Agentes uterotônicos para prevenir hemorragia pós‐parto: uma metanálise em rede
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between September 2006 and February 2009, 1964 parturients were randomised in a hospital setting in Egypt and South Africa. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with medical complications such as hypertension and diabetes, previous caesarean section, or an abdominal wall that was not thin enough to allow easy palpation of the uterus after delivery. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 1302) versus placebo or control (n = 662). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta. death. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to 1 of 3 groups by selecting the next number in a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | The allocated group was noted inside opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | In Assiut, investigators evaluated blood loss by collection with a calibrated plastic drape placed under the mother within 30 minutes of delivery. At the East London Hospital Complex, investigators evaluated blood loss by collection with a low profile plastic “fracture” bedpan placed under the mother. |
| Incomplete outcome data (attrition bias) | Low risk | Investigators were unable to collect outcome data from 14 randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN: 12609000372280). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 30) versus 400 mcg of misoprostol administered orally (n = 30). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intra‐operative blood loss by the estimation of attending physicians, and by measurement of preoperative and postoperative Hb concentration and haematocrit. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st July 2010 and 31st March 2011, 218 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with altered serum electrolytes, peritonitis, sepsis, previous bowel surgery, thyroid disease, inflammatory bowel disease, or chronic constipation. | |
| Interventions | 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 109) versus 600 mcg plus 5 IU of misoprostol plus oxytocin administered rectally plus by an intravenous bolus (n = 109). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation sequence was developed by 1 researcher (O.O.) using a computer‐generated table of random numbers with varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "The same researcher administered the drugs intra‐operation and set up the infusions in the operating room; he was the only person who was not blind to the drug allocation and he did not take any further part in the active running of the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction of labour or caesarean section, or those with haematocrit of less than 30%, pre‐eclampsia/eclampsia, grand multiparity (5 or more), multiple pregnancy, coagulopathy, or medical disorders. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 100) versus 400 mcg of misoprostol administered orally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into 2 groups, A and B, by blocked (restrictive) double‐blind randomisation using random table generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss at delivery by collection with a large kidney dish, for measurement in a graduated measuring jar. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 80 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with risk factors for excessive blood loss e.g. those with placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 40) versus 10 IU of oxytocin administered by an intravenous bolus (n = 40). | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was "single‐blind" but the identity of those blinded and the method of blinding were not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between October 2009 and February 2011, 120 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction of labour or instrumental delivery, or those with previous caesarean section, extensive perineal, vaginal or cervical lacerations, bleeding disorders, Hb less than 100 g/L, uterine malformations, grand multiparity, multiple pregnancy, polyhydramnios, intrauterine fetal death, medical problems such as pre‐eclampsia, diabetes, cardiopulmonary problems, bowel disease, or allergy to prostaglandins. | |
| Interventions | Placebo or control (n = 40) versus 200 mcg of misoprostol administered sublingually (n = 40) versus 5 IU of oxytocin administered intramuscularly (n = 40). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Investigators used closed envelopes. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Objective assessment of blood loss | Low risk | Assessor blinding was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Investigators evaluated blood loss by collection with sterile packs weighed beforehand and afterwards. |
| Selective reporting (reporting bias) | Unclear risk | "Following randomisation, 16 study participants were excluded from our analysis. Of these, 14 patients received intrapartum oxytocin, 1 patient experienced extensive vaginal laceration and another experienced a cervical laceration." |
| Intention to treat analysis | High risk | The protocol of the study was unavailable for verification. |
| Funding source | Unclear risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st December 1997 and 20th April 1998, 213 parturients were randomised in a hospital setting in Belgium. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertensive disorders, gestational age less than 32 weeks, intrauterine fetal death, uterine malformations, inflammatory bowel disease, obliterative vascular or coronary disease, sepsis, allergy to prostaglandins or alkaloids. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 105) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 108). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonic, transfusion, manual removal of placenta, nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated list and randomisation in blocks. |
| Allocation concealment (selection bias) | Low risk | The study box contained either 2 capsules of misoprostol and an ampoule containing placebo, or 2 capsules with placebo and an ampoule containing methylergometrine. The study boxes and capsules were indistinguishable in the 2 groups. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | High risk | "213 women were enrolled in the study, but the data for 13 were excluded because a caesarean section was performed after randomisation (n = 3), or because no predelivery (n = 3) or postpartum (n = 7, short hospital stay) blood sample was taken." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between May 2011 and May 2012, 200 parturients were randomised in a hospital setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, bleeding disorders, prolonged labour, placenta praevia, placental abruption, multiple pregnancy, BMI more than 30, or previous PPH. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 100) versus 800 mcg of misoprostol administered rectally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with special drapes placed under the mother until 1‐hour postpartum, and weighed beforehand and afterwards. Blood was also collected in graduated plastic bags. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between May 2009 and December 2009, 240 parturients were randomised in a hospital setting in Kuwait. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients less than 18 years old and those with known or suspected coagulopathy, grand multiparity (5 or more), uterine fibroids, polyhydramnios, multiple pregnancy, fetal macrosomia, severe anaemia, cervical tears or who required prophylactic oxytocin infusion. The presence of contraindications for the use of either Syntometrine or carbetocin that include pre‐existing hypertension, pre‐eclampsia, asthma, cardiac, renal or liver diseases, epilepsy, or history of hypersensitivity to Syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 120) versus 5 IU and 500 mcg of ergometrine plus oxytocin administered intramuscularly (n = 120). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated code prepared before the recruitment. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, consecutively‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a new plastic sheet placed under the mother following delivery of the placenta, and weighed (together with any gauzes, tampons and pads applied during the delivery) beforehand and 2 hours afterwards. A digital scale was used for weight measurement. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between November 2006 and July 2007, 377 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, gestational age less than 37 weeks performed for fetal or maternal distress where, due to time constraints, it was not possible to recruit or randomise, or those with multiple pregnancy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 188) versus 5 IU of oxytocin administered by an intravenous bolus (n = 189). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, morbidity,. additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, vomiting, headache, tachycardia, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence (1:1 ratio—blocks of 10, no stratification) was generated by computer. |
| Allocation concealment (selection bias) | Low risk | The preparation of the ampoules was undertaken by DHP Ltd. (Powys, UK) which provided sequentially numbered and labelled boxes each containing a 1 mL ampoule of the study drug. All boxes and ampoules were identically labelled, with the study number being the only differentiating feature between different drug packs. The random allocation sequence was not known to the investigators until the study had finished and the analysis was started. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Blood loss was estimated by the attending surgeon "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (EudraCT 2005‐002812‐94). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | Ferring Pharmaceuticals funded the cost of preparation of blinded medication ampoules. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 23rd September 2012 and 9th September 2013, 1140 parturients were randomised in a hospital setting in Uganda. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or elective caesarean section, or those with intrauterine fetal death, heart disease, severe malaria or acute bacterial infection, multiple pregnancy, antepartum haemorrhage, altered cognitive status or reported hypersensitivity to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 570) versus 600 mcg of misoprostol administered sublingually (n = 570). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level.. third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A study biostatistician generated a randomisation list with a block size of 10. |
| Allocation concealment (selection bias) | Low risk | The study clinical pharmacist prepared the study drugs and placebos. The midwife research assistants received opaque envelopes with affixed study codes, containing both an injection (1 mL of oxytocin 10 IU or its placebo) and 3 pills (misoprostol 600 mg or its placebo). |
| Blinding of participants and personnel (performance bias) | Low risk | "To achieve blinding of the participants and assessors, both inactive agents were manufactured and packaged to resemble actual study medicines in terms of shape, size, and colour." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a clean plastic sheet placed under the mother during and after the third stage of labour. The sheet was specifically designed and piloted for the purpose. Blood was then drained into a calibrated container to improve accuracy in blood loss measurement. Furthermore, "mothers were given pre‐weighed standard sanitary pads to place in the perineum at all times. These pads were changed and weighed hourly for the first 6 hours, and then every 6 hours until 24 hours postpartum. Blood loss was estimated as 1 mL per g of weight of the pad after subtracting the dry pad weight." Investigators added the estimated blood loss in pads, to the volume of blood already collected with the plastic sheet. To improve consistency in the estimation of blood loss, standardised electronic scales were used to weigh soiled sanitary pads. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01866241). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by scholarship funding from the Father Bash Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st April 2009 and 31st December 2009, 264 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients in the second or third stage of labour, or those with cervical lacerations or coagulopathy. | |
| Interventions | 30 IU of oxytocin administered by an intravenous bolus plus infusion (n = 132) versus 20 IU plus 600 mcg of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 132). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code produced by an independent statistician using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially numbered sealed packets made of identical opaque brown‐paper envelopes prepared by the hospital pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape "which is a sterile intrapartum blood collection mat with a calibrated receptacle" placed under the mother after the delivery of the baby and immediate clamping of the umbilical cord. The drape included ribbons tied around the abdomen of the mother to optimise blood collection. |
| Incomplete outcome data (attrition bias) | Low risk | "6 women from the misoprostol group and 3 from the oxytocin group were excluded from statistical analysis. 5 of these women in the misoprostol group and all 3 in the oxytocin group were excluded because of the occurrence of cervical lacerations in |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was conducted without external funding. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 12th June 2005 and 18th December 2006, 48 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients requiring general anaesthesia, or those with cardiac disease, hypertension or any condition predisposing to uterine atony and PPH, such as placenta praevia, multiple pregnancy, pre‐eclampsia, macrosomia, polyhydramnios, uterine fibroids, bleeding disorders, chorioamnionitis, previous uterine atony, previous PPH or allergy/hypersensitivity to oxytocin or ergot derivatives. | |
| Interventions | 250 mcg plus 20 IU of ergometrine plus oxytocin administered by an intravenous bolus (n = 24) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 24). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), nausea, vomiting, hypertension, tachycardia. hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used consecutively‐numbered opaque sealed packets or envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by measurement of haematocrit preoperatively and 48 hours postoperatively. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 550 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 275) versus placebo or control (n = 275). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, manual removal of placenta, third‐stage duration (min), vomiting, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed, opaque containers containing 400 mg misoprostol or placebo tablets. |
| Blinding of participants and personnel (performance bias) | High risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. Blinding of the midwife administering the tablets was therefore not possible." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with an absorbent plastic‐backed linen saver and a low‐profile plastic “fracture” bedpan placed under the mother. Blood collection in the plastic bedpan continued until 1 hour after delivery of the baby. At 1 hour after delivery, all the blood on the linen saver was scooped into the bedpan with the blood already collected there, and "the total blood was carefully measured." All the used linen savers and vaginal pads were weighed, and the known dry weights of these materials were subtracted from the measured total weight. |
| Incomplete outcome data (attrition bias) | Low risk | "Records of 4 of the 550 allocations (all from the placebo group) could not be traced." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 491 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 241) versus 500 mcg and 5 IU respectively of ergometrine plus oxytocin administered intramuscularly (n = 250). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Unclear risk | "About halfway through enrolment it was discovered that a small number of women had been excluded from the Syntometrine [ergometrine plus oxytocin] group because of hypertension detected after enrolment (thus contraindicating the use of Syntometrine [ergometrine plus oxytocin]. The Syntometrine envelopes had been reallocated to subsequent participants, and it was not possible to trace the women originally allocated." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 119 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 62) versus placebo or control (n = 57). | |
| Outcomes | The study recorded the following outcome: additional uterotonics. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between October 2000 and February 2004, 622 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta praevia, placental abruption, coagulopathy or unstable asthma. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 311) versus 400 mcg of misoprostol administered orally (n = 311). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation cards were produced. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "The packages were prepared by the hospital pharmacy and their active drug unknown to the physicians and nurses." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by a combination of the visual estimation of attending physicians and measurement of blood volume in a kidney dish placed under the mother during the third stage of labour. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Nova Scotia Health Research Foundation (public funding). |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between 1st October 1987 and 31st October 1988, 1429 parturients were randomised in a hospital setting in Ireland. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, vaginal breech or instrumental delivery, or those with hypertension, epidural anaesthesia, antepartum haemorrhage, placenta praevia, placental abruption, first stage of labour more than 15 hours, "quick" delivery or needing resuscitation. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 705) versus placebo or control (n = 724). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level. third‐stage duration (min), nausea, vomiting, hypertension, headache, aAbdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used. The first number was selected from the table and the numbers were then allocated in blocks of 100, following in sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "as accurately as possible, with full realisation of the well‐documented problems of clinical measuring and estimation." |
| Incomplete outcome data (attrition bias) | Low risk | No losses but dropouts for change in Hb |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by public funding, or conducted without external funding. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 652 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with medical disorders, in active labour with more than 4 cm dilatation or stillbirths. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 321) versus 10 IU of oxytocin administered intramuscularly (n = 331). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment with a 1:1 ratio using computer‐generated simple randomisation. |
