressed as a percentage; exact calculation not reported | |
| Notes | Type of publication: conference abstract Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Some outcomes (e.g. AQLQ) may be influenced by knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessor described, and not clear how adherence data were collected and calculated. Self‐report outcomes (e.g. AQLQ) may be subject to detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | High risk | Conference abstract; no trial registration identified. Study reported only as a conference abstract from 2008 and does not appear to have been published in full. Therefore, limited details about methods and outcomes, in particular, no measure of variance for the AQLQ |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, multi‐centre, parallel‐group randomised controlled trial Duration: 12 weeks Setting: 4 general practices and a hospital outpatient clinic. UK Trial registration: not reported | |
| Participants | Population: 102 adults with asthma randomised to receive a combined inhaler (n = 51) or separate inhalers (n = 51) Age: 18 to 70 years; mean age of all trial completers (36 in each group) 44 years (range 20 to 69 years) Baseline asthma severity: mean duration of illness 13.9 years (range 0.25 to 54 years). No details of baseline asthma severity given Inclusion criteria: patients with asthma, 18 to 70 years of age, who required treatment with regular inhaled steroids and beta‐agonists (as assessed by their own doctor) Exclusion criteria: not reported Percentage withdrawn: 30% from each trial arm Other allowed medication: not reported | |
| Interventions | Intervention summary: Treatment group was given 1 Turbuhaler inhaler containing a fixed combination of terbutaline (250 μg per dose) and budesonide (100 μg per dose) Control summary: Control group was given 2 Turbuhaler inhalers ‐ 1 containing terbutaline (250 μg per dose) and 1 containing budesonide (100 μg per dose) Complex intervention: no | |
| Outcomes | Outcomes measured: adherence, lung function measures (FVC and FEV1) Adherence calculation: percent adherence = number of doses taken × 100/number of doses prescribed ‐ measured using Turbuhaler Inhalation Computer | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: study funded by the Astra Clinical Research Unit, which also provided the Turbuhaler Inhalation Computer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly divided into treatment and control groups" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | Open‐label design; although outcomes (adherence with an electronic monitor and lung function) are unlikely to be highly susceptible to influence according to participants' and personnel's knowledge of group allocation. "In order to obtain as accurate a picture of "normal" behaviour as possible, patients were not told that the Turbuhalers contained TICs [Turbuhaler Inhalation Computer] or that their compliance was being monitored" |
| Blinding of outcome assessment (detection bias) | Low risk | Open‐label design, although outcomes (adherence with a covert electronic monitor and lung function) are unlikely to be highly susceptible to influence according to outcome assessors' knowledge of group allocation |
| Incomplete outcome data (attrition bias) | High risk | Approximately 30% drop‐out in both arms of the trial. Participants who dropped out were younger but otherwise did not differ from those who completed according to trial report. However, no flow diagram presented, so unclear if reasons for drop‐out were balanced |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, but all outcomes stated in methods clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: parallel‐group randomised controlled trial; blinding not stated Duration: 13 weeks Setting: private and public paediatric respiratory clinics. Australia Trial registration: not reported | |
| Participants | Population: 47 children with asthma randomised to receive a 'Funhaler' (n = 26) or a control spacer (n = 21) Age: 18 months to 7 years; mean age in the Funhaler group 3.4 years and in the control group 3.8 years Baseline asthma severity: intervention group: mean frequency of wheeze (5‐point scale) = 1.9; number with exacerbation in previous month = 8; mean fluticasone dose (mg/d) = 166. Control group: mean frequency of wheeze (5‐point scale) = 1.9; number with exacerbation in previous month = 3; mean fluticasone dose (mg/d) = 193 Inclusion criteria: children with diagnosis of asthma, 18 months to 7 years of age, taking preventive asthma medication on a daily basis Exclusion criteria: not reported Percentage withdrawn: 8% from the intervention arm and 5% from the control arm Other allowed medication: not reported | |
| Interventions | Intervention summary: small‐volume spacer that incorporates an incentive toy (spinning disk and whistle) that is driven by the child’s expired breath (the 'Funhaler') Control summary: a control spacer (Aerochamber Plus) Complex intervention: no | |
| Outcomes | Outcomes measured: adherence, symptoms (from a 'symptoms questionnaire'), exacerbations (defined as the child having received a course of prednisolone initiated by the parent in response to an escalation of symptoms requiring regular reliever medication more than 4th‐hourly for 24 hours as per asthma management plan or prescription of prednisolone by the child’s primary care physician) Adherence calculation: Adherence was evaluated as a percentage of prescribed doses registered by the Smartinhaler between midnight and midday and between midday and midnight for morning and evening doses, respectively, or at any time during the day for once‐daily dosing | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All subjects were then randomized to either the FunHaler or a control spacer using a minimization computer program (Minim) with equal weighting for age, sex and level of maternal education" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Although primary outcome ‐ adherence ‐ was measured by an electronic counter, other outcomes (such as symptoms) may be subject to performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Main outcome ‐ adherence ‐ objectively measured, but other outcomes (such as symptoms) subject to detection bias, as the unblinded parent is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Attrition low and balanced (< 10% in both arms) and all drop‐outs accounted for |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified; symptoms measured but not reported numerically so could not be included in meta‐analysis. Other outcomes reported appropriately |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 6 months Setting: participants recruited from emergency departments, followed up in community. New Zealand Trial registration: ACTRN12613001353785 | |
| Participants | Population: 220 children with asthma randomised to receive an audiovisual inhaler reminder (n = 110) or usual care (n = 110) Age: 5 to 15 years; mean age (SD) in audiovisual reminder group was 8.9 (2.5) years and in control group was 8.9 (2.6) years Baseline asthma severity: intervention group: mean (SD) asthma morbidity score 9.3 (2.2); mean (SD) Childhood Asthma Control Test score 18.8 (4.4); mean (SD) FEV1 (% predicted) 92 (17). Control group: mean (SD) asthma morbidity score 9.2 (2.5); mean (SD) Childhood Asthma Control Test score 18.8 (4.2); mean (SD) FEV1 (% predicted) 90 (17) Inclusion criteria: children and adolescents 6 to 15 years of age who attended the regional emergency department in Auckland, New Zealand, with a suspected diagnosis of asthma exacerbation and were screened for eligibility; patients with a diagnosis of acute asthma who were on treatment or needed treatment with twice‐daily inhaled corticosteroids Exclusion criteria: diagnosis of a chronic lung disease other than asthma, congenital heart disease; living outside the Auckland catchment area; diagnosis of a severe chronic medical disorder that causes impaired immunity or increased morbidity Percentage withdrawn: 2% from the intervention arm and 5% from the control arm Other allowed medication: other asthma drugs, including LABAs and theophylline | |
| Interventions | Intervention summary: covert electronic monitoring device for use with preventive inhalers (SmartTrack) with the audiovisual function enabled Control summary: covert electronic monitoring device for use with preventive inhalers (SmartTrack) with the audiovisual function disabled Complex intervention: no | |
| Outcomes | Outcomes measured: adherence to preventive inhaled corticosteroids; number of days absent from school and whether or not parents or carers were absent from work for 1 day or longer; asthma control (cACT); asthma symptoms (Asthma Morbidity Score); exacerbations since previous visit; unscheduled doctor, emergency clinic or hospital visits; rescue medication use; lung function Adherence calculation: Adherence was defined as the proportion of preventer doses taken relative to the number of doses prescribed. This proportion was calculated by measuring the degree of deviation from the prescribed dose up to the prescribed dose (i.e. non‐adherence, up to a maximum of 0% non‐adherence) and subtracting from 1 (i.e. 100% adherence) | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: Health Research Council of New Zealand and Cure Kids | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a simple, unrestricted block randomisation with block sizes of 200, we randomly assigned patients" |
| Allocation concealment (selection bias) | Low risk | "The study statistician provided the randomisation group to investigators in opaque, sealed envelopes, which were opened by investigators and research assistants in consecutive order to allocate participants to their randomisation group. Envelopes were sealed to investigators, and research assistants did not know the next allocation group" |
| Blinding of participants and personnel (performance bias) | High risk | "Participants were unaware of the adherence monitoring function of either device, but were informed that the reliever monitoring device was to be used with their reliever inhaler to enable investigators to know when the drug was running out" Primary outcome ‐ adherence ‐ was monitored covertly and objectively with an electronic device. However, other outcomes such as cACT are subject to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Primary outcome ‐ adherence ‐ was monitored covertly and objectively with an electronic device. However, other outcomes such as cACT and parent‐reported exacerbations are subject to risk of detection bias, as participant or parent is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Low drop‐out (< 5%) in both arms; all participants accounted for and ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Retrospectively registered trial. All planned outcome measures in trial registration and methods reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 24 weeks Setting: participants recruited from research volunteer databases, newspaper advertisements and informal contacts. New Zealand Trial registration: not reported | |
| Participants | Population: 110 people with asthma randomised to receive an audiovisual inhaler reminder (n = 55) or usual care (n = 55) Age: 12 to 65 years; median age (range) in audiovisual reminder group was 39 (13 to 65) years and in control group was 35 (15 to 64) years Baseline asthma severity: intervention group: baseline ICS dose: median (range) 500 (100 to 2000); PEF: mean (SD) 434 (99). Control group: baseline ICS dose: median (range) 500 (100 to 4000); PEF: mean (SD) 444 (128) Inclusion criteria: requirement to take regular ICS at a fixed dose, no exacerbation in previous month or run‐in period, not pregnant or lactating;if of child‐bearing potential, using contraception Exclusion criteria: diagnosis of chronic obstructive pulmonary disease, use of a long‐acting beta‐agonist, history of other clinically significant disease. Individuals were required to not be taking a long‐acting beta‐agonist to avoid the potential influence of such treatment on adherence to ICS therapy Percentage withdrawn: 20% from the intervention arm and 16% from the control arm Other allowed medication: not reported, apart from the criterion that participants could NOT be taking a long‐acting beta‐agonist | |
| Interventions | Intervention summary: covert electronic monitoring device for use with preventive inhalers (SmartInhaler) with the audiovisual function enabled Control summary: covert electronic monitoring device for use with preventive inhalers (SmartInhaler) with the audiovisual function disabled Complex intervention: no | |
| Outcomes | Outcomes measured: adherence to ICS, Asthma Control Questionnaire (ACQ), peak expiratory flow (PEF) Adherence calculation: adherence defined as the proportion of medication taken as prescribed over the latter half of the trial (expressed as a percentage) | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: supported by a research grant from GlaxoSmithKline, UK. The sponsor had no involvement in study design; collection, analysis or interpretation of data; writing of the report; or the decision to submit for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was by reference to a computer‐generated random code" |
| Allocation concealment (selection bias) | Low risk | "The randomization was by reference to a computer‐generated random code concealed from the researcher who opened an envelope at the time of randomization" |
| Blinding of participants and personnel (performance bias) | High risk | "Subjects were informed that the purpose of the study was to determine the outcome when patients with asthma on a wide range of ICS doses and inhaler devices were changed to standard treatment via the novel Smartinhaler MDI device. Subjects were not informed of the electronic adherence monitor placed within their FP MDI" Primary outcome ‐ adherence ‐ was monitored covertly and objectively with an electronic device. However, other outcomes such as ACQ are subject to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Primary outcome ‐ adherence ‐ was monitored covertly and objectively with an electronic device. However, other outcomes such as ACQ are subject to the risk of detection bias, as the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out moderately high (16% to 20%), although quite balanced. All participants accounted for in flow diagram. 11 participants in the intervention group and 9 participants in the control group "did not provide data" in the final 12‐week period of the study. It is not clear whether these participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, but all outcomes stated in methods clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group, multi‐centre randomised controlled trial Duration: 13 weeks Setting: '15 states of the country'. Brazil Trial registration: not reported | |
| Participants | Population: 271 people with asthma randomised to receive telephone calls to promote adherence (n = 140) or usual care (n = 131) Age: 12 years of age and older; mean age (SD) in the telephone call group was 43.3 (15) years and in the control group was 44.4 (16.6) years Baseline asthma severity: intervention group: proportion with severe persistent asthma 47.1%; proportion with history of asthma emergencies 30.7%; proportion with history of asthma hospitalisations 48.6%. Control group: proportion with severe persistent asthma 47.3%; proportion with history of asthma emergencies 38.9%; proportion with history of asthma hospitalisations 53.4% Inclusion criteria: 12 years of age or older with moderate to severe persistent asthma according to GINA criteria and the Third Brazilian Consensus on Asthma Management; residential phone number; ability to comprehend study procedures and to sign the relevant consent form Exclusion criteria: mild persistent asthma, pregnancy or breast feeding, intention to move during the study, regular use or recent past abuse of alcohol or illicit drugs, clinically significant active general medical conditions Percentage withdrawn: Report states that 293 participants were 'screened'; 4 were excluded for not fulfilling inclusion criteria, 8 for not responding to telephone calls and 10 for not returning the monitoring disk to the office. It is not clear whether these participants were excluded before or after randomisation, and if after randomisation, from which arm they were excluded. Baseline characteristics and results are given for only 271 participants Other allowed medication: not reported | |
| Interventions | Intervention summary: telephone calls every 2 weeks to reinforce asthma management and to promote adherence, delivered by a specially trained nursing student Control summary: usual care Complex intervention: no | |
| Outcomes | Outcomes measured: adherence Adherence calculation: percentage of patients taking 85% or more of prescribed doses as measured by electronic monitor | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: funded by GSK‐Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding of participants or personnel described. However, the only outcome measured ‐ adherence ‐ was monitored objectively with an electronic device |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding of participants or personnel described. However, the only outcome measured ‐ adherence ‐ was monitored objectively with an electronic device |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out not reported for each arm (22 dropped out in total); total numbers randomised at start of intervention not clear |
| Selective reporting (reporting bias) | High risk | No prospective trial registration identified. Adherence not reported in a way that can be included in a meta‐analysis (percentages per group with no measure of variance, only an inexact P value |
| Other bias | Unclear risk | None noted |
| Methods | Design: open‐label, 4‐arm cluster randomised trial Duration: 6 months Setting: 60 GPs. To minimise cross‐contamination between intervention groups, only 1 GP from a practice could participate. Australia Trial registration: ACTRN12610000854033 | |
| Participants | Population: 60 GPs (of which 55 attended training, and 43 were available to enrol patients) were randomised to be trained in 1 of the following 4 interventions: personalised adherence discussion (PAD); inhaler reminders and feedback (IRF); PAD + IRF; or usual care. GP participants then enrolled 143 patient participants between them; PAD n = 24; IRF n = 35; PAD + IRF n = 41; usual care n = 43 Age: enrolled patients 14 to 65 years of age; mean age (SD) in PAD group 42.3 (15.6) years; in IRF group 40 (30.7) years; in PAD + IRF group 39.7 (17.1) years; in usual care group 40 (14.1) years Baseline asthma severity: FEV1 % predicted mean (SD) in the PAD group 67.3 (21.3); in the IRF group 84.4 (19.4); in the PAD + IRF group 78.0 (15.2); in the usual care group 75.7 (22.0); percentage prescribed high‐dose (> 500 mcg/d) inhaled steroids: PAD group 54%, IRF group 40%, PAD + IRF group 66%, usual care group 44% Inclusion criteria: 14 to 65 years of age; suboptimal asthma control; twice‐daily ICS/LABA for at least 1 month Exclusion criteria: asthma exacerbation in the last month; use of combined inhaler as maintenance/reliever; major respiratory disease (e.g. COPD); serious uncontrolled medical conditions; clinically important visual or auditory impairment; shift workers with a variable roster; pregnant or lactating women Percentage withdrawn: 13% from the PAD arm, 0% from the IRF arm, 22% from the PAD + IRF arm, 5% from the usual care arm Other allowed medication: not reported | |
| Interventions | Intervention summary (1): PAD: GPs asked participants to complete a short questionnaire about barriers to controller inhaler use. GPs were trained to carry out a personalised discussion about the participant's key barrier(s) to adherence and to help the participant set goals and goal‐achievement strategies around an asthma issue that the participant wished to resolve, using patient‐centered materials Intervention summary (2): IRF: Participants received twice‐daily SmartTrack reminders for missed ICS/LABA doses. They could customise ringtones/ring times, cancel individual reminders or switch reminders off completely. Each month, GPs received an automated e‐mail to view a website graph of their patients' daily ICS/LABA use; the participant could log in to view his or her own graph at any time. GPs were asked to discuss the ICS/LABA use graph with the participant at the study follow‐up visit or at any subsequent appointments, at the GP's discretion. Only GPs in PAD groups were trained in specific communication strategies for discussing adherence Intervention summary (3): PAD + IRF: both PAD and IRF components as outlined above Control summary: All GPs in all groups received usual care training. This included advice on writing an asthma action plan (10 minutes), demonstration and review of inhaler technique (10 minutes) and recent changes to asthma guidelines (15 minutes) Complex intervention: yes | |
| Outcomes | Outcomes measured: ACT score; Mini‐AQLQ; HADS; MARS‐A; FEV1; exacerbations Adherence calculation: monitored with SmartTrack device on inhaler. Calculation of adherence not described | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: National Health and Medical Research Council of Australia (ID571053) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each GP’s patients represented 1 cluster. GPs were randomized separately 1:1 to active and control groups for the 2 interventions, using a 2 × 2 factorial design, allowing the effect of the 2 interventions (given in addition to UC) to be tested separately and together, in comparison with UC alone. Randomization of GPs was by a computer‐generated program prepared by an independent statistician before study start, with an automated minimization algorithm to ensure a balance of randomization across 3 stratification factors" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation concealment for GPs was maintained before study start, and revealed to each GP only during the training workshop" However, it is unclear whether allocation was concealed from investigators until randomisation had occurred |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants (GPs or their patients) described. Most of the outcomes measured are subjective and are susceptible to influence from knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | Primary outcome ‐ ACT ‐ was collected via telephone by a researcher blinded to group allocation. However, for many outcomes, measures are subject to risk of detection bias, as the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Primary analysis was by intention to treat. However, drop‐out was somewhat unbalanced, with 5% dropping out from the usual care group, 13% from the PAD group, 0% from IRF group and 21% from the IRF + PAD group |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, but all outcomes stated in methods clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: 1 year Setting: recruited at outpatient chest clinic and followed up by GPs. Norway Trial registration: not reported | |
| Participants | Population: 78 adults with asthma randomised to an asthma education intervention (n = 39) or usual care (n = 39) Age: 18 to 70 years; mean age (SD) in the intervention group 41 (12) years and in the control group 44 (12) years Baseline asthma severity: FEV1 % predicted (SD) in the intervention group 93 (13) and in the control group 95 (17). 95% were using an ICS at baseline in the intervention group and 97% in the control group Inclusion criteria: asthma, defined as prebronchodilator FEV1 ≥ 80% of predicted value; positive reversibility test;documented 20% spontaneous variability (PEF or FEV1); positive methacholine test Exclusion criteria: unstable coronary heart disease, heart failure, serious hypertension, diabetes mellitus, kidney or liver failure Percentage withdrawn: 18% from the intervention group and 0% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: patient brochure; 2 × 2 hour group sessions (separate groups for asthma and COPD patients). First session delivered by doctor, second by pharmacist; 1 or 2 individual sessions with nurse or physiotherapist; individual treatment plan Control summary: standard treatment plan; GP follow‐up for 1 year Complex intervention: yes | |
| Outcomes | Outcomes measured: patient compliance, GP visits, absenteeism, days in hospital Adherence calculation: Medication compliance was coded to Daily Defined Doses (DDD). Dispensed medication reported from local pharmacies on monthly basis. Compliance calculated as prescribed DDD/dispensed DDD × 100. Defined a priori patients as compliant at 75% | |
| Notes | Type of publication: 2 peer‐reviewed full‐text journal articles reporting different outcomes Funding: Norwegian Medical Association Fund for Quality Improvement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At inclusion they signed a written consent and were then randomized to an intervention group or a control group using random number tables" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Outcomes measured were relatively objective (e.g. exacerbations, hospitalisations, GP visits, absenteeism), but participant knowledge of group allocation may have affected health care‐seeking behaviour |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described; although some outcomes measured were relatively objective and unlikely to be affected by assessors' knowledge of group allocation, patient‐reported outcomes such as QOL may be at risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Unbalanced drop‐out: 0% in control group but 18% in intervention group |
| Selective reporting (reporting bias) | High risk | No prospective trial registration found; multiple publications, each including a different set of outcomes. Not clear if all measured outcomes have been reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: intervention delivered over 65 weeks Setting: school setting. USA Trial registration: NCT00110383 | |
