Short‐acting erythropoiesis‐stimulating agents for anaemia in predialysis patients
Information
- DOI:
- https://doi.org/10.1002/14651858.CD011690.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 09 January 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Kidney and Transplant Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
Draft the protocol: DH, EH
-
Study selection: DH, EH, NE
-
Extract data from studies: DH, EH, CE, NE
-
Enter data into RevMan: DH, CE, EH
-
Carry out the analysis: DH, EH, CE
-
Interpret the analysis: DH, EH
-
Draft the final review: DH, EH
-
Disagreement resolution: AW
-
Update the review: DH, EH
Declarations of interest
-
Deirdre Hahn: none known
-
Elisabeth M Hodson: none known
-
Angela C Webster: none known
-
Noha Elserafy: none known
-
Christopher Esezobor: none known
Acknowledgements
We acknowledge the assistance of the Cochrane Kidney and Transplant editorial office for their assistance with this review. We would also like to thank the referees for their feedback and advice during the preparation of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 09 | Short‐acting erythropoiesis‐stimulating agents for anaemia in predialysis patients | Review | Deirdre Hahn, Christopher I Esezobor, Noha Elserafy, Angela C Webster, Elisabeth M Hodson | |
| 2015 May 25 | Short‐acting erythropoiesis‐stimulating agents for anaemia in predialysis patients | Protocol | Deirdre Hahn, Christopher I Esezobor, Noha Elserafy, Angela C Webster, Elisabeth M Hodson | |
Differences between protocol and review
Removal of the comparison of short‐acting ESAs in evaluating the benefits and harms of different Hb or HCT targets in CKD patients receiving ESA treatment for anaemia as this is included in another systematic review (Strippoli 2006).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anemia [blood, *drug therapy];
- Epoetin Alfa [*administration & dosage];
- Erythropoietin [*administration & dosage];
- Hematinics [*administration & dosage];
- Hemoglobin A;
- Injections, Intravenous;
- Randomized Controlled Trials as Topic;
- Recombinant Proteins [administration & dosage];
- *Renal Dialysis;
- Renal Insufficiency, Chronic [*blood];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Epoetin alpha every 2 weeks versus weekly, Outcome 1 Change in haemoglobin level.
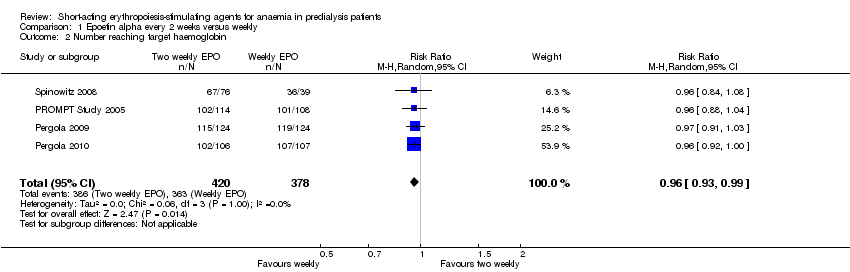
Comparison 1 Epoetin alpha every 2 weeks versus weekly, Outcome 2 Number reaching target haemoglobin.
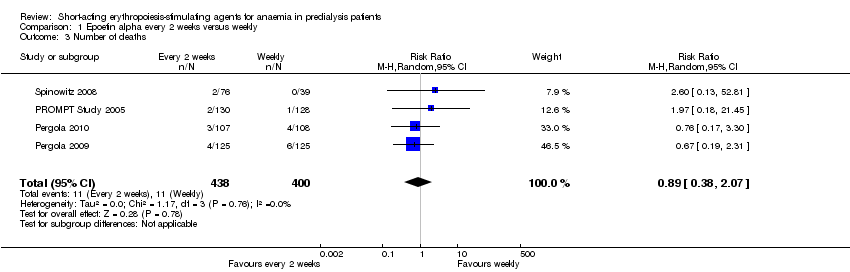
Comparison 1 Epoetin alpha every 2 weeks versus weekly, Outcome 3 Number of deaths.