| Allocation concealment (selection bias) | Low risk | The study medications and placebos were packaged in appropriately coded envelopes by administrative staff from the department of clinical pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape placed under the mother before delivery of the baby. "The calibrated blood collection receptacle was opened after delivery and drainage of amniotic fluid. The blood collected in the drape was transferred to a measuring jar with 10 mL calibrations for accuracy. Blood‐soaked swabs were weighed in g, and the known dry weight of the swabs was subtracted; this volume was added to the measured blood volume from the drape (assuming an equivalence of 1 g and 1 mL)." Blood loss was measured at 1 and 2 hours after delivery of the baby. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ClinicalTrials.gov NCT01373359). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Jawaharlal Nehru Medical College (the institution of the authors). Study medications were donated by Cipla (misoprostol) and AstraZeneca (oxytocin). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between November 1999 and June 2000, 602 parturients were randomised in a hospital setting in France. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks, previous PPH, intrauterine fetal death, previous uterine scar, multiple pregnancy or pre‐eclampsia. | |
| Interventions | Placebo or control (n = 220) versus 2.5 IU of oxytocin (n = 196) administered intramuscularly versus 600 mcg of misoprostol administered orally (n = 186). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), change in Hb level, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Slips with the words “control,” “Syntocinon,” and “Cytotec” were placed into envelopes which were then drawn at random upon admission into the delivery room to determine to which group the woman would belong. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by weighing (methods of collecting blood were not reported). |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between October 2000 and December 2002, 756 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with a bleeding disorder. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 377) versus 20 IU of oxytocin administered by an intravenous infusion (n = 379). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Agent vials were coded with a number, which had been assigned using a random number table. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque vials containing either a 200 mcg misoprostol tablet or placebo. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet." |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st September 2007 and 5th January 2008, 104 parturients were randomised in hospital settings in France and Italy. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with toxaemia, eclampsia or epilepsy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 52) versus 10 IU of oxytocin administered by an intravenous infusion (n = 52). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), vomiting, headache, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The patients were divided in 2 groups with blinding to the study medication." Blinding of caregivers was unconfirmed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "a sensitive colorimetric method." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The authors "do not have a financial relationship with the organisation that sponsored the research." No other source(s) of funding for the study were reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with heart disease or cardiac arrhythmia, hypertension or liver/renal/endocrine disease. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 29) versus 32.5 IU of oxytocin administered by an intravenous bolus plus infusion (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, death, blood loss (mL), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by a sensitive colorimetric measurement of the Hb concentration of blood loss collected "by means of aspiration from the operative field [that] began immediately after administration of the study drug and ceased at the time of skin closure. All gauzes used during this timeframe were placed in 15% Lyse solution. All aspirated blood, gauzes, and the reference blood sample were sent to the laboratory for quantification of total blood volume. Blood on gauzes was extracted with Lyse solution, and Hb content was determined with a sensitive colorimetric method adapted to the Cobas FARA analyser. Haemoglobin concentration is proportional to the absorbance of a hydrogen peroxide‐activated aminophenazone‐phenol mixture measured at a wavelength of 500 nm. The inter‐assay coefficient of variation averaged 3.3%, and the limit of detection of the assay was 14 mg/dL. The amount of blood collected in gauzes was calculated with the following formula: blood loss in dL = amount of Hb in surgical gauzes in mg /Hb concentration in mg/dL before caesarean section. Total blood loss was calculated by means of summing the volumes of blood aspirated and collected with gauzes." |
| Incomplete outcome data (attrition bias) | Low risk | "3 patients who received general instead of epidural anesthesia were excluded from the study and did not receive the study medication" but the study report did not specify whether these exclusions occurred before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 164 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients younger than 18 years old, or those without known PPH risk, known or suspected coagulopathy, heart disease or cardiac arrhythmia, chronic liver/renal/endocrine disease or hypersensitivity to study drugs. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 84) versus 10 IU of oxytocin administered by an intravenous infusion (n = 80). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), change in Hb level, nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used computer‐generated randomisation codes with a block size of 4. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used consecutively‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": "for each study subject, kits containing both the study medication and a placebo were prepared in the hospital pharmacy according to the randomisation schedule, to assure blinding of the clinical staff." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | High risk | "4 women did not receive study medication and were therefore not included in the analysis (3 were excluded as a result of caesarean births). Had these 4 women completed the study and received the medication, 1 would have received carbetocin and 3 would have received oxytocin. This factor contributed to the lower reported number of women receiving oxytocin." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 700 parturients were randomised in a hospital setting in Mozambique. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 350) versus 10 IU of oxytocin administered intramuscularly (n = 350). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Neither the investigators nor the nurses participating in the study had access to the codes until the completion of the study." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss with a metallic collector placed under the mother, from immediately after delivery of the baby until the mother was removed from the delivery room. |
| Incomplete outcome data (attrition bias) | Low risk | "A few subjects were excluded after randomisation for emergency caesarean section or incomplete data collection." |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of retained placenta were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | This study was financed by the Maputo Central Hospital (the institution of the authors) and the Special Program on Research and Research Training in Human Reproduction of the WHO (public funding). |
| Methods | 5‐arm placebo‐controlled randomised trial. | |
| Participants | Between July 2008 and April 2009, 75 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with active labour, ruptured membranes, drug allergy, multiple pregnancy, significant obstetric disease, risk factors for PPH (abnormal placentation, fibroids, previous PPH, previous classical uterine incision), coagulopathy or thrombocytopenia. | |
| Interventions | Placebo or control (n = 15) versus 5, 3, 1, or 0.5 IU of oxytocin administered by an intravenous bolus (n = 60). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), nausea, vomiting, tachycardia, hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using Microsoft Excel‐generated random number allocations. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque envelopes containing group assignments. |
| Blinding of participants and personnel (performance bias) | Low risk | "The obstetrician and anaesthetist involved in each case were blinded to the oxytocin dose assignments." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "by estimating blood collected by suction and by calculating the weight of blood on surgical swabs." |
| Incomplete outcome data (attrition bias) | Low risk | "75 patients were enrolled, and 74 patients completed the study; 1 patient was excluded due to protocol violation (obstetrician request for supplemental oxytocin despite adequate uterine tone)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the Department of Anaesthesia of the Stanford University School of Medicine (the institution of the authors). |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2000 and 1st October 2000,1633 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 407) versus 400 mcg of misoprostol administered rectally (n = 405) versus 10 IU of oxytocin administered by an intravenous infusion (n = 412) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly plus by an intravenous infusion (n = 409). | |
| Outcomes | The study recorded the following outcomes: PPH at 500. PPH at 1000, additional uterotonics, transfusion, change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "All medications were applied by midwives, but residents who treat[ed] the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Blinding of outcome assessment (detection bias) | Low risk | "All medications were applied by midwives, but residents who treat[ed] the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a sterile steel bedpan and plastic bed linen. Gauzes and pads were also collected and weighed until 1 hour after delivery of the placenta. |
| Incomplete outcome data (attrition bias) | Low risk | "The study enrolled 1633 women, but the data for 27 women were excluded because of lack of predelivery (n = 13) or postpartum (n = 14, short hospital stay) haemoglobin concentrations." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between January 2000 and October 2000, 1800 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an intravenous infusion (n = 450) versus 400 mcg of misoprostol administered orally (n = 450) versus 10 IU of oxytocin administered by an intravenous infusion (n = 450) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly plus by an intravenous infusion (n = 450). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration. The identity of medication was concealed from the resident physicians who managed the delivery and the third stage of labor. The caregivers and residents who followed up on the patient for the next 24 hours were also blind as to which patients received placebo and which received active medication. The randomisation code was not broken until the completion of the study." |
| Blinding of outcome assessment (detection bias) | Low risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration. The identity of medication was concealed from the resident physicians who managed the delivery and the third stage of labor. The caregivers and residents who followed up on the patient for the next 24 hours were also blind as to which patients received placebo and which received active medication. The randomisation code was not broken until the completion of the study." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a sterile steel bedpan and plastic bed linen from immediately after delivery. Gauzes and pads were also collected 1 hour after delivery of the placenta and weighed. |
| Incomplete outcome data (attrition bias) | High risk | "The data for 226 patients were excluded because of caesarean deliveries performed after randomisation (n = 206) and the lack of predelivery (n = 6) or postpartum (n = 14, short hospital stay) haemoglobin concentrations." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2007 and October 2008, 1410 parturients were randomised in a hospital setting in Spain. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with gestational age less than 32 weeks, coagulopathy, Hb less than 80 g/L, liver or kidney disorder, grand multiparity (5 or more), hypersensitivity or any contraindication for use of prostaglandins. | |
| Interventions | 400 mcg and 200 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually and rectally plus intramuscularly (n = 702) versus 10 IU of oxytocin administered intramuscularly (n = 698). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by computer. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes prepared by people not related to the study. This process was supervised by an analyst. Every morning a secretary received the sealed envelopes for distribution and this process was monitored by someone working on the study. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After delivery of the baby, investigators evaluated blood loss by collection with a sterile waterproof cloth placed under the mother, to channel blood into a bottle with capacity of 2 L: the volume reading was collected once beyond the third stage of labour. |
| Incomplete outcome data (attrition bias) | Low risk | "3 y 7 de las mujeres aleatorizadas a los grupos I y II, respectivamente, no recibieron ningún tratamiento por incumplimiento del protocolo y, como la información correspondiente a ellas no fue registrada en ningún momento, no forman parte de este informe": 3 women in the misoprostol plus active management group, and 7 women in the active management group, were excluded from the analysis due to protocol deviations and non‐availability of the information. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by the Science and Ethics Committee of the Hospital Eusebio Hernandez in Habana, Cuba in conjunction with the Clinica Mediterranea Medica in Valencia, Spain (the institutions of the authors). |
| Methods | 4‐arm controlled randomised trial. | |
| Participants | Between January 2005 and November 2008, 160 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with thyroid disorder, inflammatory bowel disease or other bowel diseases, previous bariatric surgery or hypersensitivity to prostaglandins. | |
| Interventions | 200 mcg, 400 mcg, or 600 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 120) versus 10 IU of oxytocin administered by an intravenous infusion (n = 40). | |
| Outcomes | The study recorded the following outcomes: fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st December 2007 and 31st May 2009, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section for cord prolapse or bradycardia, or those with cardiovascular, respiratory, liver or haematological disorders or known hypersensitivity to prostaglandins. | |
| Interventions | 800 mcg of misoprostol administered rectally (n = 100) versus 40 IU of oxytocin administered by an intravenous infusion (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer‐generated random numbers in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | The packets containing the 2 drugs were sealed and opaque, and could not be identified by the surgeons and anaesthetists. |
| Blinding of participants and personnel (performance bias) | Low risk | "The packets containing the 2 types of drug were sealed and opaque, and could not be identified by the surgeons and anaesthetist." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss by collection with a suction bottle for volumetric measurement, combined with linen savers and mops weighed before and after delivery. They added the approximate volume of the contents of the suction bottle (a) to the difference in weight between dry (b) and soaked (c) linen savers and mops (1 g equivalent to 1 mL). Amniotic fluid volume (d) was calculated by multiplying amniotic fluid index by 30 mL. Finally, intraoperative blood loss was determined by subtracting amniotic fluid volume from approximate blood loss ((a plus (c ‐ b)) ‐ d). Furthermore, investigators evaluated postoperative bleeding over the next 8 hours by weighing soaked pads and subtracting the dry weight. |
| Incomplete outcome data (attrition bias) | Low risk | "4 women in group 1 [misoprostol] and 6 women in group 2 [oxytocin] were excluded from the analysis: 4 women required conversion to general anaesthesia, 5 women had traumatic intraoperative bleeding (extension of lower segment incision or ligament hematoma), and 1 woman had placenta accreta resulting in hysterectomy." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000075). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st September 2009 and 31st August 2010, 530 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with risk factors for PPH, including BMI more than 30, grand multiparity (5 or more), polyhydramnios, fetal macrosomia, antepartum haemorrhage, prolonged labour, previous PPH, Hb less than 80 g/L, severe pre‐eclampsia, asthma or coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 265) versus 10 IU of oxytocin administered intramuscularly (n = 265). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used pre‐prepared sealed and opaque packet. |
| Blinding of participants and personnel (performance bias) | Low risk | "The misoprostol and placebo tablets were similar in size, shape, and colour. The ampoules of oxytocin and placebo were also similar. Selection, enrolment, and randomisation were done by the resident doctors, whereas preparation of packets and confidential record maintenance was done by the labour room nursing staff in charge." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with specially designed, pre‐weighed absorbent thick cotton pads with plastic lining, placed under the mother. Blood clots, if any, were expressed from the vagina into a polythene bag. Any episiotomy wound was repaired immediately, and the swabs used for the purpose of episiotomy were not included in blood loss assessment. If necessary, pads were replaced during the observational hour after delivery. Then the soaked pad(s) and the blood clots were weighed. "The specific gravity of blood being 1.08, the amount of blood lost in mL was approximately equal to the weight in g." |
| Incomplete outcome data (attrition bias) | Low risk | "2 women in the study group and 1 woman in the control group refused sublingual administration of the drug." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000672). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st October 2012 and 31st December 2013, 396 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients requiring conversion to general anaesthesia, or those with cardiovascular, hepatic, or haematological disorders or any contraindication for the use of misoprostol or oxytocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus and infusion (n = 198) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 198). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random number sequence and blocks of size 8. |