| Participants | Population: 290 children with asthma randomised to supervised ICS therapy at school (n = 145) or usual care (n = 145) Age: 5 to 18 years; mean age (SD) in the intervention group 11.1 (2) years and in the control group 10.8 (2.1) years Baseline asthma severity: intervention group: 22 had mild asthma, 113 moderate asthma and 9 severe asthma; control group: 24 had mild asthma, 115 moderate asthma and 14 severe asthma Inclusion criteria: physician‐diagnosed asthma, requiring daily controller medication Exclusion criteria: children not able to switch medications to budesonide Percentage withdrawn: 14% from the intervention group and 21% from the usual care group Other allowed medication: Children could take additional medications if their physician considered this necessary | |
| Interventions | Intervention summary: Child took inhaler medication at a set time each schoolday under the supervision of staff members. Child was provided education in using the inhaler if he or she was observed to use the inhaler incorrectly. Daily monitoring Control summary: continued usual parent or self‐supervised daily ICS treatment. Daily monitoring Complex intervention: no | |
| Outcomes | Outcomes measured: episode of poor asthma control (EPAC); rescue medications; school absences; peak flow; rescue medication use at school Adherence calculation: not applicable | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: National Institutes of Health Grant R01HL075043; AstraZeneca provided the medications (Pulmicort Turbuhaler) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random sequence of treatment codes, stratified according to school system, was generated" |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed", although no details given regarding how this was achieved |
| Blinding of participants and personnel (performance bias) | High risk | "Patients, their parents, and study staff were not blinded to intervention condition; however, physicians were blinded to their patient's Main outcome (EPAC) measured might be subject to performance bias, as participant knowledge of group allocation may have affected behaviour |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Main outcome (EPAC) measured might be subject to detection bias, as participant knowledge of group allocation may have affected behaviour, such as decision to use rescue medication or absenteeism from school |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out was somewhat higher in the control group (20.7%) than in the intervention group (13.8%), and data do not appear to have been imputed for those who did not complete the study. The length of the study explains the extent of drop‐out, although the quantity of missing data and imbalance between groups may still have affected endpoint scores |
| Selective reporting (reporting bias) | High risk | Prospectively published protocol and main outcome measure ‐ EPAC ‐ clearly reported. However, some data not reported in a way that would allow inclusion in a meta‐analysis (e.g. QOL) |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 9 weeks Setting: schools in the Rochester City School District. USA Trial registration: not reported | |
| Participants | Population: 184 children with asthma randomised to school‐based care (n = 93) or usual care (n = 91) Age: 3 to 7 years; mean age in each group not reported Baseline asthma severity: not reported Inclusion criteria: symptoms consistent with mild persistent or more severe asthma; 3 to 7 years of age; enrolled in the Rochester City School District; family had access to a working telephone for monthly follow‐up telephone calls Exclusion criteria: children scheduled to move from the school district within 6 months; Spanish‐speaking families enrolled in study year 2 only Percentage withdrawn: 4% from the intervention group and 0% from the usual care group Other allowed medication: Children using more than 1 preventive medication were instructed to continue with their other medications (in addition to the fluticasone given through school) at the discretion of their primary care provider | |
| Interventions | Intervention summary: School nurse administered fluticasone once each day the child was in school Control summary: carers and parents notified of their child's asthma severity. No medications received in school through the programme Complex intervention: no | |
| Outcomes | Outcomes measured: number of symptom‐free days during the 2 weeks before the follow‐up interview; asthma symptoms; night‐time asthma symptoms; need for rescue inhaler use; absenteeism Adherence calculation: not applicable | |
| Notes | Type of publication: peer‐reviewed journal article Funding: Halcyon Hill Foundation, Webster, NY; Robert Wood Johnson Foundation’s Generalist Physician Faculty Scholars Program. GlaxoSmithKline, Research Triangle Park, NC, donated fluticasone propionate and spacers used in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by current use of preventive medications and was blocked in groups of 6. Pairs of siblings were assigned randomly to the same group. Randomization cards were made from a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomization cards were made from a table of random numbers and were kept in sealed, opaque, sequentially numbered envelopes until after the baseline assessment was completed. Following randomization, families and primary care providers were notified of the child’s group allocation" |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Some outcomes (e.g. PAQLQ, health care‐seeking behaviour) may be subject to risk of performance bias from knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | "To ensure an unbiased assessment, an independent research group, blinded to each child’s group allocation, conducted the follow‐up interviews" However, for participant‐reported outcomes, such as symptoms and PAQLQ, the unblinded participant is the outcome assessor; therefore, these outcomes are at risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | All but 4 participants (for whom no data were available ‐ all from the intervention group) were included in the primary analysis |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods reported |
| Other bias | Low risk | None noted |
| Methods | Design: parallel‐group randomised controlled trial; blinding not stated Duration: 13 weeks Setting: not reported. UK Trial registration: not reported | |
| Participants | Population: 83 'pre‐school' children with asthma randomised to an asthma education intervention or usual care (n for each group not given) Age: 'pre‐school children'; no further details reported Baseline asthma severity: not reported Inclusion criteria: 'asthmatic pre‐school children'; no further details reported Exclusion criteria: not reported Percentage withdrawn: not reported Other allowed medication: not reported | |
| Interventions | Intervention summary: educational booklet about asthma and its treatment, and clinic consultation based on contents of booklet Control summary: usual care Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence; beliefs and anxieties about adherence Adherence calculation: medication electronically monitored; details of adherence calculation not given | |
| Notes | Type of publication: conference abstract Funding: National Asthma Campaign, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were "randomly allocated" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No description of procedures to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | No description of procedures to blind outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out not reported, so unclear how many participants completed the study |
| Selective reporting (reporting bias) | High risk | Conference abstract, so minimal details given. No prospective trial registration identified |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 6 weeks, with follow‐up to 52 weeks Setting: family home. USA Trial registration: not reported | |
| Participants | Population: 15 children with asthma randomised to adherence improvement strategies (n = 7) or usual care plus education (n = 8) Age: 7 to 12 years; mean age (SD) in the intervention group 9 (1.16) years and in the control group 8.8 (1.67) years Baseline asthma severity: not reported Inclusion criteria: children 7 to 12 years of age with diagnosis of asthma Exclusion criteria: not reported Percentage withdrawn: 0% from the intervention group and 0% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: focused education, monitoring, contingency management, discipline techniques Control summary: comprehensive asthma education covering topics from the "Air Wise" programme Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence (MDILog); pulmonary function; PedsQL Asthma module; healthcare costs Adherence calculation: (number of actuations per day/number of actuations prescribed) × 100 (mean % dose per day per child) | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: National Institute of Child Health & Human Development Grant number HD34784 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was developed by a statistics consultant before participant recruitment to assign children to a group; we assigned children to groups on the basis of this table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No description of procedures to blind participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | No description of procedures to blind outcome assessors; in the case of VAS results and QOL results, the participant/career, who was aware of group allocation, is the outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Very small study; less than 50% in each arm completed the study |
| Selective reporting (reporting bias) | High risk | No prospective trial registration identified. Such small numbers make results difficult to interpret and combine in a meta‐analysis; SDs small despite small sample sizes so will be falsely highly weighted in meta‐analysis. Unable to extract adherence data owing to statistical method (pooled series time analysis) used to analyse and no raw data presented |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group, proof‐of‐concept randomised controlled trial Duration: 9 weeks Setting: recruited through emails sent to 40 largest universities in the UK requesting that those with individuals managing their asthma with an ICS preventer should consider enrolling Trial registration: ISRCTN29399269 | |
| Participants | Population: 216 adults with asthma randomised to an online community intervention ("AsthmaVillage") (n = 99) or no online community intervention ("AsthmaDiary") (n = 117) Age: mean (SD) in the intervention group 27.2 (9.2) years and in the control group 28.8 (10.1) years Baseline asthma severity: not reported Inclusion criteria: individuals managing their asthma with an ICS preventer Exclusion criteria: failed to complete the eligibility questionnaire (n = 256) or baseline measures (n = 228), did not have asthma (n = 105), were not prescribed an ICS preventer inhaler for a weekly regimen of at least 1 dose per week (n = 87), failed to complete informed consent (n = 35), had previously participated in the pilot study (n = 9) Percentage withdrawn: 60.6% from the intervention group and 45.3% from the usual care group ('withdrawn' defined as insufficiently engaging in the intended intervention) Other allowed medication: not reported | |
| Interventions | Intervention summary: an online community in which participants could report their preventer use and write posts, comments or questions. Questions and comments needed to be answered by community members themselves because no experimenter intervention was provided once the trial had begun. The only feedback participants could receive during the trial was that received from other participants because this intervention was optimised for implementation at scale and at low cost. This trial attempted to determine the value of an online community, implemented without the added support of a community manager to engage members Control summary: Control condition comprised an online diary, AsthmaDiary. This online diary was created with the use of Google Forms. A single‐item survey was created: “How many times did you take your preventer?” Participants randomised to the control condition could report the number of puffs and, after entering their unique PIN, hit “submit”. Because participants did not need to log in with a username to fill out the form, participants used a PIN that allowed their posts to be identified by the researcher. Participants in the control condition could not see the posts of other participants and could not otherwise know whether other participants were posting on their condition Complex intervention: no | |
| Outcomes | Outcomes measured: medication adherence (SMAQ), website activity/'adherence' Adherence calculation: SMAQ was recalculated with dichotomous scoring of all variables (more than 2 missed uses was treated as non‐adherent) and reverse scoring of item 4 of the SMAQ (“Thinking about the last week, how often have you not taken your asthma preventer medicine as prescribed?”) | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: funded by a pilot grant from the University of Leeds School of Psychology. A Fulbright Scholarship from the US‐UK Fulbright Commission supported the first study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization occurred through a random number generator, yielding two unequal groups" |
| Allocation concealment (selection bias) | Unclear risk | "The experimenters then manually separated the two lists and emailed both groups log‐in instructions" It seems unlikely that allocation was not concealed given the nature of the study design (i.e. the participant is 'remote'), but this is not a standard description of an allocation procedure, so we cannot be sure exactly what the process entailed |
| Blinding of participants and personnel (performance bias) | High risk | Participants were not blinded to group allocation and knowledge of group allocation, and adherence monitoring may have affected their self‐reported adherence (e.g. those in the intervention arm systematically over‐estimating adherence) |
| Blinding of outcome assessment (detection bias) | High risk | The participant is the outcome assessor for the main outcome ‐ self‐reported adherence ‐ and as participants were aware of group allocation, we consider this outcome to be at high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | Very high and unbalanced drop‐out (60% in intervention arm and 45% in control arm). Although an ITT analysis was performed for the primary outcome ‐ self‐reported adherence ‐ it is unclear how this high level of drop‐out may have impacted the results |
| Selective reporting (reporting bias) | High risk | Trial retrospectively registered (ISRCTN 29399269), but not all outcomes reported in trial report, including AQLQ |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 6 weeks, with follow‐up to 6 weeks Setting: clinic and private practice. USA Trial registration: not reported. | |
| Participants | Population: 16 adults with asthma randomised to twice‐daily (bid) dosing (n = 8) or 4‐times‐daily (qid) dosing (n = 8) Age: over 18 years of age; mean age (SD) in the intervention group 46.9 (10) years and in the control group 42.3 (12.1) years Baseline asthma severity: intervention group: 2 on maintenance oral steroids; control group: 4 on maintenance oral steroids Inclusion criteria: clinical stable asthma, requiring regular ICS Exclusion criteria: not reported Percentage withdrawn: 0% from the intervention group and 0% from the usual care group Other allowed medication: "other asthma therapy continued throughout the study" | |
| Interventions | Intervention summary: 4 inhalations flunisolide twice daily Control summary: 2 inhalations flunisolide, 4 times daily Complex intervention: no Notes: Participants changed to flunisolide at beginning of study if necessary. Both groups used bid dosing for a run‐in period to establish a baseline | |
| Outcomes | Outcomes measured: compliance; PEFR; symptom score Adherence calculation: % days with more or less than prescribed 8 inhalations; mean inhalations per day; frequency distribution of total daily inhalation; number inhaler responses per day | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After informed consent was obtained, patients were randomized into two groups of eight each. Randomization was stratified so each group contained four clinic and four private practice patients" ‐ no further details given about how stratified random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | Unclear risk | No description of procedures to blind participants or personnel. However, primary outcome measure ‐ adherence ‐ objectively measured and unlikely to be prone to performance or detection bias. Participants were unaware that the primary aim of the study was to assess compliance. Subjective nature of secondary outcomes, such as asthma symptoms, may result in higher risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description of procedures to blind outcome assessors. However, primary outcome measure ‐ adherence ‐ objectively measured and unlikely to be prone to performance or detection bias. Subjective nature of secondary outcomes, such as asthma symptoms, may result in higher risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | "One patient did not use the NC at all for 39 of the 42 study days, but actuated the device 109 times on the day of the three‐week visit, and 56 times on the day of the six‐week visit. This patient was dropped and replaced in the study." No other withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods reported in text |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 6 months Setting: 66 community pharmacies in Belgium Trial registration: not reported | |
| Participants | Population: 201 adults with asthma randomised to adherence education (n = 107) or control (n = 94) Age: 18 to 50 years of age; mean age (range) in the intervention group 32.5 (19 to 51) years and in the control group 36.3 (17 to 51) years Baseline asthma severity: intervention group: mean (range) ACT score: 19.3 (10 to 25); 89.5% on ICS at baseline; control group: 19.7 (11 to 25); 93.9% on ICS at baseline Inclusion criteria: required to carry a prescription for asthma medication; under treatment for asthma for at least 12 months; ‘‘using’’ controller medication; making regular visits to the pharmacy Exclusion criteria: smoking history of more than 10 pack‐years, another severe disease (e.g. cancer) and an ACT score at screening < 15 (indicating seriously uncontrolled asthma; for ethical reasons, these patients were immediately referred to their GP or respiratory specialist) or = 25 (indicating complete asthma control; no room for improvement) Percentage withdrawn: 25% from the intervention group and 26% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: At the first visit, pharmacist delivered personal education about using an inhaler correctly; understanding asthma symptoms, triggers and early warnings; understanding asthma controller and reliever therapy; facilitating adherence to use of controller; and stopping smoking. At visits 2 and 3 (1 and 3 months), pharmacist gave advice based on participant's ACT score Control summary: usual pharmacy care. All participants filled in an asthma diary in the 2‐week run‐in period but had no further contact outside of usual pharmacy visits Complex intervention: yes | |
| Outcomes | Outcomes measured: Asthma Control Test (Dutch), diary card data (nocturnal awakenings, rescue medication use, PEF), asthma‐related ED visits and hospitalisations, AQLQ, Knowledge of Asthma and Asthma Medicine questionnaire (KAAM), inhalation technique checklist Adherence calculation: Adherence was measured using refill rates and self‐reporting via an adherence scale | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The sequence of allocation to either control or intervention group was predetermined by the investigators based on a randomisation |
| Allocation concealment (selection bias) | Low risk | "Serially numbered, closed envelopes were made for each participating pharmacy. The envelope with the lowest number was opened by the pharmacist upon inclusion of a new patient" |
| Blinding of participants and personnel (performance bias) | High risk | It was not possible to blind participants, so although adherence is measured objectively using pharmacy data, many other outcomes such as ACT and AQLQ are subject to potential performance bias, as participants know to which group they were assigned |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome assessors is not described, and although the primary outcome (adherence measured using pharmacy data) is not prone to detection bias, other patient‐reported outcomes (such as ACT and AQLQ) are at risk because the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Approximately 25% of participants dropped out of each arm of the trial. Although reasons were similar and baseline characteristics of those completing and not completing did not differ significantly, rate of drop‐out is high, and we cannot be sure that this did not affect the results. Secondary outcomes were analysed per protocol rather than by ITT |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods reported in text/tables |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: intervention delivered over 13 weeks; follow‐up continued to 26 weeks Setting: primary care and asthma specialty practices serving low‐income inner‐city neighbourhoods with high prevalence of asthma morbidity. USA Trial registration: NCT00115323 | |
| Participants | Population: 333 adults with asthma randomised to a problem‐solving (PS) intervention (n = 165) or an asthma education (AE) intervention (n = 168) Age: minimum age 18; mean age (SD) in PS group 49 years (13) and in AE group 49 (14) years Baseline asthma severity: sufficiently severe to require treatment with ICS. FEV1% predicted (SD) in PS group 66 (19) and in AE group 64 (19) Inclusion criteria: English‐ or Spanish‐speaking adults with moderate or severe persistent asthma according to National Heart, Lung, and Blood Institute Expert Panel Report 3 guidelines. Inclusion criteria were designed to identify patients with sufficiently severe and reversible asthma who were likely to benefit from ICS therapy. Specific criteria included the following: age ≥ 18 years; physician’s diagnosis of asthma; prescription for an ICS‐containing medication for asthma; and evidence of reversible airflow obstruction, that is, an increase ≥ 15% and 200 mL in FEV1 with asthma treatment over the previous 3 years, or an increase in FEV1 or FVC ≥ 12% and 200 mL in FEV1 within 30 minutes of inhaled albuterol. Smokers were included Exclusion criteria: severe psychiatric problems such as obvious mania or schizophrenia that would make it impossible for individuals to understand or carry out problem solving Percentage withdrawn: not specifically reported Other allowed medication: not specifically reported | |
| Interventions | Intervention summary: four 30‐minute sessions. Individualised intervention involved 4 interactive steps, usually 1 per research session, aimed at improving or maintaining adherence. Step 1: breaking problems into small achievable pieces; Step 2: brainstorming for alternative solutions; Step 3: choosing the best solution by weighing the consequences, both desirable and undesirable, of each candidate solution (between third and fourth meetings, the solution was tried); Step 4: evaluating and revising chosen solution. Intervention delivered to participants by a research co‐ordinator (college graduates interested in health‐related or education carers or further schooling, committed to working with patients and having a research experience. Co‐ordinators were diverse in race/ethnicity, as were participants) Control summary: four 30‐minute sessions, each focused on an asthma patient education topic unrelated to self‐management, adherence or ICS therapy. Topics covered included proper technique for using an albuterol‐rescue metered dose inhaler and a dry powder inhaler or spacer, depending on the patient’s medications; use of peak flow meters; common asthma triggers; and pathophysiology of asthma. These sessions did not involve discussion of problem solving or adherence, only a didactic presentation of health information. Delivered to participants by a research co‐ordinator (college graduates interested in health‐related or education carers or further schooling, committed to working with patients and having a research experience. Co‐ordinators were diverse in race/ethnicity, as were participants) Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence to ICS regimen prescribed by participant’s physician assessed by an electronic monitor; Mini‐AQLQ; ACQ; spirometry (FEV1 and FVC); hospitalisations and ED visits for asthma or any cause; patient satisfaction Adherence calculation: Daily ICS adherence was calculated as (# actuations downloaded/# prescribed) × 100 (using an electronic adherence monitor attached to the inhaler) | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: supported by grants from the National Institutes of Health (HL070392, HL088469) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomized according to a computer‐generated algorithm in 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Main outcome ‐ adherence ‐ objectively measured, but other outcomes such as ACQ and AQLQ are subject to performance bias |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Main outcome ‐ adherence ‐ objectively measured, but other outcomes such as ACQ and AQLQ are subject to detection bias as the unblinded participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial (NCT00115323). All outcomes reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group, multi‐centre randomised controlled trial Duration: 17 weeks, with follow‐up continuing to 1 year Setting: recruitment from primary and subspecialty care, inpatient and emergency department settings at 1 large paediatric tertiary care centre. USA Trial registration: NCT00149487 | |
