Aspirina (dosis única) para el dolor perineal durante el período posparto temprano
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT | |
| Participants | Setting: Cincinnati General Hospital, Ohio, USA Date: December 1965 to April 1966 Inclusion criteria: women with a painful ('moderate' or 'severe') mediolateral episiotomy, within 24 hours following an uncomplicated labour and birth. Exclusion criteria: breastfeeding; aged < 18 years; known aspirin sensitivity; 'mild' pain at interview within 24 hours of birth. | |
| Interventions | Aspirin (N = 17 randomised) 600 mg aspirin; women received a single oral dose in a black capsule Placebo (N = 18 randomised) Women received a single oral dose in a black capsule All women: women did not receive other analgesics during the 6 hours of study or during the 6 hours before entering the study | |
| Outcomes | Adequate pain relief as reported by the woman: pain intensity evaluated by 1 research nurse hourly for 6 hours; women were asked "How much do your stiches hurt you?", and answers were transposed into an ordinal scale from 0 to 3 (0 = no pain; 1 = slight pain; 2 = moderate pain; 3 = severe pain). The difference between a woman's pre‐treatment pain intensity score and each hourly post‐treatment score gave an hourly pain relief score; a total 6 hour pain relief score was calculated for each woman by adding these scores. Mean Pain Relief scores (equivalent to SPID scores) were used to calculate 'Adequate pain relief as reported by the woman' (taken over 6 hours) Maternal adverse effects: women were asked on the day following treatment whether they noticed any other effects of the treatment; if they answered 'yes' they were asked 'What were they'; no leading questions were asked | |
| Notes | Funding: the study was supported in part by USPHS grants HE 05622 and HE 07392 from the National Institutes of Health Declarations of interest: not reported (short ‘About the authors’ section describing affiliations) Additional trial arms: this was a 5‐arm trial, also assessing chlorphenesin 400 mg (N = 18), 800 mg (N = 17) and combination aspirin 300 mg and chlorphenesin 400 mg (N = 18); we included only the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned... according to a predetermined schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind"; and "All patients received a single dose of coded mediation by mouth in identical black capsules". Assumed that blinding of women and personnel was successful with the use of an identical placebo |
| Blinding of outcome assessment (detection bias) | Low risk | 1 research nurse evaluated pain intensity and side effects by interviewing women. Assumed that blinding of the research nurse and women was successful with the use of an identical placebo |
| Incomplete outcome data (attrition bias) | High risk | 88 women had moderate or severe episiotomy pain; 84 completed the 6 hours of study and "form the basis of this report"… "Four of the 88 patients entering the trial were withdrawn owing to distressing pain unrelieved by the study drugs" (1/17 from the aspirin group; 0/18 from the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | Very few outcomes reported (pain relief and side effects only); no access to trial registration or protocol to further assess selective reporting |
| Other bias | Low risk | Baseline characteristics were comparable between groups; no other obvious risk of bias identified |
| Methods | RCT | |
| Participants | Setting: Cincinnati General Hospital, University of Cincinnati College of Medicine, Ohio, USA (assumed from affiliation) Inclusion criteria: healthy, consenting, ward patients with moderate to very severe episiotomy pain (mediolateral or midline) within 48 hours of an otherwise uncomplicated birth Exclusion criteria: mild pain; under the age of 18; history of aspirin allergy; breastfeeding; given analgesics within the previous 6 hours | |
| Interventions | Aspirin Group 1* (N = 20 randomised) 1200 mg aspirin; women received a single oral dose in capsules Aspirin Group 2* (N = 20 randomised) 600 mg aspirin; women received a single oral dose in a capsules Placebo (N = 19 randomised) Lactose placebo; women received a single oral dose in a capsule All women: lactose capsules were included with medication where necessary to provide a total of 4 capsules per dose. All drugs were administered before breakfast with a full glass of water, and women were instructed to lie on their right side for 2 hours after administration. Stilbestrol and ferrous sulphate were given routinely in the postpartum period, but all other drugs except for the study drugs were avoided, and except for "cleansing: all perineal care was suspended for the 8‐hour study period. | |
| Outcomes | Adequate pain relief as reported by the woman: the same trained nurse observer interviewed women hourly for 8 hours; they were asked "How much do the stiches hurt you"; answers were transposed to an ordinal score on a scale of 0 to 4 (0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain; 4 = very severe pain):
Need for additional pain relief in the first 48 hours for perineal pain: requirement for additional known analgesic medication (codeine or propoxyphene) for inadequate response to study drugs. Maternal adverse effects: side effects were evaluated at the last interview by the question, "Did you notice any other effects from today's medicine?" If the answer was "yes", the woman was asked, "What are they?" No other leading questions were asked | |
| Notes | Funding: "Supported in part by United Stated Public Health Service Grant HE‐05622 and by Merck Sharp & Dohme Research Laboratories. Supplies of flufenisal and other coded medications were provided by Dr. A. W. Vogel, Merck Sharp & Dohme Research Laboratories" Declarations of interest: not reported Additional trial arms: this was a 5‐arm trial, also assessing flufenisal 300 mg (N = 20) and flufenisal 600 mg (N = 21); we included only the relevant arms in this review. Note: we combined the 2 aspirin groups for the main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Within each of the 3 strata of pain intensity, patients were randomly assigned under double‐blind conditions to one of the 5 treatment groups according to a predetermined balanced allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All test medications were prepackaged in individual patient‐coded vials containing a single oral dose in identical capsules. Lactose capsules were included with medication where necessary to uniformly provide a total of 4 capsules per dose" and "double‐blind conditions" |
| Blinding of outcome assessment (detection bias) | Low risk | Subjective evaluation of pain relief and side effects; the same trained nurse observer interviewed women; considered reasonable to assume nurse and women were blinded |
| Incomplete outcome data (attrition bias) | High risk | Imputation of data likely to have influenced results. Quote: "Pain relief data collected before additional analgesic was given to each of these 14 patients was included in the analysis without qualification, but interviews were discontinued. By convention each patient’s pain intensity score for each of the residual hours was adjusted to the value of her pretreatment score, and these adjusted scores were used for calculations of pain relief which were then analysed together with the earlier recorded data. Although such an adjustment was arbitrary and tended to underestimate in these 14 patients the analgesic response to the study treatments, bias in the opposite direction, i.e. tending to exaggerate analgesic response to treatments, would have occurred if all or part of the hourly data for these 14 patients would have been excluded from the analysis or no adjustment made" |