| Allocation concealment (selection bias) | Low risk | Assignments were contained in sealed, opaque and sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) | Low risk | "Randomisation and confidential record maintenance were performed by residents who were not involved in the trial, and the operation theatre midwife prepared the sealed packets and allocated and administered the drugs. Thus, clinicians, investigators, data analysts, and participants were masked to the treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss from after delivery of the placenta. Blood was collected with a suction bottle, linen savers and mops: the dry weights of these materials were subtracted from the soaked weights, and the total volume of intraoperative blood loss calculated on the basis that 1 g is equivalent to 1 mL. Investigators also evaluated postoperative blood loss by weighing soaked pads. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2013/05/003645). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 300 parturients were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with grand multiparity (more than 5), multiple pregnancy, pregnancy‐induced hypertension, antepartum haemorrhage, previous caesarean, Hb less than 80 g/L, other obstetric problems or known hypersensitivity to prostaglandins. | |
| Interventions | 100 mcg or 200 mcg of misoprostol administered sublingually (n = 200) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by "measuring blood and blood clots collected in sponges." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 991 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with medical conditions that precluded the use of ergometrine, such as pre‐eclampsia, cardiac disease or conditions that required prophylactic oxytocin infusion after delivery such as grand multiparity (4 or more) or presence of uterine fibroids. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 500) versus 10 IU of oxytocin administered by an intravenous bolus (n = 491). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "The preparation and administration of the medication was carried out by a second midwife who was not involved in the management of the patient except for the drug administration. The medical attendant who delivered the baby was not informed of the type of oxytocics used." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "by measuring the amount of blood clots and weighing the towels and swabs used." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between December 1997 and December 1998, 930 parturients were randomised in a hospital setting in Australia, Papua and China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with coagulopathy, asthma, heart disease, severe renal disease, epilepsy or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 455) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 310) versus 10 IU of oxytocin administered intramuscularly (n = 129). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by a random number list in blocks of 20, with separate randomisation for each centre. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered sealed security (opaque) envelopes containing the appropriate drug label for each centre. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by combining "estimated" and "measured" values according to the standard clinical practice of each study centre. The "estimated" blood loss was judged by the attending senior midwives and/or clinicians. The "measured" blood loss was calculated as the actual volume of blood collected in a calibrated measuring jug, combined with the difference in weight between dry and blood‐stained undersheets and sanitary pads. |
| Incomplete outcome data (attrition bias) | Low risk | Data were not collected completely from 67 study participants: "the main reasons for exclusion prior to randomisation, and following randomisation but before treatment, were the need for caesarean section and development of hypertension, either before or during labour. Two women (1 in each group) were not included in the analysis as no record was made of the primary outcome of blood loss." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between February 1992 and December 1994, 694 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing general anaesthesia or requiring a classical uterine incision, or those with heart disease, chronic hypertension requiring treatment, liver, renal, or endocrine disorders, coagulopathy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 348) versus 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 346). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated randomisation code, stratified by centre and with use of random blocks of 2. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "All physicians and nurses involved, all investigators and their staff, and all sponsor representatives were kept blinded to the treatment codes at all times." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Informed consent was obtained from 694 patients. 35 patients were withdrawn from the study before they received study drug, leaving a total of 659 patients who received [the] study drug and were included in the safety analysis." Major protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 196 parturients were randomised in a hospital setting in Indonesia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 98) versus 10 IU of oxytocin administered intramuscularly (n = 98). | |
| Outcomes | The study recorded the following outcomes: blood loss (mL), third‐stage duration (min), shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm placebo‐controlled randomised trial. | |
| Participants | Between July 1993 and July 1994, 371 parturients were randomised in a hospital and community setting in the Netherlands. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, requiring tocolysis or those who refuse to take part or with cardiac disease, multiple pregnancy, non‐cephalic presentation, polyhydramnios, coagulopathy, stillbirth, antepartum haemorrhage, Hb less than 4.8 mmol/L or previous complication in third stage. | |
| Interventions | Placebo or control (n = 143) versus 5 IU of oxytocin administered intramuscularly (n = 78). There were 4 exclusions post randomisation but it was unclear from which group. The oral ergometrine group was merged with the control group for analysis. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes. Care was taken that no difference could be seen or heard between the packages of the ergometrine/placebo tablets and the oxytocin ampoules. |
| Blinding of participants and personnel (performance bias) | High risk | The study was "double‐blind" with placebo to match ergometrine treatment, but "to allow comparison with a standard prophylactic regimen a third group receiving the standard intramuscular oxytocin was added, but for obvious reasons this could not be conducted in a blind manner." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a "fresh" perineal pad placed under the mother from immediately after birth until 1 hour after the delivery of the placenta. The difference in the weight of the pad before and after delivery was calculated on the basis that 1 g is equivalent to 1 mL of blood. "During delivery some blood was usually spattered on the drapes and gowns of the attendants, although attempts were made to minimise such losses. This gave a constant error of approximately 10%. In addition, the placental interstices contain maternal blood (about 9% of placental weight). As systematic overestimations (amniotic fluid) and underestimations (blood loss) are likely to be equally distributed among the groups, no corrections have been made for them." |
| Incomplete outcome data (attrition bias) | Low risk | "4 women with exclusion criteria were entered erroneously (3 forcipal extractions, 1 augmentation). They are considered as non‐participants." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between September 2002 and December 2005, 1620 parturients were randomised in a community setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients at high risk and inappropriate for home or community births according to India’s ministry of health guidelines including those undergoing elective caesarean section or breech vaginal delivery, or those previous caesarean section, Hb less than 80 g/L, antepartum haemorrhage, hypertension, multiple pregnancy, history of previous antepartum or PPH, retained placenta, uterine inversion, diabetes, heart disease, seizures, placenta praevia, asthma or contraindications to misoprostol. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 812) versus placebo or control (n = 808). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a randomisation list with a random block size generated by the data co‐ordinating centre and stratified by the midwife. |
| Allocation concealment (selection bias) | Low risk | The envelopes were numbered and each envelope had a 5‐digit code number assigned to it. The first 2 digits were the auxiliary nurse midwife number, followed by a sequence number beginning with 001 and ending with 100, assigned to the individual participant. Non‐distinguishable envelopes in batches of 100 were distributed to each of the midwives affiliated with the 4 selected primary‐health centres. |
| Blinding of participants and personnel (performance bias) | Low risk | "The identical placebo was specifically manufactured for the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a polyurethane blood collection drape placed under the mother from immediately after birth until 1 hour after delivery of the baby. The blood collection drape included a calibrated receptacle specifically developed for the study. In the event of persistent bleeding beyond 1 hour, the drape was removed at 1 hour, blood loss measured, and a new drape used with a second measurement made at 2 hours. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00097123). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Institute of Child Health and Human Development (public funding) and the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between December 2011 and May 2013, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (not defined), rhesus negative blood group, cardiac disease, diabetes, bleeding disorder, precipitated labour, overdistended uterus, traumatic PPH, PROM/chorioamnionitis, intrauterine death, previous caesarean section/scar on uterus or inability to obtain the informed consent. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 50) versus 200 mcg of ergometrine administered intramuscularly (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators used a systematic random sampling method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with drapes that were weighed together with mops and clots, and by measurement of Hb concentration and haematocrit of a sample of venous blood before delivery and 24 hours after birth. A sample of venous blood before delivery and 24 hours after the birth was also collected, for Hb and haematocrit measurement "as an objective index of blood loss." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 50 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 25) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 25). | |
| Outcomes | The study recorded the following outcome: Blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between the beginning of August 2007 and the end of December 2007, 100 parturients were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with multiple pregnancy, prolonged labour more than 12 h, 2 or more previous caesarean sections, previous uterine rupture, Hb less than 80 g/L, who had a history of heart, renal or liver disorders or had a coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 20 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By a simple randomisation method, patients were allocated into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection in a suction bottle, and with drapes and pads beneath the mother. Amniotic fluid was suctioned and measured, and then subtracted from the total volume of the suction bottle. Meanwhile the known dry weight(s) of drapes and pads were subtracted from the soaked weights of these materials. Measurements of blood collected in the suction bottle and on drapes and pads were added together. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2013 and 31st June 2014,180 parturients were randomised in a hospital setting in Egypt. The population comprised nulliparous women with a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients undergoing elective caesarean section, vaginal delivery or general anaesthesia, or those who were multigravida, or with malpresentation, fetal anomalies, placenta praevia, diabetes, hypertension, pre‐eclampsia or cardiac disease. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 90) versus 20 IU of oxytocin administered by an intravenous infusion (n = 90). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, headache, fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blinded": "a double dummy system for administration was used." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) | Unclear risk | "100 cases were excluded (4 had congenital fetal anomalies, 7 cases had placenta praevia, 5 cases were diabetic, 8 had hypertension, 9 had pre‐eclampsia, 3 cases were cardiac, |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 382 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with asthma, anaemia, bleeding disorders, cardiac disease, inflammatory disease, bowel disease, multiple pregnancy, pre‐eclampsia, placenta praevia, placental abruption, previous APH, previous PPH, grand multiparity (not defined), fibroids, growth restriction, fetal malformations or allergy to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus (n = 191) versus 10 IU of oxytocin administered by an intravenous infusion (n = 191). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, transfusion, death, blood loss (mL), vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo and misoprostol tables "looked identical in size, colour, and packing." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection in a suction bottle minus sonographically estimated amniotic fluid volume, together with visual estimates of the volume of blood on the floor and the weight differences between dry and used towels, linens, and swabs. Visual estimates were performed by obstetricians blinded to treatment allocation. Towels, linen and swabs were weighed with an electronic scale. Weights were added to volumetric values on the basis that 1 g is equivalent to 1 mL. Investigators evaluated postoperative blood loss by weighing bed linen, gowns and perineal pads. Furthermore, blinded investigators estimated blood loss by multiplying maternal blood volume in mL by the difference between preoperative and postoperative haematocrit measurements, all divided by preoperative haematocrit measurements. |
| Incomplete outcome data (attrition bias) | Low risk | "4 patients in the placebo group and 12 patients in the misoprostol group were excluded from the study due to loss to follow‐up or missed preoperative hematocrit data." |
| Selective reporting (reporting bias) | Unclear risk | The study report matches the study protocol that was registered retrospectively (ClinicalTrials.gov NCT01466530). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from Mansoura University (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 1996 and March 1998, 1000 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or water birth, or those with severe asthma. | |
| Interventions | 500 mcg of misoprostol administered orally (n = 501) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 499). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta. death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician using computer‐generated block randomisation with varying block size. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, sequentially‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between February 2010 and October 2012, 380 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing general anaesthesia, or those with coagulopathy, coronary artery disease, hypertension, PPH due to causes other than uterine atony or hypersensitivity to carbetocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 190) versus 100 mcg of carbetocin administered by an intravenous bolus (n = 190). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, vomiting, headache, hypotension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Drugs were in pre‐prepared sealed and opaque packets. |
| Blinding of participants and personnel (performance bias) | Low risk | "Randomisation was done by the resident doctors immediately before transfer to theatre, whereas preparation of packets and confidential record maintenance was done by the labour room nursing staff... Caesarean delivery was performed by 4 senior obstetricians who were blinded to the allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 1st January 2008 and 1st January 2009, 400 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing their first elective caesarean section, or those unsure of gestation or with hypertension, diabetes, oligohydramnios, abnormal placenta or abnormal laboratory investigations. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 200) versus 10 IU of oxytocin administered by an intravenous infusion (n = 200). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, NNU admissions, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was placed in sealed envelopes until the time of operation. |
| Blinding of participants and personnel (performance bias) | Low risk | Attending obstetricians and other caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from after uterine incision, by collection in 2 separate suction sets administered by a nurse, and by weighing surgical towels before and after each operation. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN 12611000638932). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 4th January 2004 and 30th January 2005, 864 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with pre‐eclampsia, hypertension, cardiac disease, severe anaemia, asthma, renal/hepatic disorders, grand multiparity (not defined), multiple pregnancy, polyhydramnios, previous PPH, fibroids or contraindications to misoprostol or ergometrine. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 432) versus 500 mcg of ergometrine administered intramuscularly (n = 432). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by simple random selection. An independent statistician generated sets of 4 random letters, which were in boxes, and each box contained 4 separate random allocations which was equivalent to an opaque sealed envelope stratified in a block of 4. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was "single‐blinded." The identity of those blinded was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by a combination of careful collection in a receptacle after the delivery of the baby, by visual estimation of blood loss, and by extrapolation of blood loss using the weight difference of the total perineal pad used up to 24 hours postpartum. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion, chest pain and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Postgraduate Medical College and Faculty of Obstetrics and Gynaecology of the University College Hospital in Ibadan, Nigeria (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st September 2011 and 31st May 2012, 300 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with premature labour (less than 28 weeks), multiple pregnancy, antepartum haemorrhage, hypertension in pregnancy, severe anaemia or haemoglobinopathy. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 151) versus 500 mcg of ergometrine administered intramuscularly (n = 149). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random tables. |