| Participants | Population: 141 children with asthma randomised to a problem‐solving intervention or family‐based education (n for each group not reported) Age: 5 to 17 years; mean age not reported Baseline asthma severity: not reported Inclusion criteria: African American, family income below the poverty line, physician‐based diagnosis of asthma of at least 12 months, moderate to severe asthma (moderate asthma includes daily symptoms, daily use of inhaled short‐acting beta‐agonist, exacerbations more than 2 times per week that affect activity and night‐time symptoms more often than once a week, FEV1 or PEF between 60% and 80% predicted and PEF variability > 30%; severe asthma includes continual symptoms, limited physical activity, frequent exacerbations together with frequent night‐time symptoms, FEV1 or PEF < 60% predicted and PEF variability > 30%). Likely to be on a stable and daily medication (inhaled steroid) that can be modified electronically for the time period required to participate in the study Exclusion criteria: serious comorbid chronic condition, serious developmental disability, income exceeding poverty level Percentage withdrawn: not reported Other allowed medication: not reported | |
| Interventions | Intervention summary: intervention tailored to observed adherence behaviours and identified barriers to increasing adherence in African American children and adolescents with asthma and their families Control summary: family education Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence, frequency of asthma symptoms, utilisation of healthcare services, use of reliever medication Adherence calculation: adherence defined as correspondence between medication doses taken each day and prescribed dose, tracked by electronic monitoring device during months 9 to 12 of the study | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article and NCT record with no study results provided. Full‐text publication of RCT findings not found; above data extracted from a paper describing observational data related to trial participants Funding: National Heart, Lung, and Blood Institute (NHLBI) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out not reported for each arm; 49/141 dropped out overall (35%) |
| Selective reporting (reporting bias) | High risk | Unable to identify full‐text report of the RCT. Observational study on same cohort reported. No study results posted on clinicalrials.gov. Not able to include any outcomes in meta‐analysis |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, 3‐arm, parallel‐group randomised controlled trial Duration: 2 months Setting: recruited from asthma clinics at a rural, university‐based hospital in northeastern United States and an urban‐based children’s hospital in the Midwest Trial registration: NCT00166582 | |
| Participants | Population: 55 children with asthma randomised to receive a team work intervention (n = 19), an asthma education intervention (n = 19) or usual care (n = 17) Age: 9 to 15 years; mean age (SD) 11.1 (1.9) years across the 3 groups (mean age not given for each group) Baseline asthma severity: team work intervention group: mild persistent asthma = 37.5%, moderate persistent asthma = 50.0%, severe persistent asthma = 12.5%; asthma education group: mild persistent asthma = 25.0%, moderate persistent asthma = 56.2%, severe persistent asthma = 18.8%; usual care group: mild persistent asthma = 18.8%, moderate persistent asthma = 62.5%, severe persistent asthma = 18.8% Inclusion criteria: child with diagnosis of persistent asthma for at least 6 months; fluticasone MDI taken daily; and no evidence of neurological or significant cognitive impairment (per parent report). Suspected history of medication non‐adherence not required Exclusion criteria: not reported Percentage withdrawn: 16% from both teamwork intervention and asthma education intervention groups and 6% from usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary (1): teamwork: emphasised the importance of parents and youth sharing responsibility for the patient’s asthma management and learning methods for addressing conflicts associated with increased responsibility of youth. Involved handouts on adolescent development, promoting youth independence, appropriate parental medication supervision and problem solving around asthma management conflicts. MDI Log‐II served as the primary source of adherence information for families. ICS adherence goals were set in consultation with physician (lowest 70%). Four sessions 2 to 3 weeks apart delivered by a 'therapist' Intervention summary (2): asthma education: similar to the teamwork group, families in this group received and reviewed written materials with the researcher during sessions. These materials covered topics often found in asthma education programmes. Time spent with families generally was equivalent to that of its parallel teamwork session, thereby creating an attention control condition. Four sessions 2 to 3 weeks apart delivered by a 'therapist' Control summary: Youth in the usual care group completed all assessments at the same time interval as other participants but did not receive guidance beyond usual care. On completion of follow‐up, these families were provided feedback on their child’s medication adherence and were offered an opportunity to receive either of the 2 interventions Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence to ICS using MDI Log‐II; parent–adolescent conflict (CBQ‐20); health outcomes (FSI); lung function; consumer satisfaction (CSQ) Adherence calculation: Mean daily adherence was defined as follows: total number of puffs inhaled divided by total number of puffs prescribed, multiplied by 100 | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: supported by the National Institute of Child Health and Human Development (R03‐HD039767‐02) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Initially, youth were randomly assigned (via computer‐generated number sequence) to one of three parallel groups… subsequent participants were assigned using a randomized block design to maintain group balance across variables" |
| Allocation concealment (selection bias) | High risk | The sequence was available to the research assistant who recruited participants into the study, as participants were immediately randomised; thus, the research assistant might have been aware of group allocation before participants were randomised |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. The main outcome measured ‐ adherence ‐ was monitored objectively with an electronic device, but other outcomes such as functional severity index and satisfaction may have been affected by knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. The main outcome ‐ adherence ‐ was monitored objectively with an electronic device. However, other outcomes such as functional severity index are subject to risk of detection bias, as the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out lower in the standard care group (6%) than in the 2 active groups (both 16%); all participants accounted for in the flow diagram, but uneven drop‐out may have skewed results because these people were not included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered trial (NCT00166582), although details minimal in trial registration and outcomes not specified. However, all outcome measures listed in methods of the paper are reported clearly |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: 78 weeks Setting: Paediatric ED in Baltimore. USA Trial registration: NCT00233181 | |
| Participants | Population: 250 children with asthma randomised to adherence monitoring and education (n = 83), education (n = 84) or usual care (n = 83) Age: 2 to 12 years of age; mean (SD) age in the adherence group 6.5 (3.43) years, in the education group 7.1 (3.37) years and in the control group 7.4 (3.3) years Baseline asthma severity: not reported Inclusion criteria: eligible for randomisation when between 2 and 12 years of age, physician‐diagnosed asthma, 2 ED visits or 1 hospitalisation for asthma in the preceding year, resided in Baltimore City, prescribed an asthma controller medication Exclusion criteria: not reported Percentage withdrawn: 8.4% from the adherence group, 3.5% from the education group and 8.4% from the control group Other allowed medication: not reported | |
| Interventions | Intervention summary (1): Educational content in the education group PLUS electronic adherence monitoring with feedback, asthma control and adherence goal setting, reinforcement (praise and low‐cost rewards), and strategies for self‐monitoring medication use Intervention summary (2): five 30‐ to 45‐minute home visits by trained asthma educators (AEs) 1, 2, 3, 4 and 8 weeks after randomization. ABC intervention is a home‐based asthma education programme with 5 core components: review of prescribed asthma regimen and training in medication, spacer and peak flow technique; development of an asthma action plan; identification of barriers to accessing healthcare services and problem solving to reduce barriers; discussion of beliefs and concerns about asthma and medications; and provision of written asthma education materials Control summary: asthma education booklet and resource guide that provided information about low‐cost asthma care providers, social services, legal services and other resources. Regardless of group assignment, participants were regularly encouraged to receive care from their primary care provider Complex intervention: yes | |
| Outcomes | Outcomes measured: self‐reported adherence; pharmacy‐based adherence; career reports of symptoms, night‐time awakenings, ED visits, hospitalisations and courses of OCS in the previous 6 months Adherence calculation: Pharmacy‐based adherence was calculated as number of ICS refills per quarter, converted into equivalent values; rates were defined as number of ICS canisters dispensed quarterly (where 3 = 100% adherence). Self‐reported adherence was % use/prescribed dose × 100 | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: National Heart, Lung, and Blood Institute grant HL063333 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "To mask staff to group assignment during recruitment, the statistician created block randomization schema and placed the randomization assignments into sealed envelopes, which were opened after families completed baseline surveys" |
| Allocation concealment (selection bias) | Low risk | "To mask staff to group assignment during recruitment, the statistician created block randomization schema and placed the randomization assignments into sealed envelopes, which were opened after families completed baseline surveys" |
| Blinding of participants and personnel (performance bias) | High risk | Participants were not blinded to group allocation, and knowledge of group allocation and adherence monitoring may have affected healthcare utilisation behaviour, as well as adherence behaviour, beyond the effect intended by trialists |
| Blinding of outcome assessment (detection bias) | High risk | "Trained research assistants who were blinded to study assignments conducted surveys by telephone" However, all asthma morbidity measures were career reported, and therefore were at risk of detection bias, as the career is the outcome assessor for these self‐reported outcomes |
| Incomplete outcome data (attrition bias) | Low risk | More than 80% of participants in all 3 arms completed all questionnaires and follow‐up. Results analysed as ITT, and all randomised participants included in the ITT analysis |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT00233181), but not all outcomes (e.g. QOL) reported in the published paper |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, pragmatic, parallel‐group randomised controlled trial Duration: 78 weeks Setting: conducted through 2 Kaiser Permanente research centres in Hawaii and Baltimore. USA Trial registration: NCT00414817 | |
| Participants | Population: 14,064 adults with asthma randomised to receive interactive voice response calls or usual care. Not all participants were previous ICS users. 3171 of the intervention group and 3260 of the usual care group described as the primary analysis sample ‐ previous ICS users Age: 18 years of age and older; ages reported in categories rather than as mean (SD) Baseline asthma severity: 33.3% had comorbid COPD; other characteristics included recent ED visit, hospitalisation or OCS burst for asthma; current SABA usage; and number of medications used Inclusion criteria: Target population consisted of KPNW and KPH members 18 years of age and older who were members for the 12 months before randomisation, had been seen for asthma and received at least 1 dispensing of a respiratory medication during that time frame. For study of both primary and secondary ICS adherence, target population included individuals without evidence of prior ICS use. Present analysis focuses on the subset of 6903 individuals with ICS dispensing during baseline year Exclusion criteria: Individuals meeting the above criteria were included in the final analysis sample only if they had ever received (or for usual care participants would have qualified for) an intervention call Percentage withdrawn: of 6903 previous users qualifying for analysis, 3171 were included in the intervention analysis sample and 3260 in the usual care analysis sample Other allowed medication: not reported | |
| Interventions | Intervention summary: interactive voice recognition (IVR) intervention including 3 basic IVR call types, each typically lasting 2 to 3 minutes: refill reminder call, for people whose last ICS dispensing was at least a month ago and who should have < 30 days supply left, reminded participants that they were due for a refill and offered a transfer to the automated pharmacy refill line and/or information about KP’s online refill service; tardy refill call, for people > 1 month past refill date, reminded participants they were due for an ICS refill, assessed asthma control, explored ICS adherence barriers and provided tailored educational messages; and initiator/restart call, for participants who were starting ICS for the first time or were lapsed users, included probes for asthma control and adherence barriers and offered tailored educational messages Control summary: usual care Complex intervention: no | |
| Outcomes | Outcomes measured: 8 alternative measures of pharmacy‐based adherence, described as continuous multiple‐interval measures of medication availability and gaps. Clinicaltrials.gov lists the primary outcome as days' supply of ICS available as documented in participants' pharmacy records at 19 months, and secondary outcomes as health status from survey responses (subset), utilisation of acute healthcare services from medical record data and an economic analysis. Multiple post hoc analyses provided in published reports Adherence calculation: 8 alternative measures of pharmacy‐based adherence (one of the resulting publications is a comparison of pharmacy‐based measures of medication adherence) | |
| Notes | Type of publication: multiple peer‐reviewed journal articles Funding: NHLBI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization stratified by region and the clinic facility to which each patient was paneled" ‐ but no further details about how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Although it was not possible to blind participants to group allocation, the primary outcome (adherence) was measured objectively using pharmacy data. However, as participants would have been aware that they were taking part in a trial of adherence and were being monitored, this may have affected their adherence behaviour beyond the effect intended by the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessors is not described; however, the nature of the primary outcomes (adherence measured using pharmacy data) makes them not prone to detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Owing to the trial design, it was not possible to measure attrition in the usual way |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT00414817), but many outcomes of interest not presented numerically, so unable to include it in the meta‐analysis ("We also observed no significant intervention effects on reliever medication (SABA) use, quality of life, asthma control, or the rate of acute asthma health care utilization") |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group, stratified cluster randomised trial Duration: 52 weeks Setting: primary care: 34 clusters ('practices' comprising 193 providers). USA Trial registration: NCT00459368 | |
| Participants | Population: 2698 adults and children with asthma from 34 clusters ('practices' comprising 193 providers) randomised to adherence education and individualised patient adherence information (17 practices, 88 providers, 1335 participants) or adherence education alone (17 practices, 105 providers, 1363 participants) Age: 5 to 56 years of age; mean age (SD) in the adherence education and individualised patient adherence information group 26.8 (17.4) years and in the adherence education alone group 28.8 (17.4) years Baseline asthma severity: physician diagnosis of asthma and prescription for ICS in the preceding 2 years; no other severity information given Inclusion criteria: Eligible primary care practices had to have access to electronic prescription writing. Eligible patients had to fulfil the following criteria: recent previous electronic prescription for an ICS; 5 to 56 years of age as of 30 April 2007; continuous enrolment in the affiliated health maintenance organisation (HMO) for at least 1 year before 30 April 2007; prescription drug coverage as of 30 April 2007; at least 1 physician diagnosis of asthma and no diagnosis of chronic obstructive pulmonary disease or congestive heart failure after 19 January 2005; and at least 1 visit to a primary care provider in the year before 30 April 2007 Exclusion criteria: ICS medication stopped and not restarted, left the HMO before start of the intervention Percentage withdrawn: 22% from the adherence education and individualised patient adherence information arm and 24% from the adherence education alone arm Other allowed medication: Participants continued their normal medication | |
| Interventions | Intervention summary: Physicians assigned to both groups received an audio compact disc, a digital video disc and a booklet that contained information on the most recent national asthma guidelines and methods for discussing medication non‐adherence with patients. Physicians in the intervention arm could also view their patients' individual adherence data generated by ePrescribing Control summary: as above, but physicians were not able to view their patients' individual adherence data Complex intervention: yes | |
| Outcomes | Outcomes measured: patient adherence to ICS in last 3 months of intervention (i.e. an individual‐level outcome accounting for practice clusters), time to and number of the following events during the intervention period: asthma‐related emergency department visit, asthma‐related hospitalisation and oral steroid use. Post hoc analysis revealed that the change in adherence between baseline and study end differed between participants in intervention and control arms Adherence calculation: Based on data from electronic prescribing, calculated days of supply was calculated to estimate adherence as a continuous measure of medication availability equal to the cumulative days of supply divided by the number of days of observation. This estimates the proportion of time that participants took their medication | |
| Notes | Type of publication: multiple peer‐reviewed full‐text journal articles Funding: supported by grants from the National Heart, Lung, and Blood Institute (HL79055), the National Institute of Allergy and Infectious Diseases (AI61774, AI79139), the National Institute of Diabetes and Digestive and Kidney Diseases (DK64695), National Institutes of Health; the Fund for Henry Ford Hospital; and the Strategic Program for Asthma Research of the American Asthma Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were randomised with stratification for whether the practice was a paediatric practice (i.e. paediatrics vs family medicine and internal medicine) to achieve approximately equal partitioning of children and adults in both study arms. One researcher (E.L.P.) generated the random allocation sequence within strata, and the identities of the practices were concealed at the time of randomisation |
| Allocation concealment (selection bias) | Low risk | The identities of practices were concealed at the time of randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Healthcare providers (rather than individual participants) were randomised and were aware of group allocation. It is unclear how their knowledge of group allocation may have impacted the adherence of their patients in ways unintended by the intervention itself |
| Blinding of outcome assessment (detection bias) | Low risk | Study staff was masked to the individual practice treatment assignment, and the primary outcome ‐ adherence as calculated from pharmacy data ‐ is objective |
| Incomplete outcome data (attrition bias) | Low risk | Although > 20% of participants did not complete the trial (22% in the intervention arm and 24% in the control arm), we carried forward their last 3 months of adherence and analysed data in the primary analysis |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial (NCT00459368). All outcomes reported in at least 1 of several associated publications |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 26 weeks, with follow‐up to 78 weeks Setting: children 'in our region' given diagnosis of asthma in previous 1 to 2 months. Sweden Trial registration: not reported | |
| Participants | Population: 60 children with asthma and their parents randomised to group discussions plus basic education (n = 32) or basic education (n = 28) Age: 3 months to 6 years of age; mean age (SD) in the intervention group 28.1 months and in the control group 26.1 months Baseline asthma severity: not reported Inclusion criteria: moderate or severe asthma defined by SPT II Brazillian Consensus on Asthma Management Exclusion criteria: not reported Percentage withdrawn: 9% from the intervention group and 14% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: meetings in a group setting with both parents. Afternoon sessions of 1.5 hours' duration; 3 weekly meetings soon after asthma diagnosis and a follow‐up meeting at 6 months. Session involved 3 paediatricians, 3 nurses and 2 psychologists. One nurse was present on all occasions. The method applied was based on the concept of concordance, meaning that we tried to “speak the same language” as the parents and to reach an alliance with them on how to look upon asthma and its management. Our goal was to reach their “main worry” and, apart from teaching about asthma, we asked the key question: “What is asthma to you?” Our intention was to use dialogue and peer education, whereby the group was encouraged to share personal experiences. In each group session, leaders had a list of subjects that were to be covered during the discussion. Attendees also received basic education Control summary: basic education about asthma and its treatment, including how to use the Nebunette, and information on environmental control at first visit to the clinic. Participants received a written treatment plan for which the principle was high dose initially, then, in association with URTI, stepping down therapy to the lowest possible dose according to the status of the child. Treatment was stopped if the child had no asthma symptoms for 6 months Complex intervention: yes | |
| Outcomes | Outcomes measured: presence of parents at group meetings; personal view on adherence at inclusion and after 6 and 18 months; how many days the child was hospitalised; how many times participants had to seek emergency help for asthma; exacerbations, as defined by the need for parents to stay at home to take care of their child because of asthma symptoms; objective measures of adherence; adherence according to parents Adherence calculation: diaries and weighing of MDIs used between 12 and 18 months after inclusion | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: Primary Care Unit in the county of Varmland. AstraZeneca provided the medicines | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The parents of the 60 children were randomized consecutively in groups of four to either the intervention or the control group" ‐ but no further details about how this was achieved |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | "The nurses carried out the randomization and the three doctors that were involved in the group sessions also performed the follow‐up visits. Therefore, a complete blinding procedure could not be established" |
| Blinding of outcome assessment (detection bias) | High risk | "The nurses carried out the randomization and the three doctors that were involved in the group sessions also performed the follow‐up visits. Therefore, a complete blinding procedure could not be established" |
| Incomplete outcome data (attrition bias) | Low risk | High levels of follow‐up in both arms (86% in control group, 91% in intervention group); all withdrawals accounted for in the publication |
| Selective reporting (reporting bias) | High risk | No prospective trial registration identified. Many outcomes reported narratively in text but with insufficient numerical detail for inclusion in the meta‐analyses. Within‐group or whole study population results sometimes presented rather than between‐group differences. 'N.S.' frequently reported rather than exact CIs, SDs or P values |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, pragmatic, parallel‐group randomised controlled trial Duration: 2 years Setting: 18 primary care and 2 specialty care medical offices, 2 contract hospitals and more than 800 physicians. USA Trial registration: NCT00958932 | |
| Participants | Population: 1187 children with asthma randomised to speech recognition (SR) intervention (n = 590) or usual care (UC) (n = 597) Age: 3 to 12 years of age; mean age (SE) in SR group 8.2 (0.13) years and in the UC group 8.1 (0.13) years Baseline asthma severity: diagnosed persistent asthma for which daily inhaled corticosteroid treatment was prescribed. Children in the SR group had inpatient visits/person‐year, mean (SE), number 0.04 (0.01); ED visits/person‐year, mean (SE), number 0.09 (0.02); oral steroid bursts/person‐year mean (SE), number 0.469 (0.04); primary care visits/person‐year, mean (SE), number 2.3 (0.08); and children in the UC group had inpatient visits/person‐year, mean (SE), number 0.04 (0.01); ED visits/person‐year, mean (SE), number 0.09 (0.02); oral steroid bursts/person‐year, mean (SE), number 0.383 (0.04); primary care visits/person‐year, mean (SE), number 2.4 (0.10) Inclusion criteria: 3 to 12 years of age, diagnosis of persistent asthma, 1 or more ICS prescriptions filled in the prior 6 months. Participants were limited to those enrolled in KPCO (Kaiser Permanente Colorado; a group‐model health maintenance organisation) for at least 1 year to ensure that patients were consistently given a diagnosis of persistent asthma and to establish a baseline ICS adherence rate Exclusion criteria: identified by physician as having a life‐threatening comorbid condition; sibling already included in the study; parent who declined to participate; instructed to take an ICS only intermittently or as needed; obtained medication from a non‐KPCO pharmacy Percentage withdrawn: 23% from the SR arm and 25% from the UC arm Other allowed medication: Participants continued their normal medication | |
| Interventions | Intervention summary: Speech recognition telephone calls to parents in the intervention condition were triggered when an inhaled corticosteroid refill was due or overdue. Calls were automatically tailored with medical and demographic information from the electronic health record and from parent answers to questions during the call regarding recent refills or a desire to receive help refilling, to learn more about asthma control or to speak with an asthma nurse or a pharmacy staff member. All standard asthma resources remained available to both intervention groups throughout the duration of the study Control summary: All standard asthma resources remained available to the usual care group throughout the duration of the study Complex intervention: no | |
| Outcomes | Outcomes measured: adherence to ICS, beta2‐agonist use, oral steroid use; asthma‐related visits for primary healthcare services, ED visits, hospitalisations and after‐hours visits on weekends or weekdays after 6 PM; participant satisfaction Adherence calculation: Adherence was expressed as proportion of days covered (PDC) over 24 months. PDC was calculated as total number of ICS days supplied divided by period for which medication was prescribed | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: supported by grant 1R01HL084067‐01A2 from the National Institutes of Health. The National Institutes of Health had no role in design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization will be at the level of individual patients" ‐ but no further detail given in the study report or protocol |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel described. Main outcomes are related to usage of healthcare services and may be influenced by participants' knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | No blinding of outcome assessor described. However, main outcomes are related to usage of healthcare services, which was obtained from medical records and is unlikely to be influenced by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Although drop‐out was relatively high (> 20%) owing to loss of insurance, it was balanced and trialists performed an intention‐to‐treat analysis; participants who lost insurance coverage during the 2‐year study period were included in a secondary analysis for evaluation of potential sample attrition bias |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial (NCT00958932) and protocol available online. All main outcomes of interest reported; trialists state they will report rescue medication use in the protocol, but this information does not appear in the main report |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: intervention delivered over 12 weeks. Follow‐up continued to 1 year Setting: Regional Difficult Asthma Service. Northern Ireland Trial registration: not reported | |
| Participants | Population: 20 people with asthma attending a difficult asthma service facility randomised to individualised psychoeducational nurse‐led intervention (n = 9) or usual care (n = 11) Age: age range not reported, but mean age (SD) in the intervention group was 50 (9.1) years and in the control group 45.2 (10) years Baseline asthma severity: All participants had 'difficult asthma' ‐ defined as persistent symptoms, despite treatment at BTS/SIGN step 4/5. Baseline mean (SD) % predicted FEV1 74.4 (20.5) Inclusion criteria: difficult asthma, defined as persistent symptoms, despite treatment at BTS/SIGN step 4/5; attendance at the Northern Ireland Regional Difficult Asthma Service; non‐adherence after phase 1 of the study (received a patient concordance discussion); age over 18 years Exclusion criteria: current tobacco smoking or significant other comorbidity that contributed to persistent respiratory symptoms Percentage withdrawn: 22% from the intervention group and 0% from the usual care group Other allowed medication: ICS/LABA; prednisolone | |
| Interventions | Intervention summary: individualised psychoeducational nurse‐led intervention comprising 8 visits to a respiratory nurse, plus usual care Control summary: usual care Complex intervention: yes | |
| Outcomes | Outcomes measured: change in adherence to inhaled combination therapy; daily prescribed dose of ICS; courses of rescue oral corticosteroids; total inhaled and nebulised beta‐agonist doses; hospital admissions and lung function; ACQ; AQLQ; HADS; State Trait Anxiety Scale Adherence calculation: % of inhaled combination therapy prescriptions refilled and change in number of participants in each group filling ≤ 50% of prescription refills | |
| Notes | Type of publication: single peer‐reviewed full‐text journal article Funding: Research and Development Office, Northern Ireland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to either the intervention or control group" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | None noted |
| Blinding of participants and personnel (performance bias) | High risk | Trial described as 'single blind' but blinding not further described. Seems unlikely that participants or personnel would have been masked, and many outcomes (e.g. ACQ, AQLQ) were subject to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Trial described as 'single blind' but blinding not further described. Seems unlikely that participants or personnel would have been masked, so likely outcome assessors were masked. However, many outcomes (e.g. ACQ, AQLQ) subject to risk of detection bias, as the unblinded participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out unbalanced, although small numbers in both arms (0% drop‐out in control group, 22% in intervention group) |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods reported |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: 6 weeks, with follow‐up to 52 weeks Setting: Outpatient clinic. Single site. Canada Trial registration: not reported. | |
| Participants | Population: 54 adults with asthma randomised to motivational interviewing (MI) (n = 26) or usual care (n = 28) Age: over 18 years of age; mean age (SD) in the intervention group was 52 (15) years and in the control group 49 (16) years Baseline asthma severity: intervention group: ACT score 17 (4); ACQ 1.7 (0.9); control group: ACT score 17 (4); ACQ score 2.1 (1.1) Inclusion criteria: age 18 years; primary diagnosis of moderate to severe persistent asthma; prescribed stable dose of ICS for at least 12 months before enrolment; uncontrolled asthma according to ACQ; non‐adherence (filling < 50% prescribed ICS in the past 12 months) Exclusion criteria: comorbid condition with greater risk than asthma (COPD, CVD, etc.), severe psychopathology; current substance abuse; cognitive or language difficulties; plan to become pregnant; plan to leave Quebec over course of the study Percentage withdrawn: 30.77% from the intervention group and 21.43% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: individual session based on an MI manual, with the overall goal of enhancing participant motivation to take ICS Control summary: Participants received whichever treatments were prescribed by their physician, which may include an action plan or referral to asthma education. Participants were given the opportunity to receive MI after study completion Complex intervention: yes | |
| Outcomes | Outcomes measured: ICS adherence; self‐reported adherence; asthma control; asthma‐related quality of life; asthma‐related self‐efficacy Adherence calculation: number of treatment days/total number of days (6 months and 12 months) | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: unrestricted investigator‐initiated grant from GSK, salary awards from Fonds del la Recherche Quebec (FRQS) and Canadian Institute of Health Research. Scholarship support from FRQS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following the completion of all baseline assessments, patients were randomized to MI or UC using a computer algorithm that generated a random code" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to MI or UC using a computer algorithm that generated a random code that was kept in a concealed envelope until opened by the study coordinator at the time of randomization as per the CONSORT guidelines" |
| Blinding of participants and personnel (performance bias) | High risk | No description of procedures to blind participants or personnel. Subjective nature of many secondary outcomes results in high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | "With the exception of the visual analog scales assessing patients’ impressions on the MI intervention (which were completed at baseline and immediately postintervention only), all postintervention assessments were completed in‐hospital at 6 and 12 months postintervention by a research assistant who was blinded to patient group. To increase the success of blinding, patients were instructed not to disclose their group assignment to the research assistant" However, for some outcomes (such as AQLQ and ACT), participants the outcome assessor were unblinded; therefore, these outcomes are at risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Over 20% drop‐out in both arms; however, ITT and per‐protocol analyses were performed, and results were very similar overall |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 10 weeks Setting: 3 primary care practices at Rush University Medical Center in Chicago, Illinois. USA Trial registration: not reported | |
| Participants | Population: 68 adolescents with asthma randomised to adherence messaging and group sessions (n = 34) or an "attention control" (n = 34) Age: 11 to 16 years of age; mean age (range) in the intervention group was 13.3 (11 to 16) years and in the control group 13.6 (11 to 16) years Baseline asthma severity: intervention group: 85.3% of participants had uncontrolled asthma at baseline; control group: 76.5% had uncontrolled asthma at baseline Inclusion criteria: self‐identified as African American or Hispanic, given diagnosis of persistent asthma, possessing an active prescription for a daily ICS for asthma. Persistent asthma was defined as asthma symptoms (e.g. cough, wheeze, shortness of breath, chest tightness) more than 2 days per week or night‐time awakenings more often than twice a month; or taking a prescribed daily ICS for asthma Exclusion criteria: career or child unable to speak English, comorbidities that could interfere with study participation, ≥ 48% adherence over 2 weeks during the run‐in period. Participants with ≥ 48% adherence were excluded, as the aim of the study was to target children with poor adherence Percentage withdrawn: 15% from both intervention and control groups Other allowed medication: not reported | |
| Interventions | Intervention summary: All participants received medical supervision, peak flow meters and an iPod during the run‐in. Those in the intervention group received music tracks and attended coping peer group sessions led by social workers during weeks 1 to 4 and 6 to 9. Session leaders were trained to use a motivational interviewing approach and to follow the study guide. During the session, participants developed and recorded 2 to 4 messages from the discussion to encourage daily use of ICS, to be played at random between music tracks Control summary: All participants received medical supervision, peak flow meters and an iPod during the run‐in. Those in the attention control group attended weekly individual sessions with a research assistant who did not promote adherence. They received the same number of iPod messages as those in the active intervention group, with content promoting adherence to ICS; also played at random between music tracks but recorded by an asthma doctor rather than by peers Complex intervention: yes | |
| Outcomes | Outcomes measured: ICS adherence (measured at baseline and at 5 and 10 weeks), asthma knowledge (ZAP Caregiver Asthma Knowledge Instrument), ICS knowledge, ICS self‐efficacy, social support, asthma exacerbations Adherence calculation: average daily adherence over previous 14 days, measured with the electronic medication monitor for ICS | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: National Heart, Lung, and Blood Institute grants K23 HL092292 and R21 HL098812. Support in the form of study drug was provided by a grant from GlaxoSmithKline (FLV114794) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blocked group randomization, using a computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | It was not possible to blind participants, although adherence, the only reported outcome of interest for this review, was measured objectively. However, awareness of intervention group and monitoring may have affected adherence behaviour beyond the effect intended by the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | "Outcomes data were collected at baseline and at 5 and 10 weeks post‐randomization (during the active treatment phase) by research assistants blinded to the participants’ group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | More than 80% in both arms attended at least 1 follow‐up visit (at 5 or 10 weeks) and were included in the analysis; reasons for dropping out were similar between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered trial (NCT01169883); outcomes listed on trial register clearly reported (although medians and IQR used, so unable to include it in the meta‐analysis). Several outcomes of interest in this review were listed as measured in the methods section of the published report but were not reported in the results (e.g. unscheduled visits, exacerbations) |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 6 to 8 months Setting: schools in the Rochester City School District. USA Trial registration: NCT01175434 | |
| Participants | Population: 100 children with asthma randomised to school‐based care (n = 49) or usual care (n = 51) Age: 3 to 10 years of age; mean age (SD) in each intervention group 7.5 (1.7) years and in usual care group 7.0 (1.8) years Baseline asthma severity: baseline PAQLQ in the intervention group 6.25 (0.8) and baseline QOL 5.82 (1.2) Inclusion criteria: children with physician‐diagnosed asthma with persistent symptoms based on NHLBI guidelines Exclusion criteria: career unable to speak and understand English, no access to a working phone for follow‐up surveys, plan to leave the school district within 6 months;any other significant medical conditions, including congenital heart disease, cystic fibrosis or other chronic lung disease, that could interfere with assessment of asthma‐related measures Percentage withdrawn: 2% from the intervention group and 0% from the usual care group Other allowed medication: not specifically reported; assumed children continued with usual medication | |
| Interventions | Intervention summary: systematic Web‐based screening to assess children’s asthma using guideline‐based symptom questions along with an algorithm to compute NHLBI severity or control classification; report of generation and electronic communication with primary care provider for authorisation of directly observed therapy with preventive asthma medications through school; prescription of guideline‐based preventive medications purchased through the child’s health insurance and delivered to schools and children’s homes by a local pharmacy; directly observed administration of medications at school by a school nurse or health aide; and systematic reassessment of symptoms using the same system, with guideline‐based adjustments to therapy as needed Control summary: Similar to children receiving the intervention, children in the usual care group were screened for eligibility with the online screening tool at the beginning of the school year, but reports were not sent to their primary care provider and directly observed therapy was not implemented at school Complex intervention: yes | |
| Outcomes | Outcomes measured: feasibility; mean symptom‐free days over 2 weeks, averaged over the study period; numbers of days and nights with symptoms; activity limitations; rescue medication use; school absenteeism; parent sleep interruption; change in family plans due to the child’s asthma over the prior 2 weeks; Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ); utilisation of healthcare services (office, ED visits, hospitalisations and non‐urgent visits for asthma care); fractional exhaled nitric oxide Adherence calculation: recorded as part of feasibility assessment only in children randomised to the intervention arm | |
| Notes | Type of publication: peer‐reviewed journal article Funding: funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (RC1HL099432) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by use of a preventive asthma medication at baseline. A permutated block design was used to ensure an equal balance of children in each group over time. The randomisation scheme was independently developed by the Biostatistics Center |
| Allocation concealment (selection bias) | Low risk | The randomisation scheme was independently developed by the Biostatistics Center; the interviewer called the Study Co‐ordinator, who provided the participant's ID number and treatment assignment |
| Blinding of participants and personnel (performance bias) | High risk | It was not possible to blind participants and personnel to group allocation. Although some outcomes may be more objective (such as hospitalisations), other outcomes such as unscheduled visits and patient‐reported outcomes (e.g. quality of life) may have been affected by participant (or career) knowledge of group allocation |
| Blinding of outcome assessment (detection bias) | High risk | Although the trial reports that "all follow up data were collected by a research group blinded to the child’s group allocation" for outcomes such as quality of life, the unblinded participant or career is the outcome assessor; therefore, these outcomes are still at risk of detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant from the intervention group was lost to follow‐up before starting the intervention. The remainder were analysed in the groups to which they were randomised |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT01175434); however, peer‐reviewed publication reports outcomes not prespecified (e.g. quality of life). Unclear if other outcomes of interest (such as asthma control) may have been measured but not reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 13 weeks Setting: an urban university and an affiliated medical centre, Detroit. USA Trial registration: not reported | |
| Participants | Population: 49 young adults with asthma randomised to multi‐component, technology‐based intervention (n = 25) or asthma education (n = 24) Age: 18 to 29 years; mean age (SD) in the intervention group 21.8 (4) years and in the control group 23.1 (3.4) years Baseline asthma severity: intervention group: asthma exacerbations in last month mean (SD) 1.8 (2.0), ACT mean (SD) 14.0 (3.1), FEV1 mean (SD) 80.0% (21.6). Control group: asthma exacerbations in last month mean (SD) 2.4 (4.6), ACT mean (SD) 14.4 (3.1), FEV1 mean (SD) 80.4% (15.7) Inclusion criteria: 18 to 29 years old, African American with diagnosis of persistent asthma, prescribed a controller medication. Individuals also had to have access to a cell phone with texting capability and to report < 80% adherence in the past 30 days and score ≤ 19 on the ACT Exclusion criteria: pregnancy, inability to understand written or spoken English, another serious medical condition requiring regular medication, active psychiatric disorder that would interfere with study participation Percentage withdrawn: 8% from the intervention group and 4% from the usual care group Other allowed medication: not reported | |
| Interventions | Intervention summary: Intervention consisted of 2 "Computerized Intervention Authoring Software" (CIAS)‐delivered sessions with personalised, daily text messaged reminders to take medication delivered between these sessions. "Ecological Momentary Assessment" (EMA) via text messaging was conducted before the first intervention session to gather real‐time data on participants’ medication adherence and asthma symptoms. These data were used to tailor the intervention session for each participant Control summary: Control participants completed CIAS‐delivered asthma education matched for length, location and method of delivery of the intervention session. Control session was delivered by the avatar “Peedy the parrot” and included interactive features such as quizzes and responses to poll questions. Content focused on facts and myths about asthma, control of environmental factors and pharmacological management. Control participants received text messages between sessions via CareSpeak, but message content was the same for all participants and contained general facts about asthma. Control participants also received 7 days of EMA before the first session, but data were not used to tailor the session Complex intervention: no | |
| Outcomes | Outcomes measured: adherence, asthma control (ACT), lung function (FEV1), participant satisfaction Adherence calculation: calculated from self‐reported number of doses missed compared with prescribed doses | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: supported by a grant from the National Institutes of Health (NHLBI, 1R34HL107664‐01A1 (K.K.M.)) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After completing the questionnaires, the computer automatically randomized them to receive either the intervention (n =25) or control (n =24)" Of note is a baseline imbalance in males and females with fewer males in the control arm, but this is more likely to be the result of small numbers in the trial than failure of randomisation |
| Allocation concealment (selection bias) | Low risk | "After completing the questionnaires, the computer automatically randomized them to receive either the intervention (n = 25) or control (n = 24)" |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome assessors is not described. In addition, for many outcomes in this trial (self‐reported adherence, ACT, etc.), the participant is the outcome assessor; therefore, we judge this study to be at high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Low and balanced drop‐out (< 10%) in both arms |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, but all planned outcomes clearly reported |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 12 weeks Setting: 1 university centre in New York. USA Trial registration: NCT02413528 | |
| Participants | Population: 12 adolescents with asthma planned to be randomised to adherence monitoring app and sensor or standard care with monitoring via sensor Age: 11 to 19 years Baseline asthma severity: not reported Inclusion criteria: asthma diagnosis, currently on a daily controller HFA medication for asthma, English‐speaking, access to a smartphone or tablet Exclusion criteria: pregnancy, foster care, emancipated minor Percentage withdrawn: not applicable Other allowed medication: not reported | |
| Interventions | Intervention summary: Participants would have been given an inhaler sensor to monitor medication use and a mobile phone application that would send them reminders and provide an opportunity to see their own medication use and win incentives for adherence Control summary: Participants would have been given an inhaler sensor to monitor medication use and a sham version of the mobile app that would not include reminders or incentives Complex intervention: no | |
| Outcomes | Outcomes measured: real‐time medication adherence; asthma control (ACT) Adherence calculation: not reported | |
| Notes | Type of publication: trial registration only Funding: CoheroHealth NB: Study terminated owing to "wireless connectivity challenges with device and mobile app" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" on NCT record ‐ but no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Described as open‐label but minimal details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as open‐label but minimal details given |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to assess as no study results posted; terminated owing to "wireless connectivity challenges with device and mobile app" |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess as no study results posted |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 1 year Setting: hospital clinics in Sheffield or Rotherham Trial registration: NCT02451709 | |