| Selective reporting (reporting bias) | Unclear risk | Limited number of outcomes reported; no access to trial registration or protocol to further assess selective reporting |
| Other bias | Unclear risk | Most baseline characteristics were similar among groups; quote "However, body weight was not similar in all treatment groups". |
| Methods | RCT | |
| Participants | Setting: Cincinnati General Hospital, University of Cincinnati College of Medicine, Ohio, USA (assumed from affiliation) Inclusion criteria: healthy, consenting women with mild to severe episiotomy pain within 24 hours of an otherwise uncomplicated birth Exclusion criteria: allergy to aspirin; receipt of medication during the 6 hours before treatment | |
| Interventions | Aspirin (N = 13 randomised) 1200 mg aspirin given in coded single oral dose of 4 capsules Placebo (N = 13 randomised) Identical lactose placebo given in coded single oral dose of 4 capsules All women: no additional medications were received in the 5‐hour period of pain evaluation. Medications were given after a non‐fatty breakfast, and all other foods except water were withheld until after the pain evaluations were completed | |
| Outcomes | Adequate pain relief as reported by the woman: the same trained nurse observer interviewed women hourly for 5 hours; they were asked "How much do the stiches hurt you"; answers were transposed to an ordinal score on a scale of 0 to 3 (0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain):
| |
| Notes | Funding: "This investigation was supported in part by USPHS training grant HE‐05622 and by the Special Research Fellowship HE‐34688 of the National Heart Institute" Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Within each of the three strata of pain intensity… patients were randomly assigned to one of the two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "identical lactose placebo, given in a coded single oral dose of four capsules under double‐blind conditions" |
| Blinding of outcome assessment (detection bias) | Low risk | Subjective evaluation of episiotomy pain; the same trained nurse observed interviewed women; reasonable to assume women and the nurse were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses/exclusions for pain relief |
| Selective reporting (reporting bias) | Unclear risk | Pain relief was the only outcome reported; no access to trial registration or protocol to further assess selective reporting |
| Other bias | Unclear risk | Very limited methodological detail provided; no detail of baseline characteristics |
| Methods | RCT | |
| Participants | Setting: University of Cincinnati Medical Center, Ohio, USA (assumed from affiliation) Inclusion criteria: healthy postpartum women with moderate to very severe episiotomy pain (mediolateral or midline) within 48 hours of an otherwise uncomplicated birth Exclusion criteria: mild pain; unmarried, aged < 18 years; history of aspirin allergy; given analgesics, sedatives or other psychotropic drugs within the previous 6 hours; breastfeeding; known drug dependence | |
| Interventions | Aspirin (N = 20 randomised) 900 mg aspirin; single oral dose of 3 tablets of 300 mg Placebo (N = 20 randomised) Lactose placebo; single oral dose of 3 tablets All women: stilbestrol and ferrous sulphate were given routinely during the postpartum period, however all other drugs were avoided unless necessary, and except for "cleansing", all perineal care was suspended for the 6‐hour study period; women were confined to bed for the first 2 hours and were intermittently out of bed during the last 4 hours. Tablets were administered on demand with a full glass of water at approximately the same time of the day throughout the study (2 hours before breakfast) and women were instructed to lie on their right sides for 2 hours afterwards | |
| Outcomes | Adequate pain relief as reported by the woman: the same trained nurse observer interviewed women hourly for 6 hours; women estimated the severity of 'stitch' pain on an ordinal score on a scale of 0 to 4 (0 = no pain; 1 = mild pain; 2 = medium pain; 3 = severe pain; 4 = very severe pain):
Need for additional pain relief in the first 48 hours for perineal pain: request for additional analgesic medication (codeine or propoxyphene) before the end of the 6‐hour study period Maternal adverse effects: side effects were elicited spontaneously at final interview "with a minimum use of leading questions and without invoking a checklist of possible side effects" | |
| Notes | Funding: "Supported in part by United States Public Health Service Grant No. HL‐05622 and by the Upjohn Company. Supplies of ibuprofen and other coded medications were provided by Carter D. Brooks, M.D., The Upjohn Company" Declarations of interests: not reported Additional trial arms: this was a 4‐arm trial also assessing ibuprofen 300 mg (N = 20) and 900 mg (N = 20); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "On entering the study patients were randomly allocated to one of the 4 groups according to a predetermined schedule. The randomization provided for stratification of patients on the basis of initial intensity of pain" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind conditions"... "All were in the form of film‐coated tablets identical in appearance and taste, and were prepackaged in code‐numbered individual dose vials" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Changes in pain intensity and side effects associated with the treatments were evaluated subjectively in uniformly conducted interviews"; reasonable to assume blinding of women and interviewer |
| Incomplete outcome data (attrition bias) | High risk | No losses/exclusions reported; assumed only 80 women randomised, and all included in analyses; 4 women requested additional analgesic (3 in the placebo group); their pain relief data before additional analgesics were given were included in the analysis, but interviews were then discontinued, and for the remaining hours, each woman’s pain intensity score was adjusted to the value of her pre‐treatment score. Imputation of data likely to have influenced results |
| Selective reporting (reporting bias) | Unclear risk | Very limited outcome data; no access to trial registration or protocol to further assess selective reporting |
| Other bias | Low risk | Baseline characteristics comparable for relevant groups ("Two exceptions were a preponderance of unmarried patients in the ibuprofen 300 mg group compared with the 3 other groups, and body weight, which in the group of patients receiving ibuprofen 900 mg was distinctly higher than in patients in the other 3 groups. These were chance occurrences with an uncertain influence on the results"); no other obvious risk of bias identified |
| Methods | RCT | |
| Participants | Setting: Department of Obstetrics and Gynaecology, St Christiana Clinic, Dendermonde, Belgium Inclusion criteria: primiparae who had undergone mediolateral episiotomy (3 cm to 5 cm) during the course of an otherwise uncomplicated birth within the previous 48 hours, with moderate to severe pain Exclusion criteria: a more extensive episiotomy (because of forceps birth or other procedures); multigravida women; known allergy to aspirin; breastfeeding; other analgesic therapy within the previous 6 hours; mild pain | |
| Interventions | Aspirin (N randomised was unclear; N = 32 analysed) 600 mg; single oral dose in 2 identical capsules Placebo (N randomised was unclear; N = 31 analysed) Placebo; single oral dose in 2 identical capsules | |
| Outcomes | Adequate pain relief as reported by the woman: the same trained nurse observer questioned women hourly for 6 hours; women estimated the severity of pain on a scale of 0 to 3 (0 = no pain; 1 = mild pain; 2 = moderate pain; 3 = severe pain):
Need for additional pain relief in the first 48 hours for perineal pain: request for additional analgesic medication 4 hours after administration of study drugs Maternal adverse effects: close observation was made for any "adverse reactions" | |
| Notes | Funding: "The statistical assistance of T. COOK, B. RODDA and C. DAURIO of the Merck Sharp & Dohme Research Laboratories is gratefully acknowledged" Declarations of interests: not reported; though author affiliations include "Merck Sharp & Dohme Research Laboratories" Additional arms: this was a 5‐arm trial also assessing diflunisal 125 mg (N = 33 analysed), 250 mg (N = 30 analysed) and 500 mg (N = 30 analysed); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated at random" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind"… "All test medications were prepackaged in individual patient‐coded vials containing a single oral dose in two identically appearing capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Efficacy parameters and side‐effects were recorded by the investigator or by the same trained nurse observer, who questioned the patient at hourly intervals" |
| Incomplete outcome data (attrition bias) | High risk | 5/161 women admitted to the trial were excluded from the analysis: 2 as their initial pain was not considered severe enough to meet protocol; 2 due to incomplete data; 1 due to lack of cooperation (unclear from which groups; leaving 156 in total ‐ 32 in the aspirin group and 31 in the placebo group). 3 women in the placebo group were withdrawn at 4 hours because of severe pain, and 1 woman in the aspirin group at 3 hours for reasons unrelated to pain or the drug; women who dropped out of the study were included in the analysis; they were assigned a pain score of 4 = worse than the scores of all women who remained in the study |
| Selective reporting (reporting bias) | High risk | Very limited outcome data reported; no access to trial registration/protocol to further assess selective reporting. Quote: "As pain relief was still very marked in the 500 mg diflunisal group at 6 hours, it was decided to extend the period of observation to 8 hours in 42 patients, who were approximately evenly distributed between the three groups" |
| Other bias | Unclear risk | Few baseline characteristics reported (initial pain score rating; age); limited methodological data reported |
| Methods | RCT | |
| Participants | Setting: Department of Obstetrics and Gynaecology, Washington University, St Louis, Missouri, USA (assumed from affiliation) Inclusion criteria: women, suffering moderate or severe pain following episiotomy Exclusion criteria: current or recent history of gastrointestinal bleeding; peptic ulcer; other GI disorders; alcohol or drug abuse; disorders of the nervous system, kidney, heart or blood; known allergies to aspirin or aspirin‐like analgesics; conditions likely to interfere with absorption, distribution, metabolism, or excretion of drugs; other pain requiring narcotic analgesics; acute dermatitis or other skin lesions; past or present malignancies; taking corticosteroids or other NSAIDs, anticoagulants or other drugs that may interfere with study medication; experiencing pain due to other causes; breastfeeding | |
| Interventions | Aspirin (N = 39 randomised) 650 mg aspirin Placebo (N = 40 randomised) All women: women received the study medication at the onset or recurrence of moderate or severe pain at least 16 but not more than 48 hours following induction of anaesthesia | |
| Outcomes | Adequate pain relief as reported by the woman: pain intensity and relief at 0.5 hours, then hourly for 8 hours was measured.
Maternal adverse effects: patient complaints were reported | |
| Notes | Funding: not reported Declarations of interests: not reported Additional arms: this was a 4‐arm trial also assessed etodolac 25 mg (N = 40)and 100 mg (N = 40); we have only included the relevant arms in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not detailed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double‐blind" but no description of whether the study medications were identical in appearance, taste, etc |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) | Unclear risk | No losses/exclusions reported, but %/no of women ‘remaining in study’ at 4, 6 and 8 hours reported |
| Selective reporting (reporting bias) | Unclear risk | No access to trial registration or protocol to further assess selective reporting |
| Other bias | Unclear risk | Results report patients were "well matched" for a variety of baseline characteristics, but no table of these characteristics presented |
| Methods | RCT | |
| Participants | Setting: Department of Medicine, Tulane University School of Medicine, New Orleans, Louisiana, USA (assumed from affiliation) Inclusion criteria: women with moderate to severe episiotomy pain within 48 hours of a normal vaginal birth Exclusion criteria: nursing; systemic diseases; allergic to aspirin | |
| Interventions | Aspirin (N = 30 randomised) 648 mg aspirin; single oral dose Placebo (N = 30 randomised) Placebo; single oral dose All women: the time between the test drug and previous analgesic, tranquillisers or sedatives was at least 5 hours | |
| Outcomes | Adequate pain relief as reported by the woman: a trained nurse observer rated pain intensity and relief hourly for 4 hours
The results were not reported in such a way to calculate 'Adequate pain relief as reported by the woman.' Figure 2 in manuscript provides patient self‐rating of pain (continuous, analogue scale) Need for additional pain relief in the first 48 hours for perineal pain: need for extra analgesia during the 4‐hour study period Maternal adverse effects: volunteered or observed side effects | |
| Notes | Funding: not reported Declarations of interests: not reported Additional arms: this was a 4‐arm trial also assessed piroxicam 20 mg (N = 31) and 40 mg (N = 29); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By random assignment", no other detail described |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind"; no further detail provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detail provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Did not report on losses to follow up or exclusions |
| Selective reporting (reporting bias) | High risk | No access to trial protocol; however results reported incompletely in text |
| Other bias | Low risk | Baseline characteristics were reported to be comparable across groups; no other obvious sources of bias identified |
| Methods | RCT | |
| Participants | Setting: Department of Medicine, Tulane University School of Medicine, USA (assumed from affiliation) Inclusion criteria: women with severe episiotomy pain or severe uterine cramping pain, following an uncomplicated vaginal birth Exclusion criteria: mild or moderate pain or baseline pain < 60% on a pain analogue; dependent on analgesics or tranquillisers; hypersensitive to salicylates or caffeine; gastrointestinal, hepatic, or renal history or a history of psychiatric illness; emotionally unstable or overtly anxious | |
| Interventions | Aspirin (N = 16 randomised) 650 mg aspirin; single oral dose Placebo (N = 16 randomised) Placebo; single oral dose All women: duration between previous analgesic and test medication was at least 6 hours | |
| Outcomes | Adequate pain relief as reported by the woman: a trained nurse observer rated pain intensity and relief hourly for 4 hours.
The results were not reported in such a way to calculate 'Adequate pain relief as reported by the woman' Maternal adverse effects: women questioned about adverse effects at the last interview | |
| Notes | Funding: "We wish to thank Mr. Garrett Swenson of American Home Products for the double‐blind supplies of test drug, and Dr. Ilbok lee (Ives Laboratories), Dr. Bruce Schneider (Wyeth Laboratories), and Dr. Syliva Wassertheil‐Smoller (Albert Einstein College of Medicine) for their assistance in the statistical analysis of data" Declarations of interest: not reported Additional arms: manuscript reports results of 2 randomised controlled trials; we have excluded the first, as it combined women with uterine and episiotomy pain, and did not report any results separately for the subset of women with episiotomy pain. The included trial was a 3‐arm trial also assessed 800 mg aspirin and 64 mg caffeine (N = 15); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were divided at random", no further detail provided |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind", no further detail provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete data has not been reported and published |
| Selective reporting (reporting bias) | High risk | No access to trial protocol; limited data presented, results reported incompletely in text |
| Other bias | Unclear risk | No baseline characteristics reported |
| Methods | RCT | |
| Participants | Setting: Tulane University School of Medicine, New Orleans, Louisiana, USA (assumed from affiliation) Inclusion criteria: postpartum women who had undergone episiotomy and requested analgesic medication for pain of at least moderate intensity, aged ≥ 18 years Exclusion criteria: receipt of analgesics or tranquillisers within 4 hours of stud entry; planned to breast feed; history of convulsive disorders, known peptic ulcer, renal, hepatic or haematological disease; known allergic reactions to salicylates or other NSAIDs | |
| Interventions | Aspirin (N = 30 randomised) 600 mg aspirin; single dose of 2 matching capsules Placebo (N = 30 randomised) Placebo; single dose of 2 matching capsules All women: the test drug was given in a single dose in the form of 2 matching capsules. | |
| Outcomes | Adequate pain relief as reported by the woman: a trained nurse observed recorded pain intensity and relief at 0.5 hours and hourly to 5 hours
Need for additional pain relief in the first 48 hours for perineal pain: need for supplemental analgesia in 5‐hour study period Maternal adverse effects: adverse effects reported by women or observed by the nurse were recorded | |
| Notes | Funding sources: "Supported in part by a grant from Adria Laboratories, inc., Columbus, Ohio" Declarations of interests: not reported Additional arms: this was a 4‐arm trial also assessed indoprofen 50 (N = 30) and 100 mg (N = 30); we included only the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further detail provided |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double blind" "the test drug was given in a single dose in the form of two matching capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | Not specifically stated; reasonable to assume women and the nurse were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Any patients who experienced inadequate pain relief were permitted to remedicate with an alternate analgesic. In such cases pain evaluations were discontinued and for the balance of the study, patients were assigned a pain intensity score equal to that at the time of remedication and pain relief scores of zero" … There were however, no women who required re‐medication |
| Selective reporting (reporting bias) | Unclear risk | No access to trial registration or protocol to further assess selective reporting |
| Other bias | Low risk | Baseline characteristics comparable between groups; no other obvious sources of bias identified |
| Methods | RCT | |
| Participants | Setting: Sinai Hospital of Baltimore, MD, USA (assumed from affiliation) Inclusion criteria: women with no systemic medical illness, experiencing moderate to severe episiotomy pain within 48 hours following an otherwise uncomplicated vaginal birth Exclusion criteria: "those used by Hermann et al" | |
| Interventions | Aspirin (N randomised was unclear; N = 40 analysed) 650 mg aspirin; single dose in identical capsules Placebo (N randomised was unclear; N = 40 analysed) Placebo; single dose in identical capsules | |
| Outcomes | Adequate pain relief as reported by the woman: one investigator assessed pain intensity and relief hourly for 6 hours;
Need for additional pain relief in the first 48 hours for perineal pain: frequency of re‐medication Maternal adverse effects: women were observed hourly for adverse reactions | |
| Notes | Funding: "We would like to acknowledge the support of Sandoz, Inc. in this study" Declarations of interests: not reported Additional arms: this was a 4‐arm trial also assessed fluproquazone 100 mg (N = 41 analysed) and 200 mg (N = 39 analysed); we included only the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind"… "All medication was supplied in identical capsules… packaged in individually sealed envelopes" |
| Blinding of outcome assessment (detection bias) | Low risk | Considered reasonable to assume blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | In the whole trial, there were 166 who entered, and 160 provided "valid data for analyses"; other losses/exclusions not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to further assess; scales used to assess pain intensity and relief (needed to use SPID and TOTPAR scores to calculate adequate pain relief) were not reported |
| Other bias | Unclear risk | Baseline characteristics not reported |
| Methods | RCT | |
| Participants | Setting: Sinai Hospital, Baltimore, MD, USA (assumed from affiliation); between July and December 1980 Inclusion criteria: postpartum women with moderate to severe episiotomy pain Exclusion criteria: allergy to salicylates; asthma; history of chronic use of analgesics, alcohol, tranquillisers, or other drugs; blood dyscrasia; gastrointestinal disorders; hepatic and/or renal disease; psychiatric illness | |
| Interventions | Aspirin group 1 (N randomised not reported; N = 40 analysed) 5 grains (300 mg) aspirin; 4 tablet single dose Aspirin group 2 (N randomised not reported; N = 41 analysed) 10 grains (600 mg) aspirin; 4 tablet single dose Aspirin group 3 (N randomised not reported; N = 40 analysed) 20 grains (1200 mg) aspirin; 4 tablet single dose Placebo (N randomised not reported; N = 39 analysed) Placebo; 4 tablet single dose All women: if women had been medicated previously for pain, the experimental protocol was not initiated for 4 hours | |