| Allocation concealment (selection bias) | Low risk | A person uninvolved with the study prepared the study drugs. The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "A person uninvolved with the study prepared the study drugs… The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes, such that only the computer‐generated randomisation numbers on the envelopes were available to identify the study drug during unblinding." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with "a fresh large perineal pad with plastic backing." They placed all the gauzes and perineal pads used to absorb the blood into a polythene bag, and subtracted the dry weight from the wet weight. Volume of blood loss was calculated on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered (PACTR 201105000292708). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st January 2002 and 30th June 2002, 97 parturients were randomised in a hospital setting in Turkey. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section or instrumental delivery, or those with premature labour (less than 37 weeks), postmaturity (more than 43 weeks), grand multiparity (more than 4), twin pregnancy, growth restriction, macrosomia, Hb less than 100 g/L, systemic disorder, prolonged third stage, manual removal of placenta or additional lacerations due to episiotomy or where it took longer than 30 min to repair lacerations after episiotomy. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 49) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly (n = 48). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was achieved using block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with scale vessels, and by subtraction of the dry weight(s) of cloths and pads from the soaked weight(s) of these items. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1345 parturients were randomised in a hospital setting in Nigeria. The population comprised multiparous women, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered vaginally. Exclusion criteria comprised severe allergic conditions or asthma, age below 18 years, pyrexia above 38°C, or abortion of the pregnancy. | |
| Interventions | 400 mcg of misoprostol administered sublingually plus 10 IU of oxytocin or 250 mcg to 500 mcg of ergometrine administered intramuscularly or by an intravenous bolus (n = 658) or intravenous bolus versus 10 IU of Oxytocin or 250 mcg to 500 mcg of ergometrine administered intramuscularly or intravenously (n = 660). | |
| Outcomes | Could not include in the analysis as could not separate out the patients that received oxytocin from those who received ergometrine. | |
| Notes | Contact with study authors for additional information: Yes. Additional data from authors: Yes, but data not provided separate for each drug used and could not be included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated in blocks of 6–8 women by the research nurse, who used a computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | The trial drugs were concealed in sealed, sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo was identical in shape, colour, size, and design. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Blood collection was initiated as soon as possible after administration of the trial medication. A low‐profile plastic fracture bedpan was placed below the woman's perineum to collect all subsequent blood loss for a period of 1 hour. Blood collected in the bedpan and all blood soaked small gauze swabs were emptied into a plastic measuring jar and the volume was measured. |
| Incomplete outcome data (attrition bias) | Low risk | No losses stated by authors but 27 women randomised were not included in the analysis for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No available protocol. |
| Intention to treat analysis | Unclear risk | 27 women randomised were not included in the analysis for the primary outcome. |
| Funding source | Low risk | The trial was funded by theMedical Research Council of South Africa. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified in 2009, 100 parturients were randomised in a hospital setting in Iran. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with twin pregnancy, fetal distress, pregnancy‐induced hypertension, oligohydramnios, polyhydramnios, macrosomia, grand multiparity (4 or more), HELLP syndrome, coagulopathy, asthma, heart/lung/liver disease, previous more than 1 caesarean section, previous myomectomy, previous other abdominal operations, febrile diseases or sensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 50) versus 10 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: transfusion, blood loss (mL), nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection with an isolated suction. The volume of blood collected in suction was combined with the volume of blood collected in gauzes and gowns: every small gauze soaked with blood was considered to contain 20 mL, and every large gauze soaked with blood 50 mL, and every g increase in the weight of a gown was considered as equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st March 2007 and 1st June 2007, 250 parturients were randomised in a hospital setting in Tunisia. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, or those with placenta praevia, retroplacental clot, multiple pregnancy, premature labour (less than 32 weeks), intra‐uterine death, Hb less than 80 g/L, coagulopathy, HELLP syndrome, antepartum haemorrhage, ruptured uterus, previous more than 2 caesareans or other uterine scar, prolonged labour (more than 12 hours) or pyrexia. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus and infusion (n = 125) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 125). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | A slip of paper was placed inside an opaque, sealed envelope. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated perioperative blood loss as a combination of the volume of liquid in the suction collection jar, and the weight of swabs and pads. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between May 2011 and August 2011, 75 parturients were randomised in a hospital setting in the Philippines. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with pre‐existing hypertension, pre‐eclampsia, diabetes, asthma, cardiac/renal diseases, coagulopathy, abnormal laboratory tests or allergy to the study medication. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 39) versus 10 IU of oxytocin administered by an intravenous infusion (n = 36). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics. transfusion, blood loss (mL,. change in Hb level, nausea, vomiting, headache, tachycardia, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "The patient and the principal investigator attending the delivery were blinded to the type of medication administered" [additional information from the authors]. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "In addition to these, the following were recorded by assigned personnel not blinded in this study: any adverse events or experiences from the 2 groups (carbetocin versus oxytocin); vital signs before and after drug infusion, the need for an additional uterotonic in each group, the need for a uterine massage and the intensity of the uterine contraction after infusion of the assigned drug." |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by visual estimation, not including blood loss considered to result from repair of lacerations. |
| Incomplete outcome data (attrition bias) | High risk | "9 women in the carbetocin group and 6 women in the oxytocin group failed to have a paired haemoglobin test to measure the change in haemoglobin 24 hours after delivery because they refused further blood extraction. These 15 women were excluded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between October 2002 and April 2003, 156 parturients were randomised in a hospital setting in China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 80) versus placebo or control (n = 76). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss in the 2 hours after delivery and after all amniotic fluids had been drained, by collection in a small tray and absorption into disposable, sterile, water‐resistant gauze. The contents were weighed and volume was determined on the basis that 1.05 g is equivalent to 1 mL of blood. A measuring cup was used to estimate the blood in the tray; blood that soaked into the gauze was measured on the basis that material measuring 10 cm by 10 cm holds 10 mL of blood. These 3 measurements were combined to ascertain total blood loss. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 2002 and 2003, 200 parturients were randomised in a hospital setting in India. The population comprised women of primigravidas, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 100) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, manual removal of placenta, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in 1:1 ratio by random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 100 parturients were randomised in a hospital setting in Ecuador. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with Hb less than 80 g/L, multiple pregnancy, polyhydramnios, previous uterine rupture, bleeding disorders, intrauterine death or hyperthermia (more than 38.5°C). | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 10 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), nausea, vomiting, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated postoperative blood loss by collection with "suction apparatus and sterile drapes before irrigation" and by weighing the blood collected in abdominal swabs and gauzes with a calibrated scale (Zhongshan Camry Electronic Co Ltd, model EK 4052‐E, Guangdong, China). Investigators estimated the volume of blood loss "by subtraction of amniotic fluid at 30 cc per each centimetre reported by amniotic fluid index." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 400 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with multiple pregnancy, coagulopathy, Hb less than 70 g/L, indication for caesarean section or contraindication to prostaglandin or oxytocin use. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 201) versus 20 IU of oxytocin administered by an intravenous infusion (n = 199). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an uninvolved party and determined by a random number sequence. |
| Allocation concealment (selection bias) | Low risk | The random number sequence was prepared by a third party and was concealed until the patient was enrolled. Packets were prepared in advance of randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | The random number sequence was "concealed until the patient was enrolled" and "packets were prepared in advance of randomisation." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss (a) by collection with drapes placed under the mother. Each drape included a plastic pouch and measured volume in mL. Meanwhile the dry weights of delivery linen and sponges were subtracted from bloodied weights to determine the volume of blood collected with these materials, on the basis that 1 g is equivalent to 1 mL. The volumes of blood in drapes and linen were added together. Furthermore "if amniotic fluid loss [after placement of the drape] was significant... the approximate percentage was recorded on the data sheet and blood loss was adjusted accordingly." Investigators evaluated blood loss (b) by estimation of the delivery attendant(s). Investigators evaluated blood loss (c) by measurement of Hb and haematocrit values were obtained on admission and on postpartum day 1. The differences between these 2 values were recorded. |
| Incomplete outcome data (attrition bias) | High risk | "Of the 75 women who were excluded from analysis, 73 underwent caesarean deliveries, 1 woman was discharged to |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between April 1998 and November 1999, 18530 parturients were randomised in a hospital setting in Argentina, China, Egypt, Ireland, Nigeria, South Africa, Switzerland, Thailand, and Vietnam. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective or emergency caesarean section after randomisation, or those with asthma, severe chronic allergic conditions, abortion, pyrexia (more than 38°C) or inability to give consent. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 9264) versus 10 IU of oxytocin administered intramuscularly or by an intravenous bolus (n = 9266). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation schedule was generated centrally at WHO, Geneva, Switzerland, by computer‐generated random numbers and was stratified by country. Within the strata, women were individually randomised into 1 of 2 intervention groups with randomly varying block sizes of 4–6 women. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were sealed, numbered sequentially, and could only be taken from the dispenser consecutively. |
| Blinding of participants and personnel (performance bias) | Low risk | "The treatment packs and their contents were identical in shape, colour, weight, and feel… Double‐blinding, including double placebos, ensured that ascertainment bias in the measurement of blood loss and use of additional uterotonics was unlikely. However, unblinding could have occurred because of the higher rate of shivering associated with misoprostol." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from the time of delivery of the baby until the third stage of the labour was completed, when the mother was transferred to postnatal care (usually up to 1 hour postpartum). Immediately after the cord was clamped and cut, they passed a flat bedpan or an unsoiled receiver under the mother. The collected blood was poured into a standard measuring jar provided by WHO for volumetric measurement. "To simplify the procedure... small gauze swabs soaked with blood were put into the measuring jar and included in the measurement together with the blood and clots." |
| Incomplete outcome data (attrition bias) | Low risk | Investigators excluded "37 and 34 women with emergency caesarean section, and 13 and 4 women lost to follow‐up in misoprostol and oxytocin groups, respectively, for blood loss |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training. Searle (Skokie, IL, USA) and Novartis (Basel, Switzerland) donated the active and placebo medications used in the trial. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered rectally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL). change in Hb level, third‐stage duration (min), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random tables. |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope with a code number was opened when vaginal delivery was imminent. The code was not broken till the end of the study. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind." "Each envelope contained either 3 tablets of 200 mcg misoprostol and an ampoule of normal saline or 3 identical looking placebo tablets and an ampoule of 10 IU oxytocin." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape placed under the mother. Pre‐weighed gauzes were used to clean any perineal tears or episiotomy. After 1 hour the dry weight of the sponges was subtracted from the soiled weight, and added to the volume of blood collected in the drape on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between August 2000 and May 2004, 352 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 173) versus 20 IU of oxytocin administered by an intravenous infusion (n = 179). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | The group assignments were available only to the pharmacy. The nurse selected an opaque vial from the drug cabinet that contained either a 200 mg misoprostol tablet or placebo. The vial number (which had been assigned in the pharmacy) and patient identification were sent to the pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Over 6 months between dates unspecified, 140 parturients were randomised in a hospital setting in West Indies. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous PPH, hypertension, previous caesarean, intrauterine death, sepsis/pyrexia (more than 38°C), antepartum haemorrhage or Hb less than 80 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 70) versus 400 mcg of misoprostol administered rectally (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation was used to randomly assign participants. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | "Both the patient and the midwife conducting the delivery were aware of the drug administered." |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a modified plastic drape placed under the mother from the commencement of the third stage of labour, until 1 hour after delivery. The collection drape measured 168 cm by 84 cm, and contained folded over side‐wings (to act as a chute) and a 34‐cm collection pouch made by folding the distal end of the drape. Standard sterile drapes were placed above the blood collection drape. Every effort was made to avoid soiling the sterile drapes before delivery of the baby, because they were not weighed. After delivery, overlying sterile drapes were removed to facilitate the use of the collection drape. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Mona Campus and Research Publication Committee of the University of the West Indies (the institution of the authors). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 500 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, or those with hypertension, diabetes or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 250) versus placebo or control (n = 250). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence, in balanced blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The tablets were either misoprostol 2 x 200 mcg or 2 placebo tablets similar in size and colour but not shape. Efforts to obtain identical placebo tablets were unsuccessful. This method of blinding proved to be effective. In only 1 case did the attending midwife inadvertently catch sight of the tablets." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g." After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 600 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 300) versus placebo or control (n = 300). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by computer in blocks of 18. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque test tubes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Misoprostol and placebo were similar in size and colour but not shape |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g." After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery". |
