Drenajes timpánicos (tubos de ventilación) para la otitis media aguda recurrente en niños
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | 3‐arm, non‐blinded (for grommets versus no (ear) surgery comparisons), multicentre, parallel‐group RCT with 2 years of follow‐up | |
| Participants | Location: USA, Children's Hospital of Pittsburgh Otitis Media Center and 2 private paediatric practices in Pittsburgh Setting of recruitment and treatment: secondary and tertiary care Sample size:
Participant (baseline) characteristics:
Inclusion criteria: children aged between 7 months and 35 months with at least 3 AOM episodes in the previous 6 months or more than 4 in the previous 12 months with the most recent episode having occurred in previous 6 months. At time of entry children were required to be free of OME. Exclusion criteria: OME at time of entry, asthma, chronic sinusitis or previous tonsillectomy or adenoidectomy | |
| Interventions | Intervention group: grommets (Teflon® Armstrong type) Comparator group 1: antibiotic prophylaxis; amoxicillin suspension 20 mg/kg/day once daily for 2 years Comparator group 2: placebo medication; liquid suspension of similar appearance and taste to antibiotic prophylaxis for 2 years In case of AOM episodes, amoxicillin 40 mg/kg/day divided into 3 daily doses for 10 days was prescribed and tympanocentesis was performed in the antibiotic prophylaxis and placebo medication groups. If a participant did not improve and if the culture yielded an amoxicillin‐resistant organism, a 10‐day course of erythromycin and sulfisoxazole or alternative antimicrobial drug was prescribed. In case of otorrhoea (through a tympanic membrane perforation or grommets), amoxicillin 40 mg/kg/day and neomycin/polymyxin B/hydrocortisone ear drops were prescribed for 10 days. Use of additional interventions: participants were randomly allocated to antibiotic prophylaxis or placebo medication received a nasopharyngeal and middle ear culture (through tympanocentesis) in case of new AOM or OME episodes | |
| Outcomes | Primary outcome: number of AOM episodes in the 2‐year postoperative period Secondary outcomes: proportion of children without AOM recurrences in the 2‐year postoperative period, proportion of children who had ultimate treatment failure (protocol‐defined criteria for a fourth tympanocentesis within 6 months or a fifth within 12 months; over 180 days with middle ear effusion in the same ear within 12 months; protocol‐defined criteria for a third placement of grommets within 12 months; a suppurative complication; a cholesteatoma; a significant adverse reaction to amoxicillin), persistent tympanic membrane perforation after grommet insertion, bacteriology of middle ear effusions Diagnosis of AOM was based on otoscopic signs (erythema or white opacification, fullness or bulging and decreased mobility of the tympanic membrane), or one or more symptoms (fever, otalgia, irritability) in the presence of middle ear effusions or both. | |
| Funding sources | Funded by a grant from the National Institute of Deafness and Communication Disorders, National Institute of Health. Amoxicillin and placebo medication were supplied by Beecham Laboratories, Bristol, TN | |
| Declarations of interest | No details provided | |
| Notes | Participants lost to follow‐up total: 109/243 (45%) (limited to participants with at least 1 follow‐up visit) Participants lost to follow‐up intervention group: 20/77 (26%); 6 treatment failure, 14 loss to follow‐up Participants lost to follow‐up comparator group 1: 46/86 (53%); 12 treatment failure, 34 loss to follow‐up Participants lost to follow‐up comparator group 2: 43/80 (51%); 11 treatment failure, 32 loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "The combined attrition rates for the amoxicillin, placebo and tympanostomy tube groups at the 6‐, 12‐, 18‐ and 24‐month end points were 21.2%, 28.0%, 35.2% and 38.3%, respectively." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; insufficient information to permit a judgement of low or high risk |
| Other bias | Unclear risk | Baseline characteristics: balanced Intention‐to‐treat analysis: performed Formal sample size calculations were performed Co‐interventions: different across groups |
| Methods | 2‐arm, non‐blinded, single‐centre parallel‐group RCT with 6 months of follow‐up | |
| Participants | Location: Saudi Arabia, ENT unit of King Abdel Azir University Hospital, Riyadh Setting of recruitment and treatment: tertiary care Sample size:
Participant (baseline) characteristics:
Inclusion criteria: children aged below 3 years with at least 3 AOM episodes diagnosed, documented and treated by their referring physician in the 6 months prior to referral. Presence or absence of OME did not preclude inclusion in the study Exclusion criteria: documented immune deficiency or craniofacial abnormalities such as cleft palate, Down's syndrome | |
| Interventions | Intervention group: grommets (type not described) Comparator group: antibiotic prophylaxis; sulfamethoxazole‐trimethoprim (SMZ‐T) 12 mg/kg/day once daily for 6 months Oral antibiotics were administered for individual AOM episodes; cefaclor for 10 days Use of additional interventions: none described | |
| Outcomes | Primary outcome: proportion of children who have no AOM recurrences in the 6‐month postoperative period Secondary outcomes: side effects of medication, number of re‐insertions of grommets (data provided for the treatment group only) Diagnosis of AOM was based on otoscopy findings and the acute onset of otalgia with or without otorrhoea. For those with grommets in place, diagnosis was based upon the presence of otorrhoea. | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | Participants lost to follow‐up total: 15/68 (22%); 7 non‐compliance with medication, 8 loss to follow‐up. Insufficient information to calculate the number of excluded children for the grommets and control groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods not described; 8/64 children (13%) were placed on a predetermined treatment regime on the basis of the parent's concern |
| Allocation concealment (selection bias) | High risk | Methods not described; 8/64 children (13%) were placed on a predetermined treatment regime |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | 15/68 children (22%) not included in final analyses; insufficient information to calculate the number of excluded children for the grommets and control groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; insufficient information to permit a judgement of low or high risk |
| Other bias | Unclear risk | Baseline characteristics: balanced Did not perform intention‐to‐treat analysis: children who were non‐compliant with medication were excluded from analyses Did not perform formal sample size calculations Co‐interventions: similar across groups |
| Methods | 2‐arm, non‐blinded, single‐centre, parallel‐group RCT with 6 months of follow‐up | |
| Participants | Location: USA, general ENT practice Setting of recruitment and treatment: secondary care Sample size:
Participant (baseline) characteristics:
Inclusion criteria: children aged below 3 years with at least 3 AOM episodes diagnosed and treated by their referring physician in the 6 months prior to referral. Presence or absence of OME, by history or physical examination, did not preclude inclusion in the study Exclusion criteria: cleft palate, Down's syndrome, recurrent tonsillitis associated with otitis media | |
| Interventions | Intervention group: grommets (Shepard Teflon®) Comparator group: active monitoring (Topical) antibiotics were administered for individual AOM episodes; ampicillin (or erythromycin plus a sulphonamide in case of ampicillin allergy) for 10 days; if drainage was present, and did not clear with antibiotics, Cortisporin® eardrops were administered Use of additional interventions: decongestant for URTI or nasal congestion | |
| Outcomes | Primary outcome: number of AOM episodes in the 6‐month postoperative period Secondary outcomes: proportion of children without AOM recurrences in the 6‐month postoperative period, grommets‐related adverse effects, number of re‐insertions of grommets (data provided for the treatment group only) Diagnosis of AOM was based on otoscopy findings. For those with grommets in place, diagnosis was based upon the presence of ear discharge in the external ear canal. | |
| Funding sources | This study was supported in part by a grant from the Medical Research Foundation at Riverside Methodist Hospital and in part by NIH Grant NSO 8854 | |
| Declarations of interest | No details provided | |
| Notes | Participants lost to follow‐up total: 13/108 (12%) Participants lost to follow‐up intervention group: 4/58 (7%); inadequate follow‐up in 3 children and parents of 1 child terminated study Participants lost to follow‐up comparator group: 9/50 (18%); inadequate follow‐up in 7 children and parents or the referring physician of 2 children terminated study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 13/108 (12%) children not included in final analyses; 4/58 (7%) in grommets group and 9/50 (18%) control group; reasons for non‐completion are clearly described, but bias due to differential loss to follow‐up cannot be excluded |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; insufficient information to permit a judgement of low or high risk |
| Other bias | Unclear risk | Baseline characteristics: balanced Intention‐to‐treat analysis was performed Did not perform formal sample size calculations Co‐interventions: similar across groups |
| Methods | 3‐arm, non‐blinded (for grommets versus no (ear) surgery comparisons), multicentre, parallel‐group RCT with 6 months of follow‐up | |
| Participants | Location: USA, ENT departments of Army Medical Centres Setting of recruitment and treatment: secondary care Sample size:
Participant (baseline) characteristics:
Inclusion criteria: children aged between 6 months and 10 years with at least 3 AOM episodes in the previous 6 months or more than 4 in the previous 18 months. Presence or absence of OME did not preclude inclusion in the study. Exclusion criteria: cleft palate, Down's syndrome, previous grommets or sulphonamide sensitivity | |
| Interventions | Intervention group: grommets (0.04 mm Paparella design grommet in majority of children) Comparator group 1: antibiotic prophylaxis; sulfisoxazole suspension 500 mg twice daily if under 5 years or 1 g twice daily if 5 years and older for 6 months Comparator group 2: placebo medication; liquid suspension of similar texture and appearance to antibiotic prophylaxis for 6 months Oral antibiotics for 10 days were administered for individual AOM episodes Use of additional interventions: postoperative antibiotic drops were initially used in the grommets group, but were discontinued later in the study Children in the antibiotic prophylaxis or placebo medication groups who had treatment failure (2 or more AOM episodes within 3 months) underwent grommet insertion. Children in the grommets group who had treatment failure were given a course of prophylactic sulfisoxazole. Children with OME that persisted for longer than 3 months underwent grommet insertion (but were not considered treatment failures if rAOM was controlled). | |
| Outcomes | Primary outcome: number of AOM episodes in the 6‐month postoperative period Secondary outcomes: proportion of children without AOM recurrences in the 6‐month postoperative period, proportion of children who had treatment failure (2 or more AOM episodes within 3 months), significant complications (no further details provided). Diagnosis of AOM was defined as the rapid and short onset of signs and symptoms of inflammation in the middle ear using the following criteria: otalgia (ear tugging in the infant), fever, tympanic membrane erythema or bulging, decreased tympanic membrane mobility, loss of tympanic membrane landmarks, otorrhoea. | |
| Funding sources | Sulfisoxazole and placebo medication were supplied by Hoffman‐LaRoche Inc, NJ No further details provided | |
| Declarations of interest | No details provided | |
| Notes | Participants lost to follow‐up total: unknown; the number of randomised children was not reported 19/41 children (46%) in non‐surgical groups underwent grommet insertion during follow‐up because of treatment failure 3/22 children (14%) in the grommets group received sulfisoxazole prophylaxis during follow‐up because of treatment failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "children were then randomized into three groups using a list of random numbers" Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit a judgement of low or high risk since the number of randomised children was not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; insufficient information to permit a judgement of low or high risk |
| Other bias | High risk | Baseline characteristics: balanced Intention‐to‐treat analysis: unknown Did not perform formal sample size calculations Co‐interventions: different across groups |
| Methods | 3‐arm, non‐blinded, single‐centre, parallel‐group RCT with 1 year of follow‐up | |
| Participants | Location: Finland, ENT department of Oulu University Hospital Setting of recruitment and treatment: tertiary care Sample size:
Participant (baseline) characteristics:
Inclusion criteria: children aged between 10 months and 2 years with at least 3 AOM episodes in the previous 6 months and residence within 25 miles of participating hospital. At time of entry children were required to be free of OME. Exclusion criteria: chronic OME, previous grommets or adenoidectomy, cranial abnormalities, documented immunological disorders, ongoing prophylaxis for a disease other than AOM | |
| Interventions | Intervention group: grommets (Donaldson silicon tubes, TympoVent®, Atos) Comparator group 1: active monitoring Comparator group 2: grommets plus adenoidectomy; not relevant for this review (since there is no "adenoidectomy alone" group) and therefore no further details related to this comparator reported AOM episodes were treated according to the Finnish guidelines; primary choice of antibiotics: amoxicillin 40 mg/kg/day for 5 days Use of additional interventions: not described | |
| Outcomes | Primary outcomes: treatment failure (2 AOM episodes in 2 months or 3 in 6 months or middle ear effusion for at least 2 months) and time to intervention failure Secondary outcomes: incidence density of AOM episodes and time to first AOM recurrence. Diagnosis of AOM was defined as presence of acute upper respiratory symptoms together with middle ear inflammation and effusion (bulging and/or decreased mobility of the ear drum, air‐fluid level) detected by pneumatic otoscopy, tympanometry, otomicroscopy or otorrhoea. | |
| Funding sources | Nothing to declare | |
| Declarations of interest | Nothing to declare | |
| Notes | Participants lost to follow‐up total: 20/200 (10%); grommets group: 11/100 (11%), control group: 9/100 (9%), but all randomised children were included in analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence using permutated blocks with a block size of 3 |
| Allocation concealment (selection bias) | Low risk | Treatment allocation as indicated in consecutively numbered, sealed, opaque envelopes, which were opened sequentially only after written informed consent had been received |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Total number of dropouts: 20/200 (10%). Grommets group: 11/100 (11%), control group: 9/100 (9%). All randomised children were included in analyses. |
| Selective reporting (reporting bias) | High risk | Trial protocol available at ClinicalTrials.gov (NCT00162994) Primary outcomes as listed at ClinicalTrials.gov (number of acute otitis media and quality of life issues) differed from those included in manuscript (intervention failure and time to intervention failure). Definition of intervention failure (2 AOM episodes in 2 months or 3 in 6 months, or middle ear effusion for at least 2 months as assessed by one of the team's otolaryngologists) as reported in the manuscript was not prespecified on ClinicalTrials.gov. Some of the secondary outcomes as listed on ClinicalTrials.gov (speed of recovery of each otitis media, number of days with middle ear effusion, number of upper respiratory infections, prevention of otitis media caused by pneumococcus) were not reported. |
| Other bias | Unclear risk | Baseline characteristics: balanced Did perform intention‐to‐treat analysis Did perform formal sample size calculations, but these were not prespecified on ClinicalTrials.gov Co‐interventions: similar across groups |
AOM: acute otitis media
ENT: ear, nose and throat
GP: general practitioner
OME: otitis media with effusion
rAOM: recurrent acute otitis media
RCT: randomised controlled trial
URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| PARTICIPANTS: OME not rAOM | |
| ALLOCATION AND PARTICIPANTS: Not a RCT; rAOM and OME | |
| ALLOCATION: Not a RCT | |
| PARTICIPANTS: OME not rAOM | |
| PARTICIPANTS OME not rAOM | |
| PARTICIPANTS AND INTERVENTION: rAOM and OME; RCT comparing grommets plus adenoidectomy versus grommets alone | |
| PARTICIPANTS: OME not rAOM | |
| PARTICIPANTS AND INTERVENTION: rAOM and OME; RCT with unilateral grommet insertion and in which contralateral ears were randomised to either myringotomy alone or no surgery | |
| PARTICIPANTS: OME not rAOM | |
| PARTICIPANTS: OME not rAOM | |
| INTERVENTION: RCT comparing grommets plus adenoidectomy versus grommets alone | |
| PARTICIPANTS AND INTERVENTION: Not rAOM; no grommets | |
| STUDY TYPE: Not a RCT | |
| PARTICIPANTS AND INTERVENTION: Infants at risk of rAOM; no grommets | |
| COMPARATOR: RCT comparing 4 different types of grommets |
AOM: acute otitis media
OME: otitis media with effusion
rAOM: recurrent acute otitis media
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | "The effect of ventilation tubes on recurrent acute otitis media in children 1‐6 years" |
| Methods | Allocation: randomised Design: parallel, open‐label |
| Participants | Number: 240 Eligibility criteria: children aged 1 to 6 years with rAOM defined as the occurrence of 3 AOM episodes in 6 months or 4 episodes in 12 months Exclusion criteria: previous grommets, previous adenoidectomy or tonsillectomy, plans to move from district within follow‐up time |
| Interventions | Intervention group: grommets (type not described) insertion Comparator group: active monitoring AOM recurrences will be treated with antibiotics |
| Outcomes | Primary outcomes: number of AOM recurrences during 1‐year follow‐up, disease‐specific health‐related quality of life (OM‐6 and OMO‐22) at 3, 6, 9 and 12 months post‐randomisation Secondary outcomes: structural changes in tympanic membrane at 3, 6, 9 and 12 months post‐randomisation, time grommets stay in place, adverse events (chronic otorrhoea, granulation tissue, persistent tympanic membrane perforation) |
| Starting date | Ethical approval obtained on 1 November 2011 Status 24 November 2017 ‐ not yet recruiting |
| Contact information | Peder Aabel, Akershus University Hospital ‐ [email protected] Magnus von Unge, Akershus University Hospital ‐ [email protected] |
| Notes | ACTRN12611000380998 https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336693 Sponsor: Akershus University Hospital Principal investigators: Peder Aabel and Magnus von Unge, Akershus University Hospital, Norway |
| Trial name or title | Efficacy of tympanostomy tubes for children with recurrent acute otitis media |
| Methods | Allocation: randomised Design: parallel, open‐label |
| Participants | Number: 240 Eligibility criteria: children aged 6 to 35 months with rAOM defined as the occurrence of 3 AOM episodes in 6 months or 4 episodes in 12 months with at least 1 episode in the preceding 6 months, and 2 of these AOM episodes have been documented by trained study personnel Exclusion criteria: previous grommets, chronic illness (cystic fibrosis, neoplasm, juvenile diabetes, renal or hepatic insufficiency, immune dysfunction, malabsorption, inflammatory bowel disease, severe asthma requiring at least 4 courses of oral corticosteroids during the last 12 months), allergy to amoxicillin, congenital anomaly (cleft palate, Down's syndrome), OME for at least 3 months in addition to rAOM, sensorineural hearing loss |
| Interventions | Intervention group: grommets (Teflon® Armstrong‐type) insertion Comparator group: active monitoring AOM recurrences will be treated with antibiotic eardrops in the grommets group and with oral antibiotics in the active monitoring group |
| Outcomes | Primary outcome: average number of AOM recurrences during the 2‐year follow‐up period Secondary outcomes: severity of AOM recurrences, frequency distribution of AOM recurrences during the 2‐year follow‐up period, time to first AOM recurrence, type of AOM recurrences, antibiotic consumption, adverse events (protocol defined diarrhoea, diaper dermatitis, chronic otorrhoea), antibiotic resistance of nasopharyngeal pathogens, cost‐effectiveness |
| Starting date | November 2015 (estimated completion date February 2021) |
| Contact information | Diana Kearney, RN, CCRC ‐ [email protected] Jennifer Nagg, RN ‐ [email protected] |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02567825 Sponsor and collaborators: University of Pittsburgh, George Washington University, National Institute on Deafness and Other Communication Disorders (NIDCD) Principal investigators: Alejandro Hoberman, MD ‐ University of Pittsburgh School of Medicine; Children's Hospital of Pittsburgh of UPMC; Diego Preciado, MD, PhD ‐ George Washington University; Childrens National Medical Center |
| Trial name or title | SIUTIT Trial |
| Methods | Allocation: randomised Design: parallel, single‐blind (outcome assessor blinded) |
| Participants | Number: 230 Eligibility criteria: children aged 9 to 36 months with at least one Greenland‐born parent, B‐ or C2‐type curve tympanogram at 2 visits 3 to 4 months apart or 3 episodes of AOM in 6 months or 4 in 12 months, American Society of Anaesthesiologists physical status classification class 1 and 2 Exclusion criteria: orofacial cleft, Down's syndrome or known generalised immune deficiency, American Society of Anaesthesiologists physical status classification class > 2 |
| Interventions | Intervention group: grommets (Donaldson‐type) insertion Comparator group: active monitoring AOM recurrences will be treated according to current practice in Greenland, which includes systemic antibiotic treatment as well as aural toilet and topical antibiotics. Grommet insertion during the study period is not accepted in the control group. |
| Outcomes | Primary outcome: number of visits to health clinic during the 2‐year follow‐up period (assessed by investigating medical records) Secondary outcomes: number of AOM episodes during the 2‐year follow‐up period (assessed by investigating medical records); disease‐specific quality of life at baseline, 3 months, 1 year and 2 years follow‐up (assessed by OM‐6 and Caregiver Impact Questionnaires); number of episodes where oral or intravenous antibiotics have been administered during the 2‐year follow‐up period (assessed by investigating medical records); proportion of children with uni‐ or bilateral tympanic membrane perforations at 2 years (based on otoscopic images, which will be anonymised and evaluated by an ENT specialist without knowledge of the intervention), number of ear discharge episodes during the 2‐year follow‐up period (assessed by investigating medical records); serious adverse events |
| Starting date | February 2016 (estimated completion date August 2020) |
| Contact information | Malene N Demant, MD ‐ [email protected] |
| Notes | https://clinicaltrials.gov/ct2/show/NCT02490332 Sponsor and collaborators: Zealand University Hospital; Government of Greenland, Agency for Health and Prevention; Copenhagen Trial Unit, Center for Clinical Intervention Research Principal investigator: Malene N Demant, MD ‐ Køge University Hospital Study director: Preben Homoe, MD PhD ‐ Køge University Hospital |
AOM: acute otitis media
OME: otitis media with effusion
OM‐6: Otitis Media‐6
Otitis Media Outcome‐22
rAOM: recurrent acute otitis media
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [2.38, 37.80] |
| Analysis 1.1 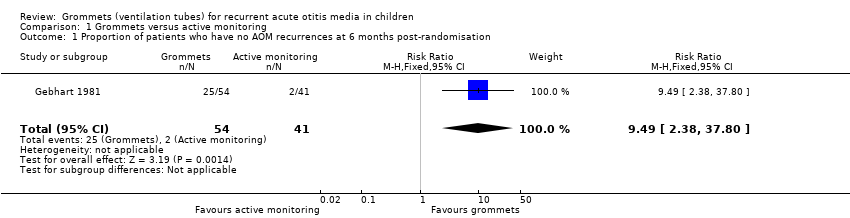 Comparison 1 Grommets versus active monitoring, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation. | ||||
| 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.00, 1.99] |
| Analysis 1.2 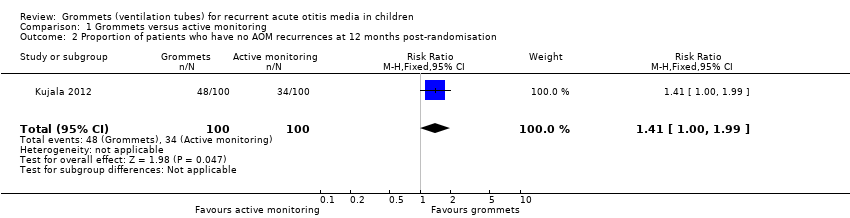 Comparison 1 Grommets versus active monitoring, Outcome 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation. | ||||
| 3 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.99, ‐1.01] |
| Analysis 1.3 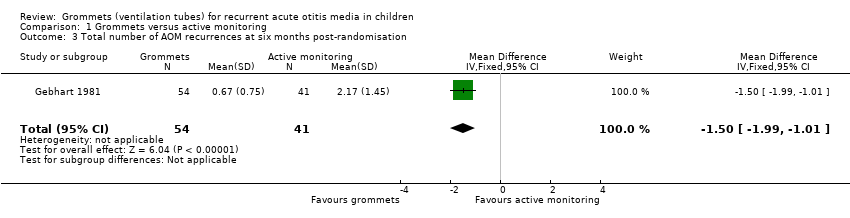 Comparison 1 Grommets versus active monitoring, Outcome 3 Total number of AOM recurrences at six months post‐randomisation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.07, 2.65] |
| Analysis 2.1  Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation. | ||||
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.37, 0.33] |
| Analysis 2.2  Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 2 Total number of AOM recurrences at six months post‐randomisation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.20, 11.04] |
| Analysis 3.1  Comparison 3 Grommets versus placebo medication, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation. | ||||
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐2.06, ‐0.22] |
| Analysis 3.2  Comparison 3 Grommets versus placebo medication, Outcome 2 Total number of AOM recurrences at six months post‐randomisation. | ||||

PRISMA flow diagram of search history.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
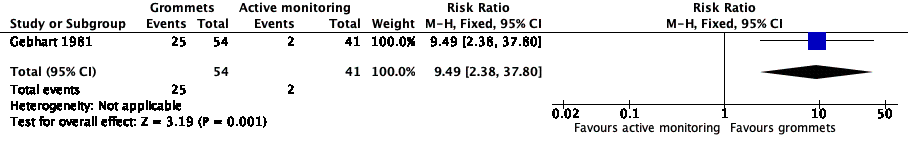
Forest plot of comparison: 1 Grommets versus active monitoring, outcome: 1.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Forest plot of comparison: 2 Grommets versus antibiotic prophylaxis, outcome: 2.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.
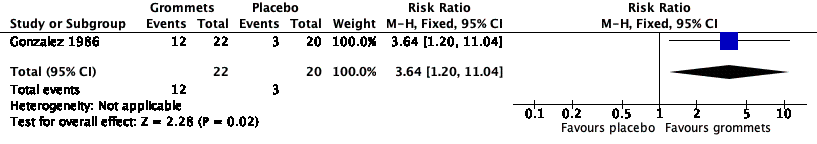
Forest plot of comparison: 3 Grommets versus placebo medication, outcome: 3.1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Comparison 1 Grommets versus active monitoring, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Comparison 1 Grommets versus active monitoring, Outcome 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation.

Comparison 1 Grommets versus active monitoring, Outcome 3 Total number of AOM recurrences at six months post‐randomisation.

Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Comparison 2 Grommets versus antibiotic prophylaxis, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.

Comparison 3 Grommets versus placebo medication, Outcome 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation.

Comparison 3 Grommets versus placebo medication, Outcome 2 Total number of AOM recurrences at six months post‐randomisation.
| Grommets versus active monitoring for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with active monitoring | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 9.49 | 95 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (463‐49)* 1000 = 2.41 | |
| 49 per 1000 | 463 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 0 (0/54) | n/a | 54 (1 RCT) | ⊕⊕⊝⊝ | — |
| Proportion of patients who have no AOM recurrences at 12 months post‐randomisation | Study population | RR 1.41 | 200 | ⊕⊕⊝⊝ | The NNTB based on the study population risk was 1/ (479‐340)* 1000 = 7.19 | |
| 340 per 1000 | 479 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 89 AOM recurrences in 41 children; mean number of AOM recurrences per child: 2.17 | 36 AOM recurrences in 54 children; mean number of AOM recurrences per child: 0.67 | MD ‐1.50, 95% CI ‐1.99 to ‐1.01 | 95 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 12 months post‐randomisation | 119 AOM recurrences in 100 children; incidence rate 1.70 | 92 AOM recurrences in 100 children; incidence rate 1.15 | Incidence rate difference ‐0.55, 95% ‐0.17 to ‐0.93 | 200 | ⊕⊕⊝⊝ | — |
| Disease‐specific health‐related quality of life of the child at 4 and 12 months post‐randomisation using the OM‐6 questionnaire | "no statistically significant differences between treatment groups were reported at 4 and 12 months for any of the six subdomains of the OM‐6 questionnaire" | 85 and 81, respectively (1 RCT) | ⊕⊕⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Grommets versus antibiotic prophylaxis for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with antibiotic prophylaxis | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 1.68 | 96 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (586‐349)* 1000 = 4.22 | |
| 349 per 1000 | 586 per 1000 | |||||
| Total number of AOM recurrences at 6 months post‐randomisation | 29 AOM recurrences in 21 children; mean number of AOM recurrences per child: 1.38 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐0.52, 95% CI ‐1.37 to 0.33 | 43 (1 RCT) | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations (when we excluded the trial with high risk of bias from the analysis, no statistically significant difference was observed between groups) and imprecise effect estimates (only two studies with small sample sizes). 2We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). | ||||||
| Grommets versus placebo medication for recurrent acute otitis media in children | ||||||
| Patients: children with recurrent acute otitis media | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo medication | Risk with grommets | |||||
| Proportion of patients who have no AOM recurrences at 6 months post‐randomisation | Study population | RR 3.64 | 42 | ⊕⊝⊝⊝ | The NNTB based on the study population risk was 1/ (546‐150)* 1000 = 2.53 | |
| 150 per 1000 | 546 per 1000 | |||||
| Significant adverse effect: a tympanic membrane perforation persisting for 3 months or longer | — | 4% (3/76) | n/a | 76 (1 RCT) | ⊕⊕⊝⊝ | — |
| Total number of AOM recurrences at 6 months post‐randomisation | 40 AOM recurrences in 20 children; mean number of AOM recurrences per child: 2.0 | 19 AOM recurrences in 22 children; mean number of AOM recurrences per child: 0.86 | MD ‐1.14, 95% CI ‐2.06 to ‐0.22 | 42 | ⊕⊝⊝⊝ | — |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence from high to very low quality due to study limitations and imprecise effect estimates (only one study with a very small sample size). 2We downgraded the evidence from high to low quality due to study limitations and imprecise effect estimates (only one study with a small sample size). | ||||||
| Study ID | Grommets | Grommets plus adenoidectomy | Active monitoring | Placebo medication | Antibiotic prophylaxis | Adenoidectomy |
| x | x | x | ||||
| x | x | |||||
| x | x | |||||
| x | x | x | ||||
| x | x | x | ||||
| Comparison pairs for this review | ||||||
| # | Intervention | Comparator | Number of trials | Study ID | ||
| 1 | Grommets | Active monitoring | 2 | |||
| 2 | Grommets | Antibiotic prophylaxis | 3 | |||
| 3 | Grommets | Placebo medication | 2 | |||
| Outcomes | |||||
| Primary outcomes | |||||
| Proportion of children who have no AOM recurrences at 3 to 6 months post‐randomisation | x | x | x | ||
| Significant adverse effect: tympanic membrane perforation persisting for 3 months or longer | x | x | |||
| Secondary outcomes | |||||
| Proportion of children who have no AOM recurrences at 6 to 12 months post‐randomisation | x | ||||
| Total number of AOM recurrences | |||||
| < 3 months | |||||
| 3 to 6 months | x | x | |||
| 6 to 12 months | x | ||||
| Disease‐specific health‐related quality of life | |||||
| < 3 months | |||||
| 3 to 6 months | x | ||||
| 6 to 12 months | x | ||||
| Generic health‐related quality of life of the child and parent | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Presence of middle ear effusion | |||||
| < 3 months | |||||
| 3 to 6 months | |||||
| 6 to 12 months | |||||
| Other adverse effects: ventilation tube misplaced in middle ear, otorrhoea within 1 week of ventilation tube placement, myringosclerosis | x | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [2.38, 37.80] |
| 2 Proportion of patients who have no AOM recurrences at 12 months post‐randomisation Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.00, 1.99] |
| 3 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.99, ‐1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.07, 2.65] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.37, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients who have no AOM recurrences at 6 months post‐randomisation Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.20, 11.04] |
| 2 Total number of AOM recurrences at six months post‐randomisation Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐2.06, ‐0.22] |

