Acupuntura para el dolor neuropático en adultos
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomisation Study duration: 10 weeks Location: Greater Manchester, UK | |
| Participants | Diagnosis: PDN Total: n = 59 Sex: 31 male, 14 female Age (years old): mean = 68, SD = 11.1 in acupuncture group; mean = 63, SD = 10.8 in control group Length of illness: not stated Inclusion criteria: people with type 1 or type 2 diabetes, aged 18‐80 years, with a clinical diagnosis of PDN and taking a prescribed drug for PDN were identified from primary and secondary care patient databases and invited to attend a screening visit held in the recruiting centre of a local district general hospital. Other inclusion criteria were patients taking a prescribed drug for their neuropathic pain; having at least one palpable pedal pulse per foot; not having previously received acupuncture treatment for PDN; being free of foot ulcers at the start of the study and having signs of peripheral sensory neuropathy, defined as the absence of any two of sharp/blunt sensations (measured using a NeuroTip); impaired light touch (10 g monofilament) or a vibration‐perception threshold on either foot > 25 V, measured with a neurosthesiometer. Exclusion criteria: not stated | |
| Interventions | 1. Acupuncture group: (n = 28) Management: A total of 5 standardised acupuncture points on the foot and lower limb of each leg (total 10) were used in the study. The chosen points were based on traditional Chinese medicine. The point location and depth of needle insertion were based on traditional acupuncture methods and good clinical practice. The depth of needle insertion varied according to point, but was usually 0.5‐1.5 cun (about 0.25‐2 cm). After insertion, the needles remained in place for 30 min and real needles were manipulated after 15 min Delivered by: acupuncturist Treatment duration: 10 weeks 2. Sham acupuncture group: (n = 31) Management: sham needle was blunt and slid into the handle rather than penetrating the skin when the needle was tapped. Before needling, a sliding plastic tube was adhered to each of the acupuncture points to mask the allocation of needles from the participants. Participants not asked whether they felt deqi to avoid the risk of participants in the placebo group becoming unblinded to their treatment allocation. After insertion, the needles remained in place for 30 min and sham needles were manipulated after 15 min, which is in keeping with normal acupuncture practice. Delivered by: acupuncturist Treatment duration: 10 weeks | |
| Outcomes | Pain intensity: VAS Withdrawals from trial due to any reason Any adverse events Quality of life: SF‐36 (physical component score, mental component score, bodily pain score) ‐‐Unable to use The Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale (measuring the likelihood of pain induced by neuromechanism), Sleep Problem Scale, MYMOP scores, Resting systolic BP, Resting diastolic BP. | |
| Notes | Study funding sources: The National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (grant reference number PBPG‐0706‐10595). "The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the recruitment, a computer‐generated randomised list of numbers was prepared allocating participants to receive either real or sham acupuncture." (p.243) Comments: the investigators describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was placed inside sequentially ordered sealed opaque envelopes, opened only after enrolment" (p.243) Comments: participants and investigators enrolling participants could not foresee assignment because of sealed, opaque envelopes used |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The treatment allocation was revealed to the acupuncturists out of sight of the participants to ensure blinding. To reduce the risk of observer bias, the acupuncture practitioners were discouraged from discussing the treatments or previous results with the patients." (p.243) Comments: trialists were not blinded to the treatment allocation but participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The treatment allocation was revealed to the acupuncturists out of sight of the participants to ensure blinding. To reduce the risk of observer bias, the acupuncture practitioners were discouraged from discussing the treatments or previous results with the patients." (p.243) Comments: the above statement indicates that observers (or assessors) were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Comments: A total of 4 participants (4/28, 14.3%) in the active group and 10 participants (10/31, 32.3%) in the sham group failed to complete the study. Missing outcome data was not balanced in numbers across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | High risk | Comments: fewer than 50 participants per treatment arm |
| Methods | Allocation: randomisation Study duration: 84 days Location: Hangzhou, Zhejiang, China | |
| Participants | Diagnosis: chemotherapy‐induced PN Total: n = 104 (6 dropouts) Sex: male 56, female 42 Age (years old): mean = 63.9 Length of illness: not stated Inclusion criteria: diagnosed multiple myeloma (MM); baseline without PN and PN appeared after chemotherapy at ≥ grade II (according to the NCI CTCAE version 3.0 neuropathy severity assessment); EMG examinations showing disturbances in median and peroneal nerve conduction; platelet count > 30 × 109/L; no history of mecobalamin allergy; having discontinued chemotherapy within 3 months and were willing to accept new therapy and sign an informed consent form Exclusion criteria: pregnancy; severe heart, liver or kidney dysfunction or other severe diseases (e.g. malignancies); neuropathy caused by tumor compression, nutritional disorders or infections or causes other than chemotherapy; refusal to sign the informed consent form | |
| Interventions | 1. Acupuncture + mecobalamin group: (n = 52) Management: participants received only 500 μg mecobalamin intramuscularly every other day, 10 times and thereafter 500 μg orally 3/day. In addition, every participant received needles bilaterally in acupoints. The first acupuncture was in prone position acupoints with needle retention, followed by supine position acupoints. An aseptic procedure was executed with disposable, stainless steel 30‐32 gauge needles, which were implanted to a depth of 0.3‐1.0 inches (about 0.76‐2.54 cm) into the acupoints until the participant felt dull pain or deqi, and were left in place for 30 min. The acupunctures were done daily for 3 days, then once every alternate day for 10 days as a treatment cycle. Each cycle was repeated every 28 days and the complete treatment included 3 cycles. Treatment duration: 84 days 2. Mecobalamin group: (n = 52) Management: participants received the same mecobalamin application as above. | |