| Incomplete outcome data (attrition bias) | Low risk | "There were no withdrawals after randomisation and all outcomes were analysed in the allocated group." However the primary outcome data of 1 study participant in the placebo group were unavailable. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding) and University of the Witwatersrand (the institution of the authors). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 6th March 2006 and 13th August 2007, 1103 parturients were randomised in a hospital setting in South Africa, Uganda, and Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those who declined participation or were unable to consent, were too ill or distressed to participate or with a not viable pregnancy. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus intramuscularly (n = 547) versus 10 IU of oxytocin administered intramuscularly (n = 556). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, manual removal of placenta, death, blood loss (mL), fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were stratified by country in blocks of 6–8. |
| Allocation concealment (selection bias) | Low risk | The trial medication was provided, and the study drug packs were prepared, by Gynuity Health Projects. When a participant enrolled, the researcher took the next study drug pack from the dispenser and immediately wrote the woman's name both on the pack and in the participant number list, which was kept separate from the case record forms. Enrolment took place when the pack was removed from the pack dispenser. The pack could not be used for another woman or returned to the dispenser. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind." "The packs were identical in shape, colour, weight, and feel, and contained either 2 tablets of 200 mcg of misoprostol (HRA Pharma, Paris, France) or 2 matching placebo tablets." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Similarly to the study team of Gulmezoglu 2001, investigators evaluated blood loss by collection with a fresh non‐absorbent sheet and low plastic “fracture” bedpan placed under the mother from as soon as possible after delivery until 1 hour postpartum. Investigators considered that "longer‐term blood loss measurement is more difficult to standardise." They transferred the blood collected in the sheet and the bedpan (together with any soaked small gauze swabs) to a measuring jar to ascertain the volume. Alternatively, they collected blood with a plastic sheet placed under the mother immediately after delivery. If bleeding continued beyond 1 hour, investigators restarted collection and measurement until bleeding subsided. Attempts were made to minimise any losses on the drapes and gowns of delivery attendants. In addition, "the placental interstices also contain maternal blood (about 9% of placental weight). Because overestimations (amniotic fluid) and underestimations (blood loss) were likely to be distributed equally between the 2 study groups, and most would have occurred before the onset of measurement, the data were not corrected. |
| Incomplete outcome data (attrition bias) | Low risk | "Data for the primary outcome were not available for 4 of the 1103 women." |
| Selective reporting (reporting bias) | High risk | The prospectively registered protocol of the study (ClinicalTrials.gov NCT 00124540) lists some secondary outcomes different to those included the study report (at least 1000 mL within the first hour only, transfusion, Hb less than 8 g/dL 24 hours after delivery). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Gynuity Health Projects through a grant from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between March 2003 and August 2004, 661 parturients were randomised in a community setting in Guinea‐Bissau. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered sublingually (n = 330) versus placebo or control (n = 331). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque envelopes that were consecutively‐numbered and filled with the study drugs. |
| Blinding of participants and personnel (performance bias) | Low risk | "Misoprostol and placebo tablets of identical form, size, colour, and packing were produced." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | After delivery of the baby and drainage of the amniotic fluid, investigators placed a clean plastic‐lined absorbent drape under the mother. They changed the drape as many times as needed. The mother stayed on the drape or was asked to wear a pad over the next 60 minutes. All drapes and pads were weighed with an electronic scale and the known dry weights were subtracted in order to ascertain the volume of blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Danish Society of Obstetrics and Gynaecology, the Illum Foundation, and the Danish International Development Agency (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 214 parturients were randomised in a hospital setting in Korea. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by caesarean (unspecified whether elective or emergency). Exclusion criteria were not specified. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 118) versus 400 mcg plus 20 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 96). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor blinding was not reported, but the use of placebo impeded knowledge of treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 100) versus unspecified of ergometrine administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: third‐stage duration (min), nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2001 and December 2002, 510 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those requiring epidural analgesia or with hypertension in pregnancy, existing hypertension, chronic renal disease, diabetes, vascular diseases, cardiac disease, anticoagulation therapy or allergy to ergometrine or oxytocin. | |
| Interventions | 500 mcg of ergometrine administered intramuscularly (n = 254) versus 10 IU of oxytocin administered by an intravenous bolus (n = 256). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), hypertension. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used numbers that were labelled on envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between November 2006 and April 2008, 1802 parturients were randomised in a hospital setting in Sweden. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those who were non‐Swedish speaking or with previous PPH, pre‐eclampsia, grand multiparity (more than 4) or intrauterine death. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 903) versus of placebo or control (n = 899). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed envelopes containing the randomisation group prepared in consecutive order and kept in another unit. At randomisation, midwives phoned the staff at the other unit who opened the envelopes and disclosed the assigned intervention and trial number. |
| Blinding of participants and personnel (performance bias) | High risk | "Because of the nature of the study, blinding was not possible for the midwives, but the parturients were not informed of which management was to be used for them." |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by removing pads soaked with amniotic fluid and placing a dry sanitary pad under the mother, immediately after the birth of the baby. They weighed all sanitary towels and pads before and after use. Blood loss was recorded (a) between the birth of the baby and the expulsion of the placenta, and (b) from expulsion of the placenta up to 2 hours postpartum. |
| Incomplete outcome data (attrition bias) | Low risk | 171 randomised women were not included in the study analysis. Among those randomised to receive oxytocin, 4 withdrew consent, 75 had caesareans, and 14 were lost to follow‐up. In the control group, 2 withdrew consent, 56 had caesareans, and 20 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | The authors excluded 131 randomised study participants from the analysis because they experienced caesarean deliveries. |
| Funding source | Low risk | The study was supported by funding from the Research and Development Board in Göteborg and Bohuslän, Baby Bag and the SU Foundation in Sweden (public funding). |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between February 2005 and March 2005, 130 parturients were randomised in a hospital setting in Tunisia. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, antepartum haemorrhage, non‐cephalic presentation, intrauterine death, grand multiparity, (more than 5), fibroids, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 65) versus placebo or control (n = 65). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, manual removal of placenta, death, change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st June 1998 and 31st December 1998,140 parturients were randomised in a hospital setting in Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 70) versus 200 mcg of ergometrine administered intramuscularly (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 238 parturients were randomised in a hospital setting in Canada. The population comprised women of parity 5 or less, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with coagulopathy, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 100) versus 5 IU of oxytocin administered by an intravenous bolus or intramuscularly (n = 113). There were 15 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, change in Hb level. third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician developed blocked randomisation tables for each centre. |
| Allocation concealment (selection bias) | Low risk | Pharmacy assembled consecutively‐numbered opaque, sealed packets that contained the group allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "13 women randomised subsequently delivered by caesarean and were excluded from analysis. 2 women were lost to follow‐up early in the trial when their packets were opened but the manoeuvre was not completed and no data were recorded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the physicians of Ontario, through the Physician Services Incorporated Foundation (public funding). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, 140 parturients were randomised in a hospital setting in Hungary. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 200 mcg of ergometrine administered by an intravenous bolus (n = 50) versus placebo or control (n = 43) versus 1 mg dinoprost administered intramuscularly (n = 47). The dinoprost arm was not included in the analysis. | |
| Outcomes | The study recorded the following outcome: third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by collection in a container placed under the mother during the third stage of labour until 2 hours postpartum. The contents of the container were transferred to a measuring cylinder. However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 1st January 1991 and 30th June 1991, 2040 parturients were randomised in a hospital setting in United Arab Emirates. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, caesarean section or instrumental delivery, or requiring general anaesthesia, epidural or diazepam, or those with antenatal hypertension (160/100 mmHg or more), hypertension on antihypertensive drugs, multiple pregnancy, cardiac disease or Hb of 90 g/L or less. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 1017) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1023). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, transfusion, manual removal of placenta, vomiting, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment was allocated as per a number code generated by the hospital pharmacist who alone was aware of the content of the ampoules. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned an opaque sealed envelope. Each envelope carried the instruction to use a numbered vial of the study drug. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "in the standard way" by measurement of blood and clots in a graduated jug, and by weighing swabs and linen. |
| Incomplete outcome data (attrition bias) | Low risk | "12 patients had to be excluded from the trial (oxytocin 5; syntometrine [ergometrine plus oxytocin] 7) after randomisation because they no longer fulfilled the inclusion criteria... [including] 2 who required caesarean section (1 in each group) and 10 who were |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 136 parturients were randomised in a hospital setting in Japan. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who were scheduled for caesarean with ASA I or II. Exclusion criteria comprised parturients that were affected by cardiovascular conditions, were scheduled for autologous blood transfusion, who had tocolytics administered or premature rupture of membranes. | |
| Interventions | 10 IU up to 20 IU of oxytocin administered by an intravenous bolus (n = 102) versus 200 mcg ergometrine administered by an intravenous bolus (n = 34). | |
| Outcomes | The study recorded the following outcome: blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no and data cannot be extracted for meta‐analysis. Manuscript translated from Japanese in full. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Methods of blinding were not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | Not reported. |
| Funding source | Unclear risk | Not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2003 and March 2004, 55 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with multiple pregnancy, hypertension or vascular diseases. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus plus infusion (n = 35) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered by an intravenous bolus plus by intravenous bolus plus infusion (n = 20). | |
| Outcomes | The study recorded the following outcome: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss by weighing compresses and rolls before and after the birth of the baby, and calculating the difference between these measurements. Pre‐weighted pads were distributed in advance to each mother, and collected at intervals of 3‐6 hours hour intervals after the aspiration of amniotic fluid. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between October 1999 and February 2000, 500 parturients were randomised in a hospital setting in Zimbabwe. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, antepartum haemorrhage, coagulopathy, multiple pregnancy, asthma or allergies to prostaglandins or oxytocin. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 243) versus 10 IU of oxytocin administered intramuscularly (n = 256). There was 1 exclusion post randomisation but it was unclear as to which group it was randomised to. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity,additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | The participant was asked to randomly pick a numbered sealed opaque envelope from the study cooler‐box. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Identical placebo tablets could not be obtained from the manufacturers. The tablets were similar in size and colour but not in shape. However, most reviewed trials on misoprostol had this similar problem although this method of blinding proved to be effective." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The data sheet was completed by the midwife supervising the delivery and collected and checked by the research assistant." |
| Objective assessment of blood loss | Low risk | After delivery, investigators evaluated blood loss by removing linen soiled with amniotic fluid, and then placing a fresh disposable incontinence pad with a plastic backing under the mother. Blood expressed from the uterus was measured with a calibrated measuring jug. The volume of blood soiling linen savers and sanitary pads was determined as the difference between dry weights and soiled weights: these measurements were added to the volume recorded by the calibrated jug. |
| Incomplete outcome data (attrition bias) | Low risk | "Data for 1 woman were excluded because she delivered undiagnosed twins after randomisation." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in China (Hong Kong SAR). The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with antepartum haemorrhage, anaemia, 2 or more surgical terminations, previous manual removal of placenta, previous PPH or previous third stage complications. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered by an intravenous bolus (n = 30) versus 600 mcg of misoprostol administered sublingually (n = 30). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, manual removal of placenta, death, fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated using a random number‐generated table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss during the third stage by visual estimation, and by objective measurement on the basis of a method previously described by Newton et al. Whilst any blood clots were collected and measured with a jug, white linen was placed under the mother during delivery and subsequently processed for 15 minutes with sodium hydroxide solution in an automatic stomacher (laboratory blender), to achieve the formation of alkaline hematin. "The optical density at 550 nm of the alkaline hematin was measured by spectrophotometry and compared with that of a known volume of a sample of the patient’s venous blood" to calculate the volume of blood loss. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 1999 and February 2002, 56 parturients were randomised in a hospital setting in Switzerland. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with fetal distress, fetal malformations, pre‐eclampsia, HELLP syndrome, coagulopathy, severe systemic disorders, an American Society of Anaesthesiologists physical status of 3 or greater, severe asthma, previous myomectomy, pyrexia (more than 38.5°C) or hypersensitivity to prostaglandins. | |
| Interventions | 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 28) versus 800 mcg plus 5 IU of misoprostol plus oxytocin administered orally plus by an intravenous bolus (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, death, blood loss (mL), nausea, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital pharmacy performed the 1:1 computer‐generated randomisation that assigned the participants to their group. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes from pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": "the study drugs and placebos [were provided by the pharmacy] in unidentifiable form." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | When the membranes ruptured before delivery, investigators evaluated intraoperative and postoperative blood loss by determining the difference in weight of cloths and pads used to absorb blood during surgery and in the intermediate care unit. When membranes did not rupture preoperatively, investigators evaluated blood loss by collection in suction bottles and subtracting estimated amniotic fluid volume. Investigators considered that 1 g is equivalent to 1 mL of blood or amniotic fluid. |
| Incomplete outcome data (attrition bias) | Low risk | "3 patients in the oxytocin group were excluded from statistical analysis because of errors in drug administration." Moreover calculated blood loss data were unavailable in 13 cases and for these women the primary outcome was estimated clinically. |
| Selective reporting (reporting bias) | High risk | The study protocol that was registered retrospectively (ClinicalTrials.gov) lists PPH as the primary outcome of the study, but the study report lists the primary outcomes as intraoperative and postoperative blood loss and drug‐related adverse effects (these items are listed only as secondary outcomes in the registration file). The study does not report the incidence of PPH at 500 mL, nor PPH at 1000 mL. |
| Intention to treat analysis | High risk | The authors excluded 3 study participants in the oxytocin group from the analysis because they incurred errors in drug administration. |
