Diferentes tipos de insulina y regímenes para pacientes embarazadas con diabetes preexistente
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
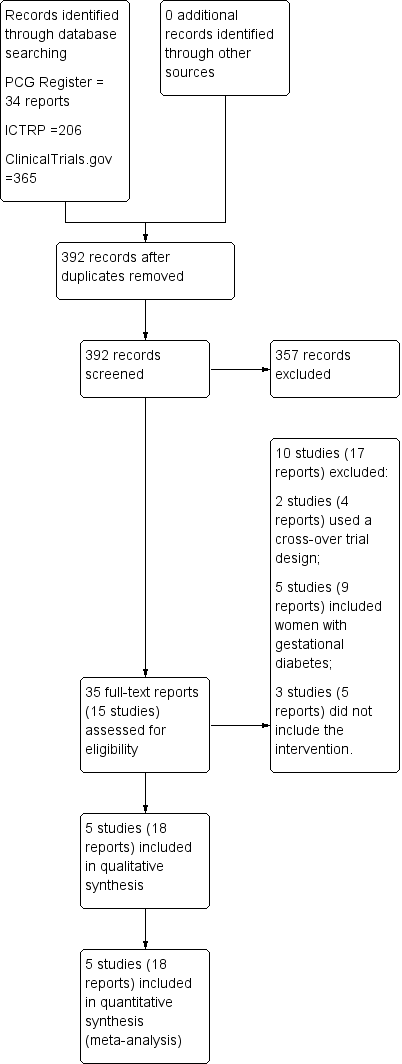
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT (open‐label, 2‐centre, 2‐arm). | |
| Participants | 43 insulin‐requiring pregnant women with diabetes (type 1 or 2). Recruited between 1983 and 1985. Setting: The Children's Hospital of San Francisco and Cornell University Medical College, New York. Inclusion criteria: pregnant women with type 1 or 2 diabetes; < 20 weeks' gestation; aged > 18 years old; treated with animal insulin for at least 24 months; bodyweight within 20% of ideal body weight as determined by the Metropolitan Life tables. Exclusion criteria: women with hypertension (blood pressure > 140/90 mmHg); serum creatine higher than the upper range of normal; advanced cardiovascular disease; history of Addison's disease or pituitary insufficiency; local or systemic allergy to animal source insulin; pre‐pregnancy insulin dose greater than 1.5 U/kg per 24 hours, history of treatment human insulin or an insulin infusion device. | |
| Interventions | Human insulin (recombinant deoxyribonucleic acid ‐ Humulin); N = 20. | |
| Outcomes | Infant Gestational age at delivery Percentile body weight Skinfold thickness Length Weight Head circumference Large‐for‐gestational age at delivery Small‐for‐gestational age at delivery C‐peptide level (pmol/mL) Glucose level (mmol) Preterm delivery Appropriate‐for‐gestational age at delivery Macrosomia (birthweight > 4000 g) Maternal Temperature Systolic blood pressure Diastolic blood pressure Resting heart rate Edema Renal function Complete blood cell count Chemistry profile Calories consumed Weight gain Glycohemoglobin levels Maternal ketonuria Mean insulin dose requirement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned to treatment with either human or their current animal insulin. However, there was no description of the method used. |
| Allocation concealment (selection bias) | High risk | Open‐label trial. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no. Open‐label trial. |
| Blinding of participants and personnel (performance bias) | High risk | Personnel: no. Open‐label trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 woman (out of 23) randomised to the animal insulin group did not complete the admission visit or return for follow‐up. She was excluded from the statistical analysis. Not all babies were included in the reporting of large‐for‐gestational age at delivery. |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from the published report. No protocol was available, so we do not know if all pre‐specified outcomes were reported. |
| Other bias | Low risk | It was reported that the baseline characteristics of groups showed a remarkably similar population of women in both groups. |
| Methods | 2 arm RCT (open‐label, parallel group, multi‐centre). | |
| Participants | Setting: 63 sites in 18 countries, mainly within Europe. Inclusion criteria: women ≥ 18 years with insulin‐treated type 1 diabetes for ≥ 12 months. Women were either pregnant with a singleton pregnancy (gestational age at delivery ≤ 10 weeks; N = 223, included in this review), or planning to become pregnant (N = 99, excluded from this review). A1c was ≤ 8% at confirmation of pregnancy. Exclusion criteria: women with multiple pregnancy, fertility treatment, clinically significant gynaecological conditions, diabetic nephropathy or medical problems, a previous child born with major congenital malformations, multiple miscarriage, or stillbirths (more than 2). Women not pregnant within 12 months of randomisation. | |
| Interventions | Experimental: prandial insulin Aspart (100 units/mL: Novo Nordisk, Basvaerd, Denmark) + NPH insulin. 1 to 4 subcutaneous injections per day (lowest available at centre) using the Novo pen. N = 113 (randomised when pregnant). Comparison: prandial human insulin (100 IU/mL; Novo Nordisk) + NPH insulin. 1 to 4 subcutaneous injections per day (lowest available at centre) using the Novo pen. N = 110 (randomised when pregnant). | |
| Outcomes | Many outcomes were reported for all women in the study: major hypoglycaemia requiring third‐party assistance, minor hypoglycaemia, maternal death, hypoglycaemic coma, inadequate glycaemic control, hyperglycaemia, pre‐eclampsia, preterm labour, emergency caesarean section, glycaemic control, A1c, plasma glucose profile breakfast, lunch, dinner, mean insulin dose), abortion, caesarean section, induced abortion, stillbirth, QoL assessments. However, few of these were reported separately for women randomised during pregnancy. | |
| Notes | SON contacted the authors to request additional data. A web site link was received, but did not allow access to the data. We received no responses to further requests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that women were 'randomised', but no further description of method was given. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no. Open‐label trial. |
| Blinding of participants and personnel (performance bias) | High risk | Personnel: no. Open‐label trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) | Unclear risk | All women in the subgroup included in the review were accounted for. Women who were not pregnant ≤ 12 months after randomisation were withdrawn from the study: potential bias in conception rates between groups affected the overall study, but not the subgroup included in the review. |
| Selective reporting (reporting bias) | High risk | Very few outcomes were reported for the subgroup of women who were randomised during pregnancy. |
| Other bias | Unclear risk | The report declared that the trial was sponsored by Novo Nordisk. It was unclear whether this conflict of interest introduced any bias. |
| Methods | 2‐arm RCT, open label, parallel group, multi‐centre. | |
| Participants | Setting: 79 different sites in 17 countries. Inclusion criteria: Women ≥ 18 years with insulin‐treated type 1 diabetes for ≥ 12 months before randomisation. They were either pregnant with a singleton pregnancy (gestational age at delivery 8 to 12 weeks; N = 162, included in this review), or planning to become pregnant (N = 148, excluded from this review). A1c was ≤ 8% at confirmation of pregnancy. Exclusion criteria: women with impaired hepatic or renal function or uncontrolled hypertension (systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or both), undergoing medical infertility treatment, or who had been previously randomised in this trial. Women not pregnant within 12 months of randomisation. | |
| Interventions | Experimental intervention: Insulin Detemir (100 units/mL) with prandial insulin Aspart (100 units/mL) in a basal bolus regimen (1:1), subcutaneous injections administered from randomisation until termination or 6 weeks postdelivery. N = 79 (randomised when pregnant). Control/Comparison intervention: NPH insulin (100 IU/mL) with prandial insulin Aspart (100 units/mL) in a basal bolus regimen (1:1), subcutaneous injections administered from randomisation until termination or 6 weeks postdelivery. N = 83 (randomised when pregnant). Basal insulin dose was titrated according to fasting or pre‐dinner capillary plasma glucose values. All bolus insulin doses were titrated according to pre‐ and postprandial plasma glucose values. Preprandial PG target of 72 to 108 mg/dL (4.0‐6.0 mmol/L) and 2‐hour postprandial glucose target < 126 mg/dL (< 7.0 mmol/L). | |
| Outcomes | Many outcomes were reported for all women in the study: hypoglycaemia, glycaemic control including A1c, insulin dose, adverse events, pregnancy outcomes. However, few of these were reported separately for women randomised during pregnancy. | |
| Notes | SON contacted the authors to request additional data. A web site link was received, but did not allow access to the data. We received no responses to further requests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | “Subjects were randomised 1:1 (using Interactive Voice/Web Response System)." |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no. Open‐label trial. |
| Blinding of participants and personnel (performance bias) | High risk | .Personnel: no. Open‐label trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Open‐label. Congenital malformations were assessed by 2 independent experts, 1 of whom was blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | All women in the subgroup included in the review were accounted for. Women who were not pregnant ≤ 12 months after randomisation were withdrawn from the study: potential bias in conception rates between groups affected the overall study, but not the subgroup included in the review. |
| Selective reporting (reporting bias) | High risk | Very few outcomes were reported for the subgroup of women who were randomised during pregnancy. |
| Other bias | Unclear risk | The report declared that the trial was sponsored by Novo Nordisk. It was unclear whether this conflict of interest introduced any bias. |
| Methods | RCT (open‐label, multi‐centre, 2‐arm). | |
| Participants | 33 pregnant women with type 1 diabetes recruited at 6 to 8 weeks' gestation and entered into the study at 15 weeks' gestation. Setting: 4 centres in Sweden. The Departments of Obstetrics and Gynaecology in Huddinge Hospital, Karolinska Hospital, Södersjukhuset in Stockholm, and Örebro Regional Hospital. Inclusion criteria: pregnant women with type 1 diabetes; duration of diabetes for a minimum of 2 years; aged 20 years or more; multiple dose regimen with regular and NPH insulin; Initial HbA1c value below 9%. Exclusion criteria: gestational or type 2 diabetes; duration of diabetes less than 2 years; aged younger than 20 years; In receipt of insulin lispro (intervention); HbA1c value greater than 9%. | |
| Interventions | Preprandial rapid‐acting insulin lispro (Humalog®) in combination with NPH in a MDI regimen with administration of lispro or regular insulin immediately before or 30 minutes before meals, respectively. Medium‐acting NPH insulin was administered at bedtime and when needed before breakfast. All women were given dietary instruction by a dietician. Blood glucose targets were pre‐ and postprandial levels of < 5.0 and < 6.5 mmol/l respectively. N = 16. | |
| Outcomes | Infant Gestational age at delivery Birthweight Length Appropriate‐for‐gestational age at delivery Small‐for‐gestational age at delivery Large‐for‐gestational age at delivery Malformation Birth trauma Asphyxia Respiratory distress Hypoglycaemia Hyperbilirubinemia Perinatal death Maternal Micoangiopathy Glycaemic control (HbA1c, blood glucose, hypoglycaemia) Retinopathy Mode of delivery Hypertension Pre‐eclampsia Polyhydamniosis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted at a central site according to 4‐patient block model (AABB, etc.). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no. Open‐label trial. |
| Blinding of participants and personnel (performance bias) | High risk | Personnel: no. Open‐label trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | The report indicated that 7 women did not satisfy the inclusion criteria and 2 were unwilling to participate. These appeared to be in addition to those randomised, but it was unclear if these women were randomised and then withdrawn from the study. |
| Selective reporting (reporting bias) | High risk | The trial was assessed from the published report, with no protocol available. It was not clear whether all prespecified outcomes were reported. Some outcomes were described as showing no differences, but these figures were not given: gestational age at delivery, birthweight, rate of large‐for‐gestational‐age infants, neonatal complications. |
| Other bias | Unclear risk | It was reported that there were no significant differences between the 2 treatment groups with regard to baseline characteristics, however, significantly more women in the lispro group had aneurysms. |
| Methods | RCT (single‐blinded, 1 centre, 4‐arm). | |
| Participants | 93 pregnant women with type 1 or 2 diabetes. Setting: University of Mississippi Medical Centre, USA. Inclusion criteria: insulin‐dependent diabetes; maternal age 15 to 44 years; < 20 weeks' gestation at entry; willingness to sign an informed consent form. Exclusion criteria: additional pregnancy complications which might affect maternal or infant outcome (hypertension, placenta praevia, fetal malformations, and glucose intolerance not requiring insulin); unwillingness to comply with prenatal care or aggressive glucose control; women's refusal to participate. | |
| Interventions | Women were enrolled into 4 groups. Intervention 1: pre‐mixed insulin (70 NPH/30 REG) administered with a needle or syringe (N = 24). Intervention 2: self‐mixed split dose regular and NPH insulin administered with a Novolin® pen (N = 22). Intervention 3: pre‐mixed insulin (70 NPH/30 REG) administered with a Novolin® pen (N = 23). Control: self‐mixed split dose regular and NPH insulin administered with a needle or syringe (N = 24). | |
| Outcomes | Infant Gestational age at delivery Preterm delivery Infant birthweight Macrosomia 1‐ and 5‐minute Apgar score Hyperbilirubinemia Hypoglycaemia Hypocalcemia Incidence of admission to the neonatal unit Maternal Caesarean delivery for cephalo‐pelvic disproportion Pregnancy‐induced hypertension Capillary glucose measurements (mg/DL) Abruption Chorioamnionitis Endometritis Maternal hospital days Number of prenatal visits Overall patient compliance (based on dietary assessment, adequate glucose monitoring, insulin usage, appropriate follow‐up with physician instructions, and visits) scored from 1 to 5, with 1 implying good compliance and 5 implying poor compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by selecting an opaque, consecutively‐numbered envelope in which computer‐generated randomisation cards were placed, to assign women into 1 of 4 groups. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by selecting an opaque, consecutively‐numbered envelope in which computer‐generated randomisation cards were placed, to assign women into 1 of 4 groups. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: no. Open‐label trial. |
| Blinding of participants and personnel (performance bias) | Low risk | Personnel: yes. Staff managing the women were unaware of the treatment regimen to which the women were assigned, during the antepartum period. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 100 women enrolled, 93 were available for outcome analysis. 2 women suffered spontaneous abortions, 2 underwent elective terminations and 3 were lost to follow‐up. These 7 women were equally distributed between the 4 groups. |
| Selective reporting (reporting bias) | Unclear risk | This trial was assessed from the published report, with no protocol available. It was not clear whether all prespecified outcomes were reported. Some outcomes were described as showing no differences, but the figures were not given: caesarean section for any indication, incidence of pregnancy‐induced hypertension, preterm labour, infant hyperbilirubinaemia, and hypoglycaemia. It was unclear whether caesarean section for cephalo‐pelvic disproportion was a pre‐specified outcome, or included because it showed a significant difference between groups. |
| Other bias | Low risk | There were no significant differences between the treatment groups with regard to baseline characteristics. |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Cross‐over trial design. | |
| Included women with gestational diabetes. | |
| Included women with gestational diabetes. | |
| Included women with gestational diabetes. | |
| Cross‐over trial design. | |
| Included women with gestational diabetes. | |
| Included women with gestational diabetes. | |
| Did not include the intervention: randomisation prior to pregnancy. | |
| Did not include the intervention: not a randomised controlled trial. | |
| Did not include the intervention: a trial of glucose monitoring not insulin regimen. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.1  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 1 Perinatal death. | ||||
| 2 Caesarean section Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.39] |
| Analysis 1.2 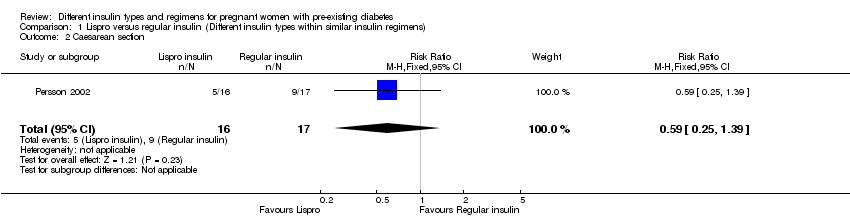 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 2 Caesarean section. | ||||
| 3 Pregnancy‐induced hypertension and pre‐eclampsia Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.30] |
| Analysis 1.3  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 3 Pregnancy‐induced hypertension and pre‐eclampsia. | ||||
| 4 Fetal anomaly Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.08] |
| Analysis 1.4  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 4 Fetal anomaly. | ||||
| 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.5  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture. | ||||
| 6 Vaginal delivery (spontaneous, ventouse, forceps) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.80, 2.67] |
| Analysis 1.6  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 6 Vaginal delivery (spontaneous, ventouse, forceps). | ||||
| 7 Blood glucose (mmol/L) week 14 (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐3.60, 1.42] |
| Analysis 1.7 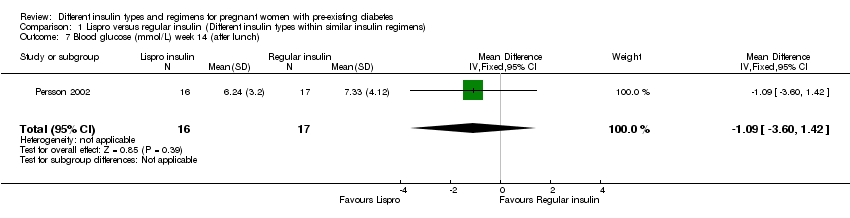 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 7 Blood glucose (mmol/L) week 14 (after lunch). | ||||
| 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐2.10, 2.02] |
| Analysis 1.8 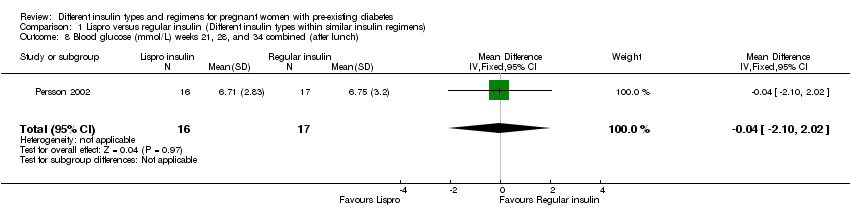 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch). | ||||
| 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.52, 3.52] |
| Analysis 1.9 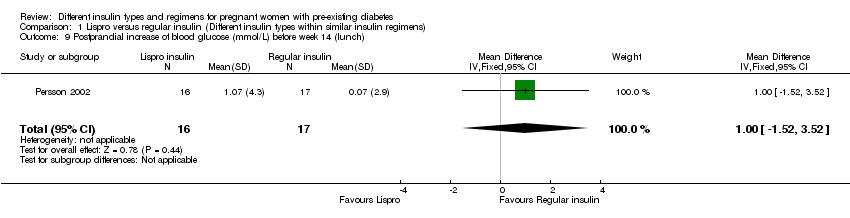 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch). | ||||
| 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.12, 2.32] |
| Analysis 1.10 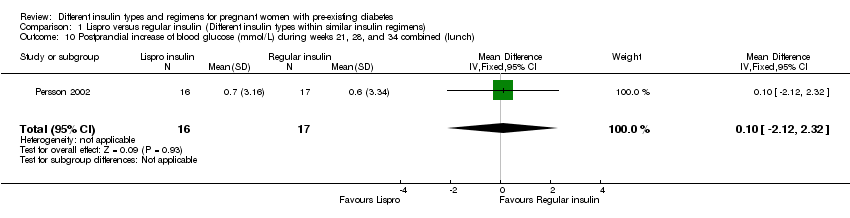 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch). | ||||
| 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| Analysis 1.11  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention. | ||||
| 12 Retinopathy Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.17, 6.67] |
| Analysis 1.12 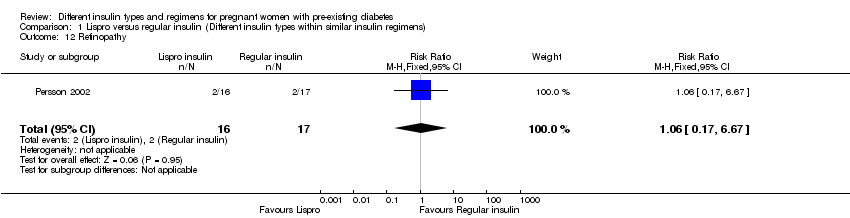 Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 12 Retinopathy. | ||||
| 13 Ventouse delivery Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.37, 27.58] |
| Analysis 1.13  Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 13 Ventouse delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Small‐for‐gestational age at delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.1 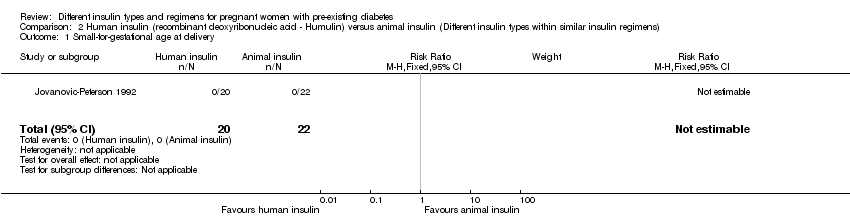 Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 1 Small‐for‐gestational age at delivery. | ||||
| 2 Preterm birth (< 37 weeks) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [0.42, 139.83] |
| Analysis 2.2  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 2 Preterm birth (< 37 weeks). | ||||
| 3 Birthweight centile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.64, 10.24] |
| Analysis 2.3  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 3 Birthweight centile (%). | ||||
| 4 Infant length (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.74, 0.14] |
| Analysis 2.4  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 4 Infant length (cm). | ||||
| 5 Skinfold thickness (mm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.10 [‐13.28, 5.08] |
| Analysis 2.5  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 5 Skinfold thickness (mm). | ||||
| 6 Body weight percentile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.74, 10.34] |
| Analysis 2.6  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 6 Body weight percentile (%). | ||||
| 7 Head circumference (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.52, ‐0.68] |
| Analysis 2.7  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 7 Head circumference (cm). | ||||
| 8 Macrosomia Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| Analysis 2.8 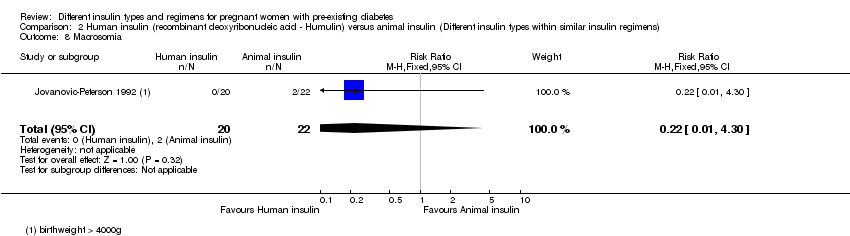 Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 8 Macrosomia. | ||||
| 9 Insulin requirement during pregnancy (U/kg/24 hour) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.45, ‐0.21] |
| Analysis 2.9  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 9 Insulin requirement during pregnancy (U/kg/24 hour). | ||||
| 10 Birthweight (g) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐591.0 [‐1066.27, ‐115.73] |
| Analysis 2.10 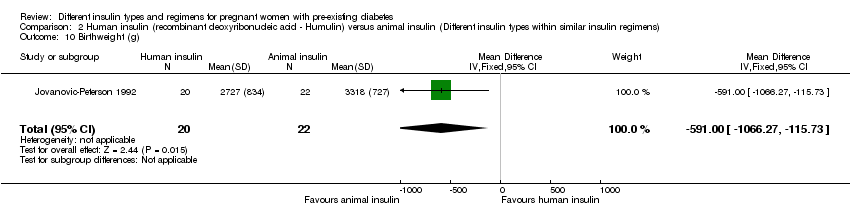 Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 10 Birthweight (g). | ||||
| 11 Infant fasting C‐peptide level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| Analysis 2.11  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 11 Infant fasting C‐peptide level at 3 months (pmol/mL). | ||||
| 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.03] |
| Analysis 2.12  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL). | ||||
| 13 Infant glucose fasting level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.62, 0.22] |
| Analysis 2.13  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 13 Infant glucose fasting level at 3 months (pmol/mL). | ||||
| 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.04, 1.04] |
| Analysis 2.14  Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL). | ||||
| 15 Gestational age at delivery (weeks) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐3.70, 4.70] |
| Analysis 2.15 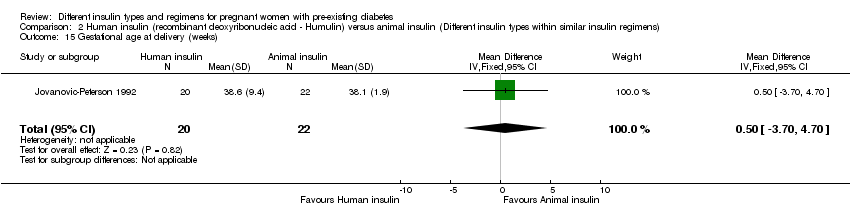 Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 15 Gestational age at delivery (weeks). | ||||
| 16 Maternal ketonuria Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.08, 1.61] |
| Analysis 2.16 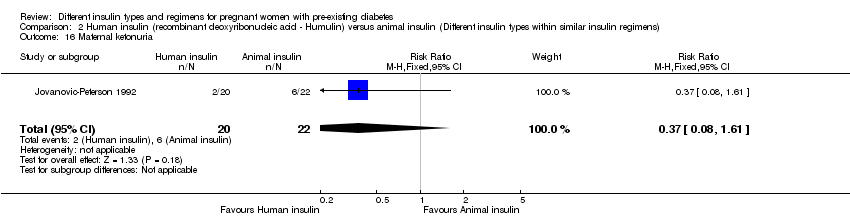 Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 16 Maternal ketonuria. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.54] |
| Analysis 3.1  Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia. | ||||
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.32] |
| Analysis 3.2 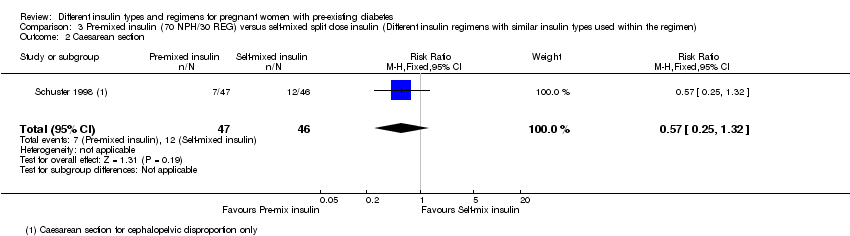 Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section. | ||||
| 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐11.25 [‐12.55, ‐9.95] |
| Analysis 3.3  Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch). | ||||
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| Analysis 3.4 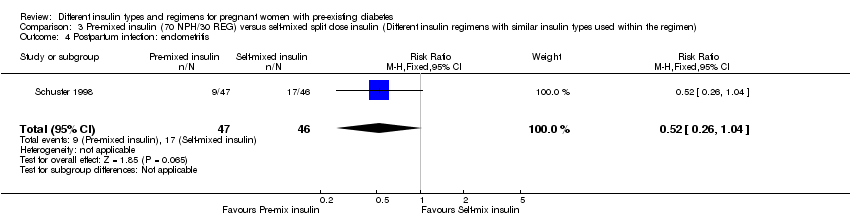 Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis. | ||||
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.40, 0.41] |
| Analysis 3.5  Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days). | ||||
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐116.56 [‐391.81, 158.69] |
| Analysis 3.6 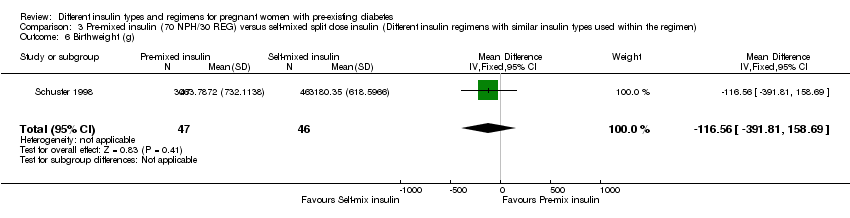 Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g). | ||||
| 7 Compliance score Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.87, 0.87] |
| Analysis 3.7  Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.76] |
| Analysis 4.1 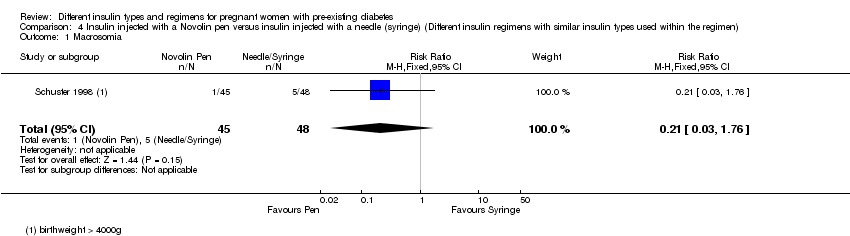 Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia. | ||||
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.97] |
| Analysis 4.2  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section. | ||||
| 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐7.23 [‐8.51, ‐5.95] |
| Analysis 4.3  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch). | ||||
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.28, 1.14] |
| Analysis 4.4  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis. | ||||
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.45, 0.33] |
| Analysis 4.5  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days). | ||||
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐162.36 [‐438.25, 113.53] |
| Analysis 4.6  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g). | ||||
| 7 Compliance score Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| Analysis 4.7  Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A1c (%) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| Analysis 5.1 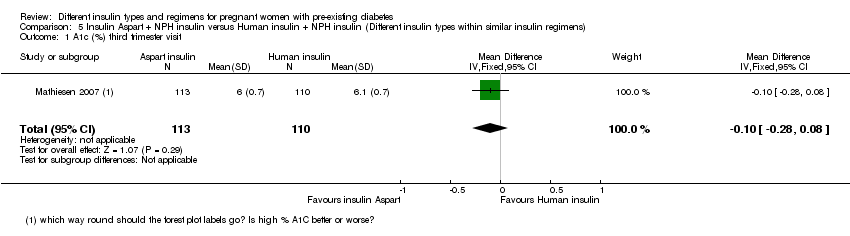 Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 1 A1c (%) third trimester visit. | ||||
| 2 Average plasma glucose (mmol/L) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.53, 0.13] |
| Analysis 5.2  Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 2 Average plasma glucose (mmol/L) third trimester visit. | ||||
| 3 Maternal hypoglycaemic episodes Show forest plot | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| Analysis 5.3  Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 3 Maternal hypoglycaemic episodes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.33, 29.67] |
| Analysis 6.1 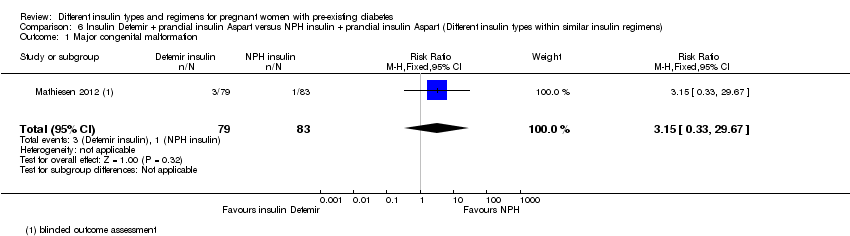 Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 1 Major congenital malformation. | ||||
| 2 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| Analysis 6.2  Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 2 Major congenital malformation. | ||||
| 3 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| Analysis 6.3  Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 3 Minor congenital malformation. | ||||
| 4 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 5.05] |
| Analysis 6.4  Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 4 Minor congenital malformation. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 1 Perinatal death.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 2 Caesarean section.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 3 Pregnancy‐induced hypertension and pre‐eclampsia.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 4 Fetal anomaly.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 6 Vaginal delivery (spontaneous, ventouse, forceps).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 7 Blood glucose (mmol/L) week 14 (after lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch).