| Participants | Population: 90 children with asthma randomised to electronic adherence monitoring with reminders and feedback (n = 47) or monitoring with no reminders or feedback (n = 43) Age: 6 to16 years of age; mean age (SD) in the intervention group 10.4 (2.9) years and in the control group 10.2 (2.9) years Baseline asthma severity: poorly controlled asthma; BTS level 3 or above Inclusion criteria: Participants had to be taking regular inhaled steroids, with no change in their medication in the last month and an ACQ score ≥ 1.5, indicating poorly controlled asthma. Electronic monitoring devices available for this trial were compatible only with Seretide or Symbicort inhalers. Therefore, all participants were at BTS level 3 at the start of the trial Exclusion criteria: could not speak English, had another significant chronic condition Percentage withdrawn: 15% from the intervention group and 9% from the control group, but all randomised participants were included in the primary ITT analysis, with the exception of 1 control group participant who was withdrawn after randomisation for not meeting eligibility criteria Other allowed medication: not reported | |
| Interventions | Intervention summary: Adherence was electronically monitored with regular feedback provided at clinic visits, during which the importance of adherence was emphasised and personalised strategies for improvement were devised. Devices also played medication reminder alarms. Alarms sounded for 5 seconds, every minute for 15 minutes (or until actuation), if the inhaler had not been actuated within the previous 6 hours of the specified time. Devices were locked to prevent tampering Control summary: Control participants had the same EMDs attached to their regular inhaler and were told that the devices monitored how often inhalers were taken, but that these data would not be reviewed. Participants were seen in their standard asthma clinic, and data were downloaded but were not reviewed. Alarms were disabled, and the devices locked Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence, change in ACQ, FEV1%, number of unplanned attendances at general practitioner (GP)/emergency department (ED) for asthma since last visit (as reported by parents), number of courses of oral steroids Adherence calculation: calculated for each 3‐month period, both morning and afternoon doses, and recorded as a percentage. This was calculated as number of doses actually taken/number of doses prescribed × 100 | |
| Notes | Type of publication: peer‐reviewed journal article Funding: Sheffield Children’s Hospital Charity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via permuted block randomisation, with allocation of 1:1 created from a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation of participants involved phoning the independent holder of the randomisation code |
| Blinding of participants and personnel (performance bias) | High risk | Owing to the nature of the intervention, neither participants nor study team members were blinded. Although adherence, lung function and oral steroid use might be considered objective outcomes, potential for performance bias remains for outcomes such as AQLQ and ACQ |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome assessors is not described; although some outcomes are relatively objective and are not prone to detection bias (adherence, lung function and oral corticosteroid use), others such as ACQ and AQLQ involve the unblinded participant as the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Less than 15% drop‐out in both arms; all but 1 participant included in the intention‐to‐treat primary analysis |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT02451709); all outcomes specified in the methods are mentioned in the paper, although some non‐numerically (e.g. mini‐PAQLQ, BMQ, SABA use, IPQ), so could not be included in the meta‐analysis |
| Other bias | Low risk | None noted |
| Methods | Design: single‐blind, parallel‐group randomised controlled trial Duration: 10 weeks Setting: 1 asthma centre at Saint Francis Hospital, Connecticut. USA Trial registration: not reported | |
| Participants | Population: 30 adults with asthma randomised to adherence monitoring and education or monitoring without feedback (n randomised to each arm not reported) Age: over 18 years of age; mean (SD) age in the intervention group 45 (11) years and in the control group 53 (14) years Baseline asthma severity: intervention group: mean FEV1 78% predicted; mean (SD) ED visits in the past year 2.3 (2.4); 80% on LABA; mean (SD) AQLQ score 4.34 (1.62). Control group: mean FEV1 63% predicted; mean (SD) ED visits in the past year 1.0 (0.8); 100% on LABA; mean (SD) AQLQ score 3.75 (1.39) Inclusion criteria: Adults with moderate to severe asthma were considered for the study if they met all of the following inclusion criteria: referral to the Asthma Center at Saint Francis Hospital and Medical Center; 1 or more markers of low socioeconomic status (Medicaid or no insurance, family income < $20,000, less than a high school education); prebronchodilator FEV1 < 80% of predicted and 15% predicted greater reversibility after bronchodilator administration; and regular use of inhaled steroids and willingness to change the schedule, if necessary, to twice‐daily dosing Exclusion criteria: not reported Percentage withdrawn: 10 participants in the intervention group and 9 in the control groups completed the trial. Numbers randomised to each arm not reported Other allowed medication: Use of long‐acting oral or inhaled bronchodilators, theophylline and oral corticosteroid was permissible | |
| Interventions | Intervention summary: Intensive asthma education and management was provided by a nurse and/or respiratory therapist over approximately a 3‐week period, with follow‐up for 7 additional weeks. Content was based on NAEPP guidelines covering goals of therapy, signs of worsening asthma, types of medications, importance of prophylactic medication, proper MDI technique, use of PEF meter, patient satisfaction and QOL and environmental evaluation and education. Data from MDI Chronologs were downloaded at each visit and were reviewed with the clinician, who emphasised techniques or strategies to improve adherence when necessary, according to type and timing of non‐adherence Control summary: Control group visited to complete outcome measures, and data were downloaded from Chronologs, but no education or adherence advice was given Complex intervention: yes | |
| Outcomes | Outcomes measured: adherence to ICS, albuterol use (mean actuations per 24 hours for each week), AQLQ, FEV1 Adherence calculation: overall mean weekly adherence and percentage of days with overuse | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: in part by an award from the University of Connecticut Health Center Research Advisory Committee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomized into 1 of 2 groups" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | "Patients were told that these instruments recorded the time and date of each MDI actuation but were blinded to the study hypothesis" Although participants were blinded to the study hypothesis, knowledge of group allocation and the fact that adherence was being monitored may have altered adherence behaviour beyond the effect intended by trialists |
| Blinding of outcome assessment (detection bias) | High risk | Although trialists went to some lengths to blind outcome assessors ("For the initial period, patients met at the first visit and three subsequent visits with a nurse and/or respiratory therapist blinded to the patients' group"), for AQLQ the participant is the outcome assessor; therefore, such outcomes are presented at risk of detection bias |
| Incomplete outcome data (attrition bias) | High risk | 10/30 participants did not complete the study; it is not clear how many were randomised to each group or whether reasons for dropping out were similar between groups |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods were reported in text/tables |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 12 weeks Setting: 143 sites in the UK Trial registration: not reported | |
| Participants | Population: 1233 participants with asthma randomised to intervention (once‐daily ICS) (n = 611) or control (twice‐daily ICS) (n = 622) Age: 12 years of age and older; mean (SD not reported) age in the adherence group 50.9 years and in the control group 50.9 years Baseline asthma severity: intervention group: mean duration of asthma 16.4 years; control group: mean duration of asthma 16.2 years Inclusion criteria: treated with beclomethasone dipropionate (BDP) hydrofluoroalkane (≤ 500 μg/d) or BDP chlorofluorocarbon (≤ 1000 μg/d) for ≥ 12 weeks, with stable BDP dosing regimen for ≥ 4 weeks immediately before study entry. Inclusion of patients who used BDP as their prior ICS therapy was justified because BDP was the ICS prescribed most commonly in the UK at the time the study was conducted, thereby providing a patient population as large and as homogeneously treated as possible. Eligible patients had no clinically significant disease that would interfere with study evaluation, and female patients of childbearing potential were required to use medically accepted birth control Exclusion criteria: ventilator support required for respiratory failure due to asthma within the previous 5 years, hospitalisation within the previous 3 months due to asthma Percentage withdrawn: 16.5% from the adherence group and 15.3% from the control group Other allowed medication: not reported | |
| Interventions | Intervention summary: mometasone furoate (MF) DPI 400 μg once daily in the evening. Participants were instructed in inhaler use and peak flow measurement to demonstrate proficiency and received salbutamol for rescue medication. They were also given diary cards and were instructed to follow an asthma action plan formulated at their first visit Control summary: mometasone furoate DPI 200 μg twice daily. Participants were instructed in inhaler use and peak flow measurement to demonstrate proficiency and received salbutamol for rescue medication. They were given diary cards and were instructed to follow an asthma action plan formulated at their first visit Complex intervention: no | |
| Outcomes | Outcomes measured: Primary outcome was adherence measured by the counter. Secondary outcomes included self‐report adherence, physician's assessment of response, quality of life on the Integrated Therapeutics Group Asthma Short Form (ITG‐ASF) (week 12), utilisation of healthcare resources and number of days missed from work or school. Adverse events were recorded at all visits, and an abbreviated physical exam was performed at visits 1 and 4. Evaluation of asthma worsening was performed at all visits, defined as increased use of rescue medication (> 12 inhalations on 2 consecutive days), a decrease in peak flow > 25% on 2 consecutive days or clinical asthma exacerbations (unscheduled doctor’s visit, hospitalisation, ER visit and/or use of additional asthma medications other than short‐acting beta‐agonists) Adherence calculation: Adherence was calculated as the number of administered doses (as determined by device counter number) times 100 divided by the number of scheduled doses. Data were not included for analysis if invalid (e.g. gross misuse of device, missing treatment end dates, device malfunction) | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: Schering Corp., a division of Merck & Co. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized to receive either MF‐DPI 400 μg once‐daily in the evening or MF DPI 200 μg twice‐daily from inhalers measuring 220 μg/actuation and delivering 200 μg/inhalation" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Trial is described as open‐label; although adherence was measured objectively with a device counter, knowledge of group allocation and monitoring may have affected adherence behaviour. In addition, patient‐reported outcomes, such as HRQOL, are susceptible to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Trial is described as open‐label; although adherence was measured objectively with a device counter, knowledge of group allocation may have affected patient‐reported outcomes, such as HRQOL, for which the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out < 20% in both groups, and reasons for discontinuation appear similar between groups. All participants included in the safety analysis |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods were reported in text/tables. Asthma worsening was not reported as planned |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 12 weeks Setting: 1 university hospital. Denmark Trial registration: not reported | |
| Participants | Population: 26 adults with asthma randomised to SMS adherence reminders (n = 12) or usual care (no reminders) (n = 14) Age: 18 to 45 years of age; mean (SD not reported) age in the intervention group 34.4 years and in the control group 30.7 years Baseline asthma severity: among the randomised participants: 8 (30.8%) were categorised as mild persistent (GINA 2), 16 (61.5%) as moderate persistent (GINA 3) and 2 (7.7%) as severe persistent (GINA 4). Before enrolment into the study, 9 participants (34.6%) had used SABA as monotherapy, 9 (34.6%) had used ICS (alone or in combination with LABA and/or SABA) and the remaining 8 (30.8%) had not used any treatment at all over the last 3 months Inclusion criteria: diagnosis of asthma based on clinical history and daily symptoms, age between 18 and 45 years, positive methacholine challenge test with PD20 ≤ 4 mmol Exclusion criteria: other medical comorbidities, smoking history of more than 10 pack‐years Percentage withdrawn: 17% from the intervention group and 14% from the control group Other allowed medication: not reported | |
| Interventions | Intervention summary: 4‐week run‐in on LABA/ICS, then 12 weeks of daily SMS reminders. SMS reminder was sent daily at 10 AM on cell phone over the following 8 weeks. All enrolled participants received a thorough education concerning the necessity of ICS treatment in asthma, and all were provided with knowledge of disease mechanisms and correct inhaler technique Control summary: 4‐week run‐in on LABA/ICS and no reminders. All enrolled participants received a thorough education concerning the necessity of ICS treatment in asthma, and all were provided with knowledge of disease mechanisms and correct inhaler technique Complex intervention: no | |
| Outcomes | Outcomes measured: mean rate of adherence to asthma treatment, reimbursement for asthma medication, change in exhaled nitric oxide levels, lung function, airway responsiveness Adherence calculation: Adherence rate was registered as the percentage of medicine actually taken by participants, calculated from medicine dose count on the Discos Seretide and number of days between clinical examinations: (60 dose‐count)/2 × days × 100% | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: GlaxoSmithKline provided a financial contribution to the service on the Internet including the short message service provided by CIM mobility NB: Only 34.6% of people had taken ICS in the last 3 months, and 30.8% no treatment at all, but everyone was put on Seretide at the start of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done by means of automatic computer generation of randomisation numbers in blocks of six" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Trial is not described as blinded; although adherence was measured objectively with a device counter, knowledge of group allocation and monitoring may have affected adherence behaviour. In addition, patient‐reported outcomes, such as ACQ and AQLQ, are susceptible to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Trial is not described as blinded; although adherence was measured objectively with a device counter, knowledge of group allocation affected patient‐reported outcomes, such as ACQ and AQLQ, for which the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out < 20% in both groups, but reasons for discontinuation not given and no participant flow diagram presented. Also unclear how many participants are included in the primary analysis, as this is presented in the text and not in a table |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified, although all outcomes listed in methods reported in text/tables |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 12 weeks Setting: 29 GSK investigational sites in Denmark and Switzerland Trial registration: NCT00351143; EudraCT no. 2005‐0003374‐48; ACE104325 | |
| Participants | Population: 274 adults with asthma randomised to adherence education and study medication (n = 140) or study medication only (n = 134) Age: over 18 years of age; mean (SD) age in the intervention group 40.5 (13.9) years and in the control group 38.7 (14.6) years Baseline asthma severity: Across the 2 groups, most randomised participants had mild persistent (51%) or moderate persistent (34%) asthma Inclusion criteria: ≥ 18 years of age; diagnosis of persistent asthma; treatment with at least 250 mg fluticasone propionate bid (or equivalent for other ICS) 4 weeks before the study and/or LABA bid or monotherapy with a short‐acting beta2‐agonist; ability to comply with use of the Asthma Monitor 2 (AM2) and the Asthma Quality of Life Questionnaire (AQLQ). Participants who had an exacerbation during the study period were allowed to remain in the study Exclusion criteria: known or suspected chronic obstructive pulmonary disease (COPD); pregnancy or lactation; smoking history > 10 pack‐years; clinical or laboratory evidence of serious uncontrolled systemic disease; microbiologically verified upper or lower respiratory tract infection within 1 month before screening visit; acute asthma exacerbation requiring hospitalisation/emergency department treatment/treatment with systemic corticosteroids within 3 months before screening visit; furthermore, for entry into treatment period 1 and treatment period 2: changes in asthma medication, including treatment with systemic corticosteroid, during the preceding period; more than 1 week of guideline‐defined asthma control before baseline visit/during treatment period 1; achieving total control in treatment period 1 (participants randomised at end of treatment period 1 and before entry into treatment period 2) Percentage withdrawn: not reported Other allowed medication: Those who had been treated with oral corticosteroids in the preceding 3 months were excluded from enrolling, and those who needed a change in asthma medication during period 1 were excluded from period 2 | |
| Interventions | Intervention summary: given salmeterol/fluticasone propionate 50/250 mg (Diskus®) bid and salbutamol prn for 12 weeks before randomisation (period 1). Then for 12 weeks (period 2), those who did not achieve total control were randomised and were given 5 patient‐centred teaching modules that included education about asthma, risk factors, prognosis, expectations of treatment, correct ways of taking controller and rescue medication and mnemonics as an aid for optimal dosing/timing of medication. Based on both written and oral information. Coaches were trained to use the standardised material at all centres Control summary: given salmeterol/fluticasone propionate 50/250 mg (Diskus®) bid and salbutamol prn for 12 weeks before randomisation (period 1). Then for 12 weeks (period 2), those who did not achieve total control were randomised and continued with the same study medication Complex intervention: yes | |
| Outcomes | Outcomes measured: total asthma control; PEF; symptom scores; rescue medication use; number of nights awakenings due to asthma; adverse events; quality of life (AQLQ); medication compliance; asthma severity; adverse events (including exacerbations, emergency visits and hospitalisations); vital signs. The asthma monitor AM2 medical device was used to collect the following data on a daily basis: FEV1; pre‐dose morning PEF; symptoms; use of rescue medication; night‐time awakenings; exacerbations; change of medication due to side effects and emergency doctor visits Adherence calculation: Treatment compliance was assessed by counting the number of doses in the returned investigational product | |
| Notes | Type of publication: single peer‐reviewed journal article Funding: GlaxoSmithKline NB: Period 2 of interest. During study period 1, all participants were treated with salmeterol/fluticasone 50/250. Those who did not achieve total asthma control in treatment period 1 were randomised to continued treatment with or without adherence education concomitantly for a further 12 weeks (period 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects who did not achieve total asthma control in treatment period 1 were randomised to continued treatment with or without compliance enhancement training concomitantly for a further 12 weeks" ‐ no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) | High risk | Trial is described as open‐label; although adherence was measured objectively with a device counter, knowledge of group allocation and monitoring may have affected adherence behaviour. In addition, patient‐reported outcomes, such as quality of life, are susceptible to risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Trial is described as open‐label; although adherence was measured objectively with a device counter, knowledge of group allocation and monitoring may have affected patient‐reported outcomes, such as quality of life, for which the participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | All efficacy analyses were performed by intent‐to‐treat (ITT) analysis on all participants with data entered into the database, who had received at least 1 single dose of trial medication in treatment period 2 (randomised portion of the trial); therefore, this was the population for analysis of the primary endpoint. Sensitivity analyses were performed |
| Selective reporting (reporting bias) | High risk | Prospectively registered trial (NCT00351143; EudraCT no. 2005‐0003374‐48). However, many outcomes of interest were not reported numerically, so could not used in the meta‐analysis |
| Other bias | Low risk | None noted |
| Methods | Design: open‐label, parallel‐group randomised controlled trial Duration: 52 weeks Setting: 5 outpatient clinics. The Netherlands Trial registration: Netherlands Trial Register NTR2583 | |
| Participants | Population: 219 children with asthma randomised to receive SMS adherence reminders (n = 108) or no reminders (n = 111) Age: 4 to 11 years of age; mean (SD) age in the intervention group 7.8 (2.2) years and in the control group 7.7 (2.1) years Baseline asthma severity: intervention group: 39.8% had poorly controlled asthma (ACT); control group: 36.5% Inclusion criteria: 4 to 11 years of age at the start of the study; doctor‐diagnosed asthma for at least 6 months; ICS use for at least 3 months; use of a pMDI; use of fluticasone, fluticasone/salmeterol or beclomethasone; at least 1 parent/career with a mobile phone Exclusion criteria: refusal to participate in the study Percentage withdrawn: not reported Other allowed medication: not reported | |
| Interventions | Intervention summary: All children received an RTMM‐device that registers time and date of administered ICS doses. Children in the intervention group received “time‐tailored” text messages that were sent only when a dose was at risk of omission Control summary: All children received an RTMM‐device that registers time and date of administered ICS doses. Those in the control group do not receive such text messages Complex intervention: no | |
| Outcomes | Outcomes measured: adherence to ICS; asthma control (ACT); frequency of asthma exacerbations and use of healthcare services (pharmacy data checked for OCS use and health records); disease‐specific quality of life; school/work absence; paediatric AQLQ; acceptance of e‐monitoring; economic evaluation Adherence calculation: proportion of all prescribed dosages taken by the child within a 6‐hour time frame around planned time of inhalation (i.e. from 3 hours before until 3 hours after) calculated from RTMM data on ICS use, attached to the inhaler | |
| Notes | Type of publication: multiple peer‐reviewed journal articles Funding: supported by a non‐conditional grant from The Netherlands Organisation for Health Research and Development (ZonMw, grand registration number 171101005). The study is also partially sponsored by the pharmaceutical company GlaxoSmithKline. The manufacturer of the RTMM devices, Evalan BV, partially sponsors the study by providing devices at cost price | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated block randomisation was used per hospital with block size of 16 patients" |
| Allocation concealment (selection bias) | Low risk | "At registration at the RTMM software interface, children were automatically assigned to the intervention or control group" ‐ suggests that allocation was concealed, as this was performed at an IT interface |
| Blinding of participants and personnel (performance bias) | High risk | "Although physicians, researchers and patients were initially blinded for randomisation, patients were generally unblinded shortly after the start of the study period, when they found out whether they received SMS reminders or not" Knowledge about group allocation may have affected performance, especially in subjective measures such as PAQLQ and cACT |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessors described. Primary outcome ‐ adherence ‐ measured objectively and not likely to be at risk of detection bias, but for other outcomes (such as PAQLQ and cACT), the unblinded participant/career is the outcome assessor; therefore, these outcomes are at risk of bias |