| Funding source | Low risk | The study was supported by funding from the Scientific Pool of Basel University Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between July 2004 and March 2005, 329 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring prophylactic oxytocin infusion, or those with pre‐existing hypertension, pre‐eclampsia, asthma, cardiac/renal/liver diseases, grand multiparity or fibroids. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 165) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 164). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, headache, tachycardia, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated code before recruitment. |
| Allocation concealment (selection bias) | Low risk | This was performed by opening a sealed, consecutively‐numbered, opaque envelope. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by visual estimation. |
| Incomplete outcome data (attrition bias) | Low risk | "15 women in the carbetocin group and 14 women in the syntometrine [ergometrine plus oxytocin] group failed to have a paired haemoglobin test to measure the change in haemoglobin 48 hours after delivery either because they had requested early home discharge or refused." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of fever were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 40 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with 2 or more previous caesarean sections or previous uterine rupture. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 20) versus 500 mcg of misoprostol administered orally (n = 20). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by means of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "The obstetrician, surgical assistant, scrub nurse and recovery midwife were blinded to the treatment. The anaesthetist and the anaesthetic assistant were not blinded as it was important for patient safety that a record was kept of all drugs administered." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative and postoperative (up to 1 hour) blood loss by visual estimation "in a standard manner (volume of blood in suction bottle plus soiling of swabs and bed sheets)." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by "assistance" from the Department of Anaesthesia at University College London Hospitals NHS Trust (the institution of the authors). |
| Methods | 3‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 597 parturients were randomised in a hospital setting in South Africa and Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section or abortion, or those with asthma, other severe chronic allergic conditions a contraindication to use of misoprostol or if they were not willing or able to give informed consent. | |
| Interventions | 600 mcg or 400 mcg of misoprostol administered orally (n = 397) versus 10 IU of oxytocin administered intramuscularly (n = 200). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), nausea,. vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random allocation sequence was generated centrally. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were consecutively‐numbered and sealed. |
| Blinding of participants and personnel (performance bias) | Low risk | "The packs were identical in shape, colour, weight and feel. Each woman received an injection and 3 tablets. Thus, the trial was double‐blinded using double placebos." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from the delivery of the baby until the mother was transferred to postnatal care. The collected blood was poured into a standard measuring jar provided by WHO for the purpose of volumetric measurement. Linen was not weighed but clots and small gauze swabs soaked with blood were included in the measurement. |
| Incomplete outcome data (attrition bias) | Low risk | Exclusion after randomisation: “8 women did not comply with treatment in the oxytocin group (6 because of emergency caesarean section, 1 was HIV positive (mistakenly excluded despite HIV sero‐positivity not being an exclusion criterion), and in another case the ampoule could not be located in the treatment pack. There was 1 woman in the misoprostol 600 mcg group who did not comply with the treatment, because the tablets could not be located after the treatment pack was opened. Finally, in the misoprostol 400 mcg group, 1 woman had an emergency caesarean section after the treatment pack was opened and could not receive the allocated treatment." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the WHO (public funding). Active and placebo medications, syringes and swabs were donated by Searle, Novartis Pharma AG and Becton Dickinson International. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between May 2013 and December 2014, 200 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, coagulopathy, pre‐eclampsia, cardiac/renal/liver disorders, epilepsy or known hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 100) versus 5 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, tachycardia, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were equally randomised using an automated web‐based randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | The study report states that investigators ensured allocation concealment, but gives no further details. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by weighing swabs and using pictorial charts. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 19th February 1990 and 20th October 1991, 3497 parturients were randomised in a hospital setting in Australia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or requiring general anaesthetic for instrumental delivery, or those with hypertension in labour (more than 150/100 mmHg), antenatal hypertension, maternal distress, advanced stage in labour, language barrier, fetal abnormality, intrauterine death or medical disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1730) versus 10 IU of oxytocin administered intramuscularly (n = 1753). There were 14 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, NNU admissions, breastfeeding, nausea, vomiting. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The ampoules were numbered by Sandoz using simple randomisation. There was no blocking or prognostic stratification. |
| Allocation concealment (selection bias) | Low risk | The ampoules were numbered by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) | Low risk | Delivery attendants were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) | Low risk | "All women allocated to receive a drug were included in that group, excluding only the 14 women for whom drug allocation was not recorded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified in 1984, 461 parturients were randomised in a hospital setting in UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with significant hypertension or cardiac disease. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 230) versus 5 IU of oxytocin administered intramuscularly (n = 231). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, manual removal of placenta, blood loss (mL), third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence: described as without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes prepared by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the standard way by graduated jug measurement plus an allowance for spillage." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Perinatal Trials Service (public funding), for the Department of Health for England and Wales, and for Birthright (the charitable arm of the RCOG). Coded medication ampoules were provided by Sandoz. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between June 2006 and June 2008, 1119 parturients were randomised in a community setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with hypertension, non‐cephalic presentation, polyhydramnios, previous caesarean, multiple pregnancy, intrauterine death, antepartum haemorrhage or Hb less than 80 g/L. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 534) versus placebo or control (n = 585). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever. shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random code in blocks of 6 was maintained by Gynuity Health Projects in New York and not revealed until data collection and cleaning were completed. |
| Allocation concealment (selection bias) | Low risk | Study medication was packed in numbered colour‐coded boxes by Gynuity Health Projects in New York. |
| Blinding of participants and personnel (performance bias) | Low risk | "Both women and TBAs were blinded to study assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | To evaluate postpartum blood loss, blood was collected with a perineal sheet and bedpan placed under the mother for a minimum of 1 hour or until active bleeding stopped (whichever occurred last). "Blood collected in the bedpan was transferred to a measuring jar, which was then closed, and the perineal sheet and cotton roll were placed in a sealed plastic bag. The closed measuring jar and sealed plastic bag were then placed inside a plastic cooler which was tightly closed and stored in a secure place in the woman’s home until the local health visitor or community health nurse arrived for weighing, 1–2 days after delivery." |
| Incomplete outcome data (attrition bias) | Low risk | "Invalid blood loss measures, which mainly occurred when monitoring visits were not possible because of poor weather conditions, were excluded from our analysis." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00120237). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 2008 and July 2008, 84 parturients were randomised in a hospital setting in Austria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients requiring general anaesthesia, or those with placenta praevia, placental abruption, multiple pregnancy, pre‐eclampsia, gestational diabetes, pre‐existing insulin‐dependent diabetes, cardiovascular/renal disorders, hypo‐/hyperthyroidism or women on cardiovascular system medications. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 28) versus 5 IU of oxytocin administered by an intravenous bolus (n = 28). There were 28 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics. change in Hb level, nausea, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated randomisation sequence in a 1:1 ratio with blocks of 10 and no stratification. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Study medication was double‐blinded to the clinical staff (obstetricians as well as anaesthesiologists) and the technicians performing the measurements." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators did not evaluate blood loss. |
| Incomplete outcome data (attrition bias) | High risk | After randomisation, investigators excluded 28 women from analysis for technical problems (n = 15), change to general anaesthesia (n = 9), recording artefacts (n = 3) and patient withdrawal (n = 1). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (EudraCT 2007‐005498‐78). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | CNSystems Medizintechnik AG in Graz, Austria provided the Task Force® Monitor 3040i system used to measure haemodynamic parameters. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 88 parturients were randomised in a hospital setting in the UK. The population comprised women of primigravidas, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 44) versus 10 IU of oxytocin administered by an intravenous bolus (n = 44). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), nausea. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the haemoglobin extraction‐dilution technique, which is acceptably accurate (Roe, Gardiner and Dudley, 1962; Thornton et al, 1963) and particularly suited to obstetric use (Moir and Wallace, 1967; Wallace, 1967). The pedometer apparatus was used and all blood and blood‐stained linen were collected." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 148 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 78) versus 5 IU of oxytocin administered by an intravenous bolus (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL), nausea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with the placenta bowl and soiled linen and swabs. "The principles of the haemoglobin extraction‐dilution technique employed have been discussed by Roe, Gardiner and Dudley (1962) and Thornton and colleagues (1963)." |
| Incomplete outcome data (attrition bias) | High risk | There were 148 study participants but blood loss data were available in only 80 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between May 2010 and October 2011, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or those with eclampsia, asthma, epilepsy, cardiac/kidney disorder or coagulopathy. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss in mL, by collection with a calibrated plastic drape, after the drainage of amniotic fluid and delivery of the baby until the third stage of labour was completed. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2013 and 30th June 2013, 235 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing planned instrumental, or those who received oxytocin and/or misoprostol other than in the third stage of labour, or those with grand multiparity (more than 4), multiple pregnancy, fibroids, polyhydramnios, pre‐eclampsia, eclampsia, hypertension, cardiac disorder, asthma, antepartum haemorrhage previous PPH, prolonged rupture of membranes or Hb less than 100 g/L). | |
| Interventions | 600 mcg of misoprostol administered orally (n = 121) versus 10 IU of oxytocin administered intramuscularly (n = 114). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, additional uterotonics, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was blocked (restrictive), using computer‐generated random numbers prepared by an independent statistician. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque envelopes but no other details provided. |
| Blinding of participants and personnel (performance bias) | Low risk | "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis." |
| Blinding of outcome assessment (detection bias) | Low risk | "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the gravimetric method" (Ambardekar 2009) until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) | High risk | 235 study participants were randomised but only 200 were analysed due to protocol deviations and missing data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (PACTR 201407000825227). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Ilorin Teaching Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 514 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with antepartum haemorrhage, coagulopathy, hypertension in pregnancy or the need for anticoagulants. | |
| Interventions | 800 mcg of misoprostol administered rectally (n = 257) versus 5 IU of oxytocin administered by an intravenous infusion (n = 257). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated random allocation system created at the Statistics Unit of Assiut University Hospital. |
| Allocation concealment (selection bias) | Low risk | Allocation codes were placed in sealed, opaque, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical‐looking." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between June 1998 and February 1999, 2058 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 1026) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1032). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation was placed in consecutively‐numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "This was not a double‐blinded study." |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between April 2000 and January 2001, 360 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 178) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 177). There were 5 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used consecutively‐numbered and sealed opaque packages. |
| Blinding of participants and personnel (performance bias) | Low risk | "The placebo was identical in size and colour but had a different shape to the misoprostol tablet. All women were asked to swallow the tablets directly from the opaque cup without looking at them. The identity of the active medication and placebo were concealed from the caregivers and the parturient." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | "5 women were excluded from the analysis because of missing post‐delivery haemoglobin level." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of tachycardia and dizziness were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 120 parturients were randomised in a hospital setting in Malaysia. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients younger than 18 years old, or those with cardiac disorder, hypertension requiring treatment, liver/renal/vascular/endocrine disorder (excluding gestational diabetes) or hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 60) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed, sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The preparation and administration of the medication was carried out by midwives who were not involved in the management of the patient except for the drug administration." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the gravimetric method" from immediately after drug administration. They used a digital scale (Soehnle, Venezia) for weight measurement. In order to minimise confounding by fluid absorbed into drapes, they collected blood with a new plastic sheet placed under the mother after delivery of the baby. They also weighed any gauzes, tampons and pads used in the first hour after delivery of the placenta, and subtracted the dry weights of these materials to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 16th December 1993 and 6th October 1994, 1000 parturients were randomised in a hospital setting in Sweden. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 513) versus placebo or control (n = 487). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Ampoules were prepared at the hospital pharmacy and consecutively‐numbered. |
| Blinding of participants and personnel (performance bias) | Low risk | "The content of the ampullas was unknown to mothers, midwives and doctors until the study was completed." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "by measuring collected blood and adding what was estimated to have been absorbed by surgical cloths and tissues." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the County Council and County Health Authority Research and Development Foundation in the County of Jämtland, Sweden (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between August 2000 and July 2001, 496 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with previous caesarean, Hb less than 80 g/L, previous PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, fibroids or precipitate labour. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 249) versus 600 mcg of misoprostol administered orally (n = 247). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random tables. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared opaque sealed sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) | Low risk | "The identity of the active medication and placebo were concealed from the caregivers and parturients." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending obstetricians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 144 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, multiple pregnancy, polyhydramnios or vaginal lacerations. | |