Comparison 1 Epoetin alpha every 2 weeks versus weekly, Outcome 4 Adverse events.
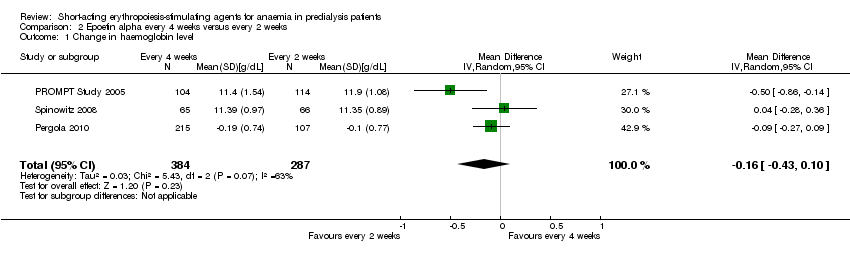
Comparison 2 Epoetin alpha every 4 weeks versus every 2 weeks, Outcome 1 Change in haemoglobin level.
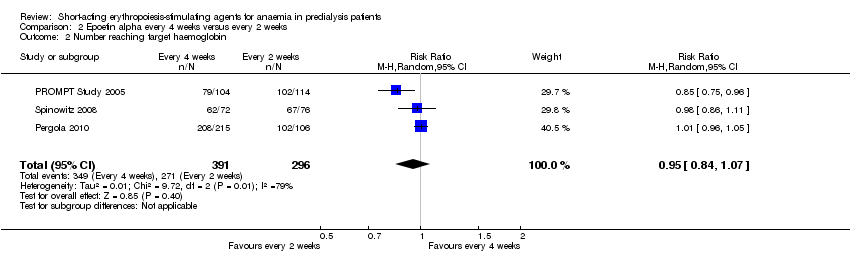
Comparison 2 Epoetin alpha every 4 weeks versus every 2 weeks, Outcome 2 Number reaching target haemoglobin.
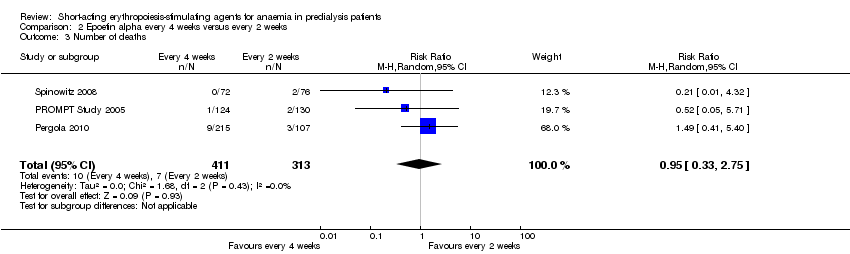
Comparison 2 Epoetin alpha every 4 weeks versus every 2 weeks, Outcome 3 Number of deaths.

Comparison 2 Epoetin alpha every 4 weeks versus every 2 weeks, Outcome 4 Adverse events.

Comparison 3 Epoetin alpha different doses given three times weekly, Outcome 1 Final haemoglobin.
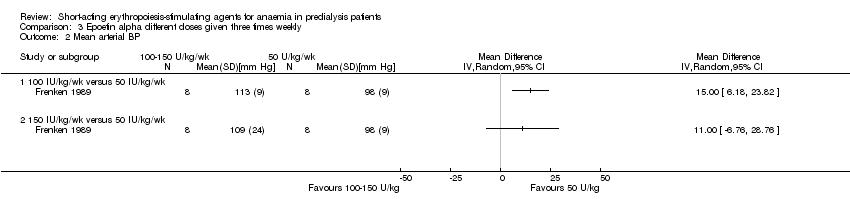
Comparison 3 Epoetin alpha different doses given three times weekly, Outcome 2 Mean arterial BP.

Comparison 3 Epoetin alpha different doses given three times weekly, Outcome 3 Final creatinine levels.

Comparison 4 Epoetin alpha different doses given every four weeks, Outcome 1 Final haemoglobin.

Comparison 4 Epoetin alpha different doses given every four weeks, Outcome 2 Number reaching target haemoglobin.

Comparison 4 Epoetin alpha different doses given every four weeks, Outcome 3 Number of deaths.

Comparison 4 Epoetin alpha different doses given every four weeks, Outcome 4 Adverse events.

Comparison 5 Epoetin alpha IV versus subcutaneous administration, Outcome 1 Final haemoglobin.
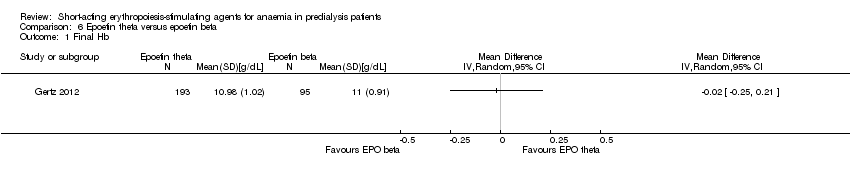
Comparison 6 Epoetin theta versus epoetin beta, Outcome 1 Final Hb.
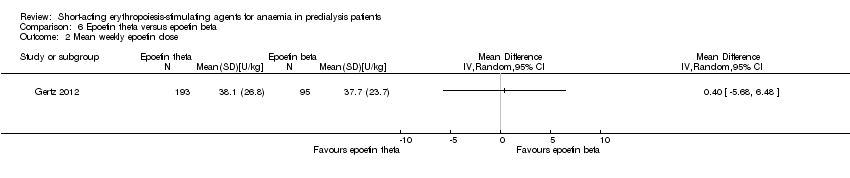
Comparison 6 Epoetin theta versus epoetin beta, Outcome 2 Mean weekly epoetin dose.
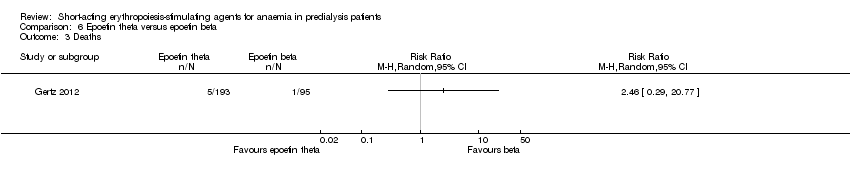
Comparison 6 Epoetin theta versus epoetin beta, Outcome 3 Deaths.

Comparison 6 Epoetin theta versus epoetin beta, Outcome 4 Adverse events.
| Epoetin alpha every 2 weeks versus to weekly for anaemia in CKD patients not receiving dialysis | ||||||
| Patient or population: anaemia in predialysis patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with weekly | Risk with Epoetin alpha every 2 weeks | |||||
| Change in Hb level | The mean change in Hb level was 0 g/dL | The mean change in Hb level in the intervention group was 0.19 g/dL lower (0.32 g/dL lower to 0.06 g/dL lower) | ‐ | 798 (4) | ⊕⊕⊝⊝ | downgraded for study limitations and indirectness |
| Number reaching target Hb | Study population | RR 0.96 | 798 (4) | ⊕⊕⊝⊝ | downgraded for study limitations and indirectness | |
| 960 per 1000 | 922 per 1000 | |||||
| Moderate | ||||||
| 947 per 1000 | 910 per 1000 | |||||
| Number of deaths | Study population | RR 0.89 | 838 (4) | ⊕⊕⊝⊝ | downgraded for study limitations and imprecision | |
| 28 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 22 per 1000 | 20 per 1000 | |||||
| Adverse events: RBC transfusions | Study population | RR 1.56 | 580 (3) | ⊕⊕⊝⊝ | downgraded for imprecision and study limitations | |
| 33 per 1000 | 52 per 1000 | |||||
| Moderate | ||||||
| 37 per 1000 | 58 per 1000 | |||||
| Adverse events: hypertension | Study population | RR 0.85 | 838 (4) | ⊕⊕⊕⊝ | downgraded for study limitations | |
| 100 per 1000 | 85 per 1000 | |||||
| Moderate | ||||||
| 95 per 1000 | 81 per 1000 | |||||
| Adverse events: thrombovascular events | Study population | RR 1.41 | 838 (4) | ⊕⊕⊝⊝ | downgraded for study limitations and imprecision | |
| 28 per 1000 | 39 per 1000 | |||||
| Moderate | ||||||
| 27 per 1000 | 38 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 allocation concealment unclear in 3 of 4 studies 2 surrogate outcome 3 few studies with low numbers and wide confidence 4 allocation concealment unclear in 2 of 3 studies | ||||||
| Epoetin alfa every four weeks versus with every two weeks in CKD patients not receiving dialysis | ||||||
| Patient or population: anaemia in predialysis patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with every 2 weeks | Risk with Epoetin alpha every 4 weeks | |||||
| Change in Hb level | The mean change in Hb level was 0 | The mean change in Hb level in the intervention group was 0.15g/dL lower (0.41 g/dL lower to 0.1g/dL more) | ‐ | 671 (3) | ⊕⊝⊝⊝ | downgraded for study limitations, heterogeneity and indirectness |
| Number reaching target Hb | Study population | RR 0.95 | 687 (3) | ⊕⊝⊝⊝ | downgraded for study limitations, heterogeneity and indirectness | |
| 916 per 1000 | 870 per 1000 | |||||
| Moderate | ||||||
| 895 per 1000 | 850 per 1000 | |||||
| Number of deaths | Study population | RR 0.95 | 724 (3) | ⊕⊕⊝⊝ | downgraded for study limitations, imprecision | |
| 22 per 1000 | 21 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 25 per 1000 | |||||
| Adverse events: RBC transfusions | Study population | RR 1.26 | 470 (2) | ⊕⊕⊝⊝ | downgraded for study limitations, imprecision | |
| 38 per 1000 | 48 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 44 per 1000 | |||||
| Adverse events: hypertension | Study population | RR 1.02 | 724 (3) | ⊕⊕⊕⊝ | downgraded for study limitations | |
| 70 per 1000 | 72 per 1000 | |||||
| Moderate | ||||||
| 62 per 1000 | 63 per 1000 | |||||
| Adverse events: arteriovenous complications | Study population | RR 1.02 | 724 (3) | ⊕⊕⊝⊝ | downgraded for study limitations, imprecision | |
| 26 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 23 per 1000 | 24 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 two of the three studies had unclear allocation concealment 2 surrogate outcome 3 unexplained heterogeneity 4 small numbers with wide confidence intervals | ||||||
| Epoetin theta versus epoetin beta in CKD patients not receiving dialysis | ||||||
| Patient or population: anaemia in predialysis patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with epoetin beta | Risk with Epoetin theta | |||||
| Final Hb | The mean final Hb was 0 g/dL | The mean final Hb in the intervention group was 0.02 g/dL lower (0.25 g/dL lower to 0.21 g/dL higher) | ‐ | 288 (1) | ⊕⊕⊕⊝ | downgraded for indirectness ‐ surrogate outcomes |
| Mean weekly epoetin dose | The mean weekly epoetin dose was 0 units/week | The mean weekly epoetin dose in the intervention group was 0.4 units per week higher (5.68 units per week lower 6.48 units/week higher) | ‐ | 288 (1) | ⊕⊕⊝⊝ | downgraded for indirectness ‐ surrogate outcomes and imprecision |
| Deaths | Study population | RR 2.46 | 288 (1) | ⊕⊕⊝⊝ | downgraded for imprecision | |
| 11 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 26 per 1000 | |||||
| Adverse events: hypertension | Study population | RR 0.35 | 288 (1) | ⊕⊕⊕⊝ | downgraded for imprecision | |
| 74 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| 74 per 1000 | 26 per 1000 | |||||
| Adverse events: RBC transfusions | Study population | RR 1.48 | 288 (1) | ⊕⊕⊝⊝ | downgraded for imprecision | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse events: discontinuation of therapy | Study population | RR 1.77 | 288 (1) | ⊕⊕⊝⊝ | downgraded for imprecision | |
| 53 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 93 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Surrogate outcome, not a patient‐centred outcome 2 Small numbers, wide confidence intervals | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in haemoglobin level Show forest plot | 4 | 785 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 2 Number reaching target haemoglobin Show forest plot | 4 | 798 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 3 Number of deaths Show forest plot | 4 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.38, 2.07] |
| 4 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RBC transfusions | 3 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.71, 3.45] |
| 4.2 Hypertension | 4 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.32] |
| 4.3 Thrombovascular events | 4 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.67, 3.00] |
| 4.4 Adverse events leading to discontinuation of therapy | 1 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in haemoglobin level Show forest plot | 3 | 671 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.43, 0.10] |
| 2 Number reaching target haemoglobin Show forest plot | 3 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 3 Number of deaths Show forest plot | 3 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.33, 2.75] |
| 4 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RBC transfusions | 2 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.53, 2.98] |
| 4.2 Hypertension | 3 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.62, 1.69] |
| 4.3 Arteriovenous complications | 3 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.39, 2.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final haemoglobin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 100 U/kg versus 50 U/kg | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 150 U/kg versus 50 U/kg | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean arterial BP Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 100 IU/kg/wk versus 50 IU/kg/wk | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 150 IU/kg/wk versus 50 IU/kg/wk | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Final creatinine levels Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 100 IU/kg/wk versus 50 IU/kg/wk | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 150 IU/kg/wk versus 50 IU/kg/wk | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final haemoglobin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Number reaching target haemoglobin Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number of deaths Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Thrombovascular events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 RBC transfusions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final haemoglobin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final Hb Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Mean weekly epoetin dose Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Deaths Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 RBC transfusions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Discontinuation of therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |


