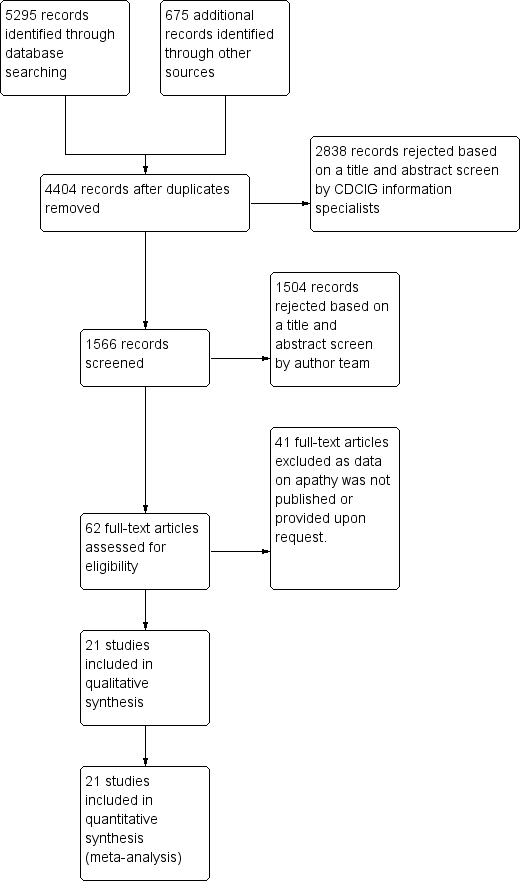
Intervenciones farmacológicas para la apatía en la enfermedad de Alzheimer
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial | |
| Participants | Participant information obtained from Porsteinsson 2014:
| |
| Interventions | Participants were randomized to receive:
During the first 3 weeks after randomization, clinicians could adjust the medication dosage according to response and tolerability. Caregivers received a standardized practical psychosocial intervention of 3 components: provision of educational materials, 24‐hour availability of crisis management services, and a 20‐ to 30‐minute counseling session at each scheduled study visit Duration: 9‐week treatment phase Enrollment: 186 participants randomized | |
| Outcomes | Outcomes were obtained from Leonpacher 2016:
Secondary:
| |
| Notes | We have selected Leonpacher 2016 to be the primary paper. However, additional information for this study was obtained from clinicaltrials.gov (NCT00898807) and Porsteinsson 2014. Study dates: July 2009 ‐ September 2013. Eight sites in the USA and Canada were included. Specific site locations not disclosed. Coordinating site: Johns Hopkins University, Baltimore, MD, USA. Funding provided by the National Institute on Aging and NIMH grant R01AG031348, and in part by HIH grant P50‐AG05142 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized to receive…” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomized to receive citalopram at a target dosage of 30 mg/day… or matching placebo.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “…there was no difference in adherence between the citalopram and placebo groups and that side effects were generally modest and consistent with those known to be associated with SSRIs (gastrointestinal complaints, respiratory tract infections and falls). The adverse effects of cognitive worsening (of unknown clinical significance) and QT prolongation, however, raise concerns about 30 mg/day dosage used…” Comment: Study withdrawals and reason for withdrawals have been reported in Porsteinsson 2014 (Safety and Adherence section and Table 3) and appear to be balanced between groups |
| Selective reporting (reporting bias) | High risk | The authors provided medians and interquartile ranges for each NPI item at baseline (Table 1) and at 9 weeks (Table 2), but did not report means ± SD scores. The authors of this meta‐analysis computed these values themselves. However, as CitAD trial disclosed that these values were not normally distributed, there may be a selective reporting bias |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, double‐blind, randomized, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Following placebo lead‐in phase (up to 14 days) participants were randomized to receive either:
Duration: 10‐week treatment phase (+ maximum 14‐day placebo lead in) Enrollment: 652 patients randomized, however 649 included in analysis. | |
| Outcomes | Primary:
Secondary:
Safety:
| |
| Notes | Study dates not reported. 61 sites in Europe, Australia, Israel, Lebanon, and South Africa were included. Specific site locations not disclosed. Corresponding author’s institution: Lily Research Laboratories, Indianapolis, IN, USA. Contract/grant sponsor: Eli Lilly and Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients randomly assigned to receive…” Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | This information has not been made available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “The proportion of patients completing the 10‐week treatment period was not significantly different among the five treatment groups (Table 1)…” Comment: Study withdrawals and reason for withdrawals have been reported in Table 1 and appear to be balanced between groups |
| Selective reporting (reporting bias) | Low risk | The authors reported means ± standard deviations on each of the 12‐item NPI‐NH scores (Table 2), BPRS (total, negative and positive) scores, and CGI scores (Table 3) |
| Other bias | Low risk | No other identified biases |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Participants were urn‐randomized into either:
Duration: 8 weeks Enrolment: 23 participants randomized, 1 participant excluded from analysis due to AE‐related drop‐out | |
| Outcomes | Primary:
Other:
| |
| Notes | Study dates: July 2005 ‐ September 2007. Study site: Butler Hospital, Providence, RI, USA. Salary support for the corresponding author provided by a National Research Service Award from the National Institute of Mental Health. Cephalon provided study medication, placebo, and $40,000 USD through an unrestricted investigator‐initiated grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A randomized, double‐blind, placebo‐controlled design was employed.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “Participants were urn‐randomized into either the experimental group (modafinil) or the control group (placebo) using apathy severity (mild, moderate, or severe), dementia severity (mild or moderate), presence of antidepressant medication, and presence of memantine as randomization factors.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..."“Both the physician (S.S.) and the clinician (L.L.F.) who performed the assessments were blind to the medication status of the participants.” Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “One experimental group participant was withdrawn from the study after 2 weeks due to an increase in motor tics.” Comment: Though no participant flow diagram was provided in text, participant withdrawal information and reasoning described in text |
| Selective reporting (reporting bias) | Low risk | Quote: “The mean scores and SDs for our 2 groups for each of the outcome measures are presented in Table 2.” Comment: Table 2 provides descriptive statistics for independent and dependent variables assessed in the study, providing means ± standard deviations for each outcome |
| Other bias | Low risk | No other identified biases |
| Methods | Data pooled from 3 multicenter, double‐blind, placebo‐controlled, parallel‐group studies | |
| Participants | Similar criteria between the 3 studies:
Data obtained by authors from Janssen‐Ortho Inc.:
| |
| Interventions | Participants were randomized to either: Duration: 3‐month treatment phase (+1 month placebo run‐in) Enrollment: 368 participants randomized Participants were randomized to either:
Duration: 5‐month treatment phase (+1 month placebo run‐in) Enrollment: 978 participants randomized Data obtained by authors from Janssen‐Ortho Inc. Participants were randomized to either:
Duration: 6‐month treatment phase (+1 month placebo run) Enrollment: 971 participants randomized | |
| Outcomes | Primary outcome for pooled analysis:
Primary outcome for included studies include changes from baseline in:
Behavioral outcome for included studies include changes from baseline in:
| |
| Notes | Study dates note reported. Sites of studies included in analyses include the USA, Canada, Great Britain, South Africa, Australia, and New Zealand. Specific site locations not disclosed. Corresponding author’s institution: Sunnybrook Health Sciences Centre, Toronto, Canada.This research was supported by a grant from the American Health Assistance Foundation‐Alzheimer's Disease Research Program (#A2003‐236) and by the Dean's Fund of the University of Toronto. All studies were supported by Jannssen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Data were pooled from 2033 subjects...who had participated in one of three randomized, double‐blind placebo‐controlled trials". |
| Allocation concealment (selection bias) | Unclear risk | This information has not been made available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 of the studies included in this pooled analysis (Tariot 2000; Rockwood 2001) have each included a figure on participant flow. However, as the data from Janssen‐Ortho Inc. has not been made available, we are unsure about the risk of bias |
| Selective reporting (reporting bias) | Low risk | The authors reported on means ± standard deviations on each of the 12‐item NPI scores, at baseline and over treatment duration |
| Other bias | Low risk | No other identified biases |
| Methods | Randomized, double‐blind, placebo‐controlled cross‐over trial | |
| Participants |
| |
| Interventions | Participants underwent a placebo washout of all psychotropic drugs based on a minimum of 5 half‐lives of the drug used Participants were then randomized to receive:
Loxapine 2.5 mg maximum 4 doses/week was available as a rescue medication Duration: 14 weeks (6‐week treatment phases of valproate or placebo + 2‐week placebo washout and tapering) Enrollment: 14 patients randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates not reported. Study site: 1) Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; 2) North York General Hospital, Toronto, Canada. Additional data and information was provided by Herrmann and Lanctôt upon request. Funding provided by the Alzheimer’s Society of Canada (grant No. 01‐07). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized to receive…” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomized to receive valproate liquid suspension or an identical placebo…” Comment: Probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Twelve patients experienced at least one adverse event during valproate treatment, compared to 8 patients during placebo treatment. Although this difference is not statistically significant, the mean number of adverse events experienced with valproate treatment (4.15 +/‐ 3.67) was significantly greater than with placebo (1.23 +/‐ 1.69) treatment (Z = ‐2.82, p = 0.005).” Comment: Withdrawal numbers and adverse events per group are provided in the Results section and are not balanced between groups |
| Selective reporting (reporting bias) | Low risk | Mean change scores ± SD scores are provided in Table 1 for NPI‐agitation subcategory, total NPI, total CMAI and MMSE scores. NPI‐apathy mean change scores ± SD scores were calculated with data provided by authors upon request |
| Other bias | Low risk | No other identified biases |
