Intervenciones para la coriorretinopatía serosa central: un metanálisis en red
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: parallel randomized controlled trial Number randomized: 16 eyes of NR participants in anti‐VEGF group 18 eyes of NR participants in PDT group Exclusions after randomization: 0 in anti‐VEGF group 0 in PDT group Number analyzed: 16 eyes of NR participants in anti‐VEGF group 18 eyes of NR participants in PDT group Unit of analysis: mixed, some participants had 1 eye included, some participants had both eyes included Losses to follow‐up: 2 eyes of participants in anti‐VEGF group 0 in PDT group How were missing data handled?: NA Power calculation: power = 80% and sample size = 34 eyes total (17 eyes in each group) | |
| Participants | Country: South Korea 50.8 (7.7) overall 15 men (83%) and 3 women (17%) in PDT group Exclusion criteria: history of treatment including PDT, focal laser photocoagulation, intravitreal injection of steroid or anti‐VEGF agent in the study eye; evidence of CNV or polypoidal choroidal vasculopathy; any other ocular diseases that can affect visual acuity, including diabetic retinopathy, retinal vascular occlusion, or ocular inflammatory diseases; media opacity that interferes with adequate image acquisition; history of any intraocular surgery except uncomplicated cataract surgery > 3 months before enrollment; history of systemic steroid or anti‐VEGF treatment in the preceding 12 months; uncontrolled glaucoma with intraocular pressure > 21 mm Hg despite treatment; uncontrolled hypertension, diabetes, or history of cerebrovascular accident or myocardial infarction; and pregnancy | |
| Interventions | Intervention: anti‐VEGF (ranibizumab) Dose: 0.5 mg/0.05 mL Frequency: baseline, 1 and 2 months Control: low‐fluence PDT Light dose: 25 J/cm2 Verteporfin concentration: 6 mg/m2 | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: Low‐fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one‐year results of a randomized trial Trial registry: NCT01325181 (clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to receive low‐fluence PDT or the intravitreal injections of ranibizumab with an equal allocation ratio by means of permuted block randomization" p. 559 |
| Allocation concealment (selection bias) | Unclear risk | "Subjects and the treating ophthalmologist (S.H.B.) were not masked to the treatment modalities" p. 559 Unclear if the allocation was masked before enrollment |
| Masking of participants and personnel (performance bias) | High risk | "Subjects and the treating ophthalmologist (S.H.B.) were not masked to the treatment modalities" p. 559 |
| Masking of outcome assessment (detection bias) | Low risk | "The investigator (J.H.) and the other examiners for BCVA measurement, OCT, FA, and ICGA were masked to treatment allocation" p. 559 |
| Incomplete outcome data (attrition bias) | Low risk | 2/34 participants (< 10%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes on trial registry entry reported in paper |
| Other bias | High risk | Received industry funding (Novartis Korea, Seoul, Korea). Stated that "the sponsor or funding organization had no role in the design or conduct of this research. No conflicting relationship exists for any author" |
| Methods | Study design: unclear ‐ refers only to eyes 8 eyes of NR participants in PDT group | |
| Participants | Country: Italy (probably) NR for overall and by group Gender (%): did not report number of men and women overall or by group Participants with chronic CSC were included as defined as: not defined Exclusion criteria: any previous treatment for CSC; evidence of other chorioretinal disorders; media opacities; and treatment with systemic steroids | |
| Interventions | Intervention: low‐fluence PDT light dose: 25 J/cm2 Verteporfin concentration: NR | |
| Outcomes | Length of follow‐up: 24 weeks | |
| Notes | Full study name: Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomized clinical trial of efficacy and safety Trial registry: not registered Contacted study authors and received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Different groups and the study described as being "blind" but no information on masking |
| Masking of outcome assessment (detection bias) | High risk | Different groups and the study described as being "blind" but no information on masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: cross‐over randomized controlled trial NR eyes of 25 participants in total, By group NR | |
| Participants | Participants with both acute or chronic CSC were enrolled Country: NR | |
| Interventions | Intervention: beta‐blocker (propranolol) Dose: NR Frequency: NR Duration: NR Dose: NR Frequency: NR Duration: NR | |
| Outcomes | Length of follow‐up: NR Secondary outcomes: no outcomes defined | |
| Notes | Full study name: Treatment of central serous chorioretinopathy with beta‐blockers and calcium antagonists Trial registry: not registered Contacted study authors and received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Both interventions were pills but no information on how similar the pills were |
| Masking of outcome assessment (detection bias) | Unclear risk | Both interventions were pills but no information on how similar the pills were |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial NR eyes of 8 participants in beta‐blocker group | |
| Participants | Country: US Mean age (SD) (years): 41.5 (NR) in total 9 men (56%) and 7 women (44%) in total Type of CSC not specified | |
| Interventions | Intervention: beta‐blocker (nadolol) Dose: 40 mg Frequency: daily Duration: NR Control: placebo | |
| Outcomes | Length of follow‐up: 4 months | |
| Notes | Full study name: Nadolol in the treatment of central serous retinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "I evaluated the effect of the nonselective beta‐blocker nadolol in a prospective, randomized, double‐masked trial" p. 770 No further information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial |
| Masking of outcome assessment (detection bias) | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 15 eyes of 15 participants in total By group NR | |
| Participants | Type of CSC not specified Country: US Mean age (SD) (years): Total and by group NR Inclusion criteria: NR | |
| Interventions | Intervention: low‐dose transpupillary thermotherapy Control: sham laser | |
| Outcomes | Length of follow‐up: 3 months Outcomes: proportion of eyes with resolved CSC | |
| Notes | Full study name: Low‐dose transpupillary thermotherapy for the treatment of central serous chorioretinopathy Trial registry: not registered Contacted study authors who were unable to provide a reference to the full‐text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Fifteen eyes of 15 patients with CSR [CSC] were randomly assigned" No further information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Control group had sham therapy but no details on how this was done |
| Masking of outcome assessment (detection bias) | Unclear risk | Control group had sham therapy but no details on how this was done |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 42 eyes of 42 participants in PDT group 0 in PDT group 39 eyes of 39 participants in PDT group 3 eyes of 3 participants in PDT group | |
| Participants | Participants with acute CSC were included and defined as: acute symptomatic CSC of ≤ 3 months' duration Country: China 41.0 (6.7) in total 54 men (86%) and 9 women (14%) in total Inclusion criteria: people with BCVA ≥ 20/200; presence of SRF involving the fovea on OCT; presence of active angiographic leakage on FA caused by CSC but not CNV or other diseases; and abnormal dilated choroidal vasculature and other features on ICGA consistent with the diagnosis of CSC | |
| Interventions | Intervention: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Control: placebo (30 mL normal saline infused instead of verteporfin, and laser applied in the same manner as in the verteporfin group) | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: Half‐dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one‐year results of a randomized controlled trial Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table kept centrally by a research nurse, and the group allocation was performed before drug preparation and infusion" p. 1757 |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table kept centrally by a research nurse, and the group allocation was performed before drug preparation and infusion. All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Masking of participants and personnel (performance bias) | Low risk | "All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Masking of outcome assessment (detection bias) | Low risk | "All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Incomplete outcome data (attrition bias) | Low risk | Overall 5/58 participants (< 10%) were lost to follow‐up; 3/39 PDT group lost to follow‐up; 2/19 of placebo group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Source of funding not reported. A conflict of interest was declared but only for 1 of the 5 authors and not the first author: "Dr Lai has served as a consultant to an advisory board of Novartis, Inc. All other authors have no financial interest to declare" |
| Methods | Study design: parallel randomized controlled trial 8 eyes of 8 participants in anti‐VEGF group 7 eyes of 7 participants in PDT group 8 eyes of 8 participants in anti‐VEGF group 7 eyes of 7 participants in PDT group | |
| Participants | Country: Turkey NR in total 46.5 (11.5) in anti‐VEGF group Participants with chronic CSC were included and defined as: eyes with symptomatic chronic CSC (duration 6 months) | |
| Interventions | Intervention: 50% PDT + anti‐VEGF (bevacizumab) Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Bevacizumab dose: 1.25 mg Bevacizumab duration: single dose, 3 days after PDT Control: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 | |
| Outcomes | Length of follow‐up: 12 months Outcomes: time to CSC resolution; BCVA; CMT | |
| Notes | Full study name: Combined half dose photodynamic therapy with verteporfin and intravitreal bevacizumab for chronic central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 32 eyes of 32 participants in Helicobacter pylori treatment group 27 eyes of 27 participants in H. pylori group 5 eyes of 5 participants in H. pylori group | |
| Participants | Country: China NR in total 43 men (81%) and 10 women (19%) in total | |
| Interventions | Intervention:Helicobacter pylori treatment Drug (dose): omeprazole 20 mg, clarithromycin 500 mg, and amoxicillin 1000 mg Frequency: twice a day Duration: 14 days | |
| Outcomes | Length of follow‐up: 12 weeks | |
| Notes | Full study name: The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned through a web‐based data entry system maintained at the Data Coordinating Center (The MEDABC Corporation, Zhengzhou, Henan, People's Republic of China), with equal probability of receiving either H. pylori eradication (referred to as the active treatment group) or placebo drugs (referred to as the control group) using a permuted‐block design with random block sizes" p. 356 |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned through a web‐based data entry system maintained at the Data Coordinating Center (The MEDABC Corporation, Zhengzhou, Henan, People's Republic of China), with equal probability of receiving either H. pylori eradication (referred to as the active treatment group) or placebo drugs (referred to as the control group) using a permuted‐block design with random block sizes" p. 356 |
| Masking of participants and personnel (performance bias) | Low risk | "The control group received an identical placebo that was the same color, size, and had the same identification name as the treatment. The placebos were taken in the same manner as the study drugs. Both drugs were also in identical opaque bottles and prepared by one nonclinician research assistant" p. 356 |
| Masking of outcome assessment (detection bias) | Low risk | "The control group received an identical placebo that was the same color, size, and had the same identification name as the treatment. The placebos were taken in the same manner as the study drugs. Both drugs were also in identical opaque bottles and prepared by one nonclinician research assistant" p. 356 |
| Incomplete outcome data (attrition bias) | Unclear risk | "A total of 64 eyes in 64 patients were enrolled and randomized equally into two groups. Eleven eyes (17.18%) were lost to follow‐up or did not yield enough data (five eyes in the active treatment group and six eyes in the control group). A total of 53 eyes (82.81%) were included in the study." p. 357 Although similar drop‐outs loss to follow‐up was approaching 20% and no information on reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest was declared: "the authors report no conflicts of interest in this work" |
| Methods | Study design: parallel randomized controlled trial 30 eyes of 30 participants in beta‐blocker group 0 in beta‐blocker group 30 eyes of 30 participants in beta‐blocker group 0 in beta‐blocker group | |
| Participants | Country: Iran 35 (8) in total 44 men (73%) and 16 women (27%) in total | |
| Interventions | Intervention: beta‐blocker (propranolol) Dose: 20 mg Frequency: twice daily Duration: NR | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: Effects of propranolol in patients with central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Allocation concealment (selection bias) | Unclear risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Masking of participants and personnel (performance bias) | Low risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Masking of outcome assessment (detection bias) | Unclear risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 This suggests that the research staff may have been unmasked to the treatment assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 25 eyes of 25 participants in anti‐VEGF group 20 eyes of 20 participants in anti‐VEGF group 5 eyes of 5 participants in anti‐VEGF group | |
| Participants | Country: South Korea 43.05 (7.46) in anti‐VEGF group 22 men (55%) and 18 women (45%) in total Exclusion criteria: people who had received any previous treatment, including PDT or focal thermal laser photocoagulation for CSC, or who had evidence of CNV, polypoidal choroidal vasculopathy, or other maculopathy on fundus examination, FA, or ICGA Equivalence of baseline characteristics: NR | |
| Interventions | Intervention: anti‐VEGF (ranibizumab) Dose: 0.5 mg/mL Frequency: single dose at baseline | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Full study name: Intravitreal ranibizumab for acute central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized into the IVRI [anti‐VEGF] group or the observation group at a ratio of 1: 1" p. 153 No details on how the random allocation generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Masking not reported and treatments different (injection vs. no treatment) |
| Masking of outcome assessment (detection bias) | High risk | Masking not reported and treatments different (injection vs. no treatment) |
| Incomplete outcome data (attrition bias) | High risk | Not reported how many were randomized by group and loss to follow‐up by group. Final numbers analyzed identical between treatment and observation group (20/20) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Low risk | Funding from non‐profit and conflict of interest was not reported. "This study was supported by 2012 Research Grant from Kangwon" |
| Methods | Study design: parallel randomized controlled trial 14 eyes of NR participants in micropulse laser group ("selective retina therapy") 0 in micropulse laser group ("selective retina therapy") 14 eyes of number of participants NR in micropulse laser group ("selective retina therapy") 0 in micropulse laser group ("selective retina therapy") | |
| Participants | Country: Germany 43.8 (5.6) in total 26 men (87%) and 4 women (13%) in total Exclusion criteria: other retinal diseases; glaucoma; cataract or other media opacities, which preclude color fundus photography and FA; previous PDT or continuous wave laser photocoagulation for CSC; systemic corticosteroid treatment, Cushing disease, renal diseases, pregnancy, and breastfeeding | |
| Interventions | Intervention: micropulse laser ("selective retina therapy") Q‐switched neodymium‐doped yttrium lithium fluoride (Nd:YLF) laser wavelength: 527 nm spot diameter; 200 µm pulse repetition rate: 100 Hz | |
| Outcomes | Length of follow‐up: 3 months | |
| Notes | Full study name: Selective retina therapy for acute central serous chorioretinopathy Trial registry: NCT00987077 (clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, opaque, sealed envelopes were prepared on the basis of the accomplished randomisation" p. 84 |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | "At all visits, BCVA was assessed using ETDRS charts at 4 m distance. The investigator was blinded" p. 84 Masking for other outcomes not reported and visual acuity assessment may be affected by the fact that the patient knew which group they were in |
| Incomplete outcome data (attrition bias) | Low risk | "All patients kept their follow‐up appointment and were included in the analysis" p. 84 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in the trials registry entry (NCT00987077; clinicaltrials.gov) |
| Other bias | High risk | Source of monetary support was not reported and conflict of interest was declared: "Johann Roider, Ralf Brinkmann and Reginald Birngruber hold patents on selective retina therapy. Carsten Klatt, Mark Saeger, Till Oppermann, Erk Porksen, Felix Treumer, Jost Hillenkamp and Elfriede Fritzer have no competing interests" |
| Methods | Study design: parallel randomized controlled trial NR eyes of 35 participants in argon laser group NR eyes of 32 participants in argon laser group NR eyes of 3 participants in argon laser group | |
| Participants | Country: UK 40.1 (NR) in total 53 men (84%) and 10 women (16%) in total Inclusion criteria: corrected visual acuity ≥ 6/12; retina detached at macula; RPE defects < 1 disc diameter; no symptomatic improvement since onset; no subretinal exudates present; no cystic retinal edema present; no associated ocular disease (e.g. drusen, congenital pit of the disc, generalized RPE dystrophy, etc.); consent to participate in the study after explanation of aims and methods | |
| Interventions | Intervention: argon laser (direct) | |
| Outcomes | Length of follow‐up: 12.1 years | |
| Notes | Full study name: Argon laser photocoagulation in the treatment of central serous retinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on how the random allocation generated: "randomised cards from sealed envelopes" p. 675 |
| Allocation concealment (selection bias) | Unclear risk | "...randomised cards from sealed envelopes" (p. 675) but not enough information on how sealed envelopes were prepared e.g. sequentially number? opaque? |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) | Low risk | 7/70 (10%) missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 32 eyes of number of participants NR in total By group NR 12 eyes of 12 participants in anti‐VEGF group 8 eyes of number of participants NR in total; by group NR | |
| Participants | Country: South Korea 43.2 (9.0) in total 20 men (83%) and 4 women (17%) in total Exclusion criteria: participants who had received any previous treatment, including PDT or focal thermal laser photocoagulation for CSC, or who had evidence of CNV, polypoidal choriovasculopathy, or other maculopathy on clinical examination, FA, or ICGA | |
| Interventions | Intervention: anti‐VEGF (bevacizumab) Dose: 1.25 mg/0.05 mL Frequency: single dose injected < 1 week after diagnosis Control: observation | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Full study name: The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table" p. 156 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) | High risk | 8/32 (25%) of participants not followed up and not reported which group they were in |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest declared: "no potential conflict of interest relevant to this article was reported" |
| Methods | Study design: parallel randomized controlled trial 16 participants were included in the study, but allocation not reported NR eyes of NR participants in carbonic anhydrase inhibitor group Number of eyes NR of 7 participants in carbonic anhydrase inhibitor group | |
| Participants | Participants with acute CSC were included and define as: participants with "symptoms for less than 20 days, no previous treatment" Country: Mexico NR in total 9 men (69%) and 4 women (31%) in total | |
| Interventions | Intervention: carbonic anhydrase inhibitors (brinzolamide) Dose: 2% Duration: twice daily Control: placebo (polyvinyl alcohol) | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Full study name: Brinzolamide for topical treatment coroidorretinopatía idiopathic central serous Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The labeling and drug control was done by third person not related with the study p. 134 |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups (both groups' treatment were eye drops), but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups (both groups' treatment were eye drops), but no information on masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 6 eyes of 6 participants in 6‐dose group 0 in 6‐dose group 6 eyes of 6 participants in 6‐dose group 0 in 6‐dose group | |
| Participants | Country: USA NR in total Participants with chronic CSC were included as defined as: "persistent CSCR [CSC] demonstrated by subfoveal fluid (SFF) on optical coherence tomography (OCT) for greater than 3 months" Exclusion criteria: concurrent progressive retinal or substantial ocular disease in the study eye; prior treatment for CSC in the study eye (anti‐VEGF, PDT, or laser) within 3 months prior to enrolment; presence of CNV or polypoidal choroidal vasculopathy on enrolment imaging; history of intraocular surgery except uncomplicated cataract surgery > 3 months prior to enrolment; prior treatment with systemic anti‐VEGF agents or steroid agents within the preceding 12 months; uncontrolled glaucoma; history of cerebrovascular accident or myocardial infarction; pregnancy | |
| Interventions | Intervention: 6‐dose group (aflibercept) Dose: 2.0 mg/0.05 mL Duration: single dose at baseline, 1, 2, 3, 4, and 5 weeks Control: 4‐dose group (aflibercept) Dose: 2.0 mg/0.05 mL Duration: single dose at baseline, 1, 2, and 4 weeks | |
| Outcomes | Length of follow‐up: 6 months Outcomes listed as: occurrence of ocular or systemic adverse events; mean change from baseline in ETDRS letter score; percentage of eyes with 20/40 or better vision; percentage of eyes with ≥ 15‐letter gain from baseline; percentage of eyes with < 15‐letter loss from baseline; mean change in CMT; mean change in SFF manually measured by a masked observer; percentage of eyes with complete resolution of macular fluid on OCT; mean change in subfoveal choroidal thickness as measured via enhanced depth imaging; and percentage of eyes with absence of leakage on FA | |
| Notes | Full study name: A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study Trial registry: NCT01710332 (clinicaltrials.gov) Study period: November 2012 to May 2013, as reported in the full‐text article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to two groups" p. 849 Did not clear describe method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Did not describe clearly how the sequence generation was assigned/stored |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | SFF height was determined by a masked observer using the digital caliper function to measure distance from the hyper‐reflective RPE to the photoreceptor outer segments on a b‐scan through the foveal center point. Choroidal thickness was determined by a masked observer using the digital caliper to measure distance from the inner border of the choroido‐scleral interface to the hyper‐reflective RPE |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Did not clearly define the primary and secondary outcomes in the published full‐text, while the trial registry had primary and secondary outcomes were clearly defined. Reported 5 additional outcomes not defined in the trial registry: "mean change in CMT; mean change in SFF manually measured by a masked observer; percentage of eyes with complete resolution of macular fluid on OCT; mean change in subfoveal choroidal thickness as measured via enhanced depth imaging; and percentage of eyes with absence of leakage on FA |
| Other bias | High risk | Industry funding |
| Methods | Study design: parallel randomized controlled trial NR eyes of 25 participants in Helicobacter pylori group 0 in H. pylori group NR eyes of 25 participants in H. pylori group 0 in H. pylori group | |
| Participants | Participants with acute CSC were included but definition not given Country: Iran NR in total 41 men (82%) and 9 women (18%) in total | |
| Interventions | Intervention:H. pylori Drugs: metronidazole and amoxicillin Dose: 500 mg Frequency: 3 times daily Duration: 2 weeks Drug: omeprazole Dose: NR Frequency: once daily Duration: 6 weeks Control: observation | |
| Outcomes | Length of follow‐up: 16 weeks Outcomes: average neuroretinal and/or pigment epithelial detachment; number of cases that reached zero subretinal fluid value; subretinal fluid level; subretinal fluid reabsorption time; mean visual acuity Adverse events reported: yes | |
| Notes | Full study name: The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy Trial registry: NCT00817245 (clinicaltrials.gov) Study period: February 2008 to January 2010, as reported in the full‐text article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were divided in two groups using random allocation software" p. 100 |
| Allocation concealment (selection bias) | Unclear risk | "The patients were divided in two groups using random allocation software" p. 100, but no information specifically on allocation concealment |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes on clinical trials registry entry (NCT00817245) were reported in the full text |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 29 eyes of 29 participants in antioxidant group 26 eyes of 26 participants in antioxidant group 3 eyes of 3 participants in antioxidant group | |
| Participants | Country: Thailand 40.4 (NR) in total 48 men (83%) and 10 women (17%) in total | |
| Interventions | Intervention: antioxidant (Icaps: vitamin A (6600 IU); vitamin C (400 mg), vitamin E (150 IU); riboflavin (10 mg); zinc (60 mg); copper (4 mg); selenium (40 mg); manganese (4 mg) and lutein/zeaxanthin (4000 μg)) | |
| Outcomes | Length of follow‐up: 3 months planned but 12 months reported (with high attrition) | |
| Notes | Full study name: High‐dose antioxidants for central serous chorioretinopathy; the randomized placebo‐controlled study Trial registry: NCT00963131 (clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Allocation concealment (selection bias) | High risk | "The corresponding author generated the allocation sequence, enrolled and assigned the patients to any additional treatments when needed" p. 2 |
| Masking of participants and personnel (performance bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Masking of outcome assessment (detection bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Incomplete outcome data (attrition bias) | High risk | 3/29 lost to follow‐up at 3 months in intervention group and 4/29 lost to follow‐up in control group but at 12 months only 7 participants seen in each group. So low risk of bias for 3 months and high risk of bias for 12 months outcomes |
| Selective reporting (reporting bias) | Low risk | Trial registry entry was available for comparison, and all outcomes were reported as per the trial registry |
| Other bias | Unclear risk | "Study and placebo drugs were contributed by Alcon Laboratories (Thailand)" but authors reported that none of the authors had any financial relationship |
| Methods | Study design: parallel randomized controlled trial 15 eyes of 15 participants in argon (direct) laser group 15 eyes of 15 participants in argon (direct) laser group "We assigned eyes in which the leakage site was in the papillomacular bundle or within 500 μm of the capillary‐free zone of the macula to Group A" | |
| Participants | Country: US NR in total 29 men (69%) and 13 women (31%) in total | |
| Interventions | Group A intervention: argon laser (indirect laser photocoagulation) | |
| Outcomes | Length of follow‐up: examined at 6 months recurrences reported to 18 months | |
| Notes | Full study name: Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on how the random allocation generated: "After the patient was positioned at the laser, a sealed envelope containing the randomized treatment assignment was opened. The assignments, worked out and kept by the statistician, directed the physician (D.M.R. in all cases) to administer direct, indirect, or sham laser photocoagulation" p. 459 |
| Allocation concealment (selection bias) | Low risk | "After the patient was positioned at the laser, a sealed envelope containing the randomized treatment assignment was opened. The assignments, worked out and kept by the statistician, directed the physician (D.M.R. in all cases) to administer direct, indirect, or sham laser photocoagulation." p. 459 |
| Masking of participants and personnel (performance bias) | Low risk | "The subject did not learn which of the three forms of treatment he or she had received until the study ended six months later" pp. 459‐460 |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups (both groups' treatment were laser), but no information on masking of outcome assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Funded by government sources but conflicts of interest were not reported |
| Methods | Study design: parallel randomized controlled trial 10 eyes of 10 participants in micropulse diode laser group 0 in micropulse diode laser group 10 eyes of 10 participants in micropulse diode laser group 0 in micropulse diode laser group | |
| Participants | Country: Brazil NR in total 10 men (67%) and 5 women (33%) in total | |
| Interventions | Intervention: micropulse diode laser Wavelength: 810 nm FastPulse laser; Opto, Brazil | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial Trial registry: NCT01327170 (clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 15 patients were randomized 2:1 through double‐masked random draw into two groups" p. 466 |
| Allocation concealment (selection bias) | Low risk | "The 15 patients were randomized 2:1 through double‐masked random draw into two groups" p. 466 |
| Masking of participants and personnel (performance bias) | Low risk | Double‐masked with placebo group |
| Masking of outcome assessment (detection bias) | Low risk | Double‐masked with placebo group |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the trial registry were reported |
| Other bias | Low risk | Source of monetary funding not reported but the conflict of interest declared: "the authors have no financial or proprietary interest in the materials presented herein" |
| Methods | Study design: parallel randomized controlled trial Total number randomized was 44 Unclear number in each group NR eyes of 20 participants in antioxidant group | |
| Participants | The type of CSC was not specified Country: Japan 49 (10) in total 35 men (90%) and 4 women (10%) in total | |
| Interventions | Intervention: antioxidant (lutein) Dose: 20 mg/day | |
| Outcomes | Length of follow‐up: 4 months | |
| Notes | Full study name: Effects of a lutein supplement on the plasma lutein concentration and macular pigment in patients with central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Masking of participants and personnel (performance bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Masking of outcome assessment (detection bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Incomplete outcome data (attrition bias) | High risk | 5 people excluded after randomization but not reported to which groups they belonged |
| Selective reporting (reporting bias) | High risk | BCVA measured but not reported. Only reported resolution of CSC for the intervention (lutein) group |
| Other bias | High risk | Funded by manufacturer of the supplement. Authors reported not having any conflict of interest |
| Methods | Study design: parallel randomized controlled trial 12 eyes of 12 participants in anti‐VEGF group 12 eyes of 12 participants in anti‐VEGF group 12 eyes of 12 participants in anti‐VEGF group 0 in anti‐VEGF group | |
| Participants | Participants with chronic CSC were included and defined as: "...either persistence of subretinal fluid detected on optical coherence tomography (OCT) for at least 3 months after diagnosis or more than 3 recurrences in at least 3 months with gravitational RPE atrophy" and "The inclusion criteria consisted of presence of CSC with chronic foveal detachment of the neuroepithelium (C3 months) and no previous treatment for CSC" Country: Italy NR in total 13 men (59%) and 9 women (41%) in total | |
| Interventions | Intervention: anti‐VEGF (bevacizumab) Dose: 1.25 mg Duration: single dose at baseline and then as needed after 4 weeks Light dose: 25 J/cm2 Verteporfin concentration: NR | |
| Outcomes | Length of follow‐up: 9 months | |
| Notes | Full study name: Intravitreal bevacizumab versus low‐fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Enrolled patients were randomly assigned to group 1 or group 2 by random block permutation in accordance with a computer‐generated randomization list" p. 609 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial NR eyes of 30 participants in yellow laser group NR eyes of 30 participants in green laser group 0 in yellow group 0 in green group Number analyzed: NR eyes of 30 participants in yellow group NR eyes of 30 participants in green group 0 in yellow group 0 in green group | |
| Participants | The type of CSC was not specified Country: China 40.0 (6.3) in total 43.7 (7.6) in yellow group 39.5 (5.0) in green group 79 men (89%) and 10 women (11%) in total NR in yellow group NR in green group Inclusion criteria: people with CSC validated by eye exam, Amsler chart examine and FFA; | |
| Interventions | Intervention 1: yellow Intervention 2: red Control: green | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: Wavelength selection in management of central serous chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | No mention of masking |
| Masking of outcome assessment (detection bias) | Unclear risk | No mention of masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial NR eyes of 15 participants in micropulse diode laser group 0 in micropulse diode laser group NR eyes of 15 participants in micropulse diode laser group 0 in micropulse diode laser group | |
| Participants | Country: India NR in total 25 men (83%) and 5 women (17%) in total | |
| Interventions | Intervention: micropulse diode laser Wavelength: 810 nm Wavelength: 514 nm | |
| Outcomes | Length of follow‐up: 12 weeks | |
| Notes | Full study name: Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial ISRCTN84128484 Trial registry: ISRCTN84128484 (ICTRP) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomly assigned into 2 groups according to the statistical random table using sequence generation" p. 2 |
| Allocation concealment (selection bias) | Unclear risk | "The allocation of patients into 2 groups was done by a person who was not involved in the study and the sequence was concealed until interventions were assigned to prevent bias" p. 2 |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups but no information on masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest declared: "the author(s) declare that they have no competing interests" |
| Methods | Study design: parallel randomized controlled trial 30 eyes of 30 participants in 30% PDT group 30 eyes of 30 participants in PDT group 0 in 30% PDT group 0 in 50% PDT group 0 in PDT group 30 eyes of 30 participants in 30% PDT group 30 eyes of 30 participants in 50% PDT group 30 eyes of 30 participants in PDT group Unit of analysis: participant, 1 eye per person 0 in 30% PDT group 0 in 50% PDT group 0 in PDT group | |
| Participants | The type of CSC was not specified Country: China NR in total 33.8 (5.5) in 30% PDT group 32.0 (4.1) in PDT group Gender (%): 25 men (83%) and 5 women (17%) in total 9 men (30%) and 21 women (70%) in PDT group history of penicillin allergy or systemic illnesses or issues with fluorescence or other systems of sexually transmitted disease that could cause intolerance of FFA treatment and PDT treatment; | |
| Interventions | Intervention: 30% PDT Light dose: 50 J/cm2 Verteporfin concentration: 2 mg/m2 Intervention: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Control: PDT Light dose: 50 J/cm2 Verteporfin concentration: 6 mg/m2 | |
| Outcomes | Length of follow‐up: 12 months Adverse events reported: no | |
| Notes | Full study name: Different doses of verteporfin photodynamic therapy for central exudative chorioretinopathy Trial registry: not registered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence specified and outlined in methods. Low risk of random sequence generation. The study used a number sequence table to assign proper randomization for groups. They used a verified and structured numbering sequence of 1‐90 that was aligned for row and column and considered from smallest value to largest. They then randomized and recorded each randomly generated assignment figure |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups but no information on masking |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
| Methods | Study design: parallel randomized controlled trial 65 eyes of 65 participants in 30% PDT group 64 eyes of 64 participants in 50% PDT group 0 in 30% PDT group 0 in 50% PDT group 61 eyes of 61 participants in 30% PDT group 56 eyes of 56 participants in 50% PDT group 4 eyes of 4 participants in 30% PDT group 9 eyes of 9 participants in 50% PDT group Power calculation: power = 80% and sample size = 112 participants (56 participants in each group) | |
| Participants | Country: China NR in total 43.1 (5.3) in 50% PDT group 87 men (74%) and 30 women (26%) in total 40 men (71%) and 16 women (29%) in 50% PDT group Exclusion criteria: previous PDT, focal photocoagulation, intravitreal injections of anti‐VEGF, or ocular surgery; other macular abnormalities such as CNV or polypoidal choroidal vasculopathy; choroidopathy that may affect choroidal thickness; any retinal vascular disease that may have fluorescein leakage during FA; history of porphyria or photosensitivity; severe impaired kidney or liver function or unstable heart condition (or a combination of these); pregnancy; inability to obtain photographs or to perform FA or ICGA; use of steroid systemically or topically in the last 6 months | |
| Interventions | Intervention: 30% PDT Light dose: 50 J/cm2 Verteporfin concentration: 1.8 mg/m2 Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Full study name: A 50% vs 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one‐year results of a randomized clinical trial Trial registry: NCT01574430 (clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table" p. 334 |
| Allocation concealment (selection bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment |
| Masking of participants and personnel (performance bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment allocation group" p. 334 |
| Masking of outcome assessment (detection bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment allocation group" p. 334 |
| Incomplete outcome data (attrition bias) | High risk | Level of lost to follow‐up was not the same in each group: 4/65 (6%) participants in 30% PDT group and 8/64 (13%) participants 50% PDT group |
| Selective reporting (reporting bias) | High risk | Primary outcome reported at clinicaltrials.gov (NCT01574430) was change from baseline in BCVA, but study primary outcome were OCT‐based improvement rate and FA‐based improvement rate at 6 and 12 months. BCVA was reported, but not defined as primary outcome |
| Other bias | Low risk | Reported no conflicts of interest and non‐industry funded |
anti‐VEGF: anti‐vascular endothelial growth factor; BCVA: best‐corrected visual acuity; CFT: central foveal thickness; CMT: central macular thickness; CNV: choroidal neovascularization; CRT: central retinal thickness; CSC: central serous chorioretinopathy; FA: fluorescein angiography; FFA: fundus fluorescein angiography; ICGA: indocyanine green angiography; logMAR: logarithm of the minimal angle of resolution; NA: not applicable as no missing data or unclear if there is missing data; NR: not reported; OCT: optical coherence tomography; p: page; PDT: photodynamic therapy; RPE: retinal pigment epithelium; SD: standard deviation; SFF: subfoveal fluid; SRF: subretinal fluid; SLO‐ICGA: scanning laser indocyanine green angiography.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Wrong treatment arms and included traditional Chinese medicine; compared Argon laser to xueshuantong | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT; randomization not clearly described | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared argon laser to traditional medication (vitamin C, E, inosine, rutin, xueshuantong, difrarel) | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared jolethin combined with argon laser (argon laser and oral jolethin, vitamin B1, inosine and venoruton tablets) to traditional medication (oral lecithin complex iodine, vitamin B1, inosine, venoruton tablets) | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared argon laser and iodine treatment to argon laser | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared krypton laser combined with danshen and inosine to krypton laser combined with anisodine | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Wrong participants. RCT enrolled participants with age‐related macular degeneration | |
| This study was terminated early due to lack of enrollment | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT; randomization not clearly described | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared therapeutic alliance group (injected subcutaneously compound anisodine injection 2 mL combined with joletion tablets taking) to joletion tablets group | |
| Wrong participants | |
| Wrong participants | |
| Wrong treatment arms and included traditional Chinese medicine; compared jolethin combined with argon laser to argon laser | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared anisodine injection to traditional medication (oral medication such as adenosine triphosphate, inosine, vitamin) | |
| Wrong treatment arms and included traditional Chinese medicine; compared argon laser combined with xueshuantong (laser combined with compound xueshuantong capsule) to argon laser | |
| Not RCT | |
| Wrong treatment arms and included traditional Chinese medicine; compared hyperbaric oxygen and iodized lecithin to hyperbaric oxygen |
RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Early Treatment of Patients with Central Serous Retinopathy: a Randomized Controlled Trial ‐ CSR & PDT |
| Methods | Study design: parallel group RCT |
| Participants | Population age: adults, elderly Gender: men and women "Poor prognostic acute CSR [CSC]" |
| Interventions | NR PDT versus observation |
| Outcomes | Primary outcomes: visual acuity (BCVA ETDRS) at 1 year of follow‐up Secondary outcomes: NR |
| Starting date | 25 March 2010 |
| Contact information | NR |
| Notes | Sponsor name: Rotterdam Eye Hospital |
| Trial name or title | Study on the Effects of Supplements Containing Lutein on Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: ≤ 40 years Gender: male and female |
| Interventions | Intervention 1: multivitamins, minerals, and lutein Control: placebo |
| Outcomes | Primary outcomes: rate of spontaneous resolution of CSC, changes in macular volume measured by OCT Secondary outcomes: NR BCVA |
| Starting date | NR |
| Contact information | Tsutomu Yasukawa Nagoya City University Graduate School of Medical Sciences Department of Ophthalmology and Visual Science |
| Notes | Sponsor name: Nagoya City University Graduate School of Medical Sciences Source of funding: Santen Pharmaceutical Co., Ltd |
| Trial name or title | Central Serous Chorioretinopathy Treated by Modified Photodynamic Therapy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 18‐75 years Gender: men and women |
| Interventions | Intervention 1: verteporfin PDT, half‐dose Intervention 2: verteporfin PDT, half‐fluence |
| Outcomes | Primary outcomes: effectiveness of both modification for the treatment of chronic CSC, fluorescent leakage as regards to BCVA OCT changes Secondary outcomes: detrimental influence on choroidal perfusion, represented by the decrease of fluorescent intensity in ICGA |
| Starting date | November 2008 |
| Contact information | Cheng‐Kuo Cheng, MD Assistant Professor and Attending Physician of Ophthalmology Shin‐Kong Wu Ho‐Su Memorial Hospital, School of Medicine, Fu‐Jen Catholic University |
| Notes | Sponsor name: Shin Kong Wu Ho‐Su Memorial Hospital Source of funding: NR |
| Trial name or title | Effect of Spironolactone in Treating Chronic Non‐Resolutive Central Serous Chorioretinitis |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 18‐60 years Gender: male and female |
| Interventions | Intervention 1: spironolactone 25 mg/day Intervention 2: placebo |
| Outcomes | Primary outcomes: change in central macular thickness at 1 and 3 months, subretinal fluid decrease of 40 microns or more Secondary outcomes: NR |
| Starting date | January 2012 |
| Contact information | Francine Behar‐Cohen, MD, PhD Hotel‐Dieu of Paris, France |
| Notes | Sponsor name: Institut National de la Santé Et de la Recherche Médicale, France Source of funding: NR |
| Trial name or title | The Safe Effective Light Dose of Photodynamic Therapy for Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 20‐70 years Gender: men and women |
| Interventions | Intervention 1: 50% group (power of PDT is applied to the participants at 50% of the full energy based on TAP study) Intervention 2: 40% group (power of PDT is applied to the participants at 40% of the full energy based on TAP study) Intervention 3: 30% group (power of PDT is applied to the participants at 30% of the full energy based on TAP study) |
| Outcomes | Primary outcomes: change in BCVA at 1, 3, 6 and months Secondary outcomes: change in central retinal thickness, success rate, recurrence rate, and complications at 1, 3, and 6 months |
| Starting date | June 2012 |
| Contact information | Min Sagong Yeungnam University College of Medicine Daegu, Republic of Korea |
| Notes | Sponsor name: Yeungnam University College of Medicine Source of funding: NR |
| Trial name or title | Prospective Randomized Controlled Treatment Trial for Chronic Central Serous Chorioretinopathy (PLACE) |
| Methods | Study design: parallel group RCT |
| Participants | Population age: ≥ 18 years Gender: men and women |
| Interventions | Intervention 1: half‐dose PDT "In the PDT treatment arm, all patients will receive an intravenous drip through which half‐dose (3 mg/m2) verteporfin (Visudyne ®) is administered, with an infusion time of 10 minutes. At 15 minutes after the start of the infusion, PDT laser treatment is performed with standard 50 J/cm2 fluency, a wavelength of 689 nm, and a treatment duration of 83 seconds. If there still is subretinal fluid on OCT scan at Evaluation Visit 1 (6‐8 weeks after Treatment Visit 1 / the first treatment with half‐dose PDT), a second treatment with half‐dose PDT will be performed (Treatment Visit 2)" Intervention 2: micropulse laser (ML) treatment ML treatment with an 810 nm diode laser will be performed of the areas identified on mid‐phase ICG angiography. Multiple laser spots will be applied, covering the leakage area on mid‐phase ICG angiography. The area(s) that has to be treated is determined based on those hyperfluorescent area(s) on mid‐phase (approximately 10 minutes) ICG‐angiography that correspond to subretinal fluid accumulation in the macula on the OCT scan and hyperfluorescent "hot spots" on the mid‐phase (3 minutes) fluorescein angiogram. If there still is subretinal fluid on OCT scan at Evaluation Visit 1 (6‐8 weeks after Treatment Visit 1 / the first ML treatment), a second ML treatment will be performed (Treatment Visit 2) |
| Outcomes | Primary outcomes: absence of subretinal fluid on OCT scan Secondary outcomes: BCVA |
| Starting date | December 2013 |
| Contact information | Camiel JF Boon, MD PhD FEBO Leiden University Medical Center, Netherlands Myrte Breukink, MD Radboud University Nijmegen Medical Centre, Institute of Ophthalmology, Netherlands |
| Notes | Sponsor name: Radboud University Source of funding: NR |
| Trial name or title | Efficacy and Safety of Intravitreal Aflibercept Injection for Subacute Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 18‐60 years Gender: men and women |
| Interventions | Intervention 1: aflibercept (Eylea) 2 mg intravitreal injection at baseline, 1 and 2 months Control: sham injection at baseline, 1 and 2 months |
| Outcomes | Primary outcomes: change in central subfield thickness from baseline to 1, 2, 3, 4, 5, and 6 months Secondary outcomes: percentage of eyes achieving complete resolution of subretinal fluid at 6 months, percentage of eyes achieving 20/20 vision at 6 months, number of aflibercept injections needed to achieve a complete resolution at 6 months, change in subfoveal choroidal thickness from baseline using EDI‐OCT at 1, 2, 3, 4, 5, and 6 months, adverse effects of intravitreal aflibercept (Eylea) injection up to 6 months |
| Starting date | October 2013 |
| Contact information | Young Hee Yoon, MD Asan Medical Center, Republic of Korea |
| Notes | Sponsor name: Asan Medical Center Source of funding: NR |
| Trial name or title | Study on the Use of Micropulse Laser to Treat Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 30‐60 years Gender: men and women "New diagnosis of CSC" |
| Interventions | Intervention 1: micropulse laser treatment "Patient's randomized to ML treatment would be treated with the following settings: 200 micron spot size, 0.2 second duration, 15% duty cycle, and 300 milliWatt power. Their eyes would be dilated prior to treatment with standard mydriatic medications, including Tropicamide and Phenylephrine" Control: no treatment "Patients randomized to this treatment arm, will not receive treatment for CSC. They will continue to be observed at month 1 and month 3. If any worsening of pathology is found during the follow‐up visits, the patient will be removed from the study and given appropriate standard of care by the attending" |
| Outcomes | Primary outcomes: resolution of fluid build‐up within 1 week to 3 months after the laser procedure is completed Secondary outcomes: NR |
| Starting date | November 2012 |
| Contact information | Khadijah Abdallah, MPH George Washington University, District of Columbia, United States |
| Notes | Sponsor name: George Washington University Source of funding: NR |
| Trial name or title | Eplerenone for the Treatment of Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: ≥ 18 years Gender: men and women |
| Interventions | Intervention 1: eplerenone 25 mg ‐ chronic CSC diagnosis "Dosing will begin at 25mg Eplerenone taken orally , one time, each day for 58 days. Throughout the 58 day treatment period dosage will be adjusted. The adjustment will be based on serum potassium and creatine levels from blood draws done at Day 12 and Day 33. From the 25 mg starting dosage, the dosage will either be increased to 50 mg a day or reduced to placebo, one time, each day" Intervention 2: placebo ‐ chronic CSC diagnosis "Dosing will begin with placebo and will stay as placebo throughout the study. The placebo pills will be taken orally, once daily, for 58 days. The placebo pills will be compounded to be of similar composition to the eplerenone tablets, without the active ingredient" Intervention 3: eplerenone 25 mg ‐ acute CSC diagnosis "Dosing will begin at 25mg Eplerenone taken orally , one time, each day for 28 days. Throughout the 28 day treatment period, dosage will be adjusted based on serum potassium and creatine levels from blood draws done on Day 12. From the 25 mg starting dosage, the dosage will either be increased to 50 mg a day or reduced to placebo, one time, each day" Intervention 4: placebo ‐ acute CSC diagnosis "Dosing will begin with placebo and will stay as placebo throughout the study. The placebo pills will be taken orally, once daily, for 28 days. The placebo pills will be compounded to be of similar composition to the eplerenone tablets, without the active ingredient" |
| Outcomes | Primary outcomes: absence of subfoveal (retinal) fluid based on spectral domain OCT measurement at 1 month in acute CSC and 2 months in chronic CSC participants Secondary outcomes: mean change in subfoveal fluid height based on OCT measurement at 1 month in acute CSC and 2 months in chronic CSC participants |
| Starting date | October 2013 |
| Contact information | Brian Burke, MPH Wills Eye Hospital |
| Notes | Sponsor name: Wills Eye Hospital, Philadelphia, United States Source of funding: NR |
| Trial name or title | Eplerenone for the Treatment of Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: 18‐65 years Gender: men and women |
| Interventions | Intervention 1: eplerenone 25 mg given daily for 1 week, followed by 50 mg given for a total of 3 months since commencement of treatment Intervention 2: placebo |
| Outcomes | Primary outcomes: decrease of at least 10% in subretinal fluid thickness as measured by OCT at 6 months Secondary outcomes: NR |
| Starting date | April 2014 |
| Contact information | Michaella Goldstein, MD Tel Aviv Souraski Medical Center, Israel |
| Notes | Sponsor name: Tel‐Aviv Sourasky Medical Center Source of funding: NR |
| Trial name or title | A Study of the Beneficial Effects of Eplerenone on Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: ≥ 21 years Gender: men and women |
| Interventions | Intervention: eplerenone 25 mg pills triturated and filled into capsules Control: sugar pill (maltodextrin filled into capsules) |
| Outcomes | Primary outcomes: difference in the number of successful treatments after 16 weeks, defined as complete absence of subretinal fluid on SD‐OCT Secondary outcomes: change in visual acuity between eplerenone and placebo at 16 weeks, change in retinal thickness between eplerenone and placebo at 16 weeks, change in retinal volume between eplerenone and placebo at 16 weeks |
| Starting date | October 2014 |
| Contact information | Oliver Findl, MD, Prof, MBA Vienna Institute for Research in Ocular Surgery, Department of Ophthalmology Hanusch Hospital Vienna, Vienna, Austria |
| Notes | Sponsor name: Oliver Findl, MD, Prof, MBA Source of funding: NR |
| Trial name or title | Short‐Term Oral Mifepristone for Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants | Population age: ≥ 18 years Gender: men and woman |
| Interventions | Intervention 1: 1 x 300 mg mifepristone tablet, taken once daily for 4 weeks Intervention 2: 3 x 300 mg mifepristone tablets (900 mg dose), taken once daily for 4 weeks Control: placebo taken once daily for 4 weeks |
| Outcomes | Primary outcomes: resolution of sub‐retinal fluid at 4 weeks after treatment, presence or absence of subretinal fluid on spectral‐domain OCT after 4 weeks of treatment with mifepristone 300 or 900 mg daily, compared with placebo Secondary outcomes: change in subretinal fluid or intraretinal fluid (or both) at weeks 1, 2, 4, and 8, BCVA at weeks 1, 2, 4, and 8, change in ETDRS BCVA compared with baseline at weeks 1, 2, 4, and 8, change in macular thickness at weeks 1, 2, 4, and 8, change in foveal thickness at weeks 1, 2, 4, and 8, change compared with baseline in thickness of subretinal fluid under the fovea on OCTat weeks 1, 2, 4, and 8, change in choroidal thickness at weeks 1, 2, 4, and 8, dye leakage in vasculature at week 4 and 8, change in OCT characteristics in the fellow eye at week 8, proportion of acute versus chronic CSC participants at week 8, proportion of acute versus chronic CSC participants as determined at baseline, with the above outcomes analyzed for each subgroup; safety and tolerability characteristics at week 8 |
| Starting date | January 2015 |
| Contact information | Roger A Goldberg, MD, MBA Bay Area Retina Associates Walnut Creek, California, United States Jeffery S Heier, MD Ophthalmic Consultants of Boston Boston, Massachusetts, United States |
| Notes | Sponsor Name: Roger Goldberg, MD, MBA Source of funding: Bay Area Retina Associates, Ophthalmic Consultants of Boston |
BCVA: best‐corrected visual acuity; CSC: central serous chorioretinopathy (also known as CSR: central serous retinopathy); ETDRS: Early Treatment Diabetic Retinopathy Study; ICGA: indocyanine green angiography; NR: not reported; OCT: optical coherence tomography; PDT: photodynamic therapy; RCT: randomized controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| Analysis 1.1  Comparison 1 Anti‐VEGF versus observation, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Mean change in CRT at 12 months Show forest plot | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [‐18.08, 35.54] |
| Analysis 1.2  Comparison 1 Anti‐VEGF versus observation, Outcome 2 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.08, 0.15] |
| Analysis 2.1  Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 3 Persistent CSC at 12 months. | ||||
| 4 Mean change in CRT at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 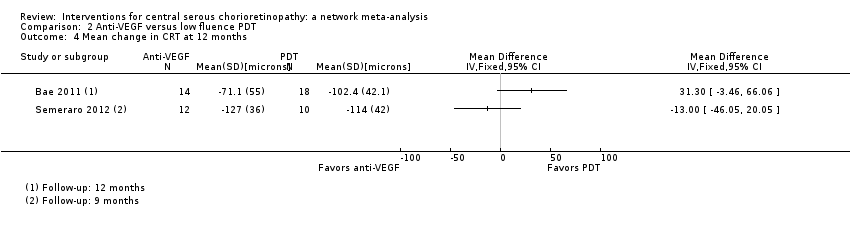 Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 4 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 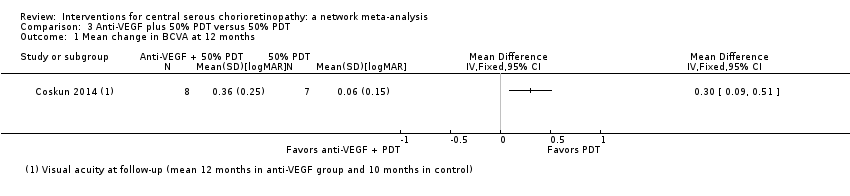 Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2 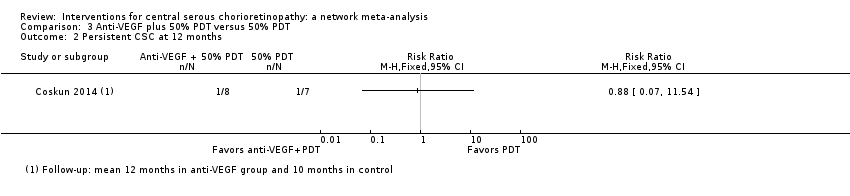 Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 2 Persistent CSC at 12 months. | ||||
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 3 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 2 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 50% PDT versus sham treatment, Outcome 1 Mean BCVA at 12 months. | ||||
| 2 Recurrence/persistence CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 50% PDT versus sham treatment, Outcome 2 Recurrence/persistence CSC at 12 months. | ||||
| 2.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3 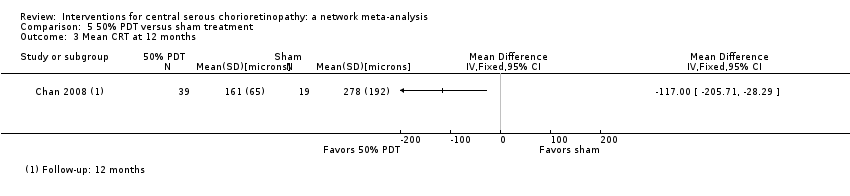 Comparison 5 50% PDT versus sham treatment, Outcome 3 Mean CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1 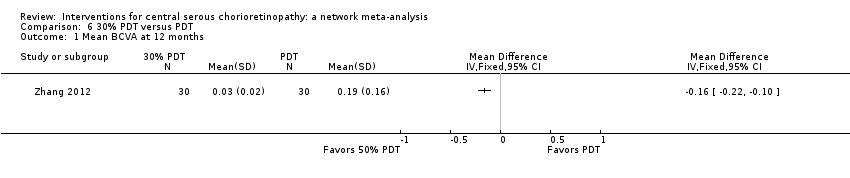 Comparison 6 30% PDT versus PDT, Outcome 1 Mean BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 30% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 30% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 50% PDT versus PDT, Outcome 1 Mean BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 50% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 50% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| Analysis 8.1  Comparison 8 30% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| Analysis 8.2  Comparison 8 30% PDT versus 50% PDT, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.3 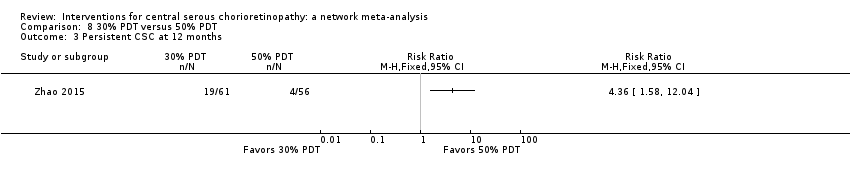 Comparison 8 30% PDT versus 50% PDT, Outcome 3 Persistent CSC at 12 months. | ||||
| 4 Mean change in CRT at 12 months Show forest plot | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | 44.90 [42.57, 47.23] |
| Analysis 8.4 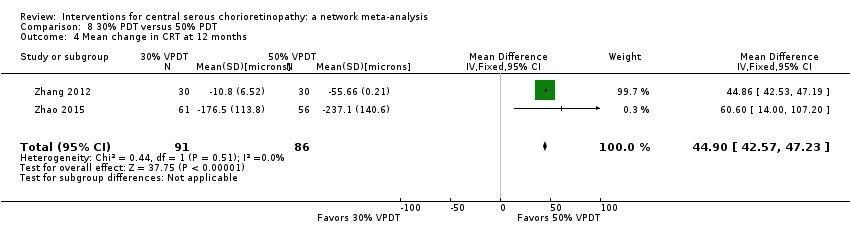 Comparison 8 30% PDT versus 50% PDT, Outcome 4 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1 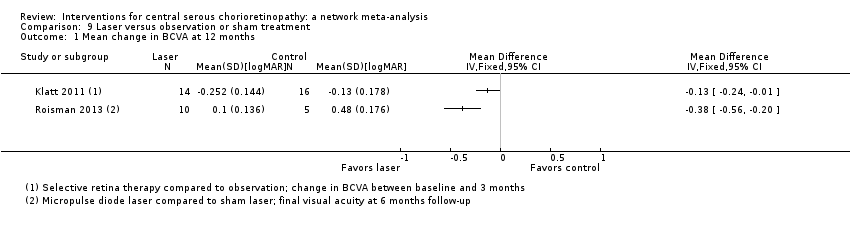 Comparison 9 Laser versus observation or sham treatment, Outcome 1 Mean change in BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.2 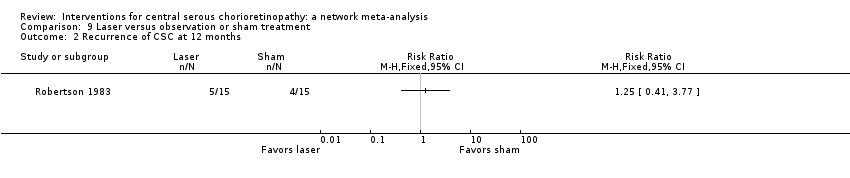 Comparison 9 Laser versus observation or sham treatment, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 Laser versus observation or sham treatment, Outcome 3 Mean change in CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 Indirect argon laser versus direct argon laser, Outcome 1 Recurrence of CSC at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Comparison of different laser wavelengths, Outcome 1 Recurrence of CSC at 12 months. | ||||
| 1.1 Yellow compared with red | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Yellow compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Red compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Antioxidant supplements versus placebo, Outcome 1 BCVA at 12 months. | ||||
| 2 Recurrence at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.2  Comparison 12 Antioxidant supplements versus placebo, Outcome 2 Recurrence at 12 months. | ||||
| 3 Persistence at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.3  Comparison 12 Antioxidant supplements versus placebo, Outcome 3 Persistence at 12 months. | ||||
| 4 CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.4 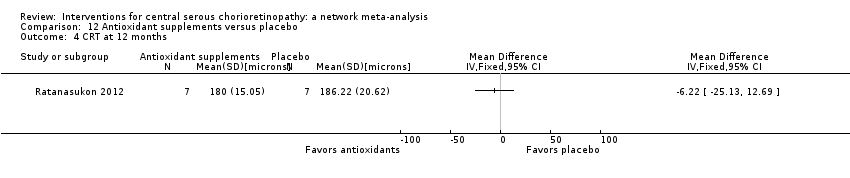 Comparison 12 Antioxidant supplements versus placebo, Outcome 4 CRT at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.1 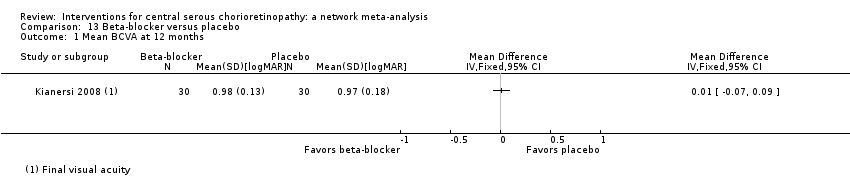 Comparison 13 Beta‐blocker versus placebo, Outcome 1 Mean BCVA at 12 months. | ||||
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.2  Comparison 13 Beta‐blocker versus placebo, Outcome 2 Recurrence of CSC at 12 months. | ||||
| 3 BCVA ≥ 20/40 at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.3  Comparison 13 Beta‐blocker versus placebo, Outcome 3 BCVA ≥ 20/40 at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent/persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.1  Comparison 14 Carbonic anhydrase inhibitors versus placebo, Outcome 1 Recurrent/persistent CSC at 12 months. | ||||
| 1.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.02] |
| Analysis 15.1  Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 1 Mean BCVA at 12 months. | ||||
| 2 Persistent CSC at 12 months Show forest plot | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.22] |
| Analysis 15.2 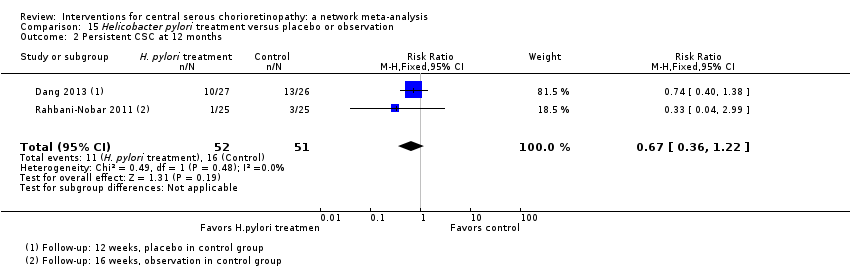 Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 2 Persistent CSC at 12 months. | ||||

Theoretical treatment network.
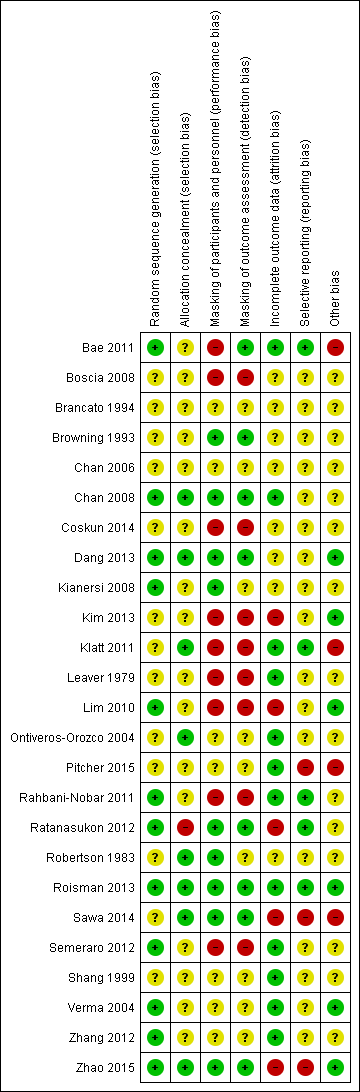
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Visual acuity network: network plot, interval plot, contribution matrix and risk of bias.
AVG: Anti‐VEGF PDT: photodynamic therapy LAS: laser AVPDT: anti‐VEGF plus PDT.
1 = control; 2 = anti‐VEGF; 3 = PDT; 4 = laser; 5 = anti‐VEGF plus PDT.
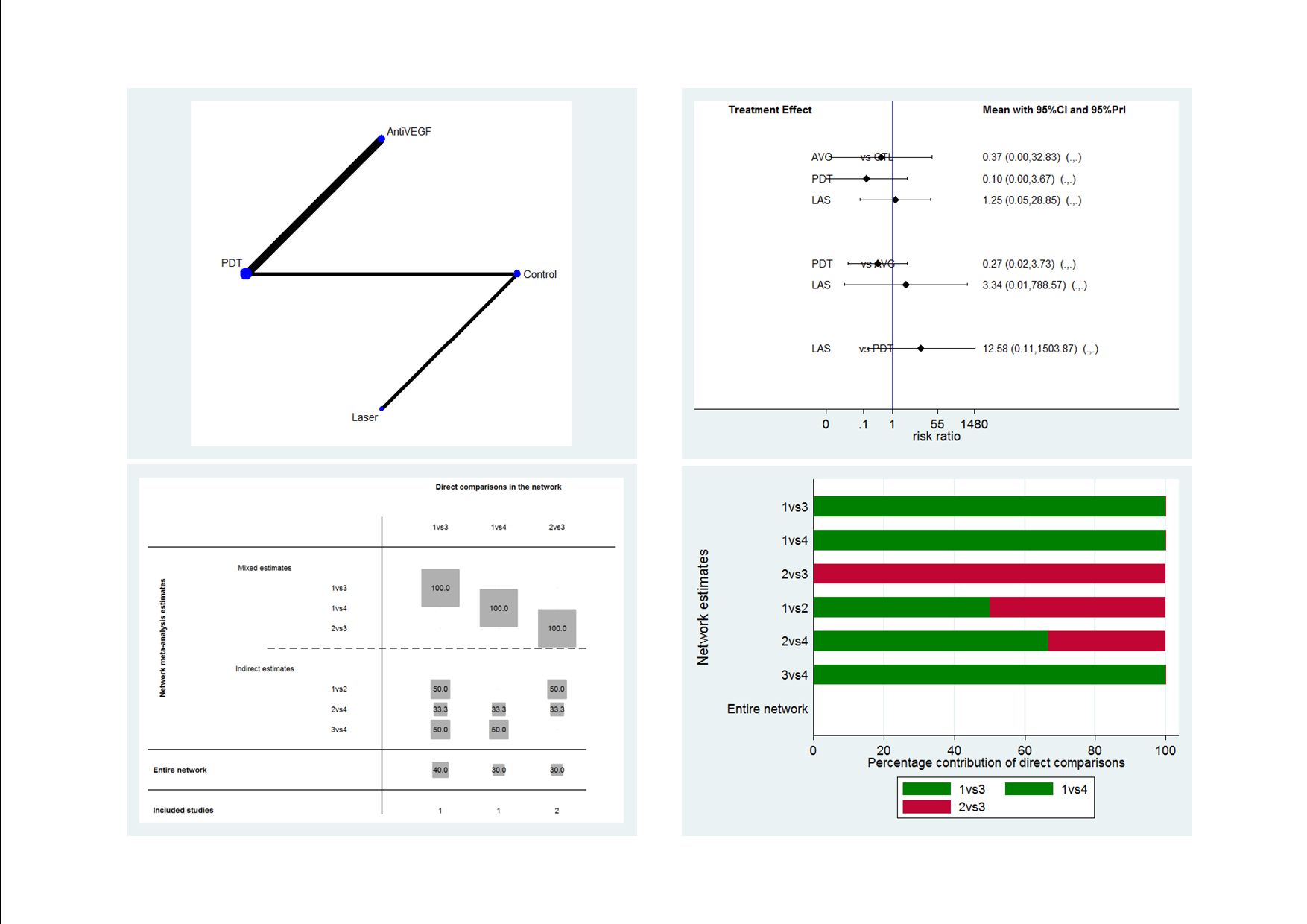
Recurrence CSC network: network plot, interval plot, contribution matrix and risk of bias.
AVG: Anti‐VEGF; PDT: photodynamic therapy; LAS: laser: CTL: control.
1 = control; 2 = anti‐VEGF; 3 = PDT; 4 = laser.

Comparison 1 Anti‐VEGF versus observation, Outcome 1 Mean change in BCVA at 12 months.

Comparison 1 Anti‐VEGF versus observation, Outcome 2 Mean change in CRT at 12 months.

Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 1 Mean change in BCVA at 12 months.

Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 2 Recurrence of CSC at 12 months.

Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 3 Persistent CSC at 12 months.

Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 4 Mean change in CRT at 12 months.

Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months.

Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 2 Persistent CSC at 12 months.

Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 3 Mean change in CRT at 12 months.

Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 1 Mean change in BCVA at 12 months.

Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 2 Mean change in CRT at 12 months.

Comparison 5 50% PDT versus sham treatment, Outcome 1 Mean BCVA at 12 months.

Comparison 5 50% PDT versus sham treatment, Outcome 2 Recurrence/persistence CSC at 12 months.

Comparison 5 50% PDT versus sham treatment, Outcome 3 Mean CRT at 12 months.

Comparison 6 30% PDT versus PDT, Outcome 1 Mean BCVA at 12 months.

Comparison 6 30% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months.

Comparison 6 30% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months.

Comparison 7 50% PDT versus PDT, Outcome 1 Mean BCVA at 12 months.

Comparison 7 50% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months.

Comparison 7 50% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months.

Comparison 8 30% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months.

Comparison 8 30% PDT versus 50% PDT, Outcome 2 Recurrence of CSC at 12 months.

Comparison 8 30% PDT versus 50% PDT, Outcome 3 Persistent CSC at 12 months.

Comparison 8 30% PDT versus 50% PDT, Outcome 4 Mean change in CRT at 12 months.

Comparison 9 Laser versus observation or sham treatment, Outcome 1 Mean change in BCVA at 12 months.

Comparison 9 Laser versus observation or sham treatment, Outcome 2 Recurrence of CSC at 12 months.

Comparison 9 Laser versus observation or sham treatment, Outcome 3 Mean change in CRT at 12 months.

Comparison 10 Indirect argon laser versus direct argon laser, Outcome 1 Recurrence of CSC at 12 months.

Comparison 11 Comparison of different laser wavelengths, Outcome 1 Recurrence of CSC at 12 months.

Comparison 12 Antioxidant supplements versus placebo, Outcome 1 BCVA at 12 months.

Comparison 12 Antioxidant supplements versus placebo, Outcome 2 Recurrence at 12 months.

Comparison 12 Antioxidant supplements versus placebo, Outcome 3 Persistence at 12 months.

Comparison 12 Antioxidant supplements versus placebo, Outcome 4 CRT at 12 months.

Comparison 13 Beta‐blocker versus placebo, Outcome 1 Mean BCVA at 12 months.

Comparison 13 Beta‐blocker versus placebo, Outcome 2 Recurrence of CSC at 12 months.

Comparison 13 Beta‐blocker versus placebo, Outcome 3 BCVA ≥ 20/40 at 12 months.

Comparison 14 Carbonic anhydrase inhibitors versus placebo, Outcome 1 Recurrent/persistent CSC at 12 months.

Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 1 Mean BCVA at 12 months.

Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 2 Persistent CSC at 12 months.
| Interventions for central serous chorioretinopathy: direct comparisons | ||||||
| Patient or population: people with central serous chorioretinopathy Settings: eye hospital | ||||||
| Comparison (intervention vs. comparator) | Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | Comments | |||
| Relative effect | No of participants | Quality | ||||
| Risk with comparator | Mean difference (95% CI) Negative values are in favor of intervention; positive values in favor of comparator | |||||
| Change in visual acuity at 12 months (logMAR) | ||||||
| Anti‐VEGF vs. observation | ‐ | 0.01 LogMAR (‐0.02 to 0.03) | ‐ | 64 (2) | Low1,2 | Both studies enrolled participants with acute CSC and reported mean change in visual acuity at 6 months |
| Anti‐VEGF vs. low‐fluence PDT | ‐ | 0.03 logMAR (‐0.08 to 0.15) | ‐ | 56 (2) | Low1,2 | Both studies enrolled participants with chronic CSC |
| Anti‐VEGF and 50% PDT vs. 50% PDT | ‐ | 0.30 logMAR (0.09 to 0.51) | ‐ | 15 (1) | Low1,2 | Participants had chronic CSC |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | ‐ | ‐0.02 logMAR (‐0.31 to 0.27) | ‐ | 12 (1) | Low1,2 | Participants had chronic CSC and were followed to 6 months |
| 50% PDT vs. observation or sham treatment | ‐ | ‐0.10 logMAR (‐0.18 to ‐0.02) | ‐ | 58 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | ‐ | ‐0.16 logMAR (‐0.22 to ‐0.10) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| 30% PDT vs. 50% PDT | ‐ | ‐0.12 logMAR (‐0.15 to ‐0.08) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| 50% PDT vs. PDT | ‐ | 0.04 logMAR (‐0.04 to 0.12 | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| Selective retina therapy vs. observation | ‐ | ‐0.13 logMAR (‐0.24 to ‐0.01) | ‐ | 30 (1) | Low1,2 | Participants had acute CSC, followed up to 3 months |
| Micropulse diode laser vs. sham laser | ‐ | ‐0.38 logMAR (‐0.56 to ‐0.20) | ‐ | 15 (1) | Low1,2 | Participants had chronic CSC |
| Antioxidant vs. placebo | ‐ | 0.01 logMAR (‐0.04 to 0.06) | ‐ | 14 (1) | Low1,2 | Lutein and acute CSC |
| Propranolol vs. placebo | ‐ | 0.01 logMAR (‐0.07 to 0.09) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| Carbonic anhydrase inhibitors vs. placebo | See comment | ‐ | ‐ | 13 (1) | ‐ | Outcome not reported |
| Helicobacter pylori treatment vs. placebo | ‐ | ‐0.04 logMAR (‐0.07 to ‐0.02) | ‐ | 103 (2) | Low1,2 | Participants had acute CSC, follow‐up 12‐16 weeks |
| Comparison (intervention vs. comparator) | Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | Comments | |||
| Risk with comparator* | Risk with intervention | Relative effect (95% CI) | No of participants | Quality | ||
| Persistent CSC at 12 months | ||||||
| Anti‐VEGF vs. observation | See comment | ‐ | ‐ | 64 (2) | ‐ | Participants had acute CSC. Both trials reported that all participants in treatment and control groups were resolved by 6 months |
| Anti‐VEGF vs. low‐fluence PDT | 111 per 1000 | 688 per 1000 (179 to 1000) | RR 6.19 (1.61 to 23.81) | 34 (1) | Low1,2 | Participants had chronic CSC |
| Anti‐VEGF and 50% PDT vs. 50% PDT | 143 per 1000 | 126 (10 to 1000) | RR 0.88 (0.07 to 11.54) | 15 (1) | Very low1,2,3 | Participants had chronic CSC |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | See comment | ‐ | ‐ | 12 (1) | ‐ | Outcome not reported |
| 50% PDT vs. sham treatment | 211 per 1000 | 25 per 1000 (2 to 215) | RR 0.12 (0.01 to 1.02) | 58 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 30% PDT vs. 50% PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 50% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| Selective retina therapy vs. observation | See comment | ‐ | ‐ | 30 (1) | ‐ | Outcome not reported |
| Micropulse diode laser | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| Antioxidant vs. placebo | See comment | ‐ | ‐ | 51 (1) | ‐ | People in the antioxidant group were less likely to have "complete resolution" at 3 months (RR 0.35, 95% CI 0.13 to 0.95; 51 participants) |
| Propranolol vs. placebo | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| Brinzolamide vs. placebo | 167 per 1000 | 48 (2 to 1000) | RR 0.29 (0.01 to 6.07) | 13 (1) | Very low2,3 | Participants had acute CSC |
| Helicobacter pylori treatment vs. placebo | 314 per 1000 | 210 (113 to 383) | RR 0.67 (0.36 to 1.22) | 103 (2) | Low1,2 | Participants had acute CSC |
| Comparison (intervention vs. comparator) | Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | ||||
| Risk with comparator* | Risk with intervention | Relative effect (95% CI) | No of participants | Quality | Comment | |
| Recurrent CSC at 12 months | ||||||
| Anti‐VEGF vs. observation | See comment | ‐ | ‐ | 64 (2) | ‐ | Outcome not reported |
| Anti‐VEGF vs. low‐fluence PDT | See comment | ‐ | ‐ | 56 (2) | Very low1,2,4 | Participants had chronic CSC. The 2 studies had different results for this outcome (I2 = 71%). In Bae 2011, there was a much higher risk of recurrence in the anti‐VEGF group (ranibizumab) compared with the PDT group (RR 19.83, 95% CI 1.19 to 330.50; 21 eyes); in Semeraro 2012, there was also an increased risk of recurrence in the anti‐VEGF (bevacizumab) group but the size of the effect was much smaller and the CIs include 1 (no effect) (RR 1.46, 95% CI 0.59 to 3.58; 22 eyes) |
| Anti‐VEGF and 50% PDT vs. 50% PDT | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | See comment | ‐ | ‐ | 12 (1) | ‐ | Outcome not reported |
| 50% PDT vs. sham treatment | 267 per 1000 | 27 per 1000 (3 to 216) | RR 0.10 (0.01 to 0.81) | 53 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 30% PDT vs. 50% PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 50% PDT vs. PDT | 270 per 1000 | 338 per 1000 (154 to 737) | RR 1.25 (0.57 to 2.73) | 60 (1) | ‐ | Type of CSC not specified |
| Selective retina therapy vs. observation | See comment | ‐ | ‐ | 30 (1) | ‐ | Outcome not reported |
| Micropulse diode laser | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| Antioxidant vs. placebo | 143 per 1000 | 46 (4 to 456) | RR 0.32 (0.03 to 3.19) | 36 (1) | Very low2,3 | Participants had acute CSC |
| Propranolol vs. placebo | 167 per 1000 | 100 (27 to 382) | RR 0.60 (0.16 to 2.29) | 60 (1) | Low1,2 | Type of CSC not reported |
| Brinzolamide vs. placebo | 314 per 1000 | 140 (20 to 953) | RR 0.21 (0.03 to 1.43 | 13 (1) | Low1,2 | Participants had acute CSC |
| Helicobacter pylori treatment vs. placebo | See comment | ‐ | ‐ | 103 (2) | ‐ | Outcome not reported |
| Adverse effects | ||||||
| All studies reported no ocular or systematic adverse effects, or did not comment on adverse effects | ||||||
| anti‐VEGF: anti‐vascular endothelial growth factor; CI: confidence interval; CSC: central serous chorioretinopathy; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy; RR: risk ratio. * Risk was estimated from the comparator group in the included studies 1 Downgraded for imprecision (‐1) 2 Downgraded for risk of bias (‐1) 3 Downgraded for imprecision (‐2) 4 Downgraded for inconsistency (‐1) | ||||||
| Treatment comparison | Study | Type of CSC | Date study conducted | Industry sponsored |
| Anti‐VEGF vs. PDT | Chronic Chronic | 2009‐2012 2009‐2010 | Yes NR | |
| PDT vs. no treatment | Acute | 2004‐2005 | NR | |
| Laser vs. no treatment | Acute | 1977‐1981 | No | |
| One additional study for the comparison PDT vs. no treatment was reported in abstract form only and no data on outcome so was not included in the network meta‐analysis (Boscia 2008). anti‐VEGF: anti‐vascular endothelial growth factor; CSC: central serous chorioretinopathy; NR: not reported; PDT: photodynamic therapy. | ||||
| Anti‐VEGF | ‐0.08 (‐0.14 to ‐0.01) | ‐0.20 (‐0.30 to ‐0.11) | 0.00 (‐0.02 to 0.03) | 0.22 (0.01 to 0.44) |
| 0.08 (0.01 to 0.14) | PDT | ‐0.13 (‐0.24 to ‐0.01) | 0.08 (0.01 to 0.15) | 0.30 (0.09 to 0.51) |
| 0.20 (0.11 to 0.30) | 0.13 (0.01 to 0.24) | Laser | 0.21 (0.11 to 0.31) | 0.43 (0.19 to 0.66) |
| ‐0.00 (‐0.03 to 0.02) | ‐0.08 (‐0.15 to ‐0.01) | ‐0.21 (‐0.31 to ‐0.11) | Anti‐VEGF and PDT | 0.22 (0.00 to 0.44) |
| ‐0.22 (‐0.44 to ‐0.01) | ‐0.30 (‐0.51 to ‐0.09) | ‐0.43 (‐0.66 to ‐0.19) | ‐0.22 (‐0.44 to ‐0.00) | Control (no treatment or sham treatment) |
| Effect estimate is the mean difference (95% confidence interval). Negative values favor the first intervention. In the lower left hand triangle, the first intervention is anti‐VEGF, PDT, laser etc. In the upper right hand triangle, the first intervention is control, anti‐VEGF and PDT, laser etc. So, for example, visual acuity with anti‐VEGF was 0.22 logMAR units better than control 95% CI 0.44 better to 0.01 better. anti‐VEGF: anti‐vascular endothelial growth factor; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy. | ||||
| Treatment comparison | Study | Type of CSC | Date study conducted | Industry sponsored |
| Anti‐VEGF vs. no treatment | Acute Acute | 2010‐2011 2008 | No No | |
| Anti‐VEGF vs. PDT | Chronic Chronic | 2009‐2012 2009‐2010 | Yes NR | |
| Anti‐VEGF + PDT vs. PDT alone | Chronic | NR (published 2014) | NR | |
| PDT vs. no treatment | Acute | 2004‐2005 | NR | |
| Laser vs. no treatment | Acute Acute Chronic | 2007‐2008 1977‐1981 NR (published 2013) | NR No NR | |
| One additional study for the comparison PDT vs no treatment was reported in abstract form only and no data on outcome so was not included in the network meta‐analysis (Boscia 2008). anti‐VEGF: anti‐vascular endothelial growth factor; CSC: central serous chorioretinopathy; NR: not reported; PDT: photodynamic therapy. | ||||
| Anti‐VEGF | 0.27 (0.02 to 3.73) | 3.34 (0.01 to 788.57) | 2.67 (0.03 to 234.08) |
| 3.77 (0.27 to 52.94) | PDT | 12.58 (0.11 to 1503.87) | 10.07 (0.27 to 371.91) |
| 0.30 (0.00 to 70.79) | 0.08 (0.00 to 9.50) | Laser | 0.80 (0.03 to 18.46) |
| 0.37 (0.00 to 32.83) | 0.10 (0.00 to 3.67) | 1.25 (0.05 to 28.85) | Control |
| Effect estimate is the risk ratio (95% CI). anti‐VEGF: anti‐vascular endothelial growth factor; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| 2 Mean change in CRT at 12 months Show forest plot | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [‐18.08, 35.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.08, 0.15] |
| 2 Recurrence of CSC at 12 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean change in CRT at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence/persistence CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 2 Recurrence of CSC at 12 months Show forest plot | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| 3 Persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean change in CRT at 12 months Show forest plot | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | 44.90 [42.57, 47.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Yellow compared with red | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Yellow compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Red compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Persistence at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 CRT at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 BCVA ≥ 20/40 at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent/persistent CSC at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean BCVA at 12 months Show forest plot | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.02] |
| 2 Persistent CSC at 12 months Show forest plot | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.22] |