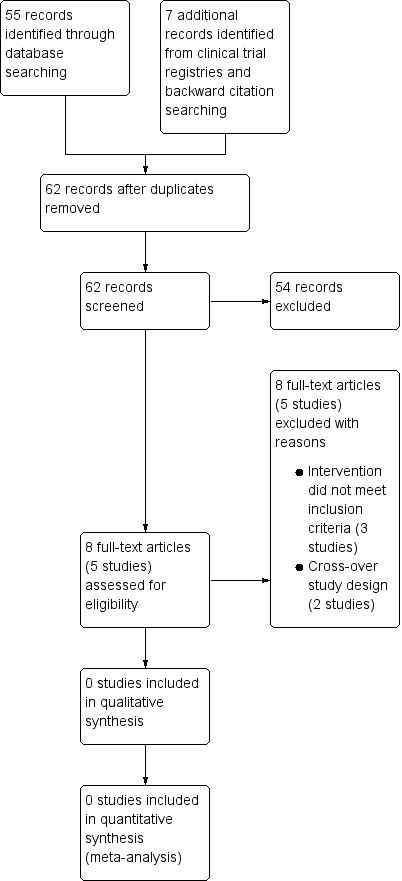
Corticosteroides orales iniciados por el paciente y por los padres para las exacerbaciones del asma
References
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Jump to:
Referencias de otras versiones publicadas de esta revisión
Jump to:
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| OCS was not part of the randomised treatment | |
| Cross‐over design | |
| OCS was administered as part of asthma action plan (i.e. co‐intervention), which was part of randomised treatment | |
| OCS could be optionally administered as part of asthma action plan, which was part of randomised treatment | |
| Cross‐over design |
OCS = oral corticosteroids.
| Patient‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: adults aged 18 years or older with asthma Settings: outpatient Intervention: patient‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days lost of study/work | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||
| Parent‐initiated steroids compared with placebo/normal care/alternative active treatment for asthma | ||
| Patient or population: children aged 5 years or older with asthma Settings: outpatient Intervention: parent‐initiated oral corticosteroids Comparison: placebo/normal care/alternative active treatment | ||
| Outcomes | Number of participants | Comments |
| Hospital admissions for asthma | 0 (0 studies) | No studies met the inclusion criteria for this review |
| Asthma control (validated scales) | 0 (0 studies) | |
| Serious adverse events (all cause) | 0 (0 studies) | |
| Unscheduled visit to a healthcare provider | 0 (0 studies) | |
| Health‐related quality of life (validated scales) | 0 (0 studies) | |
| Days off school | 0 (0 studies) | |
| Adverse events (all cause) | 0 (0 studies) | |
| GRADE Working Group grades of evidence | ||