| Outcomes | Pain intensity: VAS Withdraw from trial due to any cause Quality of life (FACT/the GOG‐Ntx questionnaire scores) ‐‐Unable to use (not in protocol) Nerve conduction velocity | |
| Notes | Study funding sources: the study was financially supported by grants from the Administration of Traditional Chinese Medicine Science and Technology Program of Zhejiang Province, Program Number: 2010ZA057, 2014ZB060; the Science and Technology Project of the Health Department of Zhejiang Province, Program Number: 2013KYA071; and the National Natural Science Foundation of China, Program Number: 81471532, 81402353. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomly divided into two groups." (p.3) Comments: the investigators describe a random component in the sequence generation process, but no details stated on random methods |
| Allocation concealment (selection bias) | Unclear risk | Comments: the author did not describe the allocation concealment. Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | High risk | Comments: although the author did not describe the blinding of participants and personnel, it would not have been possible to blind participants and personnel who were giving the intervention because one group did not receive acupuncture. |
| Blinding of outcome assessment (detection bias) | High risk | Comments: although the study author did not describe the blinding of outcome assessment, the outcomes which were participant‐reported would have detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Comments: a total of 3 participants (3/52, 5.8%) in the treatment group and 3 participants (3/52, 5.8%) in the control group left the trial or were lost to follow‐up, but reasons for dropout were not related to the intervention |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | Unclear risk | Comments: 50‐199 participants per treatment arm |
| Methods | Allocation: randomisation Study duration: 8 weeks Location: Lankao, Henan, China | |
| Participants | Diagnosis: DPN Total: n = 84 Sex: male 57, female 27 Age (years old): mean = 56.3 Length of illness: not stated Inclusion criteria: people with diabetes, accompanied by remote sense obstacle, weaker muscles, tendon slow and dyskinesia. Exclusion criteria: PN caused by liver and kidney diseases | |
| Interventions | 1. Manual acupuncture group: (n = 42) Management: participants received acupuncture once daily for 8 weeks (2 courses). Participants were maintained at supine position. Number 28 needle inserted acupoint for 0.5‐1 cun, retaining the needle for 30 min, hand‐manipulating needle twice Treatment duration: 8 weeks 2. Western medicine group: (n = 42) Management: participants received mecobalamin (500 ug, once daily) and nimodipine (40 mg, 3 times daily) for 8 weeks (2 courses) | |
| Outcomes | Any pain‐related outcome: no clinical response* ‐‐Unable to use (not in protocol) Motor nerve conduction velocity (MNCV); Sensory nerve conduction velocity (SCV) | |
| Notes | *No clinical response: no improvement or worse on pain and numbness of body, disturbance of perception (touch and thalposis), delay of response to stimulus and no increase in nerve‐conduction velocity Study funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...assigned randomly into control and observation groups according to random number table..." (p.47) Comments: the investigators described a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comments: the study author did not describe the allocation concealment. Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | High risk | Comments: although the author did not describe the blinding of participants and personnel, it would not have been possible to blind participants and personnel giving the intervention because one group did not receive acupuncture. |
| Blinding of outcome assessment (detection bias) | High risk | Comments: although the study author did not describe the blinding of outcome assessment, most of the outcomes were participant self‐reported, hence would have detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Comments: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | High risk | Comments: fewer than 50 participants per treatment arm |
| Methods | Allocation: randomisation Study duration: 12 weeks Location: Changchun, Jilin, China | |
| Participants | Diagnosis: DPN Total: n = 90 Sex: male 44, female 46 Age (years old): mean = 56.4, SD = 5.45 in acupuncture group; mean = 55.4, SD = 7.28 in Xiaoke bitong capsule group; mean = 55.8, SD = 6.46 in lipoic acid capsule group Length of illness: 3 months‐11 years Inclusion criteria: participants corresponding to diagnosis standards, strict diet control, stable amount of exercise over 2 weeks and receiving conventional glucose‐ lowering treatment (fasting blood glucose ≤ 7.0 mmol/L, 2‐hour post‐meal blood glucose ≤ 10.0 mmol/L, glycosylated haemoglobin < 7%, normotension and ortholiposis) Exclusion criteria: patients received relevant drugs for treatment of DPN within 2 weeks before enrolment; haemorrhage tendency within 2 months before enrolment; diabetic ketosis, ketoacidosis or infection within 1 month before enrolment; PN caused by other reasons; severe underlying diseases (e.g. liver and kidney dysfunction, cardiac insufficiency, myocardial infarction, cerebrovascular disease, malignant tumour); hyperglycemia caused by hyperthyroidism or hepatitis; women during gestation or lactation; systolic pressure ≥ 160 mmHg and/or diastolic pressure ≥ 100 mmHg; mentally disturbed or poor compliance; drug allergy history or allergic constitution | |
| Interventions | 1. Acupuncture + Xiaoke bitong capsule group: (n = 30) Management: participants received Xiaoke bitong capsule (1.2 g per time, 3 times daily) orally before 3 meals for 12 weeks. In addition, participants received acupuncture (retaining the needle for 30 min, hand‐manipulating of needle once before end) once daily (one course for 4 weeks, course interval was 3‐5 days) Treatment duration: 12 weeks 2. Xiaoke bitong capsule group: (n = 30) Management: participants received Xiaoke bitong capsule same as above 3. Lipoic acid capsule group: (n = 30)* Management: participants received lipoic acid capsule (0.2 g per time, 3 times daily) orally before 3 meals for 12 weeks | |
| Outcomes | Any pain‐related outcome: no clinical response** ‐‐Unable to use (not in protocol) Biochemical criterion; nerve conduction velocity; markers of oxidative stress | |
| Notes | *we did not use the data from this group, as it did not meet our inclusion criteria. **no clinical response: no improvement on the TCM symptoms (reduced score of syndrome < 30%) Study funding sources: key project of Administration of Traditional Chinese Medicine of Jilin Province (No: 2014‐ ZD2); Project of Health and Family Planning Commission of Jilin Province (No: 2015ZFZC06) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly divided into..." (p.51) Comments: the investigators described a random component in the sequence generation process, but no details stated on random methods |