| Outcomes | Adequate pain relief as reported by the woman: trained research nurse investigator questioned women at 0.5 hours and hourly for 4 hours regarding pain intensity which was recorded on a 4 point scale (0 = none; 1 = slight pain; 2 = moderate pain; 3 = severe pain). Pain intensity scores were provided in Table 1 from 0 to 4 hours and were thus used to calculate SPID scores and 'Adequate pain relief as reported by the woman' (taken over 4 hours). Need for additional pain relief in the first 48 hours for perineal pain: women re‐medicated for episiotomy pain within 4‐hour study period Maternal adverse effects: side effects observed or reported | |
| Notes | Funding: not reported Declarations of interests: not reported Note: we combined the 3 aspirin groups for the main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "prospective blind study"… "Each single four‐tablet dose was individually packaged and identified only by study and patient number, and all tablets appeared identical" |
| Blinding of outcome assessment (detection bias) | Low risk | As above; considered reasonable to assume blinding |
| Incomplete outcome data (attrition bias) | High risk | "Because of protocol ineffectiveness, participation in the study was terminated if the patient requested additional analgesic medication, topical analgesics, or Sitz baths. In such cases pain intensity was measured at all intervals up to the time of termination"… 6/121 women in the aspirin groups and 10/39 in the placebo groups required re‐medication. No clear reporting of other losses/exclusions |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to further assess risk of selective reporting |
| Other bias | Unclear risk | Baseline characteristics not reported |
| Methods | RCT | |
| Participants | Setting: LNJP Hospital, New Delhi, India (assumed from affiliation) Inclusion criteria: women from an otherwise healthy population whose chief complaint was moderate to severe pain following episiotomy on the first postoperative morning Exclusion criteria: known hypersensitivity to dipyrone and aspirin; receipt of any analgesics 8 hours before entry to the study | |
| Interventions | Aspirin (N = 90 randomised) 500 mg aspirin; single oral dose in identical tablet form Placebo (N = 88 randomised) Placebo; single oral dose in identical tablet form All women: nothing was permitted to be taken orally for the first hour after treatment administration | |
| Outcomes | Adequate pain relief as reported by the woman:
Maternal adverse effects: adverse drug reactions | |
| Notes | Funding: not reported Declarations of interests: not reported Additional arms: this was a 3 arm trial also assessed dipyrone 500 mg (N = 89); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated at random" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "approved double‐blind approach, which was strictly adhered to" "identical tablet form" |
| Blinding of outcome assessment (detection bias) | Low risk | Reasonable to assume blinding |
| Incomplete outcome data (attrition bias) | Low risk | No losses/exclusions |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to further assess risk selective reporting |
| Other bias | Low risk | Figures reported for baseline characteristics (such as pain severity, age, weight and height at baseline), and reported "all three groups were also comparable…" |
| Methods | RCT | |
| Participants | Setting: Cedars‐Sinai Medical Center, Los Angeles, California, USA (assumed from affiliation). Inclusion criteria: hospitalised women with moderate, severe or very severe pain due to uterine cramps or episiotomy within 48 hours of delivery (94 women in total with episiotomy pain) Exclusion criteria: breastfeeding; receipt of any analgesic, sedative or psychotropic medication within 6 hours before administration of the study drug | |
| Interventions | Aspirin (N = 20 randomised) 650 mg aspirin; single oral dose of 2 x 325 mg identical looking capsules Placebo (N = 18 randomised) Placebo; single oral dose of 2 identical looking capsules | |
| Outcomes | Adequate pain relief as reported by the woman: 1 nurse observer recorded pain intensity hourly for 8 hours; intensity was rated from 1 to 5 (1 = no pain; 2 = mild pain; 3 = moderate pain; 4 = severe pain; 5 = very severe pain); pain intensity differences were calculated, as were SPID scores at 4, 6, and 8 hours; the time of maximum pain relief; duration of pain relief; the proportion of women with at least 50% pain relief 1 and 2 hours after treatment Need for additional pain relief in the first 48 hours for perineal pain: proportion requiring additional analgesics Adverse effects: adverse effects mentioned by women were recorded No data included in the meta‐analyses as results were not reported separately for women with post‐episiotomy pain | |
| Notes | Funding: not reported Declarations of interests: not reported Additional arms: this was a 5‐arm trial also assessed fendosal 100 (N = 19), 200 (N = 19) and 400 mg (N = 18); we included only the relevant arms in this review Note: trial also included women with postpartum uterine cramps pain; not included in review (157/250); no data were able to be included in the meta‐analyses as results were not reported separately for women with post‐episiotomy pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assignment to treatment was randomised" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind" and "identical looking capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | As above; 1 nurse observer recorded the pain intensity scores prior and hourly after administration of medication, reasonable to assume that outcome assessment is blinded |
| Incomplete outcome data (attrition bias) | High risk | A total of 250 eligible women were "admitted to the study"… "The PI scores from patients taking another analgesic were handled as treatment failures by substituting the initial PI score for all hours after the analgesic was taken" |
| Selective reporting (reporting bias) | Unclear risk | Results (such as SPID scores) were reported incompletely in text |
| Other bias | Low risk | Baseline characteristics (such as initial intensity and type of pain; age and weight) were comparable between groups |
| Methods | RCT | |
| Participants | Setting: Hospital Maternidad Concepcion Palacios, Caracas, Venezuela. Inclusion criteria: women of legal age (aged ≥ 18 years), who were able to communicate meaningfully with the nurse‐observer, who were hospitalised and had severe post episiotomy pain after an uncomplicated birth and could tolerate oral medications Exclusion criteria: planning to breast feed within 24 hours after administration of the study medications; serious complicating illness or abnormal postpartum bleeding, with active peptic ulcer disease or other gastrointestinal disease associated with blood loss; receipt of any other investigational drug within the 1 month prior; history of drug or alcohol abuse; known allergic sensitivities to aspirin, diclofenac, or other NSAIDs | |
| Interventions | Aspirin (N = 50 randomised) 650 mg aspirin; single oral dose of 2 x 325 mg capsules; and 3 placebo tablets Placebo (N = 52 randomised) Placebo; single oral dose of 2 placebo capsule and 3 placebo tablets All women: each woman received a single unit dose consisting 2 capsules and 3 tablets, with at least 8 ounces of water; women were asked to sit up or lie on their right side for 2 hours after administration. No medications (analgesics, sedatives, hypnotics, tranquillisers) were permitted concomitantly or during the 4 hours prior to taking the medication | |
| Outcomes | Adequate pain relief as reported by the woman: the same nurse‐observer interviewed the women at 0.5 hours, and hourly for 8 hours
Need for additional pain relief in the first 48 hours for perineal pain: re‐medication within 8‐hour study period Maternal adverse effects: adverse effects were recorded if they were observed or volunteered | |
| Notes | Funding: "This work was supported in part by a grant from the Ciba‐Geigy Corporation, Summit, NJ" Declarations of interests: not reported; though first and second authors affiliated to "Analgesic Development Ltd." Additional arms: this was a 5‐arm trial also assessed diclofenac potassium 25 mg (N = 52), 50 mg (N = 50) and 100 mg (N = 51); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned… computer program generated a random permutation such that two patients received each treatment" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind" and "Each patient received a single‐unit dose consisting of 3 tablets and 2 capsules… all unit doses were identical in appearance and packaging" |
| Blinding of outcome assessment (detection bias) | Low risk | 1 nurse observer involved in outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | If a woman wished to withdraw before the first hour because of inadequate relief, a non‐study analgesic was administered, and she was discontinued from the study (none were re‐medicated in this first hour; therefore no discontinuations); if a woman required additional analgesic after the first hour, she was included, and relief scores of 0 and intensity scores equal to the pain at time of re‐medication were assumed for the duration (5/50 in the aspirin group 19/52 in the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to further assess risk of selective reporting |
| Other bias | Low risk | Baseline characteristics reported (age, weight, height, parity, days post‐delivery) were comparable between groups; no other obvious sources of bias identified |
| Methods | RCT | |
| Participants | Setting: Hospital Maternidad Concepcion Palacios, Caracas, Venezuela Inclusion criteria: women with severe post‐episiotomy pain after an uncomplicated birth; aged ≥18 years, who could tolerate oral medications Exclusion criteria: known allergic sensitivities to study medication; abnormal postpartum bleeding, or complicating illnesses; breastfeeding; history of drug dependence; receipt of other investigational drugs prior to enrolment | |
| Interventions | Aspirin (N = 30 randomised) 600 mg aspirin; single oral dose of 1 aspirin capsule and 1 placebo tablet Placebo (N = 30 randomised) Placebo; single oral dose of 1 capsule and 1 tablet All women: as a single dose; women were given the study medication by the nurse observer when their pain was severe; no medications that might alter the response to the study analgesics were permitted concomitantly or during the 4 hours before the test medication was taken | |
| Outcomes | Adequate pain relief as reported by the woman: the same nurse observer interviewed at the time of medication, 0.5 hours, and hourly for 4 hours
Need for additional pain relief in the first 48 hours for perineal pain: re‐medication within 4 hours Maternal adverse effects: adverse reactions were noted if observed or volunteered | |
| Notes | Funding: "A grant‐in‐aid and test medication from Upjohn Company made this research possible" Declarations of interests: not reported Additional arms: this was a 4‐arm trial also assessed zomepirac 100 mg (N = 30) and ibuprofen 400 mg (N = 30); we have only included the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a randomised study" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "in a double‐blind fashion. Because the study medications were not identical in appearance, a double‐dummy technique was used"; each woman received one tablet and one capsule as appropriate |
| Blinding of outcome assessment (detection bias) | Low risk | The same nurse observer interviewed the patients |
| Incomplete outcome data (attrition bias) | High risk | If before the first hour a woman reported inadequate pain relief, a conventional analgesic was given and she was removed from the study; if a woman requested ‘rescue’ medication after the first hour she was given it and was included in the evaluation; responses at the time of re‐medication were assumed for the duration of the study; all 120 women who participated in the study were included in the analysis; 5 women who received placebo required rescue medication during the study; no women receiving aspirin required re‐medication |
| Selective reporting (reporting bias) | High risk | Some incomplete reporting "The three drugs were much the same for mean onset, duration and time to peak values. The hypothesis that there is no difference among treatments was rejected at the 0.05 level or better for all variables"; patients rating of overall improvement and of study medication mentioned in methods and not reported |
| Other bias | Low risk | Baseline characteristics presented were comparable between groups; no other obvious sources of bias identified |
| Methods | RCT | |
| Participants | Setting: Hospital Maternidad Concepcion Palacios, Caracas, Venezuela Inclusion criteria: women with moderate or severe post‐episiotomy pain after an uncomplicated delivery, who could tolerate oral medication, aged ≥ 18 years Exclusion criteria: breastfeeding; any complicating illness or abnormal postpartum bleeding; receipt of any other investigational drug within 1 month prior to enrolment; history of drug dependence or known allergic sensitivities to prolonic acid derivatives or aspirin | |
| Interventions | Aspirin (N = 29 randomised) 600 mg aspirin; single oral dose of 5 identical tablets Placebo (N = 31 randomised) Placebo; single oral dose of 5 identical tablets All women: no medications that might confound the interpretation of the efficacy and/or adverse effect liability of the study analgesics were permitted concomitantly or during the 4 hours before taking the study medication | |
| Outcomes | Adequate pain relief as reported by the woman: the same nurse‐observer interviewed the women at the time medication was administered and hourly for 6 hours:
Need for additional pain relief in the first 48 hours for perineal pain: re‐medication within 6‐hour study period Maternal adverse effects: adverse reactions were noted if they were observed or volunteered | |
| Notes | Funding: "Supported by a grant from Boots Pharmaceuticals, Inc" Declarations of interests: not reported Additional arms: this was a 5‐arm trial also assessed flurbiprofen 25 (N = 32), 50 (N = 29), 100 mg (N = 31); we included only the relevant arms in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In each successive block of ten patients a computer program generated a random permutation such that, two patient received each treatment" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind… Each patient was given one dose of five tablets that were identical in appearance and packaging" |
| Blinding of outcome assessment (detection bias) | Low risk | Same nurse interviewer interviewed patients at administration and hourly afterwards; reasonable to assume blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | If women reported inadequate pain relief before the first hour, a conventional analgesic agent was given and they were removed from the study; if women requested rescue medication after the first hour, they were given the conventional analgesic and included in the analyses – baseline pain intensity and zero pain relief were assumed for the duration of scheduled observations; 168 women were enrolled in the study; 16 were "dropped from the analysis because they received concomitant oxytoxic medication that the sponsor felt might confound the interpretation of the efficacy of the study drug" 1/32 in the placebo group; 4/33 in the aspirin group; no women re‐medicated in the first hour; 14 re‐medicated in placebo group; 1 re‐medicated in the aspirin group |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to further assess selective reporting |
| Other bias | Low risk | Baseline characteristics reported were balanced between groups; no other obvious risk of bias identified |
| Methods | RCT | |
| Participants | Setting: "in an obstetric and gynecology unit"; Montreal, Quebec, Canada (assumed from affiliation) Inclusion criteria: women who had an episiotomy Exclusion criteria: receipt of tranquillisers, sedatives, hypnotics or other analgesics during the 4 hours preceding the study; breastfeeding | |
| Interventions | Aspirin (N = not reported) 1200 mg aspirin; single dose of 4 x 300 mg tablets Aspirin (N = not reported) 600 mg aspirin; single dose of 2 x 300 mg tablets and 2 placebo tablets Placebo (N = not reported) Placebo; single dose of 4 placebo tablets All women: the medication was administered 10.5 to 14.4 hours after episiotomy, upon request by the woman or when pain was judged moderate to severe by the nurse | |
| Outcomes | Adequate pain relief as reported by the woman: severity of pain was judged using a 30 cm visual analogue scale (no pain, slight, moderate, severe, unbearable); the women registered the intensity of pain by putting a stroke on the place on the scale before drug administration and every hour for 4 hours (women were not allowed to see the result of their previous assessment); the research nurse independently recorded her own evaluation of the analgesic effect of the medication (scale of 0 to 4: no pain, to worse than before) Need for additional pain relief in the first 48 hours for perineal pain: additional analgesic during 4‐hour period Maternal adverse effects: side effects reported by the women were noted on case report forms | |
| Notes | Funding: "This study was supported by a grant from Roussel Canada Inc., Montreal, Quebec, Canada" Declarations of interests: not reported; though last author affiliated to "Roussel Canada Inc." Additional arms: this was a 5‐arm trial also assessed tiaprofenic acid 200 mg (N = not reported) and 400 mg (N = not reported); we included only relevant arms in this review Note: no data could be included in the meta‐analyses as numbers for each group were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "double‐blind"… "The drugs were prepared as tablets… all were of identical appearance" |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: "double‐blind"… "The drugs were prepared as tablets… all were of identical appearance" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clearly reported (nor were the numbers of women in each group) |
| Selective reporting (reporting bias) | High risk | Results incompletely reported within text (including nurse’s evaluation of analgesic effect); numbers in each group also not reported |
| Other bias | Unclear risk | Baseline characteristics incompletely reported in text "There were no significant differences among the 5 groups with respect to age, height, weight or vital signs" |
GI: gastro‐intestinal
NSAIDs: non‐steroidal anti‐inflammatory drugs
RCT: randomised controlled trial
SPID: summed pain intensity differences
TOTPAR: total pain relief
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| RCT. Included women with "postpartum pain", not exclusively women with perineal pain; results not reported separately for women with perineal pain | |
| RCT. Did not assess aspirin for perineal pain, and rather assessed 2 agents: 1 (Darvon), containing propoxyphene with aspirin, phenacetin, and caffeine; and 1 (Fiorinal) which combined aspirin, phenacetin, and caffeine with a mild sedative, butalbital | |
| RCT. Included women with "postpartum pain", not exclusively women with perineal pain; results not reported separately for women with perineal pain | |
| RCT. Included women with "postpartum pain", not exclusively women with perineal pain; results not reported separately for women with perineal pain | |
| RCT. Did not assess aspirin for perineal pain, and rather assessed an aspirin compound (acetophenetidin acetylsalicylic acid and caffeine); codeine and an aspirin compound; dextropropoxyphene; dextropropoxyphene and an aspirin compound; and a starch placebo | |
| RCT. Did not assess aspirin for perineal pain, and rather assessed an aspirin/caffeine combination; an acetaminophen/aspirin combination; acetaminophen alone; and placebo | |
| Not RCT: "In consecutive cases of vaginal delivery with episiotomy, orders in alternating patients for analgesic 1 or analgesic 2... were written". This study assessed Darvon Compound (dextro propoxyphene and acetylsalicylic acid compound); and a preparation containing acetylsalicylic acid, acetophenetidin, caffeine and codeine phosphate. Aspirin not assessed | |
| RCT. Included women with "postpartum pain", not exclusively women with perineal pain; results not reported separately for women with perineal pain | |
| RCT. Included women with "postpartum pain", not exclusively women with perineal pain; results not reported separately for women with perineal pain | |
| Did not assess single dose aspirin, and rather assessed twice daily acetylsalicylic acid; and naproxen; it was not clear whether this was a randomised controlled trial |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Unclear |
| Participants | 100 women with post‐episiotomy pain |
| Interventions | Single oral doses of 400 mg ibuprofen, 500 mg analgin, 500 mg paracetamol, 600 mg aspirin, and placebo |
| Outcomes | Pain intensity; pain relief; side effects |
| Notes | Awaiting classification pending further details regarding allocation |