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 12 Retinopathy.

Comparison 1 Lispro versus regular insulin (Different insulin types within similar insulin regimens), Outcome 13 Ventouse delivery.

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 1 Small‐for‐gestational age at delivery.

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 2 Preterm birth (< 37 weeks).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 3 Birthweight centile (%).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 4 Infant length (cm).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 5 Skinfold thickness (mm).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 6 Body weight percentile (%).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 7 Head circumference (cm).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 8 Macrosomia.

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 9 Insulin requirement during pregnancy (U/kg/24 hour).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 10 Birthweight (g).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 11 Infant fasting C‐peptide level at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 13 Infant glucose fasting level at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 15 Gestational age at delivery (weeks).

Comparison 2 Human insulin (recombinant deoxyribonucleic acid ‐ Humulin) versus animal insulin (Different insulin types within similar insulin regimens), Outcome 16 Maternal ketonuria.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch).

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis.

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days).

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g).

Comparison 3 Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 1 Macrosomia.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 2 Caesarean section.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch).

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 4 Postpartum infection: endometritis.

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 5 Use of healthcare resources (maternal hospital days).

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 6 Birthweight (g).

Comparison 4 Insulin injected with a Novolin pen versus insulin injected with a needle (syringe) (Different insulin regimens with similar insulin types used within the regimen), Outcome 7 Compliance score.

Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 1 A1c (%) third trimester visit.

Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 2 Average plasma glucose (mmol/L) third trimester visit.

Comparison 5 Insulin Aspart + NPH insulin versus Human insulin + NPH insulin (Different insulin types within similar insulin regimens), Outcome 3 Maternal hypoglycaemic episodes.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 1 Major congenital malformation.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 2 Major congenital malformation.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 3 Minor congenital malformation.

Comparison 6 Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens), Outcome 4 Minor congenital malformation.
| Lispro versus regular insulin (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with regular insulin | Risk with Lispro | |||||
| Macrosomia | (0 studies) | Not reported | ||||
| Perinatal death | Study population | not estimable | 33 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Pre‐eclampsia | Study population | RR 0.68 | 33 | ⊕⊝⊝⊝ | ||
| 647 per 1000 | 440 per 1000 | |||||
| Caesarean section | Study population | RR 0.59 | 33 | ⊕⊝⊝⊝ | ||
| 529 per 1000 | 312 per 1000 | |||||
| Fetal anomaly | Study population | RR 0.35 | 33 | ⊕⊝⊝⊝ | ||
| 59 per 1000 | 21 per 1000 | |||||
| Birth trauma, including shoulder dystocia, nerve palsy, and fracture | Study population | Not estimable | 33 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High or unclear risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases 2 Small sample size and no events 3 One study with design limitations 4 Very wide 95% confidence intervals crossing the line of no effect 5 Small sample size with few events | ||||||
| Human insulin versus animal insulin (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with animal insulin | Risk with human insulin (Humulin) | |||||
| Macrosomia | Study population | RR 0.22 | 42 | ⊕⊝⊝⊝ | ||
| 91 per 1000 | 20 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section | (0 studies) | Not reported | ||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, and fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias was high or unclear for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting 2 One study with serious design limitations including lack of blinding for allocation concealment 3 Very wide 95% confidence intervals crossing the line of no effect 4 Small sample size and few events | ||||||
| Pre‐mixed insulin (70 NPH/30 REG) versus self‐mixed split dose insulin (Different insulin regimens with similar insulin types used within the regimen) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with self‐mixed split dose insulin | Risk with pre‐mixed insulin (70 NPH/30 REG) | |||||
| Macrosomia | Study population | RR 0.49 | 93 | ⊕⊝⊝⊝ | ||
| 87 per 1000 | 43 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section 4 | Study population | RR 0.57 | 93 | ⊕⊝⊝⊝ | ||
| 261 per 1000 | 149 per 1000 | |||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Very wide 95% confidence intervals crossing the line of no effect 2 Small sample size and few events 3 One study with serious design limitations 4 Caesarean section for cephalo‐pelvic disproportion | ||||||
| Insulin injected with a Novolin pen versus insulin injected with a needle or syringe (Different insulin regimens with similar insulin types used within the regimen) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with insulin injected with a needle or syringe | Risk with insulin injected with a Novolin pen | |||||
| Macrosomia | Study population | RR 0.21 | 93 | ⊕⊝⊝⊝ | ||
| 104 per 1000 | 22 per 1000 | |||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section 4 | Study population | RR 0.38 | 93 | ⊕⊝⊝⊝ | ||
| 292 per 1000 | 111 per 1000 | |||||
| Fetal anomaly | (0 studies) | Not reported | ||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Very wide 95% confidence intervals crossing the line of no effect 2 Small sample size with few events 3 One study with serious design limitations 4 Caesarean section for cephalo‐pelvic disproportion | ||||||
| Insulin Aspart (+ NPH) compared to human insulin (+ NPH) for pregnant women with pre‐existing diabetes (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with human insulin (+ NPH) | Risk with insulin Aspart (+ NPH) | |||||
| Macrosomia | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Pre‐eclampsia | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Fetal anomaly | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Birth trauma including shoulder dystocia, nerve palsy and fracture | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Composite outcome measure of neonatal morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Insulin Detemir + prandial insulin Aspart versus NPH insulin + prandial insulin Aspart (Different insulin types within similar insulin regimens) | ||||||
| Patient or population: pregnant women with pre‐existing diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with NPH (+ Aspart) | Risk with insulin Detemir (+ Aspart) | |||||
| Macrosomia | (0 studies) | Not reported | ||||
| Perinatal death | (0 studies) | Not reported | ||||
| Pre‐eclampsia | (0 studies) | Not reported | ||||
| Caesarean section | (0 studies) | Not reported | ||||
| Fetal anomaly (major) 1 | Study population | RR 3.15 | 162 | ⊕⊝⊝⊝ | ||
| 12 per 1000 | 38 per 1000 | |||||
| Birth trauma including shoulder dystocia, nerve palsy, or fracture | (0 studies) | Not reported | ||||
| Composite outcome measure of neonatal morbidity | (0 studies) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assessed by an expert who was blinded to the outcome 2 One study with design limitations 3 Very wide 95% confidence intervals crossing the line of no effect 4 Large effect estimate 5 Small sample size with few events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.39] |
| 3 Pregnancy‐induced hypertension and pre‐eclampsia Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.30] |
| 4 Fetal anomaly Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.08] |
| 5 Birth trauma, including shoulder dystocia, nerve palsy, and fracture Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Vaginal delivery (spontaneous, ventouse, forceps) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.80, 2.67] |
| 7 Blood glucose (mmol/L) week 14 (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐3.60, 1.42] |
| 8 Blood glucose (mmol/L) weeks 21, 28, and 34 combined (after lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐2.10, 2.02] |
| 9 Postprandial increase of blood glucose (mmol/L) before week 14 (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.52, 3.52] |
| 10 Postprandial increase of blood glucose (mmol/L) during weeks 21, 28, and 34 combined (lunch) Show forest plot | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.12, 2.32] |
| 11 Maternal hypoglycaemia and hyperglycaemia episodes requiring intervention Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 12 Retinopathy Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.17, 6.67] |
| 13 Ventouse delivery Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.37, 27.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Small‐for‐gestational age at delivery Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Preterm birth (< 37 weeks) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [0.42, 139.83] |
| 3 Birthweight centile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.64, 10.24] |
| 4 Infant length (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.74, 0.14] |
| 5 Skinfold thickness (mm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.10 [‐13.28, 5.08] |
| 6 Body weight percentile (%) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.74, 10.34] |
| 7 Head circumference (cm) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐9.52, ‐0.68] |
| 8 Macrosomia Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 9 Insulin requirement during pregnancy (U/kg/24 hour) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.45, ‐0.21] |
| 10 Birthweight (g) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐591.0 [‐1066.27, ‐115.73] |
| 11 Infant fasting C‐peptide level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| 12 Infant C‐peptide level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.19, ‐0.03] |
| 13 Infant glucose fasting level at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.62, 0.22] |
| 14 Infant glucose level 1 hour after glucose‐amino acid challenge at 3 months (pmol/mL) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.04, 1.04] |
| 15 Gestational age at delivery (weeks) Show forest plot | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐3.70, 4.70] |
| 16 Maternal ketonuria Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.08, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.54] |
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.25, 1.32] |
| 3 Antepartum capillary glucose measurement (mg/dL), 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐11.25 [‐12.55, ‐9.95] |
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.40, 0.41] |
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐116.56 [‐391.81, 158.69] |
| 7 Compliance score Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.87, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Macrosomia Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.76] |
| 2 Caesarean section Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.97] |
| 3 Antepartum capillary glucose measurement (mg/dL) 2 hours postprandial (after lunch) Show forest plot | 1 | 10218 | Mean Difference (IV, Fixed, 95% CI) | ‐7.23 [‐8.51, ‐5.95] |
| 4 Postpartum infection: endometritis Show forest plot | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.28, 1.14] |
| 5 Use of healthcare resources (maternal hospital days) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.45, 0.33] |
| 6 Birthweight (g) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐162.36 [‐438.25, 113.53] |
| 7 Compliance score Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 A1c (%) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 2 Average plasma glucose (mmol/L) third trimester visit Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.53, 0.13] |
| 3 Maternal hypoglycaemic episodes Show forest plot | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.33, 29.67] |
| 2 Major congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| 3 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 4 Minor congenital malformation Show forest plot | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 5.05] |