| Incomplete outcome data (attrition bias) | High risk | "Reasons why patients left the study prematurely were not systematically registered", and the total number of people who did not complete the study and hence had to have their data imputed is not reported. The numbers in Figure 2 suggest very low retention of around 50% in each arm |
| Selective reporting (reporting bias) | Low risk | Prospectively published protocol and all outcomes clearly reported in main publication |
| Other bias | Low risk | None noted |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AE: asthma education; AQLQ: Asthma Quality of Life Questionnaire; BDP: beclomethasone dipropionate; BMQ: Beliefs about Medication Questionnaire; BTS/SIGN: British Thoracic Society/Scottish Intercollegiate Guidelines Network; cACT: Childhood Asthma Control Test; CBQ‐20: Conflict Behaviour Questionnaire‐20; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CSQ: Consumer Satisfaction Questionnaire; CVD: cardiovascular disease; DDD: Daily Defined Doses; DPI: dry powder inhaler; ED: emergency department; EMA: Ecological Momentary Assessment; EMD: electronic monitoring device; EPAC: episode of poor asthma control; FEV1: forced expiratory volume in one second; FP: fluticasone propionate; FSI: Functional Severity Index; FVC: forced vital capacity; GINA: Global Initiative for Asthma; GP: general practitioner; GSK: GlaxoSmithKline; HADS: Hospital Anxiety and Depression Scale; HFA inhaler: hydrofluoroalkane inhaler; HMO: health maintenance organisation; HRQOL: health‐related quality of life; ICS: inhaled corticosteroids; IPQ: Illness Perceptions Questionnaire; IQR: interquartile ratio; IRF: inhaler reminders and feedback; IT: information technology; ITT: intention‐to‐treat; IVR: interactive voice response; KAAM: Knowledge of Asthma and Asthma Medicine Questionnaire; KP: Kaiser Permanente; KPCO: Kaiser Permanente Colorado; KPH: Kaiser Permanente Hawaii; KPNW: Kaiser Permanente Northwest; LABA: long‐acting beta2‐agonists; MARS‐A: Medication Adherence Report Scale; MD: mean difference; MDI: metered dose inhaler; MI: motivational interviewing; NAEPP: National Asthma Education and Prevention Program; NHLBI: National Heart, Lung, and Blood Institute; NS: not statistically significant; OCS: oral corticosteroid; PAD: personalised adherence discussion; PAQLQ: Paediatric Asthma Quality of Life Questionnaire; PDC: proportion of days covered; PedsQL: Paediatric Quality of Life Inventory; PEF: peak expiratory flow; pMDI: pressurised metered dose inhaler; PS: problem solving; QOL: quality of life; RTMM: real‐time medication monitoring; SABA: short‐acting beta2‐agonists; SD: standard deviation; SF‐36: Short Form‐36; SMAQ: Simplified Medication Adherence Questionnaire; SMS: short message service/text message; SR: speech recognition; UC: usual care; URTI: upper respiratory tract infection; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Drug trial observing adherence | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Drug trial observing adherence | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Drug trial observing adherence | |
| Trial of tailored asthma education in older adults; adherence not primary focus | |
| Wrong study design | |
| Wrong study design | |
| Wrong study design | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Drug trial observing adherence | |
| Drug trial observing adherence | |
| Drug trial observing adherence | |
| Trial of once‐daily vs twice‐daily dosing designed to demonstrate equivalent efficacy. Simplification of treatment regimen postulated to improve adherence in a real‐life setting, but this was a double‐blind, double‐dummy trial, and participants in the trial were not aware of which regimen they had been prescribed. Judged not to be an intervention to improve adherence per se | |
| < 50% taking ICS at baseline | |
| Wrong study design | |
| Adherence not primary focus | |
| Wrong study design | |
| Adherence not primary focus | |
| Written action plan (WAP) vs unformatted prescription post exacerbation. WAP was multi‐faceted and was intended to modify physician ICS prescribing behaviour as well as participant adherence, follow‐up and asthma management more generally. Adherence to ICS not primary focus of the intervention | |
| Wrong study design | |
| Adherence not primary focus | |
| Adherence not primary focus | |
| Wrong study design | |
| Adherence not primary focus | |
| Wrong study design | |
| Drug trial observing adherence | |
| Drug trial observing adherence | |
| Drug trial observing adherence | |
| Drug trial observing adherence | |
| Not asthma, or mixed population | |
| Intervention to improve adherence to asthma therapy generally. Number using ICS not reported, and ICS not mentioned anywhere in trial report. Therefore assumed not to be an intervention aimed at improving adherence to ICS | |
| Adherence not primary focus | |
| Drug trial observing adherence | |
| SMART therapy (single LABA/ICS inhaler for maintenance and reliever) vs separate inhalers for ICS and SABA, so groups received different medications. This means the effect on measures of asthma control might be a result of LABA therapy, or of improved adherence to ICS, but it is not a clear enough comparison to judge | |
| Shared decision making vs clinician decision making and usual care. Interventions led to different medication usage, which meant this is not a clear comparison by which to assess ICS adherence, and that and was not primarily aimed at improving adherence to ICS. Adherence to ICS therefore not a primary focus of intervention | |
| Wrong study design |
ICS: inhaled corticosteroid; LABA: long‐acting beta2‐agonists; SABA: short‐acting beta2‐agonists; WAP: written action plan
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial. Title: Is Compliance With Inhaled Therapy in Asthma Increased by the Use of Small‐Volume Spacers? |
| Participants | Asthma. No other details |
| Interventions | Patients are randomised to use:
|
| Outcomes | Not provided at time of registration |
| Notes | Trial end date 01/10/2003. Listed as completed and no longer recruiting. No publications identified and no results posted |
| Methods | Open‐label randomised controlled trial with parallel assignment. Title: Increasing Adherence to Asthma Medication in Urban Teens |
| Participants | Inclusion criteria: 10 to 15 years of age; resident of Baltimore City; diagnosis of asthma or reactive airway disease; current emergency department visit or hospitalisation for asthma; prescribed a daily asthma controller medication Exclusion criteria: plans to move outside of the Baltimore City area within 1 year from study entry; current participation in another asthma education study; families unwilling or unable to participate; families who were enrolled and participated in the pilot study |
| Interventions | Self‐management (standard care group) vs motivational interviewing plus self‐management |
| Outcomes | Adherence to controller therapy measured by electronic medication monitoring at baseline, 3 months and 6 months. Number of symptom‐free days, emergency department utilisation and hospitalisation, career/adolescent quality of life ‐ all measured at the same time points |
| Notes | Planned enrolment 207. Primary completion date January 2012. Listed as completed, but no publications identified and no results posted |
| Methods | Open‐label randomised controlled trial with cross‐over assignment. Title: Usage, Usability & Effect on Adherence and Clinical Outcomes of Text Message Reminders for Adolescents With Asthma |
| Participants | Inclusion criteria: between the ages of 12 and 22; diagnosis of persistent asthma; receiving care at Cincinnati Children's Hospital Medical Center or affiliate; prescription of a controller medication; must have a cell phone that receives text messages; asthma not well controlled based on Asthma Control Test (ACT) score; English speaking Exclusion criteria: no diagnosis of persistent asthma; receiving asthma care other than at a Cincinnati Children's Hospital Medical Center or affiliate; asthma well controlled based on ACT score; does not have a cell phone that receives text messages or plans to change phones within the next 6 months; not taking a daily asthma controller medication; currently receiving asthma appointment or medication reminder text messages from another source |
| Interventions | Text message reminders vs no intervention |
| Outcomes | Asthma Control Test (ACT), Pediatric Quality of Life Scale (PedsQL), adherence change from baseline |
| Notes | Planned enrolment 61. Primary completion date December 2012. Listed as completed but no publications identified and no results posted |
| Methods | Open‐label randomised controlled trial with parallel assignment. Title: Improving Asthma Control in the Real World: A Systematic Approach to Improving Dulera Adherence |
| Participants | Inclusion criteria: physician diagnosis of asthma of moderate severity; ≥ 18 years of age; currently receiving an inhaled corticosteroid medication and prescribed Dulera 100/5 as part of standard of care based on asthma severity and dosing guidelines; Asthma Control Questionnaire (ACQ) result > 1.0 at entry; demonstration of correct inhalation technique for use of meter dosed inhalers (MDIs); history of reversible airway obstruction documented by treating physician. Exclusion criteria: Intermittent asthma (asthma exacerbations or symptoms < 3 days/wk); diagnosis of emphysema in prior year; diagnosis at any time of chronic obstructive pulmonary disease (COPD), chronic bronchitis, cystic fibrosis, bronchiectasis, Churg Strauss, Wegener's, sarcoidosis, pulmonary hypertension or lung cancer; taking any medication documented to have a drug interaction with Dulera |
| Interventions | Dulera adherence monitoring with motivational interviewing vs standard asthma care |
| Outcomes | ACQ, adherence to Dulera, validation of an adult asthma adherence questionnaire (AAAQ) |
| Notes | Planned enrolment 40. Estimated study completion date December 2015. Note from clinicaltrials.gov: "The recruitment status of this study is unknown because the information has not been verified recently" |
| Methods | Open‐label randomised controlled trial with parallel assignment. Title: Adolescent Controlled Text Messaging to Improve Asthma Medication Adherence in Primary Care (ACT Me) |
| Participants | Inclusion criteria: provider‐diagnosed persistent asthma; prescription of an inhaled corticosteroid (ICS) in accordance with National Heart, Lung, and Blood Institute (NHLBI) Expert Panel Report 3 guidelines for at least 30 days before enrolment; Asthma Control Test (ACT) score < 20 (indicating lack of current control); no provider‐diagnosed exacerbation in the 30 days before enrolment; possession of a text‐enabled cell phone and plan to keep it throughout the study period; agreement by parents (or participants over 18 years old) to any charges levied by their cell phone carrier for text messages associated with the study if they do not have an unlimited texting plan; ability to speak and read English Exclusion criteria: another chronic lung disease (which would complicate measurement of asthma control); cognitive or psychiatric disorder that the treating clinician judges would impair study participation; use of Advair Diskus for ICS (for which no reliable electronic monitor exists); current enrolment in another asthma intervention study |
| Interventions | Technology‐based system that allows adolescents to compose, schedule and send 1‐time or recurring text messages to their own cell phones. Control group receives usual care |
| Outcomes | Adherence each month, feasibility, acceptability and useability of the website, asthma control (ACT), quality of life (Pediatric Quality of Life Scale (PedsQL)) |
| Notes | Planned enrolment 29. Primary completion date December 2015. Listed as completed, but no publications identified and no results posted |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Motivational Intervention for Asthma (MI‐ACT) |
| Methods | Double‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Motivational communication or control |
| Outcomes | Primary outcome measure: inhaled corticosteroid adherence |
| Starting date | January 2011 |
| Contact information | |
| Notes | Link to study registration: https://clinicaltrials.gov/ct2/show/NCT01381159 |
| Trial name or title | EmPhAsIS: Empowering Pharmacists in Asthma Management Through Interactive SMS |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Interactive SMS or usual care |
| Outcomes | Adherence to inhaled corticosteroid medication |
| Starting date | May 2015 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02170883 |
| Trial name or title | Teaching Inhaler Use With the INCA Device in a Community Pharmacy Setting |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Feedback on inhaler use, education or control |
| Outcomes | Rate of adherence |
| Starting date | February 2014 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02203266 |
| Trial name or title | Inhaler Adherence in Severe Unstable Asthma (INCA‐SUN) |
| Methods | Double‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Feedback on inhaler use or routine care |
| Outcomes | Adherence to preventer medication; cost and effectiveness of medication |
| Starting date | December 2015 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02307669 |
| Trial name or title | Intervention to Improve Inhalative Adherence |
| Methods | Single‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Audio reminders and support calls or control |
| Outcomes | Time to next asthma or COPD exacerbation; number of exacerbations; adherence |
| Starting date | January 2014 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02386722 |
| Trial name or title | Assessment of a Mobile Intervention to Increase Adherence to Asthma Medication Among Adolescents |
| Methods | Open‐label, parallel‐group randomised |
| Participants |
|
| Interventions | Medication Monitoring and Mobile App or sham comparator |
| Outcomes | Real‐time medication adherence |
| Starting date | August 2015 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02426814 |
| Trial name or title | ICS/LABA Combination With Integrated Dose Counter and Smartphone App to Improve Asthma Control |
| Methods | Open‐label, parallel‐group randomised |
| Participants |
|
| Interventions | Smartphone self management or routine care |
| Outcomes | Changes in airway inflammation profile; changes in scores of Asthma Control Questionnaire; changes in lung function parameters; numbers of rescue medications used |
| Starting date | August 2014 |
| Contact information | |
| Notes | Link to study registration: https://clinicaltrials.gov/ct2/show/NCT02556073 |
| Trial name or title | Asthma Controller Adherence After Hospitalization |
| Methods | Open‐label, parallel‐group randomised |
| Participants |
|
| Interventions | Daily text message reminders or control |
| Outcomes | Feasibility; acceptability; adherence |
| Starting date | December 2015 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02615743 |
| Trial name or title | Effectiveness of an AEP on Patient's Knowledge, Medication Adherence and Inhaler Technique |
| Methods | Single‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Asthma Education Programme (AEP) or routine care |
| Outcomes | Participant knowledge status regarding asthma; participant medication adherence status; participant inhaler technique |
| Starting date | June 2015 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02715219 |
| Trial name or title | Evaluation of a Community Pharmacist Managed Asthma Consultation Service |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Comprehensive disease management programme for asthma or control |
| Outcomes | Peak expiratory flow rate; medication adherence; asthma control |
| Starting date | May 2016 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02768623 |
| Trial name or title | A Computer‐Based ED Intervention to Improve Pediatric Asthma Medicine Adherence (ED‐AMAP) |
| Methods | Single‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Interactive tailored asthma medication adherence education on an iPad or routine care |
| Outcomes | Asthma controller medication adherence |
| Starting date | February 2016 |
| Contact information | |
| Notes | https://www.clinicaltrials.gov/ct2/show/NCT02787174 |
| Trial name or title | Development and Testing of an Adolescent Adherence Patient Tool for Asthma |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants | Adolescent patients using ICS registered at 1 of the pharmacies in the UPPER‐network
|
| Interventions | Smartphone application for patients combined with a computer management system for healthcare providers (pharmacists) |
| Outcomes | Adherence |
| Starting date | April 2015 |
| Contact information | |
| Notes | http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5061 |
| Trial name or title | Prospective Study of the Feedback From an Adherence Monitor on Asthma Control (INCA) |
| Methods | Single‐blind, parallel‐group randomised controlled trial |
| Participants |
|
| Interventions | Feedback from a computer log of inhaler use or control |
| Outcomes | Adherence rate |
| Starting date | February 2012 |
| Contact information | |
| Notes | https://clinicaltrials.gov/ct2/show/NCT01529697 |
ACT: Asthma Control Test; COPD: chronic obstructive pulmonary disease; ED: emergency department; FEV1: forced expiratory volume in one second; GINA: Global Initiative for Asthma; GP: general practitioner; HFA: hydrofluoroalkane; ICS: inhaled corticosteroids; INCA: Inhaler Compliance Assessment device; LABA: long‐acting beta2‐agonist; MDI: metered dose inhaler; SMS: short message service/text message
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence (objective measures) Show forest plot | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 20.13 [7.52, 32.74] |
| Analysis 1.1  Comparison 1 Adherence education versus controls, Outcome 1 % Adherence (objective measures). | ||||
| 1.1 Complex | 4 | 230 | Mean Difference (IV, Random, 95% CI) | 21.55 [4.71, 38.39] |
| 1.2 Simple education | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 15.40 [5.98, 24.82] |
| 2 % Adherence (all measures) Show forest plot | 10 | 1693 | Mean Difference (IV, Random, 95% CI) | 11.59 [3.72, 19.46] |
| Analysis 1.2  Comparison 1 Adherence education versus controls, Outcome 2 % Adherence (all measures). | ||||
| 2.1 Complex | 8 | 744 | Mean Difference (IV, Random, 95% CI) | 12.21 [1.26, 23.17] |
| 2.2 Simple education | 2 | 949 | Mean Difference (IV, Random, 95% CI) | 10.60 [5.17, 16.03] |
| 3 > 85% adherence Show forest plot | 1 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 2.68 [1.61, 4.46] |
| Analysis 1.3  Comparison 1 Adherence education versus controls, Outcome 3 > 85% adherence. | ||||
| 4 Exacerbations requiring OCS (people with 1 or more) Show forest plot | 3 | 349 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.99, 3.36] |
| Analysis 1.4  Comparison 1 Adherence education versus controls, Outcome 4 Exacerbations requiring OCS (people with 1 or more). | ||||
| 5 Asthma control Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Adherence education versus controls, Outcome 5 Asthma control. | ||||
| 5.1 ACQ | 4 | 455 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.49, 0.43] |
| 5.2 ACT | 3 | 333 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.82, 1.43] |
| 6 Unsheduled visits to a healthcare provider (people with 1 or more) Show forest plot | 4 | 688 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.19] |
| Analysis 1.6  Comparison 1 Adherence education versus controls, Outcome 6 Unsheduled visits to a healthcare provider (people with 1 or more). | ||||
| 6.1 Hospital | 1 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.56, 2.70] |
| 6.2 ED | 2 | 367 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.83] |
| 6.3 GP | 1 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.54] |
| 7 Quality of life (AQLQ) Show forest plot | 6 | 734 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.20, 0.23] |
| Analysis 1.7  Comparison 1 Adherence education versus controls, Outcome 7 Quality of life (AQLQ). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence (objective measures) Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| Analysis 2.1  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 1 % Adherence (objective measures). | ||||
| 1.1 Reminders/trackers | 3 | 321 | Mean Difference (IV, Random, 95% CI) | 16.29 [9.53, 23.04] |
| 1.2 With feedback | 3 | 234 | Mean Difference (IV, Random, 95% CI) | 24.98 [17.53, 32.44] |
| 2 % Adherence (all measures) Show forest plot | 8 | 762 | Mean Difference (IV, Random, 95% CI) | 18.41 [11.82, 25.00] |
| Analysis 2.2 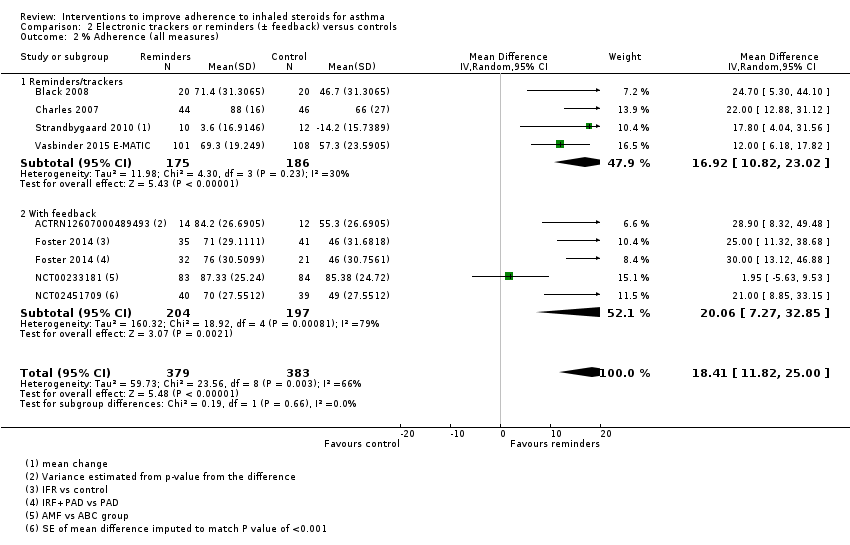 Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 2 % Adherence (all measures). | ||||
| 2.1 Reminders/trackers | 4 | 361 | Mean Difference (IV, Random, 95% CI) | 16.92 [10.82, 23.02] |
| 2.2 With feedback | 4 | 401 | Mean Difference (IV, Random, 95% CI) | 20.06 [7.27, 32.85] |
| 3 Exacerbations requiring OCS (people with at least 1) Show forest plot | 4 | 3063 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.39] |
| Analysis 2.3 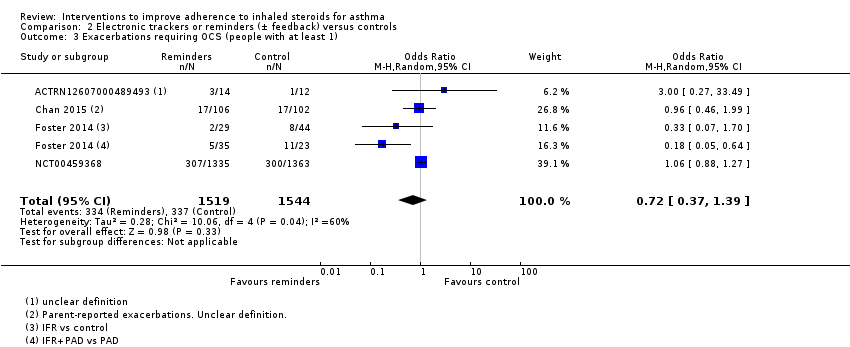 Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 3 Exacerbations requiring OCS (people with at least 1). | ||||
| 4 Asthma control Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 4 Asthma control. | ||||
| 4.1 ACQ | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.29, 0.78] |
| 4.2 ACT | 4 | 596 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.20, 1.69] |
| 5 Unscheduled visits to a healthcare provider Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 5 Unscheduled visits to a healthcare provider. | ||||
| 5.1 ED | 2 | 2918 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.88, 1.47] |
| 5.2 Hospital | 2 | 2865 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.78] |
| 6 Unscheduled visits to a healthcare provider Show forest plot | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 6 Unscheduled visits to a healthcare provider. | ||||
| 6.1 GP/ED visits | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Hospitalisations | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Absenteeism Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 7 Absenteeism. | ||||
| 8 Absenteeism Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 8 Absenteeism. | ||||
| 9 Quality of life (AQLQ) Show forest plot | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.13] |
| Analysis 2.9 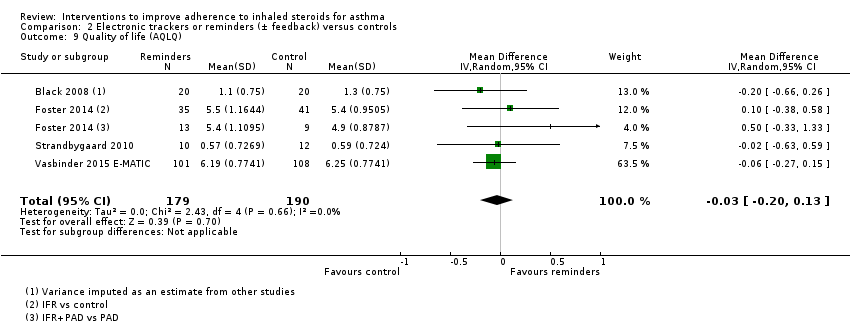 Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 9 Quality of life (AQLQ). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence Show forest plot | 3 | 1310 | Mean Difference (IV, Random, 95% CI) | 4.02 [1.88, 6.16] |
| Analysis 3.1  Comparison 3 Simplified versus usual regimens, Outcome 1 % Adherence. | ||||
| 2 Exacerbations requiring OCS Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2 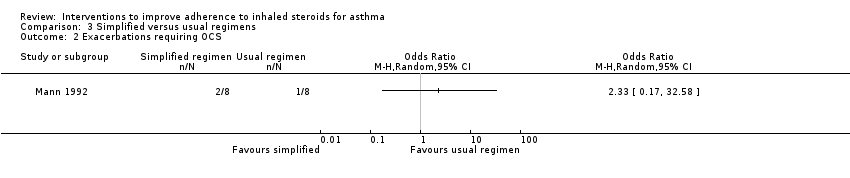 Comparison 3 Simplified versus usual regimens, Outcome 2 Exacerbations requiring OCS. | ||||
| 3 Asthma control (ACQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Simplified versus usual regimens, Outcome 3 Asthma control (ACQ). | ||||
| 4 Unscheduled visits Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.4 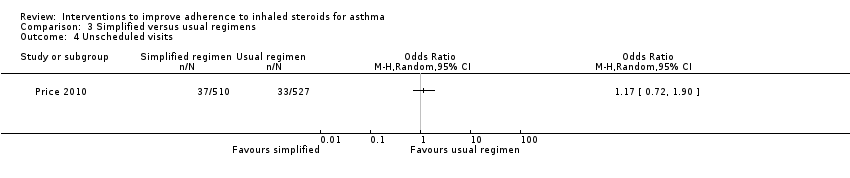 Comparison 3 Simplified versus usual regimens, Outcome 4 Unscheduled visits. | ||||
| 5 Absence from work/school Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.5 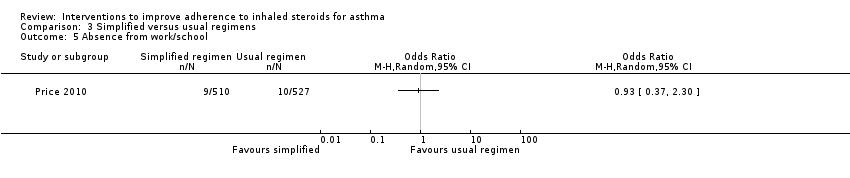 Comparison 3 Simplified versus usual regimens, Outcome 5 Absence from work/school. | ||||
| 6 Quality of life (ITG‐ASF % change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Simplified versus usual regimens, Outcome 6 Quality of life (ITG‐ASF % change from baseline). | ||||
| 7 All adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Simplified versus usual regimens, Outcome 7 All adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Unscheduled visits (1 or more hospitalisations for any cause) Show forest plot | 2 | 279 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.05] |
| Analysis 4.1  Comparison 4 School‐based ICS therapy versus controls, Outcome 1 Unscheduled visits (1 or more hospitalisations for any cause). | ||||
| 2 Quality of life (PAQLQ) Show forest plot | 2 | 279 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.01, 0.49] |
| Analysis 4.2 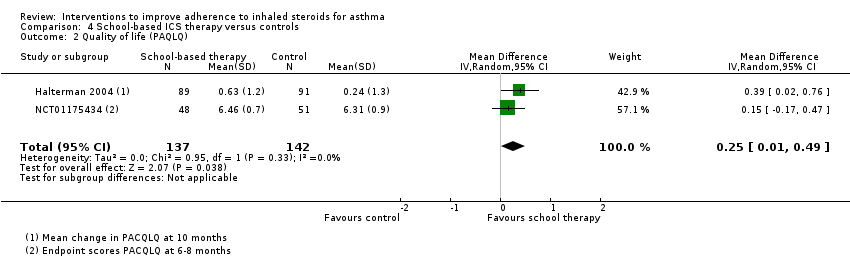 Comparison 4 School‐based ICS therapy versus controls, Outcome 2 Quality of life (PAQLQ). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1. Children vs adults Show forest plot | 10 | 1693 | Mean Difference (IV, Random, 95% CI) | 11.59 [3.72, 19.46] |
| Analysis 5.1  Comparison 5 Subgroup analyses for % adherence, Outcome 1 Comparison 1. Children vs adults. | ||||
| 1.1 Children | 4 | 1241 | Mean Difference (IV, Random, 95% CI) | 8.01 [‐4.77, 20.79] |
| 1.2 Adults/adolescents and adults | 6 | 452 | Mean Difference (IV, Random, 95% CI) | 14.43 [5.49, 23.36] |
| 2 Comparison 2. Complex vs simple interventions Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| Analysis 5.2  Comparison 5 Subgroup analyses for % adherence, Outcome 2 Comparison 2. Complex vs simple interventions. | ||||
| 2.1 Complex | 3 | 234 | Mean Difference (IV, Random, 95% CI) | 24.98 [17.53, 32.44] |
| 2.2 Simple | 3 | 321 | Mean Difference (IV, Random, 95% CI) | 16.29 [9.53, 23.04] |
| 3 Comparison 2. Children vs adults Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| Analysis 5.3  Comparison 5 Subgroup analyses for % adherence, Outcome 3 Comparison 2. Children vs adults. | ||||
| 3.1 Children | 3 | 314 | Mean Difference (IV, Random, 95% CI) | 17.29 [8.32, 26.26] |
| 3.2 Adults/adolescents and adults | 3 | 241 | Mean Difference (IV, Random, 95% CI) | 22.84 [16.66, 29.02] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Adherence education vs controls, outcome: 1.2 % Adherence (all measures).

Comparison 1 Adherence education versus controls, Outcome 1 % Adherence (objective measures).

Comparison 1 Adherence education versus controls, Outcome 2 % Adherence (all measures).

Comparison 1 Adherence education versus controls, Outcome 3 > 85% adherence.

Comparison 1 Adherence education versus controls, Outcome 4 Exacerbations requiring OCS (people with 1 or more).

Comparison 1 Adherence education versus controls, Outcome 5 Asthma control.

Comparison 1 Adherence education versus controls, Outcome 6 Unsheduled visits to a healthcare provider (people with 1 or more).

Comparison 1 Adherence education versus controls, Outcome 7 Quality of life (AQLQ).

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 1 % Adherence (objective measures).

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 2 % Adherence (all measures).

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 3 Exacerbations requiring OCS (people with at least 1).

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 4 Asthma control.

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 5 Unscheduled visits to a healthcare provider.

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 6 Unscheduled visits to a healthcare provider.

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 7 Absenteeism.

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 8 Absenteeism.

Comparison 2 Electronic trackers or reminders (± feedback) versus controls, Outcome 9 Quality of life (AQLQ).

Comparison 3 Simplified versus usual regimens, Outcome 1 % Adherence.

Comparison 3 Simplified versus usual regimens, Outcome 2 Exacerbations requiring OCS.

Comparison 3 Simplified versus usual regimens, Outcome 3 Asthma control (ACQ).

Comparison 3 Simplified versus usual regimens, Outcome 4 Unscheduled visits.

Comparison 3 Simplified versus usual regimens, Outcome 5 Absence from work/school.

Comparison 3 Simplified versus usual regimens, Outcome 6 Quality of life (ITG‐ASF % change from baseline).

Comparison 3 Simplified versus usual regimens, Outcome 7 All adverse events.

Comparison 4 School‐based ICS therapy versus controls, Outcome 1 Unscheduled visits (1 or more hospitalisations for any cause).

Comparison 4 School‐based ICS therapy versus controls, Outcome 2 Quality of life (PAQLQ).

Comparison 5 Subgroup analyses for % adherence, Outcome 1 Comparison 1. Children vs adults.

Comparison 5 Subgroup analyses for % adherence, Outcome 2 Comparison 2. Complex vs simple interventions.

Comparison 5 Subgroup analyses for % adherence, Outcome 3 Comparison 2. Children vs adults.
| Adherence education compared with controls for asthma | |||||||
| Patient or population: asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
| Risk with controls | Risk with adherence education | ||||||
| % Adherence WMD of follow‐up 71.7 weeks (all studies) | Objective measures | Mean adherence in the control group was 46.7% | Mean adherence with adherence education was 20.13% higher (7.52 higher to 32.74 higher) | ‐ | 280 (5 RCTs) | ⊕⊕⊝⊝ | Only studies in which adherence was measured with an electronic monitor |
| All measures | Mean adherence in the control group was 57.1% | Mean adherence with adherence education was 11.59% higher (3.72 higher to 19.46 higher) | ‐ | 1693 | ⊕⊕⊝⊝ | ||
| Exacerbations requiring OCS (people with 1 or more) WMD of follow‐up 30.8 weeks | 149 per 1000 | 242 per 1000 (148 to 370) | OR 1.82 | 349 | ⊕⊕⊝⊝ | ||
| Asthma control (ACQ) WMD of follow‐up 28.5 weeks | Mean ACQ score was 1.52 | Mean score with adherence education was 0.03 better (0.49 better to 0.43 worse) | ‐ | 455 | ⊕⊕⊕⊝ | Lower score indicates better control. Scale 0 to 6. MCID 0.5 | |
| Asthma control (ACT) WMD of follow‐up 29.5 weeks | Mean ACT score was 18.88 | Mean score with adherence education was 0.30 better | ‐ | 333 | ⊕⊕⊕⊝ | Higher score indicates better control. Scale 5 to 25. MCID 3 | |
| Unsheduled visits to a healthcare provider (people with 1 or more) WMD of follow‐up 67.2 weeks | 159 per 1000 | 83 per 1000 | OR 0.48 | 688 | ⊕⊝⊝⊝ | Includes visits to ED, GP, hospital for any cause | |
| Absenteeism WMD of follow‐up 63.3 weeks | We did not perform an analysis of absences because the data were heavily skewed | ‐ | 109 | Not graded | |||
| Quality of life (AQLQ) WMD of follow‐up 27.4 weeks | Mean AQLQ score was 5 | Mean score with adherence education was 0.01 better (0.20 worse to 0.23 better) | ‐ | 734 | ⊕⊕⊕⊝ | Higher score indicates better QOL. Scale 1 to 7. MCID 0.5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded once primarily owing to risk of bias from open‐label trials and some concerns regarding attrition bias, selective reporting and selection bias (‐1 risk of bias) bDowngraded once owing to inconsistency between study results (‐1 inconsistency) cFunnel plot examined; no clear evidence of publication bias (no downgrade for publication bias) dConfidence intervals include no difference and/or potential important harm or benefit of the intervention (‐1 imprecision) eConfidence intervals fall within the established MCID for this scale (no downgrade for imprecision) fStudies contributing to this analysis reported different types of unscheduled visits and some recorded visits for any cause rather than asthma alone (‐1 indirectness) gUnclear how absenteeism was defined or reported, and different participants may have different thresholds for missing work or school. One study was conducted in children and the other in adults. Combined, this makes the outcome hard to interpret | |||||||
| Electronic trackers or reminders (±feedback) compared with controls for asthma | |||||||
| Patient or population: asthma | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | ||
| Risk with controls | Risk with electronic trackers or reminders (± feedback) | ||||||
| % Adherence WMD of follow‐up 47.6 weeks | Objective measures only | Mean adherence in the control group was 53.27% | Mean adherence was 19.86% higher (14.47 higher to 25.26 higher) | ‐ | 555 (6 RCTs) | ⊕⊕⊕⊝ | Only studies in which adherence was measured with an electronic monitor |
| All measures | Mean adherence in the control group was 56.06% | Mean adherence with trackers was 18.41% higher (11.82 higher to 25.00 higher) | ‐ | 762 (8 RCTs) | ⊕⊕⊝⊝ | ||
| Exacerbations requiring OCS (people with at least 1) WMD of follow‐up 48.6 weeks | 218 per 1000 | 169 per 1000 | OR 0.72 | 3063 | ⊕⊝⊝⊝ | ||
| Asthma control (ACQ) WMD of follow‐up 43.0 weeks | Mean ACQ score in the control group was 0.89 | Mean score with trackers or reminders was 0.24 better (0.29 worse to 0.78 better) | ‐ | 109 | ⊕⊕⊝⊝ | Lower score indicates better control. Scale 0 to 6. MCID 0.5 | |
| Asthma control (ACT) WMD of follow‐up 34.0 weeks | Mean ACT score in the control group was 20.04 | Mean score with trackers or reminders was 0.74 better (0.20 worse to 1.69 better) | ‐ | 596 | ⊕⊕⊝⊝ | Higher score indicates better control. Scale 5 to 25. MCID 3 | |
| Unscheduled healthcare visits to a healthcare provider (ED) WMD of follow‐up 50.0 weeks | 84 per 1000 | 95 per 1000 | OR 1.14 | 2918 | ⊕⊕⊕⊝ | Two studies (n = 2865) also reported hospitalisations. OR 0.97 (0.53 to 1.78) | |
| Absenteeism (people with at least 1 absence) Follow‐up 26 weeks | 327 per 1000 | 409 per 1000 | OR 1.42 | 220 | ⊕⊕⊝⊝ | ||
| Quality of life (AQLQ) WMD of follow‐up 36.8 weeks | Mean AQLQ score in the control group was 5.15 | Mean score with trackers or reminders was 0.03 worse (0.13 better to 0.20 worse) | ‐ | 369 | ⊕⊕⊕⊝ | Higher score indicated better QOL. Scale 1 to 7. MCID 0.5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded once primarily owing to risk of bias from open‐label trials and some concerns regarding attrition bias, selective reporting and selection bias (‐1 risk of bias) bDowngraded once for inconsistency between study results (‐1 inconsistency) cConfidence intervals include no difference and potential important harm and benefit of the intervention (‐1 imprecision) dConfidence intervals fall within the MCID for this scale (no downgrade for imprecision) eDowngraded once owing to risk of performance and detection bias (‐1 risk of bias) | |||||||
| Simplified compared with usual regimens for asthma | ||||||
| Patient or population: asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual regimens | Risk with simplified regimens | |||||
| % Adherence (objective measures) WMD of follow‐up 12.9 weeks | Mean adherence in the control group was 86.73% | Mean adherence with simplified regimens was 4.02% higher | ‐ | 1310 | ⊕⊕⊕⊝ | Only studies in which adherence was measured with an electronic monitor |
| Exacerbations requiring OCS People with 1 or more Follow‐up 12 weeks | 125 per 1000 | 250 per 1000 | OR 2.33 | 16 | ⊕⊕⊝⊝ | |
| Asthma control (ACQ) Follow‐up 24 weeks | Mean ACQ score in the control group was 0.89 | Mean score with simplified regimens was 0.03 better (0.34 better to 0.28 worse) | ‐ | 103 | ⊕⊕⊕⊝ | Lower score indicates better control. Scale 0 to 6. MCID 0.5 |
| Unscheduled visits Follow‐up 12 weeks | 63 per 1000 | 72 per 1000 | OR 1.17 | 1037 | ⊕⊕⊝⊝ | |
| Absence from work/school Follow‐up 12 weeks | 19 per 1000 | 18 per 1000 | OR 0.93 | 1037 | ⊕⊕⊝⊝ | |
| Change in quality of life (ITG‐ASF) Follow‐up 12 weeks | Mean change in quality of life in the control group was 14 | Mean change with simplified regimens was 6 points better | ‐ | 1037 | ⊕⊕⊝⊝ | Higher score indicates better QOL. Range 0 to 100. MCID not known |
| All adverse events Follow‐up 12 weeks | 175 per 1000 | 139 per 1000 | OR 0.76 | 1233 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once primarily owing to lack of blinding and some concerns regarding attrition bias, selective reporting and selection bias (‐1 risk of bias) bOne very small trial resulting in very wide confidence intervals (‐2 imprecision) cAlthough confidence intervals fall within the MCID, only one study contributed to this outcome (‐1 imprecision) dConfidence intervals include both important potential harm and benefit of the intervention (‐1 imprecision) eConfidence intervals do not exclude no difference (‐1 imprecision) fConfidence intervals range from no difference to an important benefit of simplified regimens (‐1 imprecision) | ||||||
| School‐based ICS therapy compared with home therapy for asthma | ||||||
| Patient or population: children with asthma Settings: school Intervention: ICS given at school Comparison: ICS given at home | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | School‐based ICS therapy | |||||
| Unscheduled visits 1 or more hospitalisations for any cause WMD of follow‐up 35.8 weeks | 49 per 1000 | 29 per 1000 | OR 0.58 (0.16 to 2.05) | 279 | ⊕⊕⊝⊝ | |
| Quality of life (PACQLQ) 1 to 7; higher is better WMD of follow‐up 35.8 weeks | Mean PAQLQ score in the control group was 6.31 | Mean score in the intervention groups was | ‐ | 279 | ⊕⊕⊕⊝ MODERATEa | |
| Adverse events Follow‐up 30 weeks | No events observed in either arm | ‐ | 99 (1 RCT) | Not graded | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| No data could be meta‐analysed for adherence, exacerbations requiring OCS, asthma control or absenteeism. Some data are presented narratively in the review aBoth contributing studies considered at high risk for performance and detection bias bConfidence intervals include both potential harm and benefit of the intervention | ||||||
| Study ID ("first received" date for clinical trials registries) | Total n | Duration of intervention/follow‐up | Age | Country | Intervention | Control | Adherence measure | Outcomes |
| (2005) | 333 | 13/26 weeks | Adults | USA | Problem‐solving intervention | Asthma education | Electronic inhaler monitor | Adherence, AQLQ, ACQ, LFTs, hospitalisation, ED visits, participant satisfaction |
| 50 | 10 weeks | Adults | USA | Interactive voice response intervention | Usual care | Electronic inhaler monitor or canister weight | Adherence, AQLQ, ACT, Beliefs about Medication Questionnaire | |
| (2009) | 1187 | 2 years | Children | USA | Telephone speech recognition intervention | Usual care | Total ICS supplied/total prescribed | Adherence, beta‐agonist use, OCS use, primary care, ED and out of hours visits, hospitalisations, participant satisfaction |
| 271 | 13 weeks | Adolescents and adults | Brazil | Telephone counselling | Ususal care | "Number of inhalations recorded on the disks" | Adherence | |
| (2005) | 141 | 17 weeks/1 year | Children | USA | Problem‐solving intervention | Family‐based intervention | Electronic inhaler monitor | Adherence, symptoms, use of healthcare services, reliever medication use |
| (2009) | 55 | 2 months | Children | USA | Team work intervention | Asthma education | Electronic inhaler monitor | Adherence, Parent‐Adolescent Conflict Questionnaire, Functional Severity Index, LFTs, Consumer Satisfaction Survey |
| 60 GPs, 143 patients | 6 months | Adolescents and adults | Australia | Personalised adherence discussion (PAD) PAD + inhaler reminder feedback (IRF) | Usual care | Electronic inhaler monitor | ACT, AQLQ, Hospital Anxiety and Depression Scale, Medication Adherence Report Scale, LFTs, exacerbations | |
| 78 | 1 year | Adults | Norway | Asthma education | Usual care | Prescribed doses/dispensed doses | Adherence, GP visits, absenteeism, days in hospital | |
| (2010) | 20 | 12 weeks/1 year | Not reported, but mean age suggests adults | Northern Ireland | Nurse‐led psychoeducation | Ususal care (difficult asthma service) | Percent of prescriptions refilled | Adherence, OCS, beta‐agonist use, hospital admissions, LFTs, ACQ, AQLQ, Hospital Anxiety and Despression Scale |
| (2008) | 298 | 90 weeks | Children | Brazil | Telephone follow‐up intervention | Usual care | Percentage of actual doses/number expected | Adherence, disease control, quality of life (SF‐36) |
| 83 | 13 weeks | Children | UK | Asthma education | Usual care | Electronic inhaler monitor | Adherence, beliefs and anxieties about adherence | |
| (2007) | 60 | 26 weeks/78 weeks | Children | Sweden | Group discussion plus basic education | Basic education | Diaries and canister weight | Adherence, views on adherence, days hospitalised, ED visits, exacerbations |
| 15 | 6 weeks/52 weeks | Children | USA | Specific adherence improvement strategies (education, monitoring, etc.) | Usual care plus education | Electronic inhaler monitor | Adherence, LFTs, PedsQL, healthcare costs | |
| (2010) | 54 | 6 weeks/52 weeks | Adults | Canada | Motivational interviewing | Usual care | Prescribed treatment days/number of days | Adherence, asthma control, quality of life, asthma‐related self‐efficacy |
| 201 | 6 months | Adults | Belgium | Adherence education | Usual care | Prescription refill rates, self‐reporting | ACT, diary card, rescue medication use, ED visits, hospitalisations, AQLQ, Knowledge of Asthma and Asthma Medicine Questionnaire, inhalation technique | |
| (2010) | 68 | 10 weeks | Adolescents | USA | Adherence messaging and group sessions | "Attention control" | Electronic inhaler monitor | Adherence, asthma knowledge, ICS knowledge, ICS self‐efficacy, social support, exacerbations |
| (2015) | 12 | 12 weeks | Adolescents | USA | Adherence monitoring and incentivisation via app and sensor | Usual care | Electronic inhaler monitor | Adherence, ACT NB: study terminated |
| 30 | 10 weeks | Adults | USA | Adherence monitoring and education | Monitoring without feedback | Electronic inhaler monitor | Adherence, rescue medication use, AQLQ, LFTs | |
| (2005) | 250 | 78 weeks | Children | USA | Adherence education | Usual care | Prescription refill rates, self‐reporting | Adherence, symptoms, night‐time awakenings, ED visits, hospitalisation, OCS courses |
| 274 | 12 weeks | Adults | Denmark and Switzerland | Adherence education and study medication | Study medication alone | Dose counting in returned investigational product | Adherence, asthma control, LFTs, symptoms, rescue medication use, night‐time awakenings, adverse events, AQLQ, asthma severity, adverse events, vital signs | |
| (2006) | 14,064 (6903 previous ICS users) | 78 weeks | Adults | USA | Telephone interactive voice recognition intervention | Usual care | Pharmacy‐based adherence measures | Adherence, use of healthcare services, economic evaluation |
| ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; ED: emergency department; GP: general practitioner; ICS: inhaled corticosteroid; IRF: inhaler reminder feedback; LFTs: lung function tests; OCS: oral corticosteroid; PAD: personalised adherence discussion; PedsQL: Paediatric Quality of Life Inventory; SF‐36: Short‐Form Health Survey | ||||||||
| Study ID | Total n | Duration of intervention/follow‐up | Age | Country | Intervention | Control | Adherence measure | Outcomes |
| 40 | 2 months | Children | New Zealand | Inhaler alarm | Usual care | Electronic inhaler monitor | Adherence, AQLQ, LFTs, beta‐agonist use | |
| (2007) | 26 | 4 months | Children | Australia | Adherence feedback during consultations | Usual care | Electronic inhaler monitor | Adherence, symptoms, LFTs |
| 220 | 6 months | Children | New Zealand | Audiovisual inhaler reminder | Usual care | Electronic inhaler monitor | Adherence, school/work absences, ACT, Asthma Morbidity Score, exacerbations, unscheduled visits, beta‐agonist use, LFTs | |
| 110 | 24 weeks | Adolescents and adults | New Zealand | Audiovisual inhaler reminder | Usual care | Electronic inhaler monitor | Adherence, ACQ, LFTs | |
| 60 GPs, 143 patients | 6 months | Adolescents and adults | Australia | Inhaler reminder and feedback (IRF) | Usual care | Electronic inhaler monitor | ACT, AQLQ, Hospital Anxiety and Depression Scale, Medication Adherence Report Scale, LFTs, exacerbations | |
| (2012) | 49 | 13 weeks | Young adults | USA | Computer sessions and tailored text reminders | Asthma education | Self‐reported missed doses | Adherence, ACT, LFTs, participant satisfaction |
| (2015) | 90 | 1 year | Children | UK | Adherence monitoring with feedback | Adherence monitoring but no feedback | Electronic inhaler monitor | "Clinical outcomes", adherence, LFTs, exacerbations |
| (2005) | 250 | 78 weeks | Children | USA | Adherence monitoring and education | Adherence education | Prescription refill rates, self‐reporting | Adherence, symptoms, night‐time awakenings, ED visits, hospitalisation, OCS courses |
| 26 | 12 weeks | Adults | Denmark | SMS (text message) adherence reminders | Usual care | "Dose‐count" on the Seretide was diskus | Adherence, change in FeNO, LFTs, airway responsiveness | |
| 219 | 52 weeks | Children | The Netherlands | SMS (text message) adherence reminders | Usual care | Electronic inhaler monitor | Adherence, ACT, exacerbations, use of healthcare services, AQLQ, school/work absence, acceptance of e‐monitoring, economic evaluation | |
| (2007) | 2698 (34 clusters) | 52 weeks | Children and adults | USA | Adherence education with adherence feedback | Adherence education alone | Electronic prescribing data/refill rate | Adherence, ED visits, hospitalisation, OCS use |
| ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality of Life Questionnaire; ED: emergency department; FeNO: fractional exhaled nitric oxide; ICS: inhaled corticosteroid; LFTs: lung function tests; OCS: oral corticosteroid | ||||||||
| Study ID | Total n | Duration of intervention/follow‐up | Age | Country | Intervention | Control | Adherence measure | Outcomes |
| 102 | 12 weeks | Adults | UK | Combined inhaler | Separate inhalers | Electronic inhaler monitor | Adherence, LFTs | |
| 16 | 6 weeks/12 weeks | Adults | USA | Twice‐daily dosing | Four‐times‐daily dosing | Electronic inhaler monitor | Adherence, LFTs, symptoms | |
| (2007) | 111 | 24 weeks | Children | New Zealand | Combined inhaler | Separate inhalers | Electronic inhaler monitor | Adherence, LFTs, ACQ, OCS, unscheduled visits |
| 1233 | 12 weeks | Adolescents and adults | UK | Once‐daily ICS | Twice‐daily ICS | "Device counter number" | Adherence, physician assessment of response, quality of life, use of healthcare services, days of school/work missed, adverse events, worsening asthma | |
| ACQ: Asthma Control Questionnaire; ICS: inhaled corticosteroid; LFTs: lung function tests; OCS: oral corticosteroid | ||||||||
| Study ID | Total n | Duration of intervention/follow‐up | Age | Country | Intervention | Control | Adherence measure | Outcomes |
| 290 | 65 weeks | Children | USA | Supervised ICS therapy at school | Usual care | N/A | Episodes of poor asthma control, school absences, rescue medication use at school | |
| 184 | 9 weeks | Children | USA | Supervised ICS therapy at school | Usual care | N/A | Symptom‐free days, daytime and night‐time symptoms, rescue medication use, school absences | |
| (2010) | 100 | 6 to 8 months | Children | USA | Supervised ICS therapy at school | Usual care | N/A | Feasibility, symptom‐free days, numbers of days and nights with symptoms, activity limitation, rescue medication use, school absenteeism, parent sleep interruption, change in family plans due to the child’s asthma, PAQLQ, utilisation of healthcare services, FeNO |
| FeNO: fractional exhaled nitric oxide; ICS: inhaled corticosteroid; PAQLQ: Pediatric Asthma Quality of Life Questionnaire | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence (objective measures) Show forest plot | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 20.13 [7.52, 32.74] |
| 1.1 Complex | 4 | 230 | Mean Difference (IV, Random, 95% CI) | 21.55 [4.71, 38.39] |
| 1.2 Simple education | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 15.40 [5.98, 24.82] |
| 2 % Adherence (all measures) Show forest plot | 10 | 1693 | Mean Difference (IV, Random, 95% CI) | 11.59 [3.72, 19.46] |
| 2.1 Complex | 8 | 744 | Mean Difference (IV, Random, 95% CI) | 12.21 [1.26, 23.17] |
| 2.2 Simple education | 2 | 949 | Mean Difference (IV, Random, 95% CI) | 10.60 [5.17, 16.03] |
| 3 > 85% adherence Show forest plot | 1 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 2.68 [1.61, 4.46] |
| 4 Exacerbations requiring OCS (people with 1 or more) Show forest plot | 3 | 349 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.99, 3.36] |
| 5 Asthma control Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 ACQ | 4 | 455 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.49, 0.43] |
| 5.2 ACT | 3 | 333 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.82, 1.43] |
| 6 Unsheduled visits to a healthcare provider (people with 1 or more) Show forest plot | 4 | 688 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.19] |
| 6.1 Hospital | 1 | 250 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.56, 2.70] |
| 6.2 ED | 2 | 367 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.83] |
| 6.3 GP | 1 | 71 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.54] |
| 7 Quality of life (AQLQ) Show forest plot | 6 | 734 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.20, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence (objective measures) Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| 1.1 Reminders/trackers | 3 | 321 | Mean Difference (IV, Random, 95% CI) | 16.29 [9.53, 23.04] |
| 1.2 With feedback | 3 | 234 | Mean Difference (IV, Random, 95% CI) | 24.98 [17.53, 32.44] |
| 2 % Adherence (all measures) Show forest plot | 8 | 762 | Mean Difference (IV, Random, 95% CI) | 18.41 [11.82, 25.00] |
| 2.1 Reminders/trackers | 4 | 361 | Mean Difference (IV, Random, 95% CI) | 16.92 [10.82, 23.02] |
| 2.2 With feedback | 4 | 401 | Mean Difference (IV, Random, 95% CI) | 20.06 [7.27, 32.85] |
| 3 Exacerbations requiring OCS (people with at least 1) Show forest plot | 4 | 3063 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.39] |
| 4 Asthma control Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ACQ | 2 | 109 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.29, 0.78] |
| 4.2 ACT | 4 | 596 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.20, 1.69] |
| 5 Unscheduled visits to a healthcare provider Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 ED | 2 | 2918 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.88, 1.47] |
| 5.2 Hospital | 2 | 2865 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.78] |
| 6 Unscheduled visits to a healthcare provider Show forest plot | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 6.1 GP/ED visits | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Hospitalisations | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Absenteeism Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Absenteeism Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 9 Quality of life (AQLQ) Show forest plot | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 % Adherence Show forest plot | 3 | 1310 | Mean Difference (IV, Random, 95% CI) | 4.02 [1.88, 6.16] |
| 2 Exacerbations requiring OCS Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Asthma control (ACQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Unscheduled visits Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Absence from work/school Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Quality of life (ITG‐ASF % change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 All adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Unscheduled visits (1 or more hospitalisations for any cause) Show forest plot | 2 | 279 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.05] |
| 2 Quality of life (PAQLQ) Show forest plot | 2 | 279 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.01, 0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparison 1. Children vs adults Show forest plot | 10 | 1693 | Mean Difference (IV, Random, 95% CI) | 11.59 [3.72, 19.46] |
| 1.1 Children | 4 | 1241 | Mean Difference (IV, Random, 95% CI) | 8.01 [‐4.77, 20.79] |
| 1.2 Adults/adolescents and adults | 6 | 452 | Mean Difference (IV, Random, 95% CI) | 14.43 [5.49, 23.36] |
| 2 Comparison 2. Complex vs simple interventions Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| 2.1 Complex | 3 | 234 | Mean Difference (IV, Random, 95% CI) | 24.98 [17.53, 32.44] |
| 2.2 Simple | 3 | 321 | Mean Difference (IV, Random, 95% CI) | 16.29 [9.53, 23.04] |
| 3 Comparison 2. Children vs adults Show forest plot | 6 | 555 | Mean Difference (IV, Random, 95% CI) | 19.86 [14.47, 25.26] |
| 3.1 Children | 3 | 314 | Mean Difference (IV, Random, 95% CI) | 17.29 [8.32, 26.26] |
| 3.2 Adults/adolescents and adults | 3 | 241 | Mean Difference (IV, Random, 95% CI) | 22.84 [16.66, 29.02] |