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over trial | |
| Participants |
| |
| Interventions | Participants took a dextroamphetamine (D‐amph) challenge test (10 mg D‐amph orally). Following up to a 1‐week washout phase, participants were randomized to receive either: ‐ methylphenidate (initiated at 5 mg orally twice/day for 3 days and increased to 10 mg orally twice/day for 11 days) ‐ placebo Duration: 5 weeks (2‐week treatment phases with a 1‐week placebo washout between phases) + at least 1 week washout after the D‐amph challenge test Enrollment: 13 participants randomized | |
| Outcomes | Primary:
Other:
| |
| Notes | Study dates: October 2003 ‐ October 2006. Study site: Sunnybrook Health Sciences Centre, Toronto, Canada. This research was supported by a grant from the American Health Assistance Foundation‐Alzheimer’s Disease Research Program (#A2003‐236) and by the Dean’s Fund of the University of Toronto. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We conducted a double‐blind, randomized, placebo‐controlled crossover trial...” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomized to receive methylphenidate or an identical placebo...” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind…” Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Eleven of the 13 patients completed the study, with both dropouts occurring during the methylphenidate treatment phase. One dropout completed all placebo assessments and the baseline methylphenidate assessment, which was carried forward for the efficacy analysis. The second patient dropped out after 8 days of methylphenidate treatment but completed a retrieved dropout assessment. This patient did not participate in the placebo phase. Results are therefore available for 13 patients treated with methylphenidate and 12 patients treated with placebo.” Comment: Though no participant flow diagram was provided in text, withdrawal information and reasoning described in text |
| Selective reporting (reporting bias) | Low risk | Table 1 provides means ± standard deviations for treatment change scores (end of treatment‐ baseline) for AES total, NPI apathy, NPI total and MMSE for participants during the methylphenidate and placebo treatment phases |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 8 weeks Enrolment: 40 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates: July 2010 ‐ September 2015. Study sites: 1) Sunnybrook Health Sciences Centre, Toronto, Canada; 2) North York General Hospital, Toronto, Canada. Additional data provided by Herrmann et al. This study was funded by the Alzheimer's Society of Canada (#:12‐74) and the Coleman Fund (internal funding) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...patients were randomized with a 1:1 balanced by ChEI to continue receiving their ChEI (continuation)...or to receive an identical‐looking placebo substitution.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was completed independently by the pharmacy...in permuted blocks using a computer‐generated code.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: 'Patients, family members, nurses, clinicians, outcome assessors, and investigators were unaware of treatment group assignments or block size'. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." |
| Incomplete outcome data (attrition bias) | Low risk | The reasons for withdrawal were provided in the participant flow diagram (figure 1). Number of early terminations and time to early termination were balanced between groups |
| Selective reporting (reporting bias) | Low risk | Mean and ± SD of the baseline, endpoint and change scores of all primary and secondary outcome measures were reported in table 3 |
| Other bias | Unclear risk | This was a discontinuation study completed in people who had been receiving long‐term ChEI treatment |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 26 weeks Enrollment: 408 patients randomized, 393 were included in the analysis as they were a part of the valid intention‐to‐treat population. | |
| Outcomes | Primary:
| |
| Notes | Study dates not reported. 25 sites in the USA. Specific site locations not disclosed. Corresponding author’s institution: University of Pittsburgh School of Medicine, Pittsburgh, PA, USA. No funding support reported in paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Subjects were randomized to either placebo...or metrifonate...treatment groups'. |
| Allocation concealment (selection bias) | Unclear risk | This information has not been made available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: '...double‐blind...' |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: '...double‐blind...' |
| Incomplete outcome data (attrition bias) | Unclear risk | This information has not been made available |
| Selective reporting (reporting bias) | High risk | LSM changes in NPI total and NPI‐subitem scores were provided in table 1. As LSM are adjusted for covariates, there is a risk of bias. Additionally, no standard error or standard deviation was reported in this paper. As a result, the authors of this meta analysis computed a standard deviation based on other studies (Raskind 1999) investigating the use of metrifonate in people with AD |
| Other bias | Low risk | No other identified biases |
| Methods | Multicentre, double‐blind, randomized, placebo‐controlled cross‐over study | |
| Participants |
| |
| Interventions |
Participants were then randomized to:
Duration: 9‐week treatment phase (4 weeks of first treatment phase + 1 week placebo washout, and cross over to 4 weeks of second treatment phase) + approximately 1‐week placebo run‐in Enrollment: 22 participants randomized | |
| Outcomes | The following are primary outcomes: Behavior:
Function:
Cognition:
| |
| Notes | Study dates not provided. Study sites: 1) Sunnybrook Health Sciences Centre, Toronto, Canada; 2) Baycrest Centre for Geriatric Care, Toronto, Canada; 3) North York General Hospital, Toronto, Canada. Additional data requested, and provided by Dr. Krista Lanctôt.This study was funded by Physicians' Services Incorporated Foundation (96‐06), Alzheimer Society of Canada Research Program, and Kunin Lunenfeld Applied Research Unite of Baycrest Centre for Geriatric Care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...administered in a randomized, double‐blind trial." |
| Allocation concealment (selection bias) | Unclear risk | This information has not been made available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double blind..." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double blind..." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of those 26, three patients dropped out before randomization and one Comment: Although no participant flow diagram was included, attrition was described in detail in the paper |
| Selective reporting (reporting bias) | Low risk | Lanctôt 2002 reported on treatment responders in this paper. Dr. Lanctôt provided relevant data for this meta‐analysis. We were able to extract the mean ± standard deviations, and frequencies for all relevant outcome measures |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 26‐week double‐blind period (+ 2‐week screening period at beginning of the study + follow‐up visit at 8 weeks post‐treatment; 36 weeks total) Enrolment: 408 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates not reported. 24 sites in the USA included. Specific site locations not disclosed. Corresponding author’s institution: Washington University, St. Louis, MO, USA. The data in this report were collected from protocol D95‐018, sponsored by Bayer Corporation. For the purposes of this meta‐analysis, the authors have collected information from the double‐blind phase only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the time of enrolment, the 408 patients in this study were randomized to the placebo (N=135) or the metrifonate (N=237) group..." |
| Allocation concealment (selection bias) | Low risk | Quote: "…according to a randomization code with blocks of six generated by computer at Bayer Corporation…" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “...double‐blind...” “Only the statistician…had access to the randomization code…” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “...double‐blind...” “…investigators were masked as to random code assignment…” |
| Incomplete outcome data (attrition bias) | Low risk | The reasons for withdrawal were provided in the participant flow diagram (figure 1) |
| Selective reporting (reporting bias) | High risk | LSM changes in NPI total and NPI‐subitem scores were extracted from figure 4. As LSM are adjusted for covariates, there is a risk of bias. Additionally, no standard error or standard deviation was reported in this paper. As a result, the authors of this meta‐analysis computed a standard deviation based on other studies (Raskind 1999) investigating the use of metrifonate in people with AD |
| Other bias | Low risk | No other identified biases |
| Methods | Multi‐center, randomized, double‐blind, placebo controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 24 weeks Enrolment: 290 participants randomized | |
| Outcomes | Outcomes were obtained from Gauthier 2002:
| |
| Notes | We have selected Gauthier 2002 as the primary paper, but this group (MSAD investigators) have also reported on the study in two other published papers (Feldman 2001; Feldman 2005). Study dates not reported. 32 sites including 22 in Canada, 6 in Australia, and 4 in France. Specific site locations not disclosed. Corresponding author’s: McGill Centre for Studies in Aging, Montreal, Canada. The results of this study are supported by Pfizer, Inc. (New York, NY) and Eisai, Inc. (Teaneck, NJ). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients...were randomized to receive...". Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote from Feldman et al "At baseline, eligible patients were randomized in a 50/50 split using a computerized randomization schedule..." Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Quote from Feldman et al "Blinding was established with identical film‐coated tablets within a blister packaged card." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Study withdrawals and reason for withdrawals have been reported in Figure 1 in Feldman 2001, and appear to be balanced between groups |
| Selective reporting (reporting bias) | High risk | "The outcome measure of interest was 12‐item Neuropsychiatric Inventory (NPI)." The authors reported on baseline least‐square means (LSM) ± standard errors on each of the 12‐item NPI scores. As LSM are adjusted for covariates, there is a risk of bias. They also included LSM change scores in figure 1. However, they did not include standard errors of these mean change scores. As such, we approximate standard deviation of change scores by calculating the standard deviation from the standard error of baseline scores |
| Other bias | Low risk | No other identified biases |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Participants were randomized to receive either:
Duration: 12‐week treatment period (+ 2 week discontinuation phase) Enrollment: 60 participants randomized initially | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates: August 2007 ‐ June 2010. Study site: VA Medical Center, Omaha, NE, USA. This study was funded by a VA Merit Review Entry Program grant to Dr. Prasad Padala | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…randomized double‐blind, placebo‐controlled trial...” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomized to methylphenidate (N=30) or placebo (N=30) groups using a random block design developed by a statistician using sealed envelopes." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “…double‐blind…” Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “One subject dropped off from the placebo group.” Comment: Although no participant flow diagram was provided in text, withdrawal information and reasoning described in text |
| Selective reporting (reporting bias) | Low risk | The authors reported mean ± standard deviations for apathy scores in each intervention arm |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 26 weeks (+ 8‐week post‐treatment follow‐up visit) Enrollment: 264 patients randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates not reported. Multicenter study in the USA. Specific number and site locations not disclosed. Corresponding author’s institution: Northwest Mental Illness Research, Education and Clinical Center, Washington, DC, USA.The data in this report were collected from protocol D96‐010, sponsored by Bayer Corporation. For the purposes of this meta‐analysis, we have collected information from the double‐blind phase only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: '...The 264 patients enrolled in this study were randomly assigned to the placebo...or the metrifonate group...'Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: '...patients...were randomly assigned...according to a computer‐generated randomization code..." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: '...double‐blind...'. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'The investigators were blinded as to random code assignment' |
| Incomplete outcome data (attrition bias) | Low risk | The reasons for withdrawal were provided in text (page 322) |
| Selective reporting (reporting bias) | High risk | LSM changes in NPI total and NPI‐subitem scores were provided in figure 2. As LSM are adjusted for covariates, there is a risk of bias. SD was computed from provided SE of change scores |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Participants were randomized to receive either:
Duration: 6 weeks Enrollment: 60 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates: June 2010 ‐ August 2012. Study sites: 1) Johns Hopkins University Baltimore, MD, USA; 2) Medical University of South Carolina, Charleston, SC, USA; 3) Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada. Funding was provided by the National Institute on Aging (R01 AG033032‐01 and 1 K08 AG029157‐01A1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…randomized, double‐blind, placebo‐controlled multicenter trial...” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization scheme, stratified by clinical center with permuted length blocks, assigned participants to methylphenidate or placebo in a 1:1 ration. The coordinating center generated the treatment assignment schedule using a documented, auditable SAS program.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Study drug was supplied as identical‐appearing capsules containing either 5 mg methylphenidate or lactose (placebo).” Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The time to early termination did not differ significantly by group". |
| Selective reporting (reporting bias) | Low risk | Table 2 provides means ± standard errors for measures for the scores, change, and treatment effects of apathy (AES, ADCS‐CGI‐C and NPI) at 6 weeks of methylphenidate and placebo groups |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Participants were randomly assigned to either:
Duration: 4‐week intervention Enrollment: 55 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates not reported. 13 sites in Bergen and Oslo, Norway. Specific site locations not disclosed. Corresponding author’s institution: University of Kalfarveien, Bergen, Norway. Funding: No funding information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were consecutively assigned to antipsychotic drug discontinuation (intervention group, IG) or no discontinuation (reference group, RG) by means of computer generated, random, permuted blocks of four.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “All study medications were provided by an independent pharmacy… to maintain blindness.”,“…patients received identically looking capsules…” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was a multicenter double‐blind, controlled four week intervention." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "…double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Seven patients completed the study prematurely (IG, n = 4; RG, n = 3; X2 = 0.20, p = 0.70), due to un‐blinding for randomization code (IG, n = 1; RG, n = 2), behavioural deterioration (IG, n = 2), restless legs (IG, n = 1) or delirium (RG, n = 1).” Comment: Study withdrawals and reason for withdrawals are described and appear to be balanced between groups |
| Selective reporting (reporting bias) | Low risk | The authors reported the number of IG participants still on antipsychotics at study completion. Mean NPI total score difference were provided from baseline to Week 4. Changes in behavioral symptoms between groups are presented in Table 2. Means ± standard deviations of differences in change in BPSD between groups are presented in Table 3 for NPI total and factor scores. Additional information regarding NPI‐subscores were requested by the authors, and were provided by Dr. Sabine Ruths |
| Other bias | Unclear risk | This is a discontinuation study |
| Methods | Multinational, randomized, double‐blind, placebo‐controlled trial | |
| Participants | Participant information obtained from Doody 2013:
| |
| Interventions | Participants were randomized to receive:
Duration: 76‐week treatment phase Enrolment: 1537 patients randomized | |
| Outcomes | Outcomes were obtained from Rosenberg 2016:
Other:
| |
| Notes | Study dates: March 2008 ‐ May 2011. Multicenter study in the USA. Specific number and site locations not disclosed. Although we have chosen Rosenberg 2016 as the primary paper, additional information regarding participant population was obtained from Doody 2013. Funding provided by Eli Lilly and the University of California at San Diego (the latter as a fiduciary for the Alzheimer’s Disease Cooperative Study), a clinical trials consortium established by the National Institute on Aging. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were randomly assigned to receive…” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | This information has not been made available |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Adverse events were more common in the two semagacestat groups than in the placebo group…The percentage of patients who discontinued the study drug because of adverse events was higher with semagacestat than with placebo (26% with 100 mg and 30% with 140 mg vs. 11% with placebo, P>0.001 for both comparisons).” This quote is from Doody 2013 Comment: Withdrawal percentages by group are provided in Doody 2013 and are not balanced between groups. AEs are presented in Table 3 |
| Selective reporting (reporting bias) | Low risk | Change in apathy subscores are presented by treatment group in Figure C on p. 377 of Rosenberg 2016, but means ± SD scores needed to be computed by the authors of this meta‐analysis |
| Other bias | Low risk | No other identified biases |
| Methods | Randomized, double‐blind, placebo‐controlled cross‐over trial | |
| Participants |
| |
| Interventions | Participants were randomized to receive:
Duration: 8 weeks (1‐week baseline + 3‐week placebo period + 1‐week washout + 3‐week treatment phase with sodium valproate). Extension of the baseline period was allowed once for 1 week in participants who did not show a score ≥ 3 on one of the items of the SDAS‐9 Enrollment: 42 participants randomized | |
| Outcomes | Primary:
Secondary:
Other:
| |
| Notes | Study dates not reported. Study site: Parnassia Psycho Medical Center, The Hague, The Netherlands. Funding provided by a grant from the Van Helten Foundation, Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands (grant number SHV94/AANV/5), a grant from the National Fund for Mental Health, Utrecht, The Netherlands (grant number 4145), and a grant from the Stichting tot Steun VCVGZ, Bennekom, The Netherlands (grant number ST07064BB. VE) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The sequence of the treatment periods was assigned at random. The code was not accessible for the investigators.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “During the ‘treatment period with placebo’ and during the wash‐out period a placebo suspension was given, identical to the active medication in appearance, quantity, smell and taste.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “…there were three drop‐outs. One patient had high fever of unknown origin during treatment with placebo, one patient was hit by a stroke during treatment with placebo, and one patient broke his hip during the wash‐out period. None of the dropouts could be associated with the intake of sodium valproate.” Comment: Withdrawal numbers and reasons for withdrawal are provided in the Results section |
| Selective reporting (reporting bias) | Low risk | Mean change scores ± SD scores are presented in Table 2, demonstrating the effects of sodium valproate compared to placebo on aggressive behavior and other types of disturbed behavior (including the GIP apathetic behavior subscore) |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial | |
| Participants |
| |
| Interventions | Participants were randomized initially (2:2:2:3 ratio) to either:
Treating physician selected the number of low‐ or high‐dose capsules for initial treatment and could adjust the dosage, as indicated clinically, over 36 weeks of trial. At any time after the first 2 weeks of treatment, the clinician could discontinue the initially‐assigned (Phase 1) medication based on their clinical judgment. Phase 1 would end and the participant could enter Phase 2 and be assigned randomly to masked treatment with an atypical antipsychotic medication not assigned to them in Phase 1 or with citalopram. Participants could also go directly to an open‐choice treatment Duration: Phase 1: 12 weeks; Phase 2: 24 weeks (36 weeks total) Enrolment: 421 participants randomized | |
| Outcomes | Psychiatric and behavioral symptoms:
Cognitive skills, functional abilities, care needs and quality of life:
| |
| Notes | For the purposes of this meta‐analysis, data from Phase 1 of the study were used. Phase 2 was not included as there was no placebo control. Study dates: March 2001 ‐ October 2004. 42 sites included. Site locations not disclosed. Principal Investigator institutions: University of Southern Carolina, Columbia, SC, USA; University of Rochester, Rochester, NY, USA. Funding was provided by the National Institute of Mental Health (N01 MH9001) and in part by the Department of Veterans Affairs. Astra‐Zeneca Pharmaceuticals, Forest Pharmaceuticals, Janssen Pharmaceutical and Eli Lilly provided medications for the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “This protocol is fundamentally a randomized‐treatment assignment…” Comment: Probably done. This quote is from Schneider 2003, where the CATIE‐AD research design and methods were originally described |
| Allocation concealment (selection bias) | Low risk | Quote: “Medication has been prepared into identically appearing ‘low strength’ capsules containing risperidone 0.5 mg, olanzapine 2.5 mg, quetiapine 25mg, citalopram 10 mg or placebo, or ‘higher strength’ capsules containing 1 mg,5 mg, 50 mg, 20 mg or placebo, respectively, in order to preserve the blind.” Comment: Probably done. This quote is from Schneider 2003 |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double blind..." Comment: Probably done. This quote is from Schneider 2003 |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done. This quote is from Schneider 2003 |
| Incomplete outcome data (attrition bias) | Low risk | Quote:“There were no significant overall differences among treatment groups with regard to the time to discontinuation of treatment for any reason” “There were no significant differences among the groups with regards to the proportion of patients who had at least one serious adverse event and the proportion who had any adverse event” Comment: Time to treatment discontinuation and adverse events are reported in Tables 2 and 3 and appear to be balanced between groups. These quotes are from Schneider 2006, where the CATIE‐AD time to treatment discontinuation and adverse events data are originally described |
| Selective reporting (reporting bias) | Low risk | Quote: “Two sets of clinical outcomes were measured: 1) psychiatric and behavioural symptoms… 2) cognition, functional skills, care needs and quality of life” Comment: Baselines scores on clinical measures provided, along with mean change ± standard deviation scores for clinical symptoms from baseline to last observation in Phase 1. Mean change ± standard deviations on clinical symptom measures between baseline and treatment week 12 are provided for cognitive measures. |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
| |
| Interventions | Participants were randomized to either:
Duration: 24 weeks Enrolment: 208 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates not reported. 27 sites across the USA. Specific site locations not disclosed. Corresponding author’s institution: Departments of Psychiatry, Medicine and Neurology, University of Rochester Medical Center, Monroe Community Hospital, Rochester, NY, USA. The results of this study are supported by Pfizer, Inc. (New York, NY) and Eisai, Inc. (Teaneck, NJ). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients...were randomized..." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomized in blocks of four, using a computerized randomization schedule..." Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Blinding was achieved using identical appearing film‐coated tablets of donepezil and placebo..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Study withdrawals and reason for withdrawals have been reported in the first paragraph of the Results section of Tariot 2001 |
| Selective reporting (reporting bias) | High risk | LSM changes in NPI‐subitem scores were provided in figure 1. As LSM are adjusted for covariates, there is a risk of bias. SD was computed from provided SE of change scores |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled, flexible‐dose trial | |
| Participants |
| |
| Interventions | Participants were assigned to either:
Dose reduction was permitted if clinically warranted, and the target dose could be resumed if appropriate. Adherence of 80% was required Duration: 24‐month double‐blind treatment phase + 2‐month single‐blind placebo treatment period Enrolment: 313 participants randomized | |
| Outcomes | Primary:
Secondary:
Other:
Safety and tolerability:
Volumetric Magnetic Resonance Image:
| |
| Notes | Study dates: October 2003 ‐ December 2009. 46 sites included. Site locations not disclosed. Study Director site: University of Rochester Medical Center, Rochester, NY, USA. Data for this study were obtained from the University of California, San Diego Alzheimer's Disease Cooperative Study Legacy Database. Funding provided by National Institute on Aging (U01AG010483). Additional support provided by a research grant and material support from Abbott Laboratories (NCT00071721). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were assigned to 1 of 2 treatment groups in permuted blocks of 4, according to a randomization list created and maintained by the ADCS Data Core.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The trial used a 125‐mg enteric‐coated extended‐release divalproex sodium formulation or identical‐appearing placebo…” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “In the valproate group, 61.40% discontinued treatment prematurely, and in the placebo group, 60.6% did so; reasons are shown in Figure 1.” Comment: Study withdrawals and reason for withdrawals have been reported in Figure 1 and all AEs experienced in each group are outlined in Table 3 |
| Selective reporting (reporting bias) | Low risk | The authors reported means ± SD scores of each outcome over time for each study visit in Table 3. Additional data were provided upon request |
| Other bias | Low risk | No other identified biases |
| Methods | Multicenter, randomized, double‐blind, placebo‐controlled trial. | |
| Participants |
| |
| Interventions | Participants were randomly assigned to either:
After 1‐week of treatment twice a day with either 3 mg mibampator or placebo, a one‐time dose reduction to 1 mg twice daily due to intolerability was permitted, which remained their dose for the remainder of the study Duration: 12‐week double‐blind treatment phase (+ 3 ‐ 28 day screening period and 1‐week single‐blind washout) Enrolment: 132 participants randomized | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Study dates: February 2009 ‐ June 2011. Multicentre study in USA. Specific number and site location details are not disclosed. Research supported by Eli Lilly and Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The interactive voice response system (IVRS) was used to assign blisterpacks containing double‐blind study drug to each patient.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The interactive voice response system (IVRS) was used to assign blisterpacks containing double‐blind study drug to each patient.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "…double‐blind..." Comment: Probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "…double‐blind..." Comment: Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “There were no significant differences between groups for SAEs, discontinuation due to AEs, TEAEs or TEAEs possibly related to study drug as deemed by the investigator...” Comment: Authors describe all SAEs and reasons for discontinuation reasons due to AE for each treatment group in the ‘Safety Evaluation’ section p. 8 |
| Selective reporting (reporting bias) | High risk | The authors used MMRM analysis to assess the primary outcome, NPI‐4‐A/A least square mean change from baseline after treatment, (Figure 2), as well as secondary efficacy measures including FrSBe total and subscale least square means change from baseline after treatment (Figure 3. |
| Other bias | Low risk | No other identified biases |
AChEI: anti‐cholinesterase inhibitor; AD: Alzheimer’s disease; ADAS‐Cog: Alzheimer’s Disease Assessment Scale‐Cognitive subscale; ADAS‐Cog11: Alzheimer’s Disease Assessment Scale‐ 11‐item cognitive subscale; ADAS‐Cog14: Alzheimer’s Disease Assessment Scale‐ 14‐item cognitive; ADAS‐Noncog: Alzheimer’s Disease Assessment Scale‐Noncognitive subscale; ADCS: Alzheimer’s Disease Cooperative Study; ADCS‐ADL: Alzheimer’s Disease Cooperative Study – Activities of Daily Living; ADCS‐ADL‐sev: Alzheimer’s Disease Cooperative Study‐Activities of Daily Living severity scale; ADCS‐CGI‐C: Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change; ADLQ: Activities of Daily Living Questionnaire; ADRQL: Alzheimer’s Disease Related Quality of Life; AE: adverse events; AES: Apathy Evaluation Scale; AIMS: Abnormal Involuntary Movement Scale; ARCI: Addiction Research Centre Inventory; BEHAVE‐AD: Behavioural Pathology in Alzheimer’s Disease Rating Scale; BID: twice daily; BPRS: Brief Psychiatric Rating Scale; BPSD: behavioral and psychological symptoms of dementia; CAS: Caregiver Activity Scale; CATIE‐AD: Clinical Antipsychotic Trials of Intervention Effectiveness‐Alzheimer’s Disease trial; CDR‐SOB: Clinical Dementia Rating sum of boxes; CGI: Clinical Global Impression scale; CGI‐C: Clinical Global Impression‐Change scale; CGI‐S: Clinical Global Impression‐Severity scale; CGI‐S‐AA: Clinical Global Impression‐Severity of Symptoms of Agitation/Aggression; CGI‐S‐GF: Clinical Global Impression‐Severity of Symptoms of Global Functioning; ChEI: Cholinesterase inhibitors; CIBIC‐plus: Clinician’s Interview‐Based Impression of Change plus Caregiver Input; CMAI: Cohen‐Mansfield Agitation Inventory; CPT: Conners’ Continuous Performance Task; CSDD: Cornell Scale for Depression in Dementia; CT: Computerized Tomography; DAD: Disability Assessment in Dementia; DAFS: Direct Assessment of Functional Status; D‐amph: dextroamphetamine challenge; DS: Dependence Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; FAST: Functional Assessment Staging of Alzheimer’s Disease; FrSBe: Frontal Systems Behaviour Scale; GDS: Global Deterioration Scale; HIS: Hachinski Ischemic Score; IADL: Instrumental Activities of Daily Living; ICD‐10: International Classification of Diseases‐ Tenth Revision; IG: intervention group; IR: immediate release; IVRS: interactive voice response system; kg: kilogram; lbs: pounds; L.L.F.: study neuropsychologist; LSM: least‐square means; LTC: long‐term care; mg: milligram; MMRM: mixed‐effects model repeated measures analysis; MMSE: Mini‐Mental State Exam; MRI: Magnetic Resonance Imaging; MSAD: moderate‐to‐severe AD; N: number; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; NJ: New Jersey; NPI: Neuropsychiatric Inventory; NPI‐4 A/A: Neuropsychiatric Inventory‐4 domain subscale, which combines the following domains: agitation/aggression, aberrant motor behavior, irritability/emotional lability and disinhibition; NPI‐10: Neuropsychiatric Inventory‐10 domains; NPI‐NH: Neuropsychiatric Inventory‐Nursing Home Version; NRS: Neurobehavioural Rating Scale; NY: New York; PO: by mouth, in Latin per os; POMA: Performance‐Oriented Mobility Assessment‐II; POMS: Profile of Mood States; QOL‐AD: Quality of Life‐AD; QUALID: Quality of Life in Late‐Stage Dementia; RG: reference group; SAEs: serious adverse events; *SAS: Simpson‐Angus Scale; SAS: SAS version 9.2 (SAS Institute Inc, Cary, North Carolina); SDAS: Social Dysfunction and Aggression Scale; SD: standard deviation; SE: standard error; SIB: Severe Impairment Battery; sMMSE: Standardized Mini‐Mental State Exam; SNRI: serotonin and norepinephrine reuptake inhibitor; S.S.: study neurologist; SSRI: selective serotonin reuptake inhibitor; TEAEs: treatment emergent adverse events; ZBI: Zarit Burden Interview
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated nor reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not investigated or reported | |
| Apathy not reported | |
| Apathy not investigated nor reported | |
| Apathy not reported |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the AES Show forest plot | 3 | 145 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐9.55, ‐0.43] |
| Analysis 1.1  Comparison 1 Methylphenidate, Outcome 1 Change in apathy from baseline as measured by the AES. | ||||
| 1.1 < 12 weeks | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐4.80, ‐0.44] |
| 1.2 ≥ 12 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐9.90 [‐13.50, ‐6.30] |
| 2 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐3.85, 3.69] |
| Analysis 1.2  Comparison 1 Methylphenidate, Outcome 2 Change in apathy from baseline as measured by the NPI‐apathy subscore. | ||||
| 3 Adverse Events Show forest plot | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.67, 2.42] |
| Analysis 1.3  Comparison 1 Methylphenidate, Outcome 3 Adverse Events. | ||||
| 3.1 < 12 weeks | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.44, 3.72] |
| 3.2 ≥ 12 weeks | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.86] |
| 4 Change in NPS from baseline as measured by the NPI Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐7.89, 8.21] |
| Analysis 1.4  Comparison 1 Methylphenidate, Outcome 4 Change in NPS from baseline as measured by the NPI. | ||||
| 5 Change in cognition from baseline as measured by the MMSE Show forest plot | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [1.06, 2.91] |
| Analysis 1.5  Comparison 1 Methylphenidate, Outcome 5 Change in cognition from baseline as measured by the MMSE. | ||||
| 5.1 < 12 weeks study duration | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.49, 2.49] |
| 5.2 ≥ 12 weeks study duration | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [1.43, 3.77] |
| 6 Change in functional permance from baseline as measured by the ADL Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.39, 1.39] |
| Analysis 1.6  Comparison 1 Methylphenidate, Outcome 6 Change in functional permance from baseline as measured by the ADL. | ||||
| 7 Change in functional performance from baseline as measured by the IADL Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [0.74, 3.86] |
| Analysis 1.7  Comparison 1 Methylphenidate, Outcome 7 Change in functional performance from baseline as measured by the IADL. | ||||
| 8 Change in global disease severity from baseline as measured by the CGIC and the ADCS‐CGIC Show forest plot | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.11] |
| Analysis 1.8  Comparison 1 Methylphenidate, Outcome 8 Change in global disease severity from baseline as measured by the CGIC and the ADCS‐CGIC. | ||||
| 9 Dropouts due to adverse events Show forest plot | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.64, 7.45] |
| Analysis 1.9  Comparison 1 Methylphenidate, Outcome 9 Dropouts due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the FrSBe‐apathy subscale Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.51, 4.05] |
| Analysis 2.1  Comparison 2 Modafinil, Outcome 1 Change in apathy from baseline as measured by the FrSBe‐apathy subscale. | ||||
| 2 Change in functional performance from baseline as measured by the ADL‐Q Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.40, 0.32] |
| Analysis 2.2  Comparison 2 Modafinil, Outcome 2 Change in functional performance from baseline as measured by the ADL‐Q. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with licensed versus unlicensed ChEIs) Show forest plot | 6 | 3598 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.80, ‐0.00] |
| Analysis 3.1  Comparison 3 Cholinesterase inhibitors, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with licensed versus unlicensed ChEIs). | ||||
| 1.1 Licensed ChEIs (and ≤ 24 weeks study duration) | 3 | 2531 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.85, 0.43] |
| 1.2 Unlicensed ChEIs ( and > 24 weeks study duration) | 3 | 1067 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.98, ‐0.29] |
| 2 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with disease severity) Show forest plot | 6 | 3598 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.80, ‐0.00] |
| Analysis 3.2  Comparison 3 Cholinesterase inhibitors, Outcome 2 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with disease severity). | ||||
| 2.1 Moderate AD (MMSE ≥ 18) | 4 | 3100 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.79, ‐0.07] |
| 2.2 Severe AD (MMSE < 18) | 2 | 498 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.82, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.88, 3.10] |
| Analysis 4.1  Comparison 4 Discontinuation of cholinesterase inhibitors, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and the BPRS withdrawn depression factor score Show forest plot | 2 | 1070 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.00, 0.28] |
| Analysis 5.1  Comparison 5 Atypical antipsychotics, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and the BPRS withdrawn depression factor score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.51, 0.03] |
| Analysis 6.1  Comparison 6 Discontinuation of antipsychotics, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.44, ‐1.04] |
| Analysis 7.1  Comparison 7 Antidepressants, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the FrSBe‐apathy T score Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.94, ‐0.46] |
| Analysis 8.1  Comparison 8 Mibampator, Outcome 1 Change in apathy from baseline as measured by the FrSBe‐apathy T score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and GIP‐apathy subscore Show forest plot | 3 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.23, 0.26] |
| Analysis 9.1  Comparison 9 Valproate, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and GIP‐apathy subscore. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 939 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.15, 0.25] |
| Analysis 10.1  Comparison 10 Semagacestat, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore. | ||||
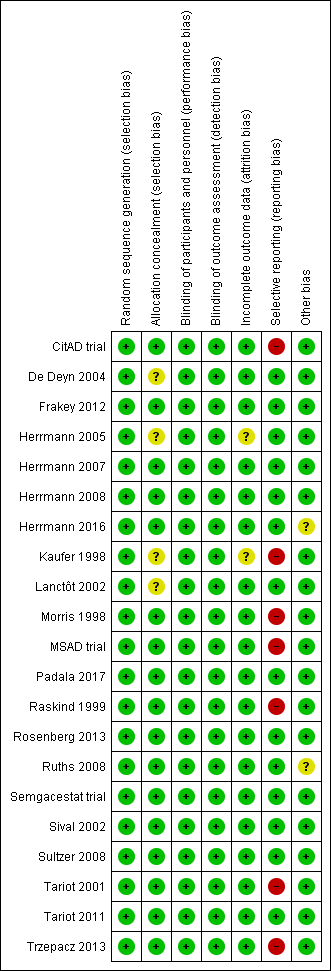
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
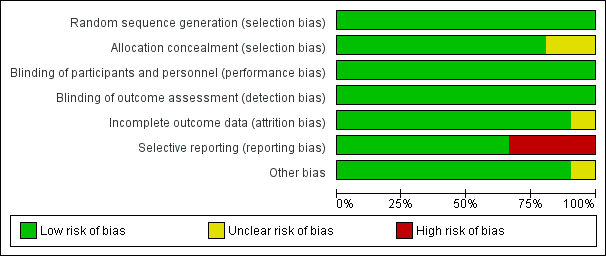
Figure 3 CaptionRisk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
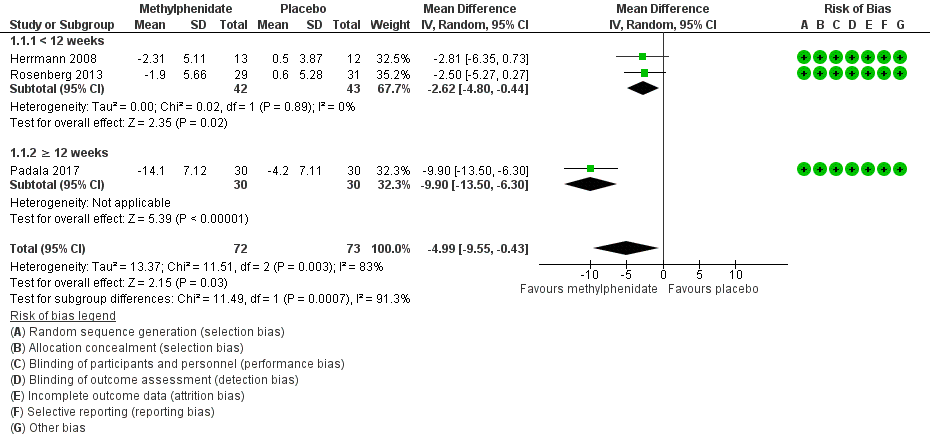
Forest plot of comparison: 7 Methylphenidate, outcome: 7.1 Apathy (AES only).
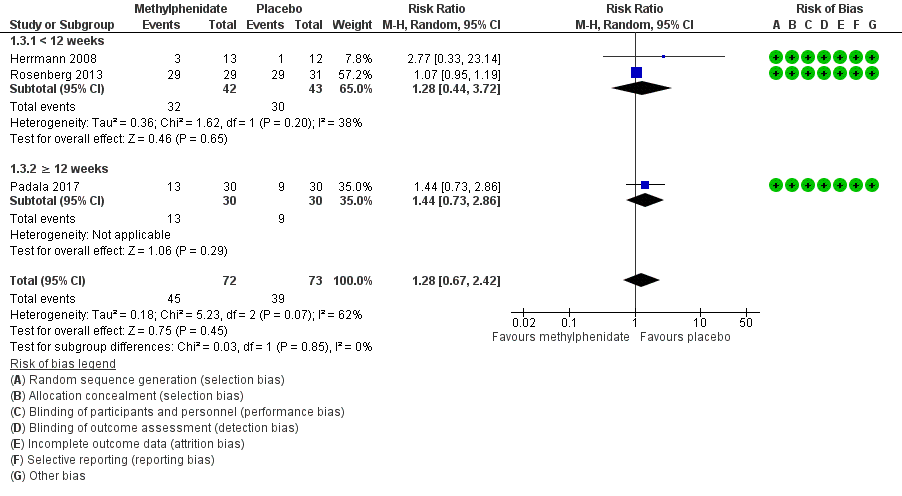
Forest plot of comparison: 7 Methylphenidate, outcome: 7.3 Adverse Events.
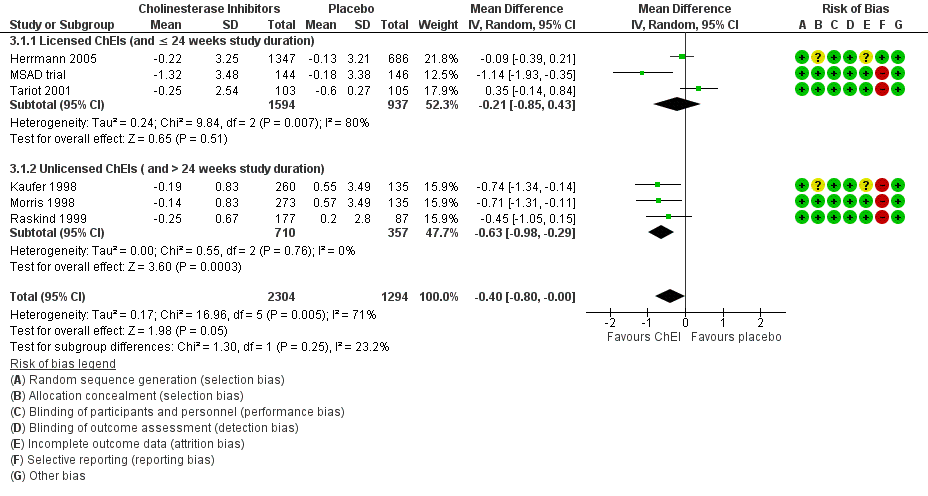
Forest plot of comparison: 3 Cholinesterase Inhibitors, outcome: 3.1 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with licensed versus unlicensed ChEIs).

Comparison 1 Methylphenidate, Outcome 1 Change in apathy from baseline as measured by the AES.

Comparison 1 Methylphenidate, Outcome 2 Change in apathy from baseline as measured by the NPI‐apathy subscore.

Comparison 1 Methylphenidate, Outcome 3 Adverse Events.

Comparison 1 Methylphenidate, Outcome 4 Change in NPS from baseline as measured by the NPI.

Comparison 1 Methylphenidate, Outcome 5 Change in cognition from baseline as measured by the MMSE.

Comparison 1 Methylphenidate, Outcome 6 Change in functional permance from baseline as measured by the ADL.

Comparison 1 Methylphenidate, Outcome 7 Change in functional performance from baseline as measured by the IADL.

Comparison 1 Methylphenidate, Outcome 8 Change in global disease severity from baseline as measured by the CGIC and the ADCS‐CGIC.

Comparison 1 Methylphenidate, Outcome 9 Dropouts due to adverse events.

Comparison 2 Modafinil, Outcome 1 Change in apathy from baseline as measured by the FrSBe‐apathy subscale.

Comparison 2 Modafinil, Outcome 2 Change in functional performance from baseline as measured by the ADL‐Q.

Comparison 3 Cholinesterase inhibitors, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with licensed versus unlicensed ChEIs).

Comparison 3 Cholinesterase inhibitors, Outcome 2 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with disease severity).

Comparison 4 Discontinuation of cholinesterase inhibitors, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore.

Comparison 5 Atypical antipsychotics, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and the BPRS withdrawn depression factor score.

Comparison 6 Discontinuation of antipsychotics, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore.

Comparison 7 Antidepressants, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore.

Comparison 8 Mibampator, Outcome 1 Change in apathy from baseline as measured by the FrSBe‐apathy T score.

Comparison 9 Valproate, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and GIP‐apathy subscore.

Comparison 10 Semagacestat, Outcome 1 Change in apathy from baseline as measured by the NPI‐apathy subscore.
| Methylphenidate compared to placebo for apathy in Alzheimer's disease | ||||||
| Patient or population: Apathy in people with mild‐to‐moderate Alzheimer's disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Methylphenidate | |||||
| Change in apathy (AES score) | The mean change from baseline in apathy was ‐4.2 to 0.6 | MD 4.99 lower | ‐ | 145 | ⊕⊕⊝⊝ | AES: Limited data on clinically meaningful changes |
| Change in apathy (NPI‐apathy subscale score) | The mean change from baseline in apathy ‐2.6 to ‐1.69 | MD 0.08 lower | ‐ | 85 | ⊕⊕⊝⊝ | 1‐ to 2‐point change suggested to be clinically significant in people with a clinically significant apathy (Rosenberg 2013) |
| Adverse events | Study population | RR 1.28 | 145 | ⊕⊕⊝⊝ | ‐ | |
| 534 per 1000 | 684 per 1000 | |||||
| Change in NPS | The mean change from baseline in NPS was ‐2.08 | MD 0.16 higher | ‐ | 25 | ⊕⊕⊝⊝ | 4‐point change suggested to be clinically significant |
| Change in cognition | The mean change from baseline in cognition was ‐1.08 to ‐0.3 | MD 1.79 higher | ‐ | 145 | ⊕⊕⊕⊝ | MMSE: 2‐ to 4‐point change suggested to be clinically significant |
| Change in functional performance assessed with: ADL scale | The mean change from baseline in functional performance was 0.4 | MD 0.50 higher | ‐ | 60 | ⊕⊕⊕⊝ | Limited data on clinically meaningful changes |
| Change in functional performance assessed with: IADL scale | The mean change from baseline in functional performance was ‐0.6 | MD 2.30 higher | ‐ | 60 | ⊕⊕⊕⊝ | Limited data on clinically meaningful changes |
| Change in global disease severity | Study population | RR 0.56 | 85 | ⊕⊕⊕⊝ | ‐ | |
| 116 per 1000 | 65 per 1000 | |||||
| Dropouts | Study population | RR 2.10 | 145 | ⊕⊕⊝⊝ | ‐ | |
| 41 per 1000 | 86 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AD: Alzheimer's disease; AEs: Adverse Events; MMSE: Mini‐Mental Status Examination; MD: Mean Difference; NPS: Neuropsychiatric Symptom, SMD: Standardized Mean Difference, CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due imprecision (wide 95% confidence interval). | ||||||
| Modafinil compared to placebo for apathy in Alzheimer's disease | ||||||
| Patient or population: Apathy in people with mild‐to‐moderate Alzheimer's disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Modafinil | |||||
| Change in apathy | The mean change from baseline in apathy was ‐6.82 | MD 0.27 higher | ‐ | 22 | ⊕⊕⊝⊝ | Limited data on clinically meaningful changes on the FrSBe apathy score |
| Adverse Events ‐ reported, but not analyzed in this review | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in NPS ‐ not investigated | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in cognition ‐ not investigated | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Change in functional performance | The mean change from baseline in functional performance was 0 | MD 0.54 lower | ‐ | 22 | ⊕⊕⊝⊝ | Limited data on clinically meaningful changes |
| Change in global disease severity ‐ not investigated | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dropouts ‐ reported, but not analyzed in this review | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded two levels due to small sample size and imprecision (wide 95% confidence interval). | ||||||
| STUDY DURATION | N OF PARTICIPANTS | Diagnosis | MEAN AGE (YRS) | MEAN MMSE (SD) | MEAN BL NPI‐APATHY (SD) score | COUNTRY | NUMBER OF SITES | TREATMENT GROUPS | |
| METHYLPHENIDATE | |||||||||
| 2 weeks Cross‐over design: 2 treatment phases of 2 weeks with a 1‐week washout between phases | 13 total | Possible or probable AD (NINCDS‐ADRDA), and apathy (NPI‐apathy subscale ≥ 1) | 77.9 (7.8) | 19.9 (4.7) | 5.9 (3) | Canada | 3 | Group 1: Methylphenidate (10 mg twice a day) | |
| 6 weeks | 60 participants | Possible or probable AD (NINCDS‐ADRDA), and clinically significant apathy for at least 4 weeks | 76 (8) | 20 (5) | Group 1: 7 (2) | USA, Canada | 3 | Group 1: Methylphenidate (target: 20 mg daily) | |
| 12 weeks | 60 participants | Dementia of the AD type (DSM‐IV‐TR), and presence of apathy (AES > 40) | 76.6 (7.9) | 23.8 (2.5) | Not reported | USA | 1 | Group 1: Methylphenidate (target: 20 mg daily) | |
| MODAFINIL | |||||||||
| 8 weeks | Group 1: 11 | Possible or probable AD (NINCDS‐ADRDA criteria) and clinically significant apathy (FrSBe Tscore ≥ 65) | Group 1: 75.3 (8.3) | Not disclosed | Not reported | USA | 1 | Group 1: Modafinil (200 mg daily) | |
| CHOLINESTERASE INHIBITORS | |||||||||
| 24 weeks | Group 1: 103 Group 2: 105 | Possible or probable AD with cerebrovascular disease (but not vascular dementia) (NINCDS‐ADRDA criteria) | Group 1: 85.4 | Group 1: 14.4 (5.4) | Not reported | USA | 27 | Group 1: Donepezil ‐ 5 mg/day for 28 days. 10 mg/day after 28 days based on tolerability. | |
| 24 weeks | Group 1: 144 | AD (DSM‐IV and NINCDS‐ADRDA criteria) | 73.6 | Group 1: 11.7 (0.35) | Group 1: | Canada, Australia, France | 32 | Group 1: Donepezil ‐ 5 mg/day for 28 days. 10 mg/day after 28 days based on tolerability. | |
| Range: 3 ‐ 6 months (12 ‐ 24 weeks) | Group 1: 1347 | Probable AD (NINCDS‐ADRDA criteria) | 76 | 18 | Group 1: | USA, Canada, Great Britain, South Africa, Australia, and New Zealand | Multicenter, but number not disclosed | Group 1: Galantamine | |
| 26 weeks | Group 1: 273 | Probable AD (NINCDS‐ADRDA criteria) | Not reported | Not reported | Not reported | USA | 25 | Group 1: Metrifonate (2 weeks – 2.0 mg/kg, followed by 0.65 mg/kg) | |
| 26 weeks | Group 1: 273 | Probable AD (NINCDS‐ADRDA criteria) | Group 1: 73.5 (8.1) | Group 1: 18.8 (5) | Not reported | USA | 24 | Group 1: Metrifonate (2 weeks – 2.0 mg/kg, followed by 0.65 mg/kg) | |
| 26 weeks | Group 1: 177 | Probable AD (NINCDS‐ADRDA criteria) mild‐moderate AD | Group 1: 74.6 (8.3) | Group 1: 18.7 (4.76) | Not reported | USA (additional sites are not disclosed) | Multicenter, but number not disclosed | Group 1: | |
| CHOLINESTERASE DISCONTINUATION | |||||||||
| 8 weeks | Group 1: 21 | Probable AD (NINCDS‐ADRDA criteria) | 89.3 | Group 1: 8.1(5.2) | Group 1: | Canada | 2 | Group 1: Donepezil, | |
| ATYPICAL ANTIPSYCHOTICS | |||||||||
| 10 weeks | Group 1: 132 | Possible or probable AD (NINCDS‐ADRDA criteria and DSM‐IV‐TR), and clinically significant psychotic symptoms | 76.6 (10.4) | 13.7 (5.1) | Group 1: 3.2 (3.9) | Europe, Australia, Israel, Lebanon, and South Africa | 61 | Group 1: 7.5 mg OLZ | |
| Up to 36 weeks (12 weeks of treatment) data available | Group 1: 100 | Dementia of the AD type (DSM‐IV) or probable AD (NINCDS‐ADRDA) and daily delusions, hallucinations, agitation, or aggression over 4 weeks prior to study entry | 77.9 (7.5) | Group 1: 15 (5.4) | Not reported | USA | 42 | Group 1: OLZ | |
| ANTIPSYCHOTIC DISCONTUATION | |||||||||
| 4 weeks | Group 1: 28 | Dementia diagnosis according to ICD‐10 | 83.4 (6.9) | Not provided | Group 1: | Norway | 9 | Group 1: Antipsychotics (haloperidol, risperidone, or olanzapine) | |
| ANTIDEPRESSANTS | |||||||||
| 4 weeks: Cross‐over design: 2 treatment phases of 4 weeks with a 1‐week washout between phases | 22 total | Primary degenerative dementia (DSM‐IV) and probable AD (NINCDS‐ADRDA), and significant behavioral problems (NPI ≥ 8) | 82 (6) | 4.1 (4.7) | Group 1: | Canada | 3 | Group 1: Sertraline (100 mg daily) | |
| 9 weeks | Group 1: 94 | Probable AD (NINCDS‐ADRDA), and significant behavioral problems (NPI ≥ 8), and clinically significant agitation on the NPI > 3 | Group 1: 78 (9) | Group 1: 17 (6.2) | Group 1: | USA | 6 | Group 1: Citalopram (30 mg daily) | |
| MIBAMPATOR | |||||||||
| 12 weeks | Group 1: 63 | Probable AD (NINCDS‐ADRDA) (DSM‐IV‐TR), and clinically significant agitation/aggression | Group 1: 77.2 (8.2) | Group 1: 16.0 (6.1) Group 2: 18 (5.3) | Not reported | USA | Multicenter, but number not disclosed | Group 1: Mibampator (target dose: 3 mg daily) | |
| VALPROATE | |||||||||
| 6 weeks: Cross‐over design: 2 treatment phases of 6 weeks with a 2‐week washout between phases | Group 1: 14 | Probable AD (NINCDS‐ADRDA), primary degenerative dementia (DSM‐IV) | 85.6 (4.5) | 4.5 (4.6) | Group 1: 2.4 (3.8) | Canada | 2 | Group 1: Valproate (mean dose: 1134.6 (400.1) mg daily) | |
| 3 weeks: Cross‐over design: 2 treatment phases of 3 weeks with a 1‐week washout between phases | Group 1: 42 | Senile dementia (NINCDS‐ADRDA)(DSM‐IV) | 80.4 (6.8) | 11.4 (5) | Not reported | Netherlands | 1 | Group 1: Valproate (2 x 240 mg) | |
| 24 months (+ 2‐month single‐blind placebo phase) | Group 1: 153 | Possible or probable AD (NINCDS‐ADRDA) | Group 1: 74.9 | Group 1: 16.9 (3.0) | Group 1: | USA | 46 | Group 1: Valproate (flexible‐dose) (mean modal dose: 250 mg daily) | |
| SEMAGACESTAT | |||||||||
| 76 weeks | Group 1: 463 | Mild‐moderate AD (NINCDS‐ADRDA) | Group 1: 72.7 (7.9) | Group 1: 20.9 (3.5) | Not reported | USA | 91 | Group 1: LY100 | |
| AD: Alzheimer's disease, BL: baseline, DSM: Diagnostic and Statistical Manual of Mental Disoders, FrSBe: Frontal Systems Behavior Scale, ICD: International Classification of Diseases, LY: LY450319 (Eli Lillyand Company study drug), NINCDS‐ADRDA: National Institute of Neurological and Communicative disorders and the Alzheimer's Disease and Related Disorders Association, NPI: Neuropsychiatric Inventory, OLZ: olanzapine, QUE: quetiapine, RIS: risperidone, SD: standard deviation. | |||||||||
| Study | Apathy | AE reported | NPS | Cognition | Function | Global Change | Dropouts due to AEs reported |
| METHYLPHENIDATE | |||||||
| AES‐Informant | Yes | NPI‐total | MMSE | N/A | CGI‐C | Yes | |
| AES‐Informant | Yes | NPI‐total (not reported) | MMSE | N/A | ADCS‐CGIC | Yes | |
| AES‐Clinician | Yes | N/A | MMSE | ADL | N/A | Yes | |
| MODAFINIL | |||||||
| FrSBe‐apathy subscale | Yes | N/A | N/A | ADLQ | N/A | Yes | |
| CHOLINESTERASE INHIBITORS | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| NPI‐apathy subscale | |||||||
| NPI‐apathy subscale | |||||||
| NPI‐apathy subscale | |||||||
| NPI‐apathy subscale | |||||||
| NPI‐apathy subscale | |||||||
| CHOLINESTERASE DISCONTINUATION | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison. | ||||||
| ATYPICAL ANTIPSYCHOTICS | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| BPRS‐withdrawn depression factor score | |||||||
| ANTIPSYCHOTIC DISCONTINUATION | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| ANTIDEPRESSANTS | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| NPI‐apathy subscale | |||||||
| MIBAMPATOR | |||||||
| FrSBe‐apathy T score | These outcomes were not investigated for this drug comparison | ||||||
| VALPROATE | |||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| GIP‐apathetic behavior subscore | These outcomes were not investigated for this drug comparison | ||||||
| NPI‐apathy subscale | These outcomes were not investigated for this drug comparison | ||||||
| SEMAGACESTAT | |||||||
| NPI‐apathy | These outcomes were not investigated for this drug comparison | ||||||
| ADCS‐CGIC: Alzheimer's Diserase Cooperative Study ‐ Clinical Global Impression of Change, ADL: Activities of Daily Living scale, ADLQ: Lawton and Brody Fucntional Assessment, AES: Apathy Evaluation Scale, CGI‐C: Clinical Global Impression of Change, FrSBe: Frontal Systems Behavior Scale, GIP: The Behavior Observation Scale for Intramural Psychogeriatric Patients, IADL: Instrumental Activities of Daily Living Scale, MMSE: Mini‐Mental State Examination, NPI: Neuropsychiatric Inventory. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the AES Show forest plot | 3 | 145 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐9.55, ‐0.43] |
| 1.1 < 12 weeks | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐4.80, ‐0.44] |
| 1.2 ≥ 12 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐9.90 [‐13.50, ‐6.30] |
| 2 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐3.85, 3.69] |
| 3 Adverse Events Show forest plot | 3 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.67, 2.42] |
| 3.1 < 12 weeks | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.44, 3.72] |
| 3.2 ≥ 12 weeks | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.86] |
| 4 Change in NPS from baseline as measured by the NPI Show forest plot | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐7.89, 8.21] |
| 5 Change in cognition from baseline as measured by the MMSE Show forest plot | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [1.06, 2.91] |
| 5.1 < 12 weeks study duration | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.49, 2.49] |
| 5.2 ≥ 12 weeks study duration | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [1.43, 3.77] |
| 6 Change in functional permance from baseline as measured by the ADL Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.39, 1.39] |
| 7 Change in functional performance from baseline as measured by the IADL Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [0.74, 3.86] |
| 8 Change in global disease severity from baseline as measured by the CGIC and the ADCS‐CGIC Show forest plot | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.11] |
| 9 Dropouts due to adverse events Show forest plot | 3 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.64, 7.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the FrSBe‐apathy subscale Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.51, 4.05] |
| 2 Change in functional performance from baseline as measured by the ADL‐Q Show forest plot | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.40, 0.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with licensed versus unlicensed ChEIs) Show forest plot | 6 | 3598 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.80, ‐0.00] |
| 1.1 Licensed ChEIs (and ≤ 24 weeks study duration) | 3 | 2531 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.85, 0.43] |
| 1.2 Unlicensed ChEIs ( and > 24 weeks study duration) | 3 | 1067 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.98, ‐0.29] |
| 2 Change in apathy from baseline as measured by the NPI‐apathy subscore (subgroup analysis with disease severity) Show forest plot | 6 | 3598 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.80, ‐0.00] |
| 2.1 Moderate AD (MMSE ≥ 18) | 4 | 3100 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.79, ‐0.07] |
| 2.2 Severe AD (MMSE < 18) | 2 | 498 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.82, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.88, 3.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and the BPRS withdrawn depression factor score Show forest plot | 2 | 1070 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.00, 0.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.51, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.44, ‐1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the FrSBe‐apathy T score Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.94, ‐0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore and GIP‐apathy subscore Show forest plot | 3 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.23, 0.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in apathy from baseline as measured by the NPI‐apathy subscore Show forest plot | 1 | 939 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.15, 0.25] |