| Interventions | 200 mcg or 500 mcg of ergometrine administered intramuscularly (n = 96) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 48). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, manual removal of placenta, blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators performed restricted random allocation. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The identity of the various drugs was not known to the investigators until after completion of the trial." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by collection in a dish pressed against the vulva for 3 minutes: the contents were carefully measured. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2006 and September 2007, 600 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertension in pregnancy, packed cell volume less than 30%, previous PPH, haemoglobinopathy or cardiac disorder. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 297) versus 250 mcg of ergometrine administered by an intravenous bolus (n = 303). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done by sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by "using a pre‐weighed gauze that was weighed again after delivery." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion and PPH at least 1000 mL were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 156 parturients were randomised in a hospital setting in Spain. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with comorbidities, refractory hypotension due to neuraxial blockage, vasoactive drugs needed to control haemodynamic issues or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 52) versus 61 IU of oxytocin administered by an intravenous bolus plus infusion (n = 104). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, nausea, vomiting, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by the estimation of delivery attendants, but blood loss data were not reported in a format way that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between June 2006 and April 2007, 100 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, antepartum haemorrhage, cardiac/renal/liver disorders, coagulopathy, asthma, glaucoma, pre‐eclampsia, eclampsia, prolonged labour or contraindications to administration of prostaglandins. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 50) versus 400 mcg of misoprostol administered sublingually (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, headache, hypotension, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation sequence was developed by a statistician who was not otherwise involved with the study using computer‐generated table of random numbers and varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "The anaesthetist was blind to the allocation until he opened each participant’s envelope at surgery… The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection in a suction bottle, and by weighing delivery drapes and gauzes on the basis that 1 g is equivalent to 1 mL of blood. "Both the surgeon and anaesthetist estimated blood loss independently... The scrub nurse weighed the drapes and gauze before and after the operation, noted the amount of blood in the suction bottle, and recorded these... The postoperative care nurse also recorded the blood loss during the first 4 hours after surgery." Finally a research assistant (not part of the medical team) calculated the mean estimated blood loss from all these values. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and October 2002, 450 parturients were randomised in a hospital setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 225) versus 800 mcg of misoprostol administered orally (n = 225). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "We acknowledge that unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and December 2002, 450 parturients were randomised in a hospital setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 226) versus 800 mcg of misoprostol administered rectally (n = 224). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "Unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) | Low risk | Estimated blood loss data were unavailable in 9 cases (misoprostol 7; oxytocin 2) and Hb measurements (misoprostol 4; oxytocin 6) were unavailable in 10 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between 29th October 2000 and 6th November 2000, 78 parturients were randomised in a hospital setting in Colombia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, multiple pregnancy, intrauterine death, coagulopathy, cervical tear or water in the blood collector. | |
| Interventions | 50 mcg of misoprostol administered sublingually (n = 25) versus 16 mLU/min of oxytocin administered by an intravenous infusion (n = 25) versus 200 mcg of ergometrine administered intramuscularly (n = 25). There were 3 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss from cord clamping until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) | Low risk | 3 women were excluded from the analysis after entering the study: "se excluyeron 3 pacientes por obito fetal, desgarro de cervix severo, vertimiento de agua en el recipiente recolector de sangre." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between 1st January 1986 and 31st January 1987, 1695 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with cardiac disorder, antepartum haemorrhage, non‐cephalic presentation, multiple pregnancy, intrauterine death but after change in the protocol multiple other exclusion criteria were introduced. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 846) versus of placebo or control (n = 849). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, change in Hb level, NNU admissions, breastfeeding, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South Western Regional Health Authority of the United Kingdom (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 400 parturients were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, placental abruption, coagulopathy, previous caesarean, macrosomia (more than 4 kg), polyhydramnios or uncontrolled asthma. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 200) versus 400 mcg of misoprostol administered orally (n = 200). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), change in Hb level, hypotension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated using simple randomisation with computer‐generated numbers in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": "for blinding the study, identical‐appearing solutions and tablets corresponding to the 2 pharmacological groups were prepared by the pharmacy and kept in the fridge until required." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss during the first hour after delivery, by collection with pads weighed before and after absorbance of blood. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered (ClinicalTrials.gov NCT01863706) but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of diarrhoea, nausea and vomiting were not completely reported). Moreover, the study publication reports outcomes (hypotension, nausea, transfusion) not listed in the registered protocol. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Hormozgan University of Medical Sciences (the institution of the authors). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, an unspecified number of parturients were randomised in an unspecified setting and country. The population comprised primiparous women, with singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients that were multiparous, severely anaemic, and hypertensive conditions during pregnancy. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus versus 200 mcg ergometrine administered by an intravenous bolus vs placebo or control. | |
| Outcomes | The study recorded the following outcome: Change in Hb level. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Abstract only available and data provided could not be used for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Methods of blinding were not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | Not reported. |
| Funding source | Unclear risk | Not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2003 and December 2003, 686 parturients were randomised in a hospital setting in Saudi Arabia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, hypertension on treatment, antepartum haemorrhage, pre‐term labour (less than 37 weeks), post maturity (more than 42 weeks) or Hb less or equal to 90 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 340) versus 10 IU of oxytocin administered by an intravenous infusion (n = 346). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "clinically in a standard way" by collection with a plastic sheet that was subsequently drained (with clots) into a graduated measuring jug, and by weighing swabs and towels. "Any delayed haemorrhage within 24 hours after delivery was calculated." |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of requirement for additional syntometrine [ergometrine plus oxytocin] were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with pre‐term labour (less than 32 weeks), prolonged labour, antepartum haemorrhage, pre‐eclampsia, intrauterine death, multiple pregnancy, epilepsy, asthma, cardiac/kidney disorder, coagulopathy or anaemia. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 100) versus an unspecified dose of ergometrine administered by an unspecified injectable method (n = 100). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, hypertension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss in the first 2 hours after delivery of the placenta, by "clinical estimation." However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2008 and August 2009, 144 parturients were randomised in a hospital setting in Panama. The population comprised women of parity 5 or more, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with coagulopathy, unknown parity or known allergy to carbetocin. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 45) versus 20 IU of oxytocin administered by an intravenous infusion (n = 90). There were 9 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, breastfeeding, nausea, vomiting. Headache. Shivering. Abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "Se pudieron reclutar 144 pacientes, en quienes se dio la aleatorización en dos grupos (carbetocina:oxitocina) en una proporción 1:2. Cualquier causal de falla en el seguimiento del protocolo establecido obligaba a la exclusión de la paciente del estudio. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of PPH were omitted). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Ferring Pharmaceuticals donated carbetocin. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between July 2010 and September 2010, 57 parturients were randomised in a hospital setting in Panama. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both caesarean and vaginal delivery. Exclusion criteria comprised parturients with HELLP syndrome, blood dyscrasia or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 26) versus 10 IU of oxytocin administered by an intravenous infusion (n = 29). There were 2 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, third‐stage duration (min), breastfeeding. Vomiting. Headache. Fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was "double‐blind": "because the 2 drugs are administered differently, a double dummy system for administration was used." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | 2 women were excluded from the study analysis after randomisation ("1 given drug before expulsion of placenta; 1 ampoule of the drug broken before use"). |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between June 1993 and December 1995, 1512 parturients were randomised in a hospital setting in the UK. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour or instrumental delivery or requiring epidural analgesia, or those with placenta praevia, previous PPH, antepartum haemorrhage, Hb less than 100 g/L or mean corpuscular volume less than 75 fL, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, anticoagulation therapy, pre‐term labour (less than 32 weeks) or contraindications to any of the drugs. | |
| Interventions | An unspecified dose of ergometrine plus oxytocin administered by an unspecified route (n = 748) versus placebo or control (n = 764). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000.,Aaditional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, breastfeeding, nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule used variably sized balanced blocks, and the randomisation envelopes were prepared in advance in the National Perinatal Epidemiology Unit (NEPU). |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending midwives. |
| Incomplete outcome data (attrition bias) | Low risk | Blood loss data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Public Health and Operational Research Committee of the Anglia and Oxford Regional Health Authority, UK (public funding). |
| Methods | 3‐arm placebo‐controlled randomised trial. | |
| Participants | Between November 2009 and September 2011, 76 parturients were randomised in a hospital setting in Norway. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with pre‐eclampsia, placenta praevia, placenta accreta, von Willebrand disease or other bleeding disorder or preoperative systolic arterial pressure less than 90 mmHg. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 26) versus 100 mcg of carbetocin administered by an intravenous bolus (n = 25) versus placebo or control (n = 25). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated list of random numbers. The block size varied between 6 and 9, with stratification into 2 strata: BMI less than 30 and BMI of 30 or more. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blinded": "to maintain blinding of the participants and investigators, the test medicine was delivered to the Department of Anaesthesiology in 10 mL syringes containing 5 mL of solution marked only with trial identification and randomisation numbers. The 10 mL syringes with the test medicines were prepared by a staff anaesthesiologist, who was otherwise uninvolved in the study." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss with the following formula: (0.75 x height in inches x 50) plus (weight in pounds x 50) x ((predelivery haematocrit measurement ‐ postdelivery haematocrit measurement)/predelivery haematocrit measurement). |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00977769). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 1721 parturients were randomised in a hospital setting in France. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an intravenous bolus (n = 863) versus 10 IU of oxytocin administered by an intravenous bolus (n = 857). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | "The study excluded 57 women from the misoprostol group and 61 from the placebo group because they had caesareans." The study report did not specify whether exclusions occurred before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors excluded 119 study participants from the analysis because they experienced caesarean deliveries: it was unclear from the study report whether these exclusions occurred before or after randomisation. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between November 2009 and September 2011, 1865 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with diabetes, non‐cephalic presentation, anaemia, antepartum haemorrhage, multiple pregnancy, grand multiparity (more than 6) or known allergy. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 900) versus 600 mcg of misoprostol administered orally (n = 900). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by dice‐box. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss at delivery by collection with pre‐calibrated kidney dishes. |
| Incomplete outcome data (attrition bias) | Low risk | "46 of the administered questionnaires were invalidated leaving a total of 1819 valid questionnaires (912 for oxytocin and 907 for misoprostol). The data were further reduced through a process of computer randomisation so as to have [an] equal study population in the 2 medication groups: oxytocin group (900 subjects) and misoprostol group (900 subjects)”. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Maiduguri Teaching Hospital. Study medications were donated by Emzor Pharmaceutical Industries. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between March 2011 and June 2011, 216 parturients were randomised in a hospital setting in Iran. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with hypertension, pre‐eclampsia, uterine rupture, cervical tear, asthma, cardiovascular/renal/liver disorders, grand multiparity (not defined), fibroids or previous PPH. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 109) versus 200 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 107). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, death,.change in Hb level, nausea, vomiting, tachycardia, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients and medical personnel were blinded to the type of drug." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | At 24 hours postpartum, blood samples could not be collected from 16 women (9 in the carbetocin group and 7 in the ergometrine plus oxytocin group). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (Iranian registry of clinical trials number 138810212854N2). |
| Intention to treat analysis | High risk | The authors excluded 16 study participants from the analysis because postpartum Hb measurements were not available. |
| Funding source | Unclear risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st September 2009 and 28th February 2010, 200 parturients were randomised in a hospital setting in Nepal. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with polyhydramnios, chorioamnionitis, preterm labour, previous caesarean, asthma, cardiac disorder or contraindication/hypersensitivity to the use of prostaglandin and uterotonics. | |
| Interventions | 1000 mcg of misoprostol administered rectally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, death, blood loss (mL), change in Hb level, third‐stage duration (min), fever, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was randomly allocated as per the lottery technique. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss in the 48 hours postpartum, by collection with pre‐weighed sterile pads and a calibrated bucket. All the soaked drapes and pads were weighed and the dry weights of these materials were subtracted to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 300 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, or those with intrauterine death, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disorder, rhesus‐negative mother, hypertension, Hb less than 70 g/L or hypersensitivity/contraindication to prostaglandins. | |
