Esternotomía parcial versus total para el reemplazo de la válvula aórtica
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: prospective randomised controlled study Duration: 4 months No. of centres: single Location: Spain Setting: cardiac surgical centre Withdrawals: none Dates: not stated | |
| Participants | 40 consecutive participants undergoing first‐time elective isolated aortic valve replacement. Exclusion criteria: none Demographics [limited / full sternotomy] Number of participants: 40 [20 / 20] Mean age (± SD) (range): 64 ± 11 years (26 to 76 years) Gender: not stated Pathophysiology: 31 AS, 9 AR Severity of disease: not stated Mean risk score: [11.6 ± 5.0 / 11.4 ± 5.5] Mean left ventricular ejection fraction: [62.3 ± 11 / 64.9 ± 13] Diabetes mellitus: not stated Preoperative lung function % predicted FEV1 : [79 ± 14 / 81 ± 21] Preoperative lung function % predicted FVC: [79 ± 14 / 80 ± 20] Smoking status: not stated | |
| Interventions | Limited sternotomy: reversed L‐ or reversed J‐shaped mini‐sternotomy Modifications from full sternotomy: none stated | |
| Outcomes | Primary outcomes: cross‐clamp and pump times, time to extubation, chest drainage (24 hours), number of blood transfusions, ICU stay, and total postoperative length of stay Secondary outcomes: pain scores (daily) and cosmetic evaluation (discharge) Other reported outcomes: none | |
| Standard care | Standard care was aortic and right atrial cannulation, aprotinin, antegrade cold blood cardioplegia (through coronary ostia), and no left ventricular vent. Mechanical prostheses in most participants. No transoesophageal echocardiography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Envelope opened at time of surgery |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates were unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant demographics |
| Methods | Study design: prospective randomised controlled study Duration: 2 years No. of centres: single Location: Italy Setting: cardiac surgery centre Withdrawals: none Dates: January 1999 to July 2001 | |
| Participants | 80 consecutive participants with aortic valve pathology undergoing elective aortic valve replacement. Exclusion criteria: emergent surgery, concomitant coronary revascularisation, left ventricular ejection fraction < 25% or heavily calcified aorta Demographics [limited / full sternotomy] Number of participants: 80 [40/40] Mean age (± SD): [62.6 ± 9.5 years / 64 ± 12.4 years] Gender: not stated Pathophysiology (AS:AR:mixed): [12:8:20 / 10:7:23] Severity of disease (NYHA status): [2.7 ± 0.9 / 2.5 ± 0.7] Mean risk score: not stated Mean left ventricular ejection fraction: [57 ± 12 / 56 ± 13] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated | |
| Interventions | Limited sternotomy: reversed C‐ or reversed L‐shaped sternal incision with < 10‐cm skin incision Modifications from full sternotomy: none stated | |
| Outcomes | Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: in‐hospital death, re‐exploration for bleeding, mean mediastinal drainage or bleeding > 800 mL, blood transfusion, atrial fibrillation, atelectasis, respiratory insufficiency, sternal wound infection, sternal instability, mechanical ventilation time, oxygen requirements (pre‐ and postextubation), pain scores (1 and 12 hours), analgesia requirements, ICU stay, hospital stay, spirometry (5 days and 1 to 2 months) (follow‐up time in parentheses) | |
| Standard care | Standard care was normothermic CPB and aortic cross‐clamping with aortic and right atrial 2‐stage venous cannulation. Retrograde and ostial antegrade cold blood cardioplegia were given. A right superior pulmonary vent was used in all cases. Transverse or oblique aortotomies were utilised depending on valve choice rather than surgical approach. Transoesophageal echocardiography was employed in all cases. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and staff blinded to surgical incision |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants reported on |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant characteristics |
| Methods | Study design: prospective randomised controlled study Duration: 9 months No. of centres: 5 centres Location: Germany Setting: cardiac surgical centres Withdrawals: 6 (5 in minimally invasive group, 1 in full sternotomy group) Dates: May 2012 to Feb 2013 | |
| Participants | 100 participants with AS in 5 German centres. Inclusion criteria: logistic EuroSCORE < 20, NYHA ≥ 2 Exclusion criteria: pure AR, previous cardiac surgery, congenital true bicuspid valve (Sievers type 0), emergency surgery, left ventricular ejection fraction < 25%, recent myocardial infarction (≤ 90 days), or stroke or TIA ≤ 6 months Demographics [limited / full sternotomy] Number of participants: 100 randomised [46 / 48]; 6 dropouts: 1 randomised to full sternotomy withdrew; 5 randomised to minimally invasive surgery were unable to have the procedure Mean age (± SD): [73.0 ± 5.3 years / 74.2 ± 5.0 years] Male gender: [27 (58.7%) / 21 (43.7%)] Pathophysiology: AS with or without aortic insufficiency Severity of disease (NYHA ≥ III): [31 (67.4%) / 29 (60.4%)] Mean STS risk score: [1.6 ± 0.7 / 1.7 ± 0.6] Mean left ventricular ejection fraction: not stated Diabetes mellitus: [15 (32.6%) / 11 (22.9%)] Preoperative COPD: [6 (13.0%) / 7 (14.9%)] Smoking status: [22 (47.8%) / 12 (25.5%)] | |
| Interventions | Limited sternotomy: upper hemi‐sternotomy into 3rd or 4th intercostal space. Modifications from full sternotomy: percutaneous femoral venous cannulation if right atrial cannulation not possible. Use of rapid deployment aortic valve prosthesis ‐ Edwards Intuity valve (a stented, trileaflet bovine pericardial bioprosthesis with a balloon‐expandable cloth covered skirt frame). | |
| Outcomes | Primary outcomes: cross‐clamp and CPB time Secondary outcomes: haemodynamic performance, quality of life (EQ‐5D), NYHA class Safety outcomes: cardiac reoperation, thromboembolism, renal failure, paravalvular leak, permanent pacemaker insertion, resternotomy, major bleeding events, endocarditis, myocardial infarction, deep sternal wound infection, cerebrovascular accident, respiratory failure | |
| Standard care | Standard care was full sternotomy with ascending aortic and right atrial cannulation. Normothermic or mild hypothermic CPB with antegrade crystalloid, cold or warm blood cardioplegia was given. Transverse aortotomies were employed in all cases. CO2 field flooding was used. In all full‐sternotomy participants, the valve choices were conventional stented valves. | |
| Notes | Disclosure: sponsored by Edwards Lifesciences LLC. Manuscript facilitated by Edwards Lifesciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates were unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | High risk | Quote: "three [patients] who were randomized to MIS‐RADVR [minimally invasive surgical rapid‐deployment aortic valve replacement] eventually received a conventional valve because of problems with their anatomy". Comment: these participants appeared to have been excluded following randomisation and an intention‐to‐treat analysis may have identified difficulty with the minimally invasive approach. |
| Selective reporting (reporting bias) | Low risk | Relevant outcome measures reported. 4 secondary outcome measures described in pretrial protocol were not described in the final study publication, but these were not considered clinically important measures. |
| Other bias | High risk | Significant confounder as mini‐sternotomy utilised rapid‐deployment valve and full‐sternotomy employed standard surgical valves. Study funded by manufacturer. |
| Methods | Study design: prospective randomised controlled study Duration: 4 years No. of centres: single Location: France Setting: university hospital Withdrawals: 1 from full sternotomy group Dates: 2003 to 2007 | |
| Participants | 78 participants undergoing aortic valve replacement for stenotic, regurgitant, or mixed aortic valve disease by a single surgeon Inclusion criteria: adults, ASA grade ≤ 3, informed consent, left ventricular ejection fraction > 40% Exclusion criteria: redo, combined surgery, ASA ≥ 4, acute pulmonary oedema, COPD, endocarditis, chronic renal failure, antiplatelet use < 7 days before surgery, haemostatic abnormality Demographics [limited / full sternotomy] Number of participants: 78 randomised [38 / 39] Mean age (± SD): [70.9 ± 11.4 years / 70.8 ± 10.2 years] Male gender: [23 (60.5%) / 27 (69.2%)] Pathophysiology: 75% AS, 24% AR, 1% mixed Severity of disease: not stated Mean risk score: [5.4 ± 1.9 / 5.2 ± 1.8] Left ventricular ejection fraction > 50%: [36 (94.7%) / 34 (87.2%)] Diabetes mellitus: not stated Preoperative % predicted FEV1 : [73.9 ± 18.2 / 78.8 ± 21] Preoperative % predicted FVC: [81.1 ± 16.1 / 83.6 ± 19.4] Smoking status: not stated | |
| Interventions | Limited sternotomy: minimal sternotomy access via 6‐ to 10‐cm mid‐line skin incision and reversed L sternal incision Modifications from full sternotomy: none | |
| Outcomes | Primary outcomes: respiratory parameters Secondary outcomes: bleeding, transfusion, and pain status Other reported outcomes: intraoperative and postoperative blood loss, transfusion rates, CPB and cross‐clamp times, operation time, mechanical ventilation time, ICU stay, hospital stay, systemic inflammatory response syndrome, re‐exploration for bleeding, death, spirometry (1, 2, and 7 days), pain scores, cardiac output studies (follow‐up time in parentheses) | |
| Standard care | Standard care included routine anaesthesia, aprotinin prophylaxis, right atrial appendage and ascending aortic cannulation, and Bretschneider's cardioplegia solution. Aortic root vent only was employed. | |
| Notes | Funding: French Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 computer generated 6‐per‐block randomisation, designed by a statistician. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Unclear risk | Limited description of preoperative participant characteristics |
| Methods | Study design: prospective randomised controlled study Duration: not stated No. of centres: single Location: Germany Setting: university hospital Withdrawals: none Dates: not stated | |
| Participants | 40 consecutive participants scheduled for elective aortic valve replacement Exclusion criteria: stentless valves or pulmonary autograft, carotid stenosis > 50%, severe ascending aortic calcification, history of TIA or stroke, Alzheimer's or Parkinson's disease Demographics [limited / full sternotomy] Number of participants: 40 [20 / 20] Mean age (± SD): [65.7 ± 1.9 years / 64.3 ± 2.9 years] Male gender: [9 (45%) / 11 (55%)] Pathophysiology (AS:AR:mixed): [8:3:9 / 6:1:13] Severity of disease mean gradient: [57 ± 14 / 63 ± 15] Mean risk score: not stated Mean left ventricular ejection fraction: [64 ± 3 / 65 ± 2] Diabetes mellitus: [4(20%) / 3(15%)] Preoperative FEV1 : [2.3 ± 0.9 / 2.6 ± 0.8] Preoperative FVC: [3.0 ± 1.0 / 3.2 ± 1.0] Smoking status: not stated | |
| Interventions | Limited sternotomy: limited median skin incision (7 to 9 cm) and reversed L‐shaped upper partial sternotomy into 4th or 5th right intercostal space Modifications from full sternotomy: the venting and cardioplegia strategies in the minimally invasive cases were different. Different surgeons performed minimally invasive and full‐sternotomy operations. | |
| Outcomes | Primary outcomes: operative time, CPB and cross‐clamp time, postoperative ventilation, 24‐hour chest tube drainage, ICU stay, and hospital stay Secondary outcomes: spirometry (postoperative day 6 or 7), pain scores (days 2 to 3 and 6 to 7), neuropsychological and biochemical tests Other reported outcomes: none (follow‐up time in parentheses) | |
| Standard care | Standard care was propofol anaesthesia, ascending aorta and right atrial cannulation, apical left ventricular vent, antegrade and retrograde cold blood cardioplegia. Right temporary pacing wires. | |
| Notes | No conflict of interest or funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Unclear risk | Some confounding aspects of surgical techniques differing between 2 groups (vent and cardioplegia techniques) |
| Methods | Study design: prospective randomised controlled study Duration: not stated No. of centres:single Location: Egypt Setting: university hospital Withdrawals: none Dates: not stated | |
| Participants | 60 consecutive participants undergoing first‐time elective aortic valve replacement for either AS or AR Exclusion criteria: emergency surgery, left ventricular ejection fraction < 25%, heavily calcified ascending aorta, redo valve surgery, other associated valve lesions Demographics [limited / full sternotomy] Number of participants: 60 [30 / 30] Mean age (± SD): [22.9 ± 2.4 / 23.8 ± 3.5] Male gender: [16 / 15] Pathophysiology (AS:AR): [15:15 / 15:15] Severity of disease: not stated Mean risk score: not stated Mean left ventricular ejection fraction: [56 ± 2.3 / 55 ± 2.6] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated | |
| Interventions | Limited sternotomy: reversed L‐shaped mini‐sternotomy to the 3rd intercostal space Other modifications from full sternotomy: venous drainage not specified in methods but noted to be different for mini‐sternotomy group | |
| Outcomes | Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: pulmonary function tests (1 week and 1 month post), length of incision, operating time, CPB time, ventilation time, chest drainage at 24 hours, blood transfusions, ICU stay, total hospital stay, participant survey of cosmetic effect, analgesia use (follow‐up time in parentheses) | |
| Standard care | Standard care was aortic and right atrial cannulation, coronary ostial and root antegrade cold blood cardioplegia, main pulmonary artery or left atrial appendage venting. All participants received a St Jude Medical mechanical bileaflet prosthesis. | |
| Notes | No conflict of interests declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Closed envelope method |
| Allocation concealment (selection bias) | Low risk | Closed envelope method |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All cited and relevant outcome measures reported |
| Other bias | Low risk | |
| Methods | Study design: prospective randomised controlled study Duration: 18 months No. of centres:single Location: Austria Setting: university hospital Withdrawals: none Dates: July 1996 to December 1997 | |
| Participants | 120 adults requiring aortic valve procedures Exclusion criteria: acute endocarditis, concomitant procedures, reoperation Demographics [limited / full sternotomy] Number of participants: 120 [60 / 60] Median age (IQR): [65 (56 to 70) years / 65 (55 to 72) years] Male gender: [35 / 36] Pathophysiology (AS:AR): [55:5 / 54:6] Severity of disease AVA (IQR): [0.6 (0.5 to 0.7) / 0.6 (0.5 to 0.8)] Mean risk score: not stated Median left ventricular ejection fraction (IQR): [67 (60 to 71) / 63 (48 to 70)] Diabetes mellitus: not stated Preoperative lung function: not stated Smoking status: not stated | |
| Interventions | Limited sternotomy: mid‐line 8‐ to 10‐cm incision, L‐shaped sternotomy to 3rd or 4th right intercostal space Other modifications from full sternotomy: none | |
| Outcomes | Primary outcomes: not stated Secondary outcomes: not stated Other reported outcomes: cross‐clamp time, CPB time, operation time, postoperative ejection fraction, duration of ventilation, chest tube drainage at 24 hour, reoperation requirements, pericardial effusions, conversion to full sternotomy, arrhythmias, strokes, wound infection, sternal instability, sternal pain | |
| Standard care | Standard care was isoflurane anaesthesia with bolus fentanyl, ascending and right atrial cannulation, 30 to 32 °C hypothermia on CPB, right superior pulmonary vein or pulmonary artery venting, ostial antegrade St. Thomas' cardioplegia and transvenous pacing wires if required only. | |
| Notes | Only the first 10 participants had echocardiography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignation to surgeons, but no clear randomisation |
| Allocation concealment (selection bias) | Unclear risk | Concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Mortality, blood loss, deep sternal wound infection, re‐exploration, and postoperative atrial fibrillation rates are unlikely to be affected by absence of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported |
| Other bias | Low risk | |
AR: aortic regurgitation; AS: aortic stenosis; ASA: American Society of Anesthesiologists; AVA: aortic valve area; CPB: cardiopulmonary bypass; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICU: intensive care unit; IQR: interquartile range; NYHA: New York Heart Association; SD: standard deviation; TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomised | |
| Not randomised | |
| Not randomised | |
| Duplicate data | |
| Intervention group was robotic surgery. | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study (propensity matched) | |
| Observational study | |
| Observational study | |
| Prospective but not randomised | |
| Observational study | |
| Prospective but not randomised | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study (propensity matched) | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study Redo surgeries | |
| Observational study | |
| Mini‐thoracotomy | |
| Observational study | |
| Mini‐thoracotomy | |
| Observational study | |
| Observational study (propensity matched) | |
| Observational study | |
| Observational study (propensity matched) | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Port access | |
| Observational study | |
| Observational study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: randomised controlled trial Duration: 35 months No. of centres: single Location: UK Setting: cardiac surgical centre Dates: February 2014 to January 2017 |
| Participants | Adults receiving first‐time, non‐emergency, isolated AVR. Exclusion criteria: requiring concomitant cardiac procedure(s); haemoglobin level < 90 g/L; pregnant; unable to stop currently prescribed treatment affecting clotting; history of thrombophilia, thrombocytopenia, or other haematological conditions that would affect participation in the trial; infective endocarditis; prevented from having red blood cells and blood products according to a system of beliefs |
| Interventions | Limited sternotomy: manubrium‐limited mini‐sternotomy (intervention arm) involves a mid‐line incision in which the manubrium is divided from the sternal notch to just below the manubrio‐sternal junction. |
| Outcomes | Primary outcomes: proportion of participants who receive a red blood cell transfusion postoperatively within 7 days of AVR surgery. Secondary outcomes: proportion of participants who receive a red blood cell transfusion during the intraoperative period, postoperative period (from admission to cardiac intensive care unit to 7 days); number of red blood cell transfusion units per participant within the 7 days following AVR surgery; proportion of participants receiving platelet transfusion or receiving fresh frozen plasma transfusion within the 7 days following AVR surgery; total number of participants receiving any blood products and the number of units transfused within the 7 days following AVR surgery and during the entire hospital stay; mean and range of postoperative blood loss within 6 and 12 hours after surgery; reoperation rates following the end of index surgery; quality of life EuroQol (EQ‐5D‐3L, EQ‐VAS) measured at baseline, day 2, 6 weeks, and 12 weeks; mean day and range of days upon which participants are deemed 'fit for discharge' from hospital; healthcare utilisation to 12 weeks postsurgery; cost and cost effectiveness; adverse event profiles related to study procedures for each arm. |
| Notes | Currently in data analysis phase |
| Methods | Study design: randomised interventional treatment trial No. of centres: single |
| Participants | Participants aged > 18 years at the time of surgery; either sex; elective, first‐time, isolated AVR Exclusion criteria: documented poor left ventricular function or LVEF 30%; documented chest wall deformities; documented severe emphysema or COPD; current body mass index 35 kg/m2; concomitant cardiac surgery; redo surgery; median sternotomy indicated |
| Interventions | Comparing upper mini‐sternotomy to full median sternotomy as a surgical approach to first‐time isolated AVR. |
| Outcomes | Primary outcomes: total length of stay in hospital for the index AVR operation measured in days Secondary outcomes: fitness for discharge; health‐related quality of life and participant satisfaction at baseline, 6 weeks, 6 months, and 12 months using the 36‐item short form health survey (SF‐36) and Coronary Revascularization Outcome Questionnaire ‐ Coronary Artery Bypass Graft (CROQ‐CABG); heart function (LVEF) by echocardiography at baseline, day of discharge, and 6 months postsurgery; procedure time: total theatre time, cross‐clamp time, cardiopulmonary bypass time, blood loss, blood transfusion; respiratory function (forced expiratory volume in 1 second) by hand‐held spirometry at baseline, day 4, day of discharge, 6 weeks, and 6 months |
| Notes | Data collection completed in October 2015. Principle Investigator contacted (April 2016) to request results. |
| Methods | Study design: open‐label, randomised controlled trial Duration: 22 months No. of centres: single Location: Sweden Setting: cardiac surgical centre Dates: October 2013 to July 2015 |
| Participants | 40 consecutive participants undergoing first‐time elective isolated AVR. Exclusion criteria: LVEF < 0.45; coexisting severe valvular disorder; previous cardiac surgery; urgent or emergent surgery |
| Interventions | Limited sternotomy: either mini‐sternotomy or anterior right‐sided mini‐thoracotomy |
| Outcomes | Primary outcomes: tricuspid annular systolic plane excursion; right ventricular fractional area change; right ventricular dimensions; pulsed wave tissue Doppler right ventricular velocity (all at postoperative days 4 and 40) Secondary outcomes: not stated (follow‐up time in parentheses) |
| Notes | Principle investigator contacted for results (October 2016). |
AVR: aortic valve replacement; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Surgical Trauma After Partial Upper Hemisternotomy Versus Full Sternotomy Aortic Valve Replacement |
| Methods | Study design: open‐label, randomised, controlled trial Duration: 20 months No. of centres: single Location: Sweden Setting: cardiac surgical centre Dates: April 2014 to December 2016 |
| Participants | 40 participants scheduled for aortic valve replacement Inclusion criteria: aged ≥ 18 years; severe aortic stenosis defined as aortic valve area of < 1 cm2 or index area of 0.6 cm2/m2 by echocardiography; referred for medically indicated aortic valve replacement; sinus rhythm; provide written informed consent Exclusion criteria: left ventricular ejection fraction < 0.45; presence of any coexisting severe valvular disorder; previous cardiac surgery; urgent or emergent surgery |
| Interventions | Partial upper hemi‐sternotomy |
| Outcomes | Primary outcomes: interleukin‐6; interleukin‐8; interleukin‐10; tumour necrosis factor‐alpha. All postoperatively at 0 to 3 days |
| Starting date | April 2015 |
| Contact information | Peter Svenarud, MD, PhD +46 (0) 8 517 708 12 |
| Notes |
| Trial name or title | Minimally Invasive Versus Conventional Aortic Valve Replacement: a Long Term Registry (SATURNO) |
| Methods | Study design: prospective registry No. of centres: single Location: Italy Setting: cardiac surgical centre Dates: October 2014 to November 2018 |
| Participants | Estimated 1000 participants undergoing aortic valve replacement Inclusion criteria: participants undergoing isolated aortic valve surgery; written informed consent to the use of personal data Exclusion criteria: other associated cardiac surgery; emergency surgery |
| Interventions | Upper J‐ or T‐ mini‐sternotomy or right mini‐thoracotomy |
| Outcomes | Primary outcomes: cardiopulmonary bypass time during surgery; total duration of intensive care unit stay during hospital stay, usually lasting 1 to 2 weeks; blood transfusions during hospital stay, usually lasting 1 to 2 weeks Secondary outcomes: renal insufficiency (need for haemofiltration) during hospital stay, usually lasting 1 to 2 weeks; prolonged ventilation (longer than 24 hours) during hospital stay; re‐exploration for bleeding (need of surgical revision for bleeding) during hospital stay, usually lasting 1 to 2 weeks; sepsis during hospital stay, usually lasting 1 to 2 weeks; neurological complications (stroke or transient ischaemic attacks, or both) during hospital stay, usually lasting 1 to 2 weeks; in‐hospital mortality during hospital stay, usually lasting 1 to 2 weeks; 30‐day mortality 30 days after surgery. |
| Starting date | October 2014 |
| Contact information | Elisa Mikus, MD |
| Notes |
| Trial name or title | Quality of Life After Ministernotomy Versus Full Sternotomy Aortic Valve Replacement (QUALITY‐AVR) |
| Methods | Study design: single‐blind, all‐comer, randomised controlled trial Duration: 36 months No. of centres: single Location: Spain Setting: cardiac surgical centre Dates: March 2016 to March 2019 |
| Participants | 96 participants with isolated aortic valve replacement due to aortic stenosis Inclusion criteria: severe aortic stenosis referred for medically indicated isolated aortic valve replacement due to aortic stenosis in participants > 18 years Exclusion criteria: left ventricular ejection fraction < 40%, previous cardiac surgery, urgent/emergent surgery, infective endocarditis, need of concomitant procedures other than isolated Morrow miectomy and thorax deformity |
| Interventions | Minimally invasive aortic valve replacement with partial "J" upper hemi‐sternotomy through right 4th intercostal space |
| Outcomes | Primary outcomes: change from baseline Questionnaire EQ‐5D‐5L Index at 1, 6, and 12 months Secondary outcomes: change from baseline Questionnaire EQ‐5D‐5L visual analogue scale for pain at 1, 6, and 12 months; early postoperative combined end point of 6 complications at 1 month (all‐cause mortality, acute myocardial infarction, cerebrovascular or transient ischaemic accident, acute renal failure (Acute Kidney Injury Classification ≥ 2), nosocomial infections (pneumonia, early endocarditis, mediastinitis, sepsis) and need of any reintervention); SATISCORE Questionnaire (satisfaction in cardiac surgery) at 1 to 6 months; change from baseline Questionnaire EQ‐5D‐5L severity index at 1, 6, and 12 months; change from baseline Questionnaire EQ‐5D‐5L health index (severity index inverse) at 1, 6, and 12 months; late postoperative combined end point of 6 complications at 1 to 5 years (all‐cause mortality, acute myocardial infarction, cerebrovascular or transient ischaemic accident, acute renal failure (Acute Kidney Injury Classification ≥ 2), nosocomial infections (pneumonia, early endocarditis, mediastinitis, sepsis), and need of any reintervention); total in‐hospital and intensive care unit stay (in days) from date of surgery until the date of discharge or date of death from any cause, whichever came first, assessed up to 1 year; cardiopulmonary bypass time in minutes and cross‐clamp ischaemic heart time in minutes needed in the surgery day 1 after surgery; mechanical ventilatory support time needed after surgery in hours at 7 days; transfusional requirements (number of red packed cells, fresh frozen plasma, and platelets) for first 72 hours after surgery; New York Heart Association functional class scale for heart failure at baseline and 1, 6, and 12 months; heart failure status between participants (number of participants alive (survival)) at 6 to 12 months; first‐year mortality (number of participants alive (survival) at 5 years; 5‐year mortality; early postoperative combined end point of 4 complications at 1 month (all‐cause mortality, acute myocardial infarction, cerebrovascular or transient ischaemic accident, and acute renal failure (Acute Kidney Injury Classification ≥ 2); late postoperative combined end point of 4 complications at 1 to 5 years (all‐cause mortality, acute myocardial infarction, cerebrovascular or transient ischaemic accident, and acute renal failure (Acute Kidney Injury Classification ≥ 2) |
| Starting date | March 2016 |
| Contact information | Emiliano A Rodriguez‐Caulo, MD, PhD, FECTS +34 951032054 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
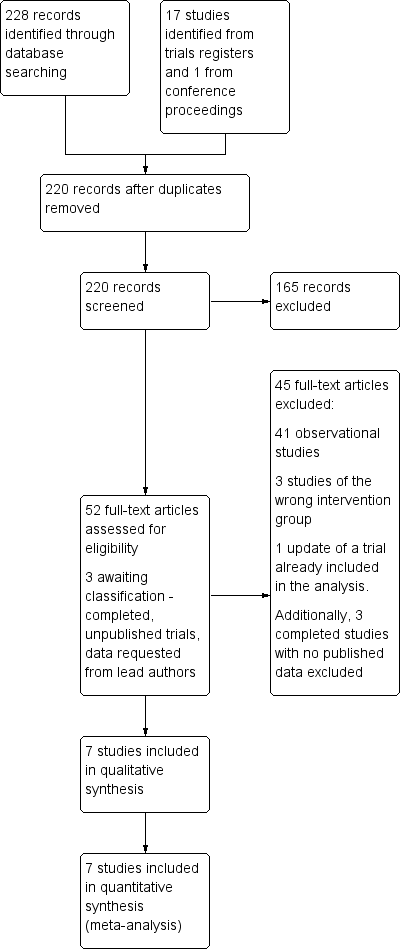
| 1 Mortality Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.82] |
| Analysis 1.1 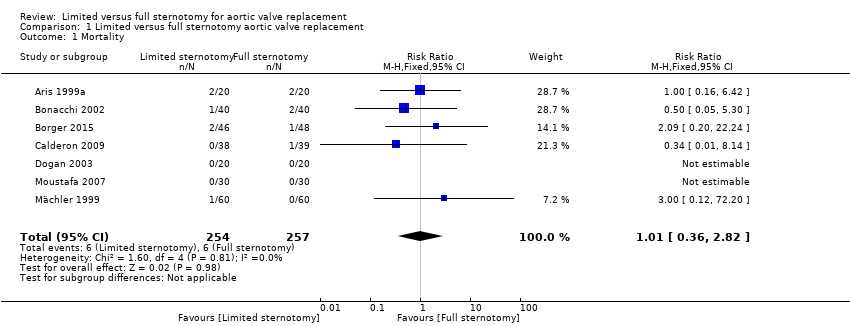 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 1 Mortality. | ||||
| 2 Cardiopulmonary bypass time (minutes) Show forest plot | 5 | 311 | Mean Difference (IV, Random, 95% CI) | 3.02 [‐4.10, 10.14] |
| Analysis 1.2 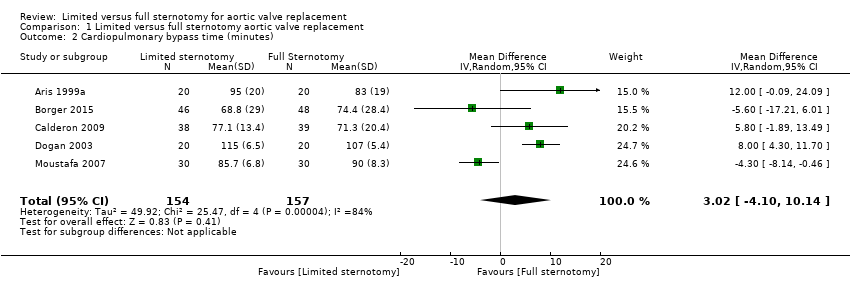 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 2 Cardiopulmonary bypass time (minutes). | ||||
| 3 Aortic cross‐clamp time (minutes) Show forest plot | 6 | 391 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐3.45, 5.35] |
| Analysis 1.3 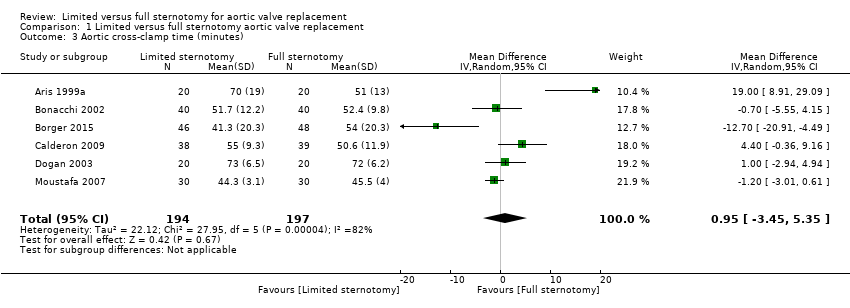 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 3 Aortic cross‐clamp time (minutes). | ||||
| 4 Length of hospital stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.63, 0.01] |
| Analysis 1.4  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 4 Length of hospital stay (days). | ||||
| 5 Postoperative blood loss (mL) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐158.00 [‐303.24, ‐12.76] |
| Analysis 1.5 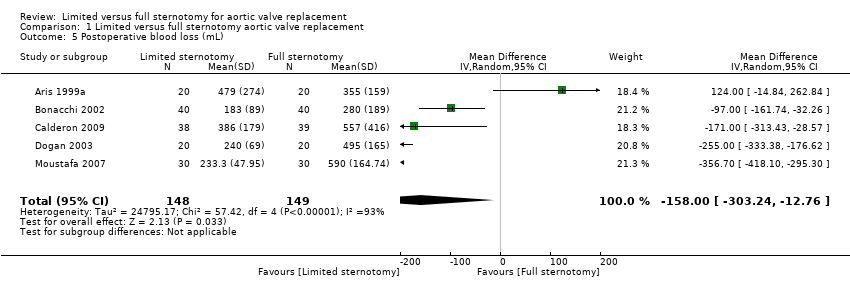 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 5 Postoperative blood loss (mL). | ||||
| 6 Deep sternal wound infection Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.30] |
| Analysis 1.6  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 6 Deep sternal wound infection. | ||||
| 7 Pain scores Show forest plot | 3 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| Analysis 1.7 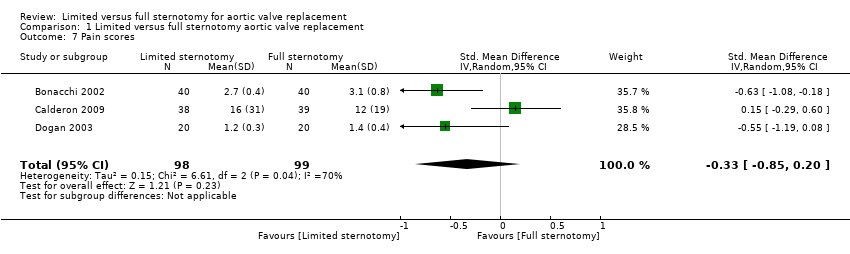 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 7 Pain scores. | ||||
| 8 Quality of life Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| Analysis 1.8  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 8 Quality of life. | ||||
| 9 Intensive care unit length of stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.93, ‐0.20] |
| Analysis 1.9  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 9 Intensive care unit length of stay (days). | ||||
| 10 Postoperative pulmonary function tests (% FEV1) Show forest plot | 4 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [0.62, 3.33] |
| Analysis 1.10  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 10 Postoperative pulmonary function tests (% FEV1). | ||||
| 11 Re‐exploration Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.13] |
| Analysis 1.11 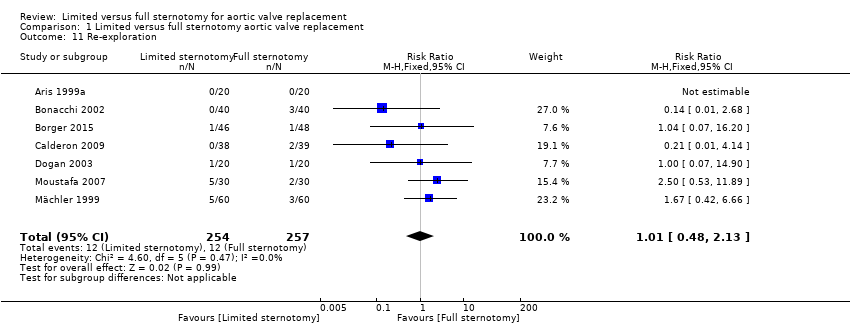 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 11 Re‐exploration. | ||||
| 12 Postoperative atrial fibrillation Show forest plot | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.07, 4.89] |
| Analysis 1.12 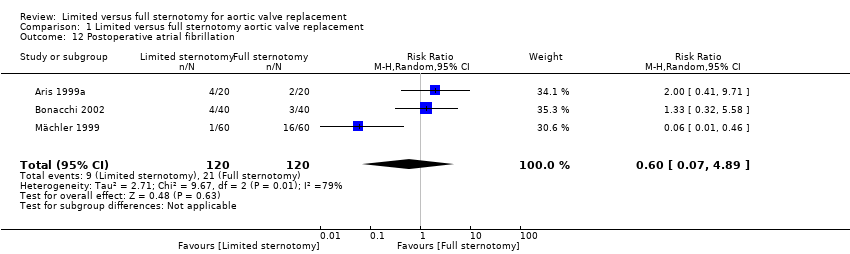 Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 12 Postoperative atrial fibrillation. | ||||
| 13 Postoperative ventilation time (hours) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐3.43, 1.19] |
| Analysis 1.13  Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 13 Postoperative ventilation time (hours). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
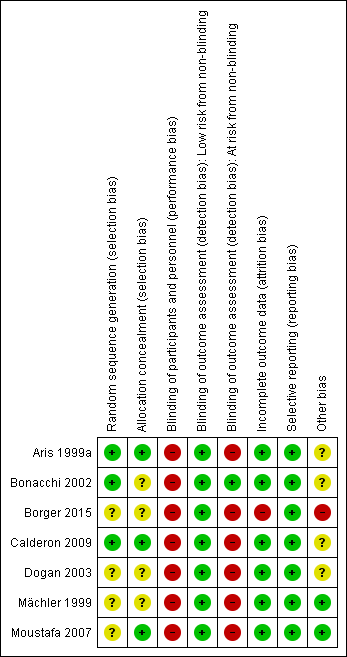
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 1 Mortality.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 2 Cardiopulmonary bypass time (minutes).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 3 Aortic cross‐clamp time (minutes).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 4 Length of hospital stay (days).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 5 Postoperative blood loss (mL).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 6 Deep sternal wound infection.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 7 Pain scores.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 8 Quality of life.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 9 Intensive care unit length of stay (days).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 10 Postoperative pulmonary function tests (% FEV1).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 11 Re‐exploration.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 12 Postoperative atrial fibrillation.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 13 Postoperative ventilation time (hours).
| Limited upper hemi‐sternotomy versus full median sternotomy for aortic valve replacement | ||||||
| Patient or population: participants requiring aortic valve replacement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with Full Sternotomy | Risk with Limited Sternotomy | |||||
| Mortality Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 24 per 1000 | |||||
| Cardiopulmonary bypass time | The mean cardiopulmonary bypass time ranged from 71 to 107 minutes | The mean cardiopulmonary bypass time in the intervention group was 3.02 minutes more (4.1 fewer to 10.14 more) | ‐ | 311 | ⊕⊕⊝⊝ | Cardiopulmonary bypass times tend to have high variability between surgeons according to surgical technique. Differences of up to 15 minutes are unlikely to have clinical significance. |
| Aortic cross‐clamp time | The mean aortic cross‐clamp time ranged from 46 to 72 minutes | The mean aortic cross‐clamp time in the intervention group was 0.95 minutes more (3.45 fewer to 5.35 more) | ‐ | 391 | ⊕⊕⊝⊝ | Ischaemic times tend to have high variability between surgeons according to surgical technique. Differences of up to 10 minutes are unlikely to have clinical significance. |
| Length of hospital stay Follow‐up: in‐patient stay | The mean length of hospital stay ranged from 6.0 to 9.3 days | The mean length of hospital stay in the intervention group was 1.31 days lower (2.63 lower to 0.01 higher) | ‐ | 297 | ⊕⊝⊝⊝ | Expediency of discharge is a quality marker in some healthcare systems, but not universally. |
| Postoperative blood loss Follow‐up: until removal of operative drains | The mean postoperative blood loss ranged from 280 mL to 590 mL | The mean postoperative blood loss in the intervention group was 158 mL lower (303 lower to 12 lower) | ‐ | 297 | ⊕⊕⊕⊝ | ‐ |
| Deep sternal wound infection Follow‐up: not specified | Study population | RR 0.71 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 17 per 1000 | |||||
| Pain scores Follow‐up: 12 hours | The mean pain scores ranged from 1.2 to 16 standard deviations | The mean pain scores in the intervention group was 0.3 standard deviations fewer (0.85 fewer to 0.2 more) | ‐ | 197 | ⊕⊝⊝⊝ | The assessment of pain within and across studies was insufficiently standardised to make strong conclusions about effect on pain |
| Intensive care unit length of stay | The mean intensive care unit stay was 1.4 to 2.1 days | The mean intensive care unit stay in the intervention group was 0.57 days lower (0.93 lower to 0.2 lower) | ‐ | 297 | ⊕⊕⊝⊝ | ‐ |
| Postoperative pulmonary function tests Follow‐up: 5 to 7 days | The mean pulmonary function tests ranged from 53% to 82% predicted FEV1 | The mean pulmonary function tests in the intervention group was 1.98% predicted FEV1 higher (0.62 higher to 3.33 higher) | ‐ | 257 | ⊕⊕⊝⊝ | ‐ |
| Re‐exploration Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 47 per 1000 | 47 per 1000 | |||||
| Postoperative atrial fibrillation Follow‐up: in‐patient stay | Study population | RR 0.60 | 240 | ⊕⊕⊝⊝ | ‐ | |
| 175 per 1000 | 105 per 1000 | |||||
| Postoperative ventilation time | The mean postoperative ventilation time ranged from 5.3 to 13.2 hours | The mean postoperative ventilation time in the intervention group was 1.12 hours lower (3.43 lower to 1.19 higher) | ‐ | 297 | ⊕⊝⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 4600 (to determine 1% difference using α 0.05, β 0.20). Studies all had fewer than 100 participants. 2 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 120 (to determine 15‐minute difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 3 Downgraded for inconsistency: use of rapid deployment valves in one study and other variations in surgical technique lead to high heterogeneity. 4 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 100 (to determine 10‐minute difference in mortality using α 0.05, β 0.80). Studies all had fewer than 100 participants. 5 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 140 (to determine 1‐day difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 6 Downgraded for indirectness: length of stay is a surrogate marker of quality and national variations exist in discharge criteria. 7 Downgraded for high risk of bias: outcome measure sensitive to lack of blinding in study. 8 Downgraded for inconsistency: variations in surgical or anaesthetic technique lead to high heterogeneity. 9 Downgraded for indirectness: different measures of pain used across studies. 10 Downgraded for inconsistency: different timing of postsurgical lung function tests across studies lead to high heterogeneity. 11 Downgraded for imprecision: wide 95% confidence intervals overlapping no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.82] |
| 2 Cardiopulmonary bypass time (minutes) Show forest plot | 5 | 311 | Mean Difference (IV, Random, 95% CI) | 3.02 [‐4.10, 10.14] |
| 3 Aortic cross‐clamp time (minutes) Show forest plot | 6 | 391 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐3.45, 5.35] |
| 4 Length of hospital stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.63, 0.01] |
| 5 Postoperative blood loss (mL) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐158.00 [‐303.24, ‐12.76] |
| 6 Deep sternal wound infection Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.30] |
| 7 Pain scores Show forest plot | 3 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| 8 Quality of life Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9 Intensive care unit length of stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.93, ‐0.20] |
| 10 Postoperative pulmonary function tests (% FEV1) Show forest plot | 4 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [0.62, 3.33] |
| 11 Re‐exploration Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.13] |
| 12 Postoperative atrial fibrillation Show forest plot | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.07, 4.89] |
| 13 Postoperative ventilation time (hours) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐3.43, 1.19] |