| Allocation concealment (selection bias) | Unclear risk | Comments: the study author did not describe the allocation concealment. Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | High risk | Comments: although the study author did not describe the blinding of participants and personnel, it would not have been possible to blind participants and personnel who delivered the intervention because one group did not receive acupuncture |
| Blinding of outcome assessment (detection bias) | High risk | Comments: although the study author did not describe the blinding of outcome assessment, those outcomes that were participant self‐reported would have detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Comments: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | High risk | Comments: fewer than 50 participants per treatment arm |
| Methods | Allocation: randomisation Study duration: 3 months Location: Liaoning, China | |
| Participants | Diagnosis: DPN Total: n = 65 Sex: male 28, female 37 Age (years old): mean = 52.5 Length of illness: 0.5‐5 years Inclusion criteria: participants conformed to the diagnostic criteria stipulated by WHO in 1999: FBG (fast blood glucose) ≥ 7.0 mmol/L, in the OGTT test 2 h BG ≥ 11.1 mmol/L or the random BG ≥ 11.1 mmol/L (all taking venous blood). The symptoms and signs were sustained pain and/or abnormal sensation in the four limbs (at least in the lower limbs), weakened reflex in 1 or both ankles, weakened sensation of vibration (sensation of vibration in inner ankle was weaker than that in entocnemial condyle), and decreased nervous conductive velocity (NCV) on the main side in electroneuro‐physiological examination. Exclusion criteria: PN caused by other factors (such as heredity, alcoholism, uraemia, infection, malnutrition, drug intoxication and metal intoxication) | |
| Interventions | 1. Conventional treatment of diabetes + acupuncture group: (n = 32) Management: participants were conventionally treated with FBG < 7.0 mmol/L and 2 h BG below 11.1 mmol/L. For those with diabetes complicated with hypertension and hyperlipaemia, their BP and blood lipid were controlled to the normal range. Diet was rationally controlled. Number 30 1‐1.5 cun filiform needles were used for acupuncture with the uniform reinforcing‐reducing method. After the needles had been inserted into the points, evenly lifting, thrusting and twirling was performed until the participants felt needling sensation. Then, the needles were retained for 25 min, and manipulated twice. Treatment duration: once/day, with 14 sessions as 1 course of treatment, for 5 consecutive courses with a 4‐day interval between courses 2. Conventional treatment of diabetes + inositol group: (n = 33) Management: the same conventional treatment as above. Participants received oral‐taken Inositol. Treatment duration: 2 g/day in 3 times for 3 months. | |
| Outcomes | Any pain‐related outcome: no clinical response* | |
| Notes | *no clinical response: subjective symptoms were not improved or even aggravated Study funding sources: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into two groups" (p.13) Comments: the investigators described a random component in the sequence generation process, but no details stated on random methods |
| Allocation concealment (selection bias) | Unclear risk | Comments: the study author did not describe the allocation concealment. Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | High risk | Comments: although the study author did not describe the blinding of participants and personnel, it would not have been possible to blind participants and personnel who delivered the intervention because one group did not receive acupuncture |
| Blinding of outcome assessment (detection bias) | High risk | Comments: although the study author did not describe the blinding of outcome assessment, those outcomes that were participant‐reported would have detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Comments: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | High risk | Comments: fewer than 50 participants per treatment arm |
| Methods | Allocation: randomisation Study duration: 8 weeks Location: Weinan, Shaanxi, China | |
| Participants | Diagnosis: Type 2 diabetes, DPN Total: n = 60 Sex: male 35, female 25 Age (years old): mean = 53, SD = 9.2 Length of illness: 3 months to 27 months Inclusion criteria: not stated Exclusion criteria: not stated | |
| Interventions | 1. Acupuncture group: (n = 30) Management: participants received acupuncture once daily for 8 weeks (2 courses). Participants were maintained at supine position. Number 28 needle inserted acupoint for 0.5‐1 cun, retaining the needle for 30 min, hand‐manipulating of needle twice Treatment duration: 8 weeks 2. Western medicine group: (n = 30) Management: participants received mecobalamin (500 μg, once daily) and nimodipine (30 mg, 3 times daily) for 8 weeks (2 courses) | |
| Outcomes | Any pain‐related outcome: no clinical response* ‐‐Unable to use (not in protocol) motor nerve conduction velocity; Sensory nerve conduction velocity | |
| Notes | *no clinical response: no improvement or worse on pain and numbness of body, disturbance of perception (touch and thalposis), delay of response to stimulus and no increased in nerve‐conduction velocity Study funding sources: special research project of Department of Education of Shaanxi Province (14JK1256) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly divided into..." (p.97) Comments: the investigators describe a random component in the sequence generation process, but no details stated on random methods |
| Allocation concealment (selection bias) | Unclear risk | Comments: the study author did not describe the allocation concealment. Insufficient information to permit judgement of low risk or high risk |
| Blinding of participants and personnel (performance bias) | High risk | Comments: although the study author did not describe the blinding of participants and personnel, it would not have been possible to blind participants and personnel who delivered the intervention because one group did not receive acupuncture |
| Blinding of outcome assessment (detection bias) | High risk | Comments: although the study author did not describe the blinding of outcome assessment, those outcomes that were participant‐reported would have detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Comments: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comments: the protocol of this study was not available. Insufficient information to permit judgement of low risk or high risk |
| Size of study (biases confounded by small size) | High risk | Comments: fewer than 50 participants per treatment arm |
BP: blood pressure; cun: measure of patient's thumb width at the knuckle to derive acupoint; DPN; diabetic peripheral neuropathy; EMG: electromyography; MYMOP: Measure Yourself Medical Outcome Profile; n: number; PDN: painful diabetic neuropathy; PN: peripheral neuropathy; SD: standard deviation; TCM: traditional Chinese medicine; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The intervention was local anaesthetic injection | |
| Treatment duration < 8 weeks | |
| Participants had carpal tunnel syndrome symptoms, no indication of neuropathic pain in full text | |
| Treatment duration < 8 weeks | |
| Participants had tension neck syndrome, no indication of neuropathic pain in full text | |
| Treatment duration < 8 weeks | |
| Quasi‐randomised study, randomisation based on the admission sequence | |
| Treatment duration < 8 weeks | |
| Treatment duration < 8 weeks | |
| Participants had adhesive capsulitis, no indication of neuropathic pain in full text | |
| Compared TCM + acupuncture with carbamazepine | |
| The intervention was needle scalpel, not acupuncture | |
| Compared acupuncture + acupuncture point injection with amitriptyline | |
| Treatment duration < 8 weeks | |
| Participants had chronic neck pain, no indication of neuropathic pain in full text | |
| Terminated study with no publication | |
| Treatment duration < 8 weeks | |
| Non‐randomised, non‐blinded study | |
| Quasi‐randomised study, randomisation based on the admission sequence | |
| Treatment duration < 8 weeks | |
| Participants had rheumatoid arthritis, no indication of neuropathic pain in full text | |
| Quasi‐randomised study, randomisation based on the admission sequence | |
| Compared different acupuncture (miniscalpel‐needle vs trigger‐point injection) | |
| Treatment duration < 8 weeks | |
| Compared combined therapy mainly based on acupuncture (electroacupuncture + acupoint injection + He‐Ne laser therapy) with Western medicine | |
| Treatment duration < 8 weeks | |
| Quasi‐randomised study, randomisation based on the admission sequence | |
| Treatment duration < 8 weeks | |
| Quasi‐randomised study, randomisation based on the admission sequence | |
| Compared acupuncture + manipulation with nerve block | |
| Treatment duration < 8 weeks |
TCM: traditional Chinese medicine
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomised, parallel, controlled trial Study duration: not stated Location: Zhejiang, China |
| Participants | Diagnosis: Multiple myeloma with PN Total: n = 104 Sex: male and female Age: range: 32‐81 years old Length of illness: not stated Inclusion criteria: diagnosed MM; baseline without peripheral neuropathy and peripheral neuropathy appeared after chemotherapy (including thalidomide and bortezomib therapy) with ≥ level 2 (according to the NCI CTCAE version 3.0 neuropathy severity assessment) and EMG examinations showing disturbances of the median and peroneal nerve conductions; platelet count > 30 × 109/L and no history of mecobalamin allergy; discontinued bortezomib and thalidomide within 3 months; met the above criteria and willing to accept this therapy and signed the informed consent Exclusion criteria: pregnancy; severe heart, liver, kidney dysfunctions, or other severe diseases (e.g. malignancies); 3neuropathy caused by tumor compression, nutritional disorders or infections and other than the chemotherapy; refused to sign the informed consent |
| Interventions | 1. Acupuncture combined with mecobalamin group: (n = 52) 2. Mecobalamin alone group: (n = 52). |
| Outcomes | Pain intensity: neuralgia score Quality of life: daily activities score ‐‐Unable to use Conduction velocities (not in protocol) |
| Notes | Awaiting classification due to unclear treatment duration |
| Methods | Allocation: RCT Study duration: 28 weeks Location: Germany |
| Participants | Diagnosis: drug‐induced polyneuropathy Total: not stated Sex: male and female Age: > 18 years Length of illness: not stated Inclusion criteria: clinically diagnosed chemotherapy‐induced PN, pathologic results of the sural nerve in NCS. Exclusion criteria: current chemotherapy treatment or restart of chemotherapy due to tumor recurrence; other diseases that may cause PN; history of epilepsy; coagulopathy or use of anticoagulants with bleeding time > 3 min, prothrombin time < 40%, platelet count < 50.000/µL or partial thromboplastin time > 50 s; bacterial infection or other skin diseases at the lower extremities; bone fracture of the lower extremities during the last 3 months; alcohol, opiate, analgesic, or drug abuse; psychiatric illnesses other than mild depression; incapable of following the study instructions; (severe language disturbances, serious cognitive deficits, lack of time); pregnant or breast‐feeding women; current participation in other clinical studies |
| Interventions | 1. Acupuncture group: Management: 10 acupuncture treatments during the first study period. NCS are performed before and after the treatment period. In the second study period, participants do not receive specific treatment but NCS at the end of the period. 2. Wait‐list group: Management: wait‐list without specific treatment during the first study period. NCS are performed before and after the period. During the second study period, participants receive 10 acupuncture treatments. NCS are repeated after the treatment period |
| Outcomes | Pain intensity: Total Neuropathy Score; Symptom‐related numerical rating scale questionnaire ‐‐Unable to use (not in protocol) Sensory sural nerve action potential amplitude (SNAP) as measured by NCS; motor tibial nerve action potential amplitude; sural and tibial nerve conduction velocity as measured by NCS |
| Notes | Awaiting classification due to unclear treatment duration |
| Methods | Allocation: randomised Study duration: bot stated Location: USA |
| Participants | Diagnosis: Carpal tunnel syndrome Total: n = 59 Sex: male 10; female 49 Age: mean ˜ 49.1 years; SD ˜ 9.8 years Length of illness: > 3 months Inclusion criteria: all participants were examined for eligibility by a psychiatrist at Spaulding Rehabilitation Hospital, which included a physical exam for Phalen's maneuver and Durkan's sign and testing of median and ulnar sensory nerve conduction (NCS: Cadwell Sierra EMG/NCS Device, Kennewick, WA). NCS inclusion criteria consisted of median nerve sensory latency > 3.7 milliseconds or median nerve sensory latency > 0.5 milliseconds compared to ulnar nerve. Exclusion criteria: contraindications to MRI, history of diabetes mellitus, cardiovascular, respiratory, or neurological illnesses, rheumatoid arthritis, wrist fracture with direct trauma to median nerve, current usage of prescriptive opioid medication, thenar atrophy, previous acupuncture treatment (manual, EA, and TENS) for carpal tunnel syndrome, nerve entrapment other than median nerve, cervical radiculopathy or myelopathy, generalised PN, blood dyscrasia or coagulopathy or current use |
| Interventions | 1. Local verum electroacupuncture group: (n = 22) 2. Distal verum electroacupuncture group: (n = 18) 3. Sham electroacupuncture group: (n = 19) |
| Outcomes | Pain intensity: VAS; the intensity of acupuncture‐evoked sensations after the scan session using ‐‐Unable to use (not in protocol) Functional imaging (functional MRI) data |
| Notes | Awaiting classification due to unclear treatment duration |
| Methods | Allocation: randomised Study duration: 28 weeks Location: Beijing, China |
| Participants | Diagnosis: discogenic sciatica Total: estimated enrolment = 60 Sex: male and female Age: Range: 18‐75 years Length of illness: not stated Inclusion criteria: unilateral leg pain diagnosed as discogenic sciatica; sciatica patients with an average leg pain VAS of ≥ 40 mm in the last 24 h; aged 18‐75 years; leg pains that correlated with CT or MRI findings of lumbar disc herniation; agreed to follow the trial protocol. Exclusion criteria: severe cases with central or giant or ruptured lumbar disc herniation, cauda equina syndrome, foot drop, or surgery requirements; progressive neurological symptoms after 3 months of strict conservative treatment (e.g. nerve root adhesion, crossed straight‐leg testing, or obvious muscle atrophy); severe cardiovascular, liver, kidney, hematopoietic system diseases, autoimmune diseases, or poor nutritional status; cognitive impairment; pregnancy; subjects who received acupuncture for sciatica within the past month |
| Interventions | 1. Acupuncture group: (n = 30) 2. Sham Acupuncture group: (n = 30) |
| Outcomes | Pain intensity: change in mean weekly VAS of leg pain and low back pain; Oswestry disability index; Serious adverse events Quality of life: patients' global impressions of improvement; ‐‐Unable to use (not in protocol) Participants' expectations for acupuncture; blinded evaluation as measured by participant questioning of whether they believed they received real acupuncture at week 4 |
| Notes | Awaiting classification due to unclear treatment duration |
| Methods | Allocation: randomised Study duration: 3 months Location: Tianjin, China |
| Participants | Diagnosis: chemotherapy‐induced PN Total: estimated enrolment = 36 Sex: male and female Age: Range: 18‐80 years old Length of illness: not stated Inclusion criteria: histopathological and/or cellular pathology results prove malignancy of the tumour and the participant has received chemotherapy treatment before; 15 weeks after the completion of chemotherapy, the limbs are still feeling abnormal and the symptoms fulfil WHO grade 2 or more; Zubrod ‐ Eastern Cooperative Oncology Group‐WHO (ZPS) grade 0‐2, cardiac function, liver function and renal function are not significantly abnormal, the survival period of the participant is expected to be > 6 months; gender unrestricted, aged 18‐80 years; voluntary participation in the study, willing to sign informed consent, willing to comply with randomised grouping, willing to follow‐up. Exclusion criteria: suffering from PN due to infection, radiotherapy, HIV, chronic alcoholism, hypothyroidism, diabetes, paraneoplastic syndrome or other diseases or are suffering from nervous system diseases; being treated with other drugs that may lead to neurotoxicity; blood coagulation disorder; pregnancy and lactating women; infection, scarring or defects near the acupoint sites; received intervention for the prevention and treatment of peripheral neuropathy 2 weeks before screening or has received TCM (acupuncture, moxibustion, cupping, Chinese medicine therapy 1 month before |
| Interventions | 1. Electroacupuncture group 2. No intervention |
| Outcomes | Quality of life: questionnaire to assess chemotherapy‐induced PN (QLQ‐CIPN20); Functional Assessment of Cancer Treatment ‐ General scale (FACT‐G) |
| Notes | Awaiting classification due to unclear treatment duration |
| Methods | Allocation: randomised, parallel, controlled trial Blinding: unclear Study duration: 7 months Location: Spain |
| Participants | Diagnosis: myofascial pain Total: n = 21 |
| Interventions | 1. Acupuncture group: (n = 11) 2. Lidocaine infiltrations: (n = 10) |
| Outcomes | Pain intensity (VAS) Quality of life |
| Notes | This reference was waiting for translation to obtain clear information |
| Methods | Allocation: randomised Study duration: unclear Location: Shangqiu, Henan, China |
| Participants | Diagnosis: idiopathic trigeminal neuralgia Total: n = 80 Sex: male 45; female 35 Age: mean ˜ 59.57 years; SD ˜ 6.27 years Length of illness: more than 4 months Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 1. Manual acupuncture group: (n = 40) 2. Treatment as usual group (carbamazepine tablets): (n = 40) |
| Outcomes | Pain intensity (VAS) Any pain‐related outcome: no clinical response*, frequency of pain, duration of pain Specific adverse events |
| Notes | Awaiting classification due to unclear treatment duration *no clinical response: no improvement or even worse after treatment |
| Methods | Allocation: randomised Study duration: not stated Location: Neimenggu, China |
| Participants | Diagnosis: diabeteic PN Total: n = 44 Sex: male 25; female 19 Age: mean ˜ 38.9 years; SD ˜ 8.2 years Length of illness: 5‐25 years Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 1. Acupuncture + western medicine group: (n = 22) 2. Western medicine group: (n = 22) |
| Outcomes | Pain‐related outcome: no clinical response* |
| Notes | Awaiting classification due to unclear treatment duration *no clinical response: no definition |
CT: Computed Tomography; EA: Electric Acupuncture; EMG: Electromyography; MRI: Magnetic Resonance Imaging; n: number of participants; NCS: nerve conduction studies; PN: peripheral neuropathy; RCT: randomised controlled trial; SD: standard deviation; TCM: traditional Chinese medicine; TENS: Transcutaneous Electrical Nerve Stimulation; VAS: Visual Analogue Scale; WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Acupuncture study for the prevention of taxane induced myalgias and neuropathy |
| Methods | Allocation: randomised Estimated duration: December 2010‐December 2015 Location: USA Length of follow‐up: 16 weeks |
| Participants | Diagnosis: breast cancer Total: n = 50 Sex: female Age: > 21 years Length of illness: not stated Inclusion criteria: age > 21 years; history of stage I‐III breast cancer; scheduled to be receiving weekly adjuvant paclitaxel for 12 weeks; signed informed consent Exclusion criteria: previous treatment with acupuncture; diabetic neuropathy or other neurological conditions; inflammatory, metabolic or neuropathic arthropathies; current narcotic use; severe concomitant illnesses; severe coagulopathy or bleeding disorder; dermatological disease within the acupuncture area |
| Interventions | 1. Electroacupuncture group (n = 25) 2. Sham group (n = 25) Treatment duration: 12 weeks |
| Outcomes | Pain: difference in neuropathic pain between the 2 arms (measured by the mean Brief Pain Inventory‐Short Form (BPI‐SF)) Quality of life: FACT‐Tax quality of life assessment Neurologic dysfunction (Grooved Pegboard test) Change in pro‐inflammatory cytokines |
| Starting date | December 2010 |
| Contact information | Dawn L. Hershman, Columbia University |
| Notes | No results have been published |
| Trial name or title | Randomised controlled pilot trial of adjunct group acupuncture vs usual care among patients with painful diabetic neuropathy |
| Methods | Allocation: randomised Estimated duration: March 2015‐June 2016 Location: USA Length of follow‐up: 12 weeks |
| Participants | Diagnosis: PDN Total: n = 60 Sex: both Age: > 18 years Length of illness: > 3 months Inclusion criteria: English or Spanish speaking; diagnosed with type 2 DM; distal lower limb pain present for ≥ 3 months; score of ≥ 4 on the 11‐point Pain Intensity Numerical Rating Scale (PI‐NRS) for the pain of diabetic PN ≥ 4 days/week before randomisation; pain characterised as burning, shooting, or stabbing in nature; ability to understand study procedures and willingness to comply with them for the entire length of the study; score of < 8 on the Semmes‐Weinstein monofilament test; stable use of pain control medications for PDN in the 1 month prior to screening (e.g. no change in prescription) or no use of pain control medications for PDN within the past month Exclusion criteria: substance abuse (as assessed by the Simple Screening Instrument for Substance Abuse); unstable medical condition (e.g. severe pulmonary disease, myocardial infarction, severe depressive symptoms); electrical therapy (e.g. TENS unit) or patch treatment (e.g. lidocaine or capsaicin) for PDN used within the past 2 weeks; acupuncture, moxibustion, cupping or herbal medicine for PDN used within the past 2 weeks; pregnancy, planning a pregnancy or breast‐feeding; inability or unwillingness to comply with this study protocol, assessed prior to randomisation |
| Interventions | 1. TAU (treatment as usual) + acupuncture group (n = 20): receive usual care with adjunctive acupuncture once/week for 12 weeks 2. TAU + acupuncture group (n = 20): receive usual care with adjunctive acupuncture twice/week for 12 weeks 3. TAU group (n = 20): receive usual care with no acupuncture Treatment duration: 12 weeks |
| Outcomes | Percentage of recruited participants retained, change from baseline in average weekly pain on the 11‐point Pain Intensity Numerical Rating Scale (PI‐NRS), Pain Qualities Assessment Scale, health‐related quality of life, depressive symptoms using the Patient Health Questionnaire, participant rating of global improvement using the Patient Global Impression of Change scale, patient‐centered symptom severity using the Measure Yourself Medical Outcome Profile, NIH PROMIS Sleep Disturbance Scale, Protective sensation of the feet using a 5.07 Semmes‐Weinstein monofilament, patient satisfaction, use of medications |
| Starting date | March 2015 |
| Contact information | Maria T Chao, [email protected] |
| Notes | No results have been published |
| Trial name or title | A randomised controlled trial to assess the effectiveness and cost‐effectiveness of acupuncture in the management of chemotherapy‐induced peripheral neuropathy |
| Methods | Allocation: randomised Estimated duration: September 2015‐May 2017 Location: Hong Kong Length of follow‐up: 20 weeks |
| Participants | Diagnosis: chemotherapy‐induced PN Total: n = 98 Sex: both Age: child, adult, senior Length of illness: not stated Inclusion criteria: diagnosis of lung cancer receiving chemotherapy with curative intent, and breast or gynaecological cancer, head & neck and colorectal cancer stage I, II or III; currently receiving neurotoxic chemotherapy (taxanes, cisplatin, carboplatin, etc); reporting tingling in hands/feet and other indications of chemotherapy‐induced PN after initiation of cancer treatments, confirmed to be indicative of chemotherapy‐induced PN by a consultant; not using any medication for the prevention or treatment of chemotherapy‐induced PN for the past 31 months; willing to participate and be randomised to one of the study groups; no previously established PN. Exclusion criteria needle phobia; low platelet count (< 50,000); comorbidity with a bleeding disorder; pregnancy; received acupuncture treatment in the past three months. In addition, the ipsilateral arm of participants who have undergone axillary dissection also excluded from needling as well as lymphoedematous limbs |
| Interventions | 1. Acupuncture group (n = 49) 2. Standard care group (n = 49) Treatment duration: 8 weeks |
| Outcomes | Pain measured using the Brief Pain Inventory, Grade of chemotherapy‐induced PN, severity of neuropathy, quality of life measured using Functional assessment of cancer therapy (FACT/GOG‐Ntx), sensory examination, measurement of costs, consumption of analgesics, motor nerve conduction |
| Starting date | September 2015 |
| Contact information | Po Ling CHENG, +85227664132, [email protected] |
| Notes | No results have been published |
| Trial name or title | Evaluating the effects of acupuncture in the treatment of taxane induces peripheral neuropathy (TIPN) |
| Methods | Allocation: randomised Estimated duration: May 2016‐December 2016 Location: USA Length of follow‐up: 12 weeks |
| Participants | Diagnosis: taxane‐induced PN Total: n = 18 Sex: female Age: > 18 years Length of illness: not stated Inclusion criteria: histologically confirmed primary invasive carcinoma of the breast (stage I, II, or III); completed active chemotherapeutic with taxane therapy (taxotere, Taxol, Abraxane) within the last 24 months; established diagnosis of motor and sensory neuropathy ≥ 2 according to the CTCAE v 4.03 scale in spite of previous treatment with Neurontin, Cymbalta and/or Lyrica; read, understand, and speak English Exclusion criteria: currently undergoing active treatment with chemotherapy (not including TKI's or other targeted therapy); any acupuncture treatment for any indication within the 30 days of enrolment; cardiac pacemaker; deformities that interfere with accurate acupuncture point locations; local infection at or near the acupuncture site; pregnant or currently lactating; medical history of chronic alcohol use; mental incapacitation or significant emotional or psychological disorder |
| Interventions | 1. Acupuncture group (n = 9) 2. Control group (no intervention) (n = 9) Treatment duration: 12 weeks |
| Outcomes | Change in taxane‐induced PN symptoms measured by the Patients' Global Impression of Change (PGIC) scale, Evaluate the mechanism of acupuncture as a treatment of taxane‐induced PN through quantification of inflammatory biomarkers and circulation levels of mitochondrial DNA (mtDNA) Change in quality of life using the FACT/GOG‐NTX questionnaire Evaluate if neuropathic mechanisms are contributing to pain measured by the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale Change in taxane‐induced PN‐related pain measured by the Brief Pain Inventory (BPI) |
| Starting date | May 2016 |
| Contact information | Mark A O'Rourke, [email protected]; Renee J LeClair, [email protected] |
| Notes | No results have been published. |
| Trial name or title | Electroacupuncture to treat painful diabetic neuropathy: study protocol for a three‐armed, randomised, controlled pilot trial |
| Methods | Allocation: random numbers will be generated using a computerised random number generator through the stratified block randomisation method of the SAS package with a random block size of 3 prepared by a statistician who is blinded to this trial. Estimated duration: recruitment is expected to be completed from June 2012‐ July 2013 Location: Daejeon University Hospital in Daejeon, Korea Length of follow‐up: 16 weeks |
| Participants | Diagnosis: PDN Total: n = 45 Sex: both Age: 18‐75 years old Length of illness: ≥ 6 months Inclusion criteria: men and women aged 18‐75 years; diagnosis of type 1 or 2 DM; distal symmetric lower limb pain present for ≥ 6 months; ≥ 4 on the 11‐point Pain Intensity Numerical Rating Scale (PI‐NRS) for the pain of diabetic PN ≥ 4 days/week before the randomisation; ≥ 3 scores on the history and physical examination portion of the Korean version of the Michigan Neuropathy Screening Instrument (MNSI); ≥ 2 abnormalities on the following measures: (1) vibration perception by a 128 Hz tuning fork; (2) 10 g monofilament test; (3) ankle reflexes; stable use (variation of a major drug ≥ 25%) of pain control medications for PDN in the three months prior to screening or no use of pain control medications for PDN within the past month. Exclusion criteria: substance abuse or dependence; cardiovascular disorder (e.g. arrhythmia) or a pacemaker; neuropsychiatric conditions (e.g. epilepsy, depression or panic disorder); other diabetic microvascular complications (for example, diabetic nephropathy or diabetic retinopathy) within the past 3 months; HbA1c > 11%; change in antihyperglycemic medications in the 3 months prior to screening; diagnosis of diabetic foot ulcer; presence of severe pain other than that induced by PDN (for example, arthritis, back pain or headache); abnormal blood test (HbA1c, blood urea nitrogen, creatinine, thyroid‐stimulating hormone, triiodothyronine, free thyroxine, vitamin B12) or urine test (proteinuria); neuropathic pain caused by a condition other than DM (for example, malignant disease, tarsal tunnel syndrome, neurothlipsis, vitamin B12 deficiency, hypothyroidism, neurotoxicity (e.g. lead, alcohol or smoking), medication (e.g. chemotherapy or isoniazid), transient ischaemic attack, stroke, multiple sclerosis, chronic inflammatory demyelinating polyneuropathy, uraemic neuropathy, sub‐acute combined spinal cord degeneration, phantom limb pain or atherosclerosis obliterans); known hypersensitivity reaction after acupuncture treatment or an inability to co‐operate with the acupuncture procedure; electrical therapy or patch treatment (e.g. lidocaine or capsaicin) for PDN used within the past 2 weeks; acupuncture, moxibustion, cupping or herbal medicine for PDN used within the past 2 weeks; participation in other clinical trials within the past 3 months; pregnancy, planning a pregnancy or breast‐feeding; unwillingness to comply with this study protocol |
| Interventions | 1. Electroacupuncture group (n = 15) 2. Sham group (n = 15) 3. Usual care group (n = 15) Treatment duration: 8 weeks |
| Outcomes | Pain Intensity: PI‐NRS; Quality of life: SF‐MPQ, Sleep disturbance score, SF‐36, Beck Depression Inventory; PGIC (patient global impression of change) Adverse events |
| Starting date | June 2012 |
| Contact information | Sun‐mi Choi, Korea Institute of Oriental Medicine; [email protected] |
| Notes | No results have been published |
DM: diabetes mellitus; MD: mean difference; MD: mean difference; n: number; PDN: painful diabetic neuropathy; PN: peripheral neuropathy
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pain‐related outcomes: no clinical response ‐ defined by original study Show forest plot | 3 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.51] |
| Analysis 1.1  Comparison 1 Acupuncture alone versus other active therapy, Outcome 1 Any pain‐related outcomes: no clinical response ‐ defined by original study. | ||||
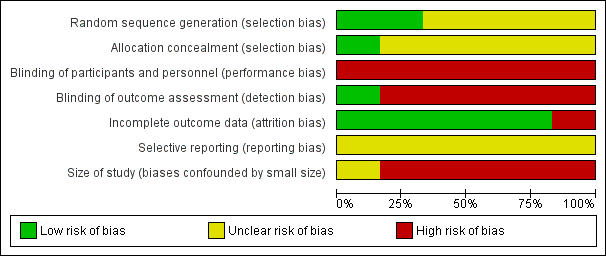
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
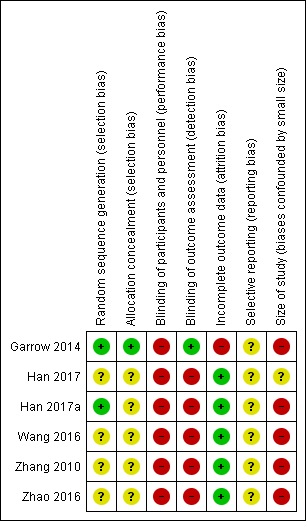
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Acupuncture alone versus other active therapy, Outcome 1 Any pain‐related outcomes: no clinical response ‐ defined by original study.
| Acupuncture versus sham acupuncture for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: sham acupuncture | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | Mean 6.2 | Mean 5.8 | The mean participant‐reported pain intensity in the intervention group was | 45 | ⊕⊝⊝⊝ | Acupuncture has no clinical significant beneficial effects on pain intensity compared to sham acupuncture. |
| Participant‐reported pain relief substantial (at least 50% pain relief over baseline) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | Mean 27.7 | Mean 37.7 | The mean bodily pain component of quality of life in the intervention groups was 10 higher | 45 | ⊕⊝⊝⊝ | Acupuncture has no beneficial effects on bodily pain compared to sham acupuncture. |
| CI: confidence interval; MD: mean difference; SF‐36: Short Form (36) Health Survey (SF‐36); VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGarrow 2014 recruited 59 participants initially; there were 14 withdrawals and only the 45 participants that completed treatment were included in the study's final results. | ||||||
| Acupuncture versus treatment as usual for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: treatment as usual | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect (Not applicable) | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participant‐reported pain relief | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| GRADE Working Group grades of evidence | ||||||
| Acupuncture versus other active therapy for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: other active therapy | ||||||
| Outcomes | Sham acupuncture | Acupuncture | Relative effect (Not applicable) | No of Participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participant‐reported pain relief | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| GRADE Working Group grades of evidence | ||||||
| Acupuncture combined with other active therapy versus other active therapy for neuropathic pain in adults | ||||||
| Patient or population: adults with neuropathic pain Comparison: other active therapy alone | ||||||
| Outcomes | Other active therapy | Acupuncture combined with other active therapy | Relative effect | No of participants | Quality of the evidence | Comments |
| Participant‐reported pain intensity | Mean 4.25 | Mean 3.23 | The mean participant‐reported pain intensity in the intervention groups was | 104 | ⊕⊝⊝⊝ | Acupuncture combined other active therapy has no clinical significant beneficial effects on pain intensity compared to other active therapy alone. |
| Participant‐reported pain relief substantial (at least 50% pain relief over baseline) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Participants experiencing any serious adverse event | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome so no evidence to support or refute benefits of intervention. |
| Quality of life | Mean 35.17 | Mean 32.98 | The mean bodily pain component of quality of life in the intervention groups was 2.19lower | 104 | ⊕⊕⊝⊝ | Acupuncture combined other active therapy improved the quality of life compared to other active therapy alone. |
| CI: confidence interval; FACT/the GOG‐Ntx: Functional Assessment of Cancer Therapy/Gynaecologic Oncology Group/Neurotoxicity; MD: mean difference; VAS: Visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded twice for study limitations (risk of bias) due to high risk of performance and detection bias. | ||||||
| Acupuncture points used | Study ID |
| Taixi (KI3); Hegu (LI4); Taichong (LR3); Sanyinjiao (SP6); Zusanli (ST36) | |
| Shenmai (B62); Zulinqi (GB41); Zhaohai (K6); Lieque (L7); Neiguan (P6); Houxi (SI3); Waiguan (SJ5); Gongsun (SP4) | |
| Feishu (BL13); Geshu (BL17); Feiyang (BL58); Zulinqi (GB41); Zhiyang (GV9); Shendao (GV11); Shenzhu (GV12); Dazhui (GV14); Taichong (LR3); Sanyinjiao (SP6); Xuehai (SP10); Tianshu (ST25); Zusanli (ST36); Xiangu (ST43) | |
| The main points: Huantiao (GB30); Yanglingquan (GB34); Sanyinjiao (SP6); Zusanli (ST36); The auxiliary points (selected 2‐3from following): Shenshu (BL23); Kunlun (BL60); Guanyuan (CV4); Qihai (CV6); Huantiao (GB30); Taixi (K3); Taichong (LIV3); Pishu (PL20) | |
| The main points: Ganshu (BL18); Pishu (BL20); Shenshu (BL23); Yishu; Feishu (BL58); Zusanli (ST36); Sanyinjiao (SP6), Taibai (SP3); Zutonggu; Qihai (CV6); Guanyuan (CV4); Fenglong(ST40) and Yanglingquan (GB34); The auxiliary points: Jianyu (LI15); Quchi (LI11); Shousanli (LI10); Hegu (LI4); Biguan (ST31); Futu (ST32); Liangqiu (ST34); Xiangu (ST43) and Neiting (ST 44); Added for blood stasis points: Geshu (BL17) and Xuehai (SP10); Added for severe numbness of the hands and feet points: Bafeng(EX‐LE10) and Baxie (EX‐UE9). |
| Outcomes | Scales | Description of scales | Relevant Studies |
| Participant‐reported pain intensity | Visual Analogue Scale (VAS) | The VAS is a visual analogue scale for pain intensity, in which 0 means no pain and 10 (or 100) means the worst pain ever experienced. | |
| Quality of life | Short Form (36) Health Survey (SF‐36) | The SF‐36 is a 36‐item, patient‐reported survey of patient health and consists of 8 scaled scores, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0‐100 scale on the assumption that each question carries equal weight. The lower the score, the more disability. The 8 sections are: vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning and mental health. Summary scores for the SF‐12, version 2 (SF‐12v2) health status measure are based on scoring coefficients derived for version 1 of the SF‐36. The higher score is better. | |
| Functional Assessment | The FACT/GOG‐Ntx questionnaire is used to investigate patients' daily activities and evaluate the degree of neuropathy. The questionnaire includes 7 questions about physical well‐being, 7 questions about social/family well‐being, 6 questions about emotional well‐being, 7 questions about functional well‐being and 9 questions about additional concerns. Where in each question, 0 = not at all and 4 = very much, lower is better. |
| Acupuncture versus sham acupuncture | ||||||||||
| Outcome | Specific measurement | Study | Manual acupuncture group | Sham acupuncture group | Effect measure | Statistical test | ||||
| Mean | SD | Total | Mean | SD | Total | MD (95%CI) | P value | |||
| Pain intensity | VASa | 5.8 | 2.6 | 24 | 6.2 | 2.3 | 21 | ‐0.40 (‐1.83 to 1.03) | 0.58 | |
| Quality of life | SF‐36b: physical health score | 31.9 | 9.2 | 24 | 32.1 | 9.8 | 21 | ‐0.20 (‐5.78 to 5.38) | 0.94 | |
| SF‐36: mental health score | 39.2 | 14 | 24 | 35.7 | 12.6 | 21 | 3.50 (‐4.17 to 11.27) | 0.38 | ||
| SF‐36: bodily pain score | 37.7 | 27.4 | 24 | 27.7 | 16.9 | 21 | 10.00 (‐3.13 to 23.13) | 0.14 | ||
| Acupuncture + other active therapies versus other active therapies | ||||||||||
| Outcome | Specific measurement | Study | Acupuncture + other active therapies group | Other active therapies group | Effect measure | Statistical test | ||||
| Mean | SD | Total | Mean | SD | Total | MD (95%CI) | P value | |||
| Pain intensity | VAS | 3.23 | 0.17 | 52 | 4.25 | 0.197 | 52 | ‐1.02 (‐1.09 to ‐0.95) | < 0.00001 | |
| Quality of life | FACT/the GOG‐Ntxc | 32.98 | 0.542 | 52 | 35.17 | 0.518 | 52 | ‐2.19 (‐2.39 to ‐1.99) | < 0.00001 | |
| MD: mean difference; SD: standard deviation | ||||||||||
| Acupuncture versus sham acupuncture | ||||||||
| Outcome | Study | Manual acupuncture group | Sham acupuncture group | Effect measure | Statistical test | |||
| Events | Total | Events | Total | RR (95%CI) | NNTB | P value | ||
| Withdraw from trial due to any cause | 4 | 28 | 10 | 31 | 0.44 (0.16 to 1.25) | NNTB = 6 | 0.53 | |
| Adverse events: any cases | 1 | 28 | 2 | 31 | 0.55 (0.05 to 5.78) | NNTB = 34 | 0.62 | |
| Acupuncture + other active therapies versus other active therapies | ||||||||
| Outcome | Study | Acupuncture + other active therapies group | Other active therapies group | Effect measure | Statistical test | |||
| Events | Total | Events | Total | RR (95%CI) | NNT | P value | ||
| Any pain‐related outcomes: no clinical response | 4 | 30 | 10 | 30 | 0.40 (0.14 to 1.14) | NNTB = 5 | 0.09 | |
| Withdraw from trial due to any cause | 3 | 52 | 3 | 52 | 1.00 (0.21 to 4.73) | NA | 1.00 | |
| NA: not applicable; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any pain‐related outcomes: no clinical response ‐ defined by original study Show forest plot | 3 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.51] |