| Interventions | 400 mcg or 600 mcg of misoprostol administered sublingually (n = 150) versus 5 IU of oxytocin administered by an intravenous bolus (n = 75) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 75). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug packets were sealed and coded by the same individual using a computer‐generated random number chart. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed drug packets. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical" and investigators were "thus blinded." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a disposable and absorbent pre‐weighed linen saver sheet with a pre‐weighed polythene bag under the mother to collect blood from the uterine cavity. Any blood clots were expressed from the vagina into the polythene bag, which was then removed and weighed. A fresh pre‐weighed sanitary napkin was applied. Separate swabs were not included in the final calculation (addition of the various gravimetric measurements), that was performed 1 hour after delivery. "The specific gravity of blood being 1.08, the amount of blood lost in mL was equal to the weight in grams." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (changes in Hb measurements were unspecified beyond textual summary that "all groups showed a slight decrease in mean haemoglobin concentration 24 hours postpartum [maximum decrease of 0.6 g/dL]; however, the difference was not significant [ANOVA, P > 0.05]"). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and February 2003, 1200 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, blood disorders, chorioamnionitis, placenta praevia or placental abruption. | |
| Interventions | 200 mcg of ergometrine administered intramuscularly (n = 300) versus 600 mcg to 1000 mcg of misoprostol administered sublingually (n = 900). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, closed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | High risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a graduated plastic bag, and by weighing towels, linen and gauzes. |
| Incomplete outcome data (attrition bias) | High risk | "144 women were excluded from analysis because they were exposed to trauma to the perineum, vagina or cervix during labour and had traumatic excessive bleeding." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between June 2003 and July 2005, 174 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 90) versus 20 IU of oxytocin administered by an intravenous infusion (n = 84). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes made at pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection with suction apparatus and sterile drapes before irrigation, and by evaluating the blood in abdominal swabs and gauzes. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm cluster controlled randomised trial. | |
| Participants | Between 21st April 2011 and 30th November 2012, 1586 parturients were randomised in a community setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 689) versus placebo or control (n = 897). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, death. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 52 CHOs were randomly allocated equally to either the intervention or the control group; this allocation was stratified by both district and distance (at least 10 km, or less than 10 km) to emergency obstetric care. The randomisation sequence was determined using Stata (version 12). |
| Allocation concealment (selection bias) | Unclear risk | .Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | "The random allocation was not masked." |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated postpartum blood loss by collection with a BRASS‐V calibrated plastic drape placed under the mother, who was asked to remain recumbent for 1 hour following delivery of the baby, or for 2 hours if active bleeding persisted. "Fluids, urine, and faeces were excluded from the blood loss measure by sweeping them to the side and into a receptacle." |
| Incomplete outcome data (attrition bias) | Low risk | "7 and 9 enrolled women in the oxytocin and control arms, respectively, lacked a blood‐loss measure." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01108289). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 2005 and April 2008, 370 parturients were randomised in a hospital setting in Singapore. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with multiple pregnancy, previous PPH, coagulopathy, coronary artery disease, hypertension or hypersensitivity/contraindications for the use of Syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 185) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 185). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was blocked and stratified by parity. The randomisation list with the allocation of the mode of intervention was forwarded from the Biostatistics Unit to the Department of Pharmacy at National University Hospital, where the purchased medications were kept. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque packages made at pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | "The identities of the medications were not known to the midwives, obstetricians and the participants. The medication codes were only broken following completion of the trial." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the visual estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered 2 years after beginning recruitment (ClinicalTrial.gov NCT00499005). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Healthcare Group of Singapore (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2003 and December 2003, 400 parturients were randomised in a hospital setting in Bangladesh. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 210) versus 10 IU of oxytocin administered intramuscularly (n = 190). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians after collection in a plastic bowl. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between May 1997 and April 1998, 65 parturients were randomised in a Hospital setting in Switzerland. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with multiple pregnancy, pre‐eclampsia, previous PPH or antepartum haemorrhage. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 31) versus placebo or control (n = 34). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random tables. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐masked": "for proper masking, the study drugs were prepared by the hospital pharmacy as 3 identical gelatine capsules." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between March 2010 and February 2011, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (more than 4), anaemia, malpresentation, polyhydramnios, antepartum haemorrhage, liver/renal disorder, previous caesarean, previous PPH, uterine anomaly, traumatic PPH or contraindications to use misoprostol or oxytocin. | |
| Interventions | 10 IU of oxytocin administered by an intravenous infusion (n = 50) versus 600 mcg of misoprostol administered sublingually (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting. fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | .Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "Due to [the] nature of administration of the drugs, [the] patient or clinical care team could not be blinded. However, [the] statistician was unaware of the group allocation." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a calibrated plastic bag under the mother to collect blood from the uterine cavity. After delivery of the placenta, a pre‐weighed pad was placed high up in vagina until 1 hour afterwards. In cases of episiotomy, a separate pad was applied to the episiotomy site, and the fluid collected by this pad was not included in blood loss measurements. |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between January 1988 and February 1990, 193 parturients were randomised in a hospital setting in the UK. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those with grand multiparity (not defined), malpresentation, multiple pregnancy, previous caesarean, previous PPH, antepartum haemorrhage, hypertension in pregnancy, intrauterine death, preterm rupture of membranes, cervical lacerations or third degree perineal tears. | |
| Interventions | Placebo or control (n = 90) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 103). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was randomly allocated using standard randomisation tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | The study was conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 21st April 2011 and 31st March 2012, 120 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, pre‐eclampsia, eclampsia, undiagnosed vaginal bleeding, prolonged labour, prolonged obstructed labour, cardiac/renal/liver disorders or fever. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 60) versus 20 IU of oxytocin administered by an intravenous infusion (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocations were generated by random tables. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | "There were no look‐alike placebo tablets for women who had oxytocin alone... The obstetricians and the scrub nurses were unaware of which oxytocic was given to each patient, as they were screened off during the surgery... No member of the obstetric team had knowledge of which agent the patient received”. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "There were no look‐alike placebo tablets for women who had oxytocin alone... The obstetricians and the scrub nurses were unaware of which oxytocic was given to each patient, as they were screened off during the surgery... No member of the obstetric team had knowledge of which agent the patient received”. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative and postoperative blood loss by collection in a suction bottle. Furthermore, soiled drapes, abdominal packs and pieces of gauze were weighed and the known dry weights subtracted. Finally, vulva pads applied during the 4 hours post‐operation, were also weighed and the known dry weights subtracted. Measurements obtained by these 3 methods were added together. Weight measurements were performed with a weighing scale made in China, of total weighing capacity of 5 kg and graduations of 0.25 g. Investigators considered that 1 g is equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of nausea, vomiting, diarrhoea, headaches, fatigue, dizziness, chills, flatulence and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st January 2012 and 30th June 2012, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 2 to 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous PPH, multiple pregnancy, previous caesarean, macrosomia, pre‐eclampsia, diabetes, cardiac/lung/bleeding/clotting disorders or taking anticoagulants. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 50) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 50). | |
| Outcomes | The study recorded the following outcome: PPH at 500. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants (patients) were divided by a lottery system in the 2 groups, each group comprising 50 patients. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss after the delivery of baby "by squeezing the soaked pads and quantifying the amount of blood clots in a kidney tray of standard size to be equal to 500 mL." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 5‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, 248 parturients were randomised in a hospital setting in Turkey. The population comprised women of parity 5 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta praevia, previous PPH, antepartum haemorrhage, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, pre‐eclampsia or anticoagulation therapy. | |
| Interventions | Placebo or control (n = 49) versus 400 mcg to 800 mcg of misoprostol administered orally, vaginally or rectally (n = 199). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, third‐stage duration (min), shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by random tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with cardiac disorder in pregnancy, uterine tumour in pregnancy, secondary PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, anaemia, coagulopathy, antepartum haemorrhage, previous PPH, prolonged labour, precipitate labour or known allergic or hypersensitivity reaction to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered by an intravenous infusion (n = 100) versus 800 mcg of misoprostol administered rectally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators used simple random sampling. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Over 10 months between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (more than 4), multiple pregnancy, preterm labour (less than 32 weeks), HELLP syndrome, polyhydramnios, coagulopathy, asthma, cardiac/renal disorder, epilepsy, hypertension, Hb less than 80 g/L or known drug allergy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 66) versus 200 mcg of ergometrine administered intramuscularly (n = 67). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, Additional uterotonics, transfusion, manual removal of placenta, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators evaluated blood loss by collection with a sterile calibrated BRASS‐V drape placed under the mother. The drape remained in place for 1 hour. Furthermore, "blood loss in gauze pieces was calculated by subtracting the weight of dry gauze from the weight of blood‐soaked gauze pieces." |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 2005 and 2006, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 100) versus 200 mcg of ergometrine administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical‐looking." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "accurately with a specially designed calibrated blood collection drape (BRASS‐V drape)." |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between October 2002 and January 2003, 120 parturients were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with preterm labour (less than 37 weeks), grand multiparity (more than 5), multiple pregnancy, hypertension in pregnancy, Hb less than 80 g/L or known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 60) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by random tables. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Treatments were administered via different routes and the authors did not report any double dummy. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by the estimation of attending nurses and obstetricians. After delivery of the baby, amniotic fluid was allowed to drain away, and amniotic fluid‐soaked bed linens were covered with dry disposable ‘linen‐savers’. A wedge‐shaped plastic bedpan was placed under the mother for 1 hour. Blood and clots from the bedpan were decanted into a measuring cylinder and measured. Blood‐soaked swabs and linen‐savers were weighed; the known dry weights were subtracted, for the weight of blood contained within them to be added to the value indicated by the measuring cylinder. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2004 and April 2005, 100 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with multiple pregnancy, antepartum haemorrhage, polyhydramnios, prolonged labour (more than 12 hours), previous more than 1 caesarean, previous uterine rupture, cardiac/liver/renal disorder, coagulopathy or Hb less than 80 g/L. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 20 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level, vomiting, headache,fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss intraoperatively and in the first hour postoperatively "in a standard manner." They measured the volume of blood in the suction bottle, and weighed blood‐soaked sponges and linen savers. Then they added the difference between dry and blood‐soaked weights of sponges and linen savers, to the volume measured in the suction bottle. |
| Incomplete outcome data (attrition bias) | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Division of Reproductive Health and Nutrition, Indian Council of Medical Research (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 15th June 1998 and 15th May 1999, 401 parturients were randomised in a hospital setting in Ghana. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with grand multiparity (more than 5), multiple pregnancy, preterm labour (less than 32 weeks), hypertension in pregnancy, HELLP syndrome, polyhydramnios, previous PPH, coagulopathy, precipitate labour, chorioamnionitis, Hb less than 80 g/L or a known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 203) versus 10 IU of oxytocin administered intramuscularly (n = 198). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), Change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque packets made by administrative staff. |
| Blinding of participants and personnel (performance bias) | Low risk | "The identity of the placebo and active medications were concealed from caregivers and participants." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) | Low risk | Of those women randomised, blood loss measurements were unavailable in 3 cases, and postpartum Hb samples were unavailable in 9 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from MaterCare International and the Canadian International Development Agency (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified, 58 parturients were randomised in a hospital setting in Australia. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients undergoing elective caesarean section or requiring general anaesthesia, or those with vascular/liver/renal disorders, preterm labour (less than 37 weeks), placenta praevia, placental abruption, previous more than 2 caesareans or an adverse reaction to carbetocin/oxytocin. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 30) versus 5 IU of oxytocin administered by an intravenous bolus (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used computer‐generated randomisation at pharmacy level, and none of the operating or anaesthetic doctors had access to this. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | The study was "double‐blinded": women received "a blinded bolus of carbetocin" or "a blinded oxytocin bolus." |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intra‐operative blood loss by the estimation of attending physicians. Excess blood was collected in measuring container by suction, and weighed together with any swabs soaked in blood. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ACTRN 12612000466842). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Frankston Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between February 1993 and March 1993, 1000 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia or cardiac disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 496) versus 10 IU of oxytocin administered intramuscularly (n = 495). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, change in Hb level, nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | "When a patient entered the study, a nursing officer who was not involved in the management of the patient drew up the indicated medication and handed this to the patient’s attendants." Study participants and caregivers were thus blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss during delivery "by measuring the amount of blood clots and weighing the towels used." |
| Incomplete outcome data (attrition bias) | Low risk | "9 [randomised participants] were excluded: 3 had a twin pregnancy, 1 had blood transfusion during labour, and the other 5 had unavailable records." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Over 8 months between dates unspecified, 2023 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with asthma, cardiac disorder, rhesus factor incompatibility or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 730) versus 10 IU of oxytocin administered intramuscularly (n = 617) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 676). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators evaluated blood loss by collection with a large sterile plastic bag placed under the mother until she was transferred to the postnatal department. The blood collected in the plastic bag was then transferred to a measuring jar. Mops were not used in the labour room, and gauze