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | No access to trial publication; awaiting classification pending availability of manuscript |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 13 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.69, 2.42] |
| Analysis 1.1 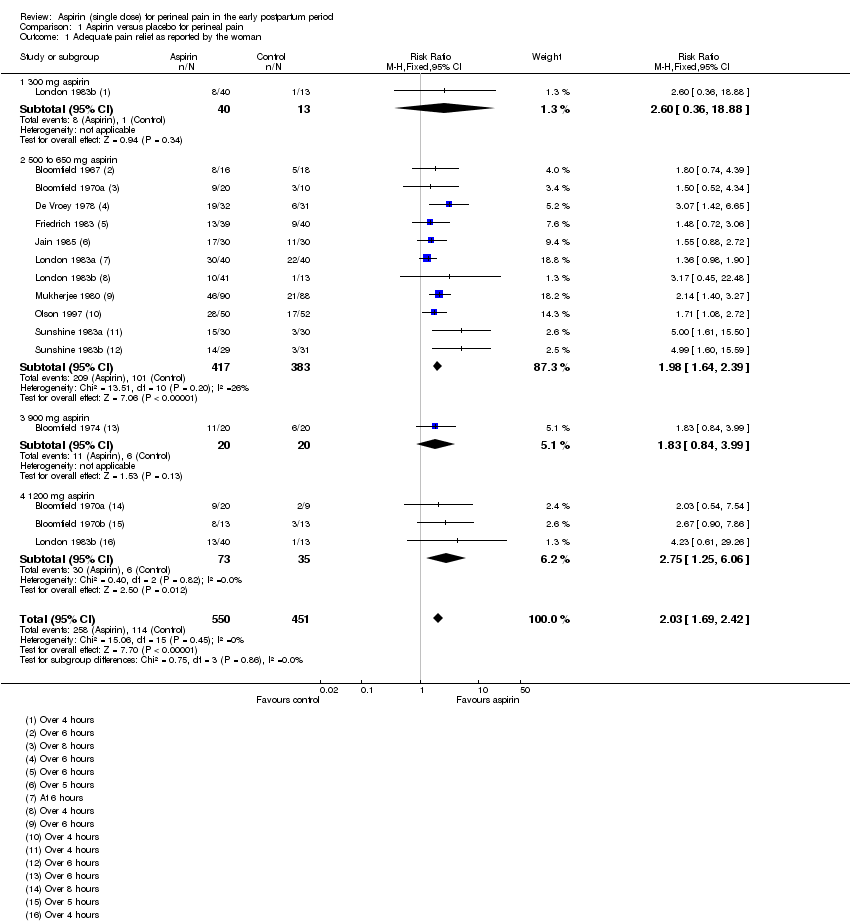 Comparison 1 Aspirin versus placebo for perineal pain, Outcome 1 Adequate pain relief as reported by the woman. | ||||
| 1.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.36, 18.88] |
| 1.2 500 to 650 mg aspirin | 11 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.64, 2.39] |
| 1.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.84, 3.99] |
| 1.4 1200 mg aspirin | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.25, 6.06] |
| 2 Need for additional pain relief Show forest plot | 10 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.17, 0.37] |
| Analysis 1.2 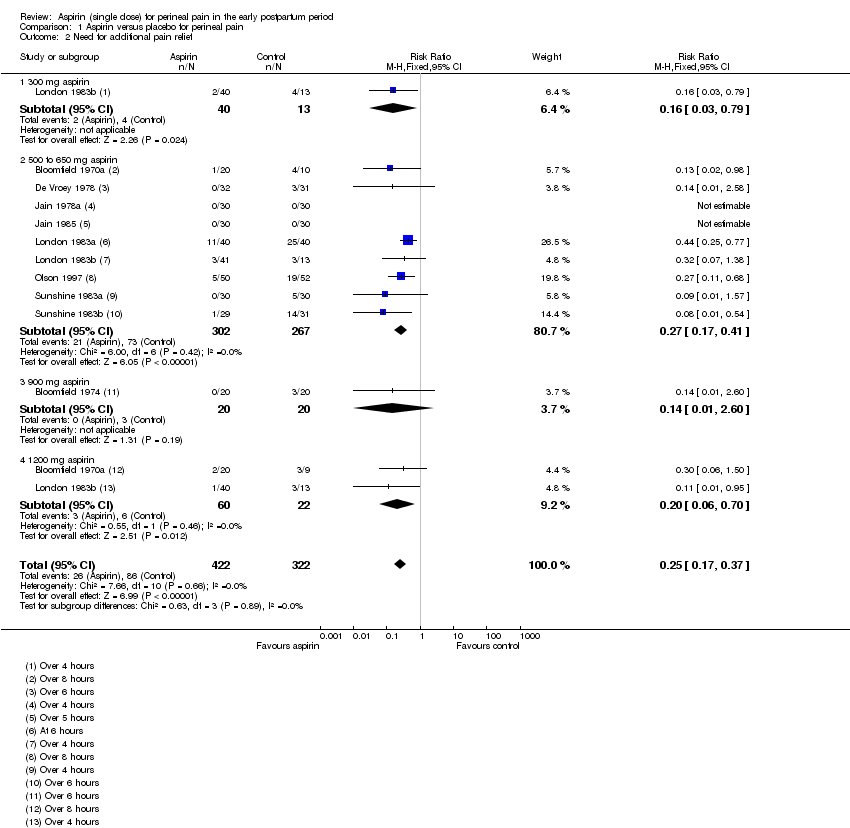 Comparison 1 Aspirin versus placebo for perineal pain, Outcome 2 Need for additional pain relief. | ||||
| 2.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.79] |
| 2.2 500 to 650 mg aspirin | 9 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.17, 0.41] |
| 2.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| 2.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.70] |
| 3 Maternal adverse effects Show forest plot | 14 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.57, 2.06] |
| Analysis 1.3  Comparison 1 Aspirin versus placebo for perineal pain, Outcome 3 Maternal adverse effects. | ||||
| 3.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 500 to 650 mg aspirin | 13 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.51, 2.53] |
| 3.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.55, 11.41] |
| 3.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman. | ||||
| 2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief. | ||||
| 3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.39] |
| Analysis 3.1  Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman. | ||||
| 2 Need for additional pain relief Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.68] |
| Analysis 3.2  Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief. | ||||
| 3 Maternal adverse effects Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| Analysis 3.3 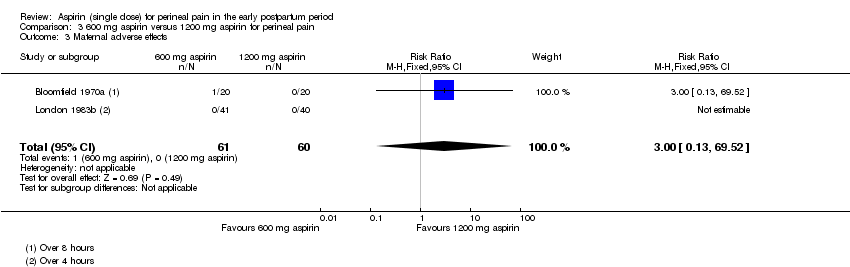 Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1 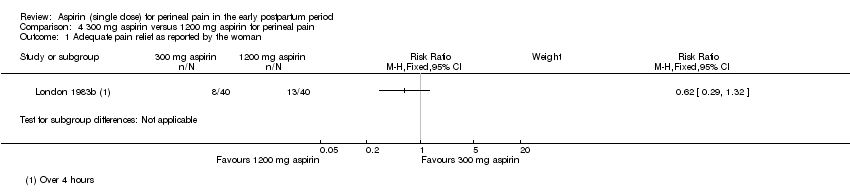 Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman. | ||||
| 2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2 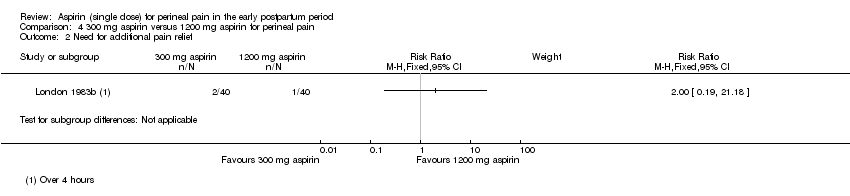 Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief. | ||||
| 3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3 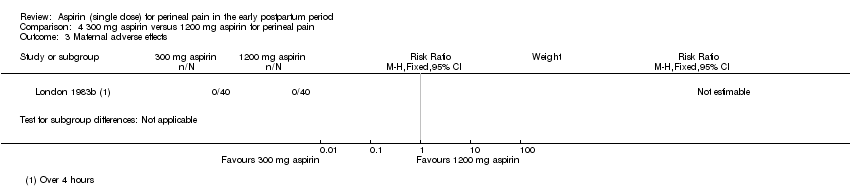 Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.1 Adequate pain relief as reported by the woman
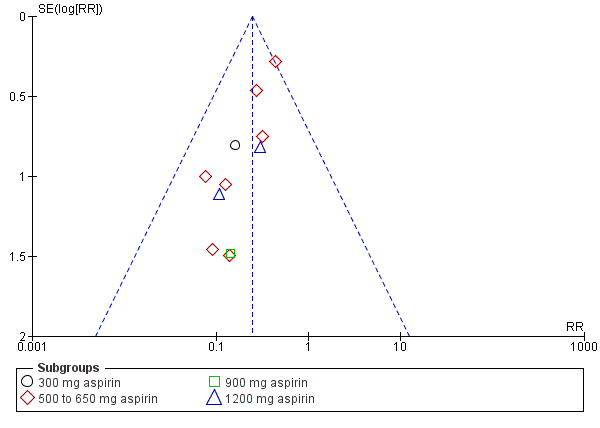
Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.2 Need for additional pain relief

Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.3 Maternal adverse effects

Comparison 1 Aspirin versus placebo for perineal pain, Outcome 1 Adequate pain relief as reported by the woman.

Comparison 1 Aspirin versus placebo for perineal pain, Outcome 2 Need for additional pain relief.

Comparison 1 Aspirin versus placebo for perineal pain, Outcome 3 Maternal adverse effects.

Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman.

Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief.

Comparison 2 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects.

Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman.

Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief.

Comparison 3 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects.

Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1 Adequate pain relief as reported by the woman.

Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2 Need for additional pain relief.

Comparison 4 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3 Maternal adverse effects.
| Aspirin compared with placebo for perineal pain in the early postpartum period | ||||||
| Patient or population: women with perineal pain in the early postpartum period Settings: 17 RCTs published from 1967 to 1997 (11 RCTs conducted in USA, 3 in Venezuela, 1 each in Belgium, Canada and India) Intervention: aspirin (single dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Aspirin | |||||
| Adequate pain relief as reported by the woman (4 to 8 hours) | Study population | RR 2.03 (1.69, 2.42) | 1001 (13 RCTs) | ⊕⊕⊝⊝ | ||
| 253 per 1000 | 513 per 1000 (427 to 612) | |||||
| Need for additional pain relief (4 to 8 hours) | Study population | RR 0.25 (0.17, 0.37) | 744 (10 RCTs) | ⊕⊝⊝⊝ | ||
| 267 per 1000 | 67 per 1000 (45 to 99) | |||||
| Maternal adverse effects (4 to 8 hours) | Study population | RR 1.08 (0.57, 2.06) | 1067 (14 RCTs) | ⊕⊝⊝⊝ | ||
| 27 per 1000 | 29 per 1000 (15 to 55) | |||||
| Neonatal adverse effects | (0 RCTs) | Not reported by any of the included RCTs | ||||
| Perineal pain at six weeks postpartum | (0 RCTs) | Not reported by any of the included RCTs | ||||
| *The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Study limitations: downgraded two levels due to the serious risk of bias 2Publication bias: downgraded by one level based on visual inspection of funnel plot which indicates likely publication bias 3Imprecision: downgraded one level due to few events and wide 95% CI around the pooled estimate which includes no effect | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 13 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.69, 2.42] |
| 1.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.36, 18.88] |
| 1.2 500 to 650 mg aspirin | 11 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.64, 2.39] |
| 1.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.84, 3.99] |
| 1.4 1200 mg aspirin | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.25, 6.06] |
| 2 Need for additional pain relief Show forest plot | 10 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.17, 0.37] |
| 2.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.79] |
| 2.2 500 to 650 mg aspirin | 9 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.17, 0.41] |
| 2.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| 2.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.70] |
| 3 Maternal adverse effects Show forest plot | 14 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.57, 2.06] |
| 3.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 500 to 650 mg aspirin | 13 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.51, 2.53] |
| 3.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.55, 11.41] |
| 3.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.39] |
| 2 Need for additional pain relief Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.68] |
| 3 Maternal adverse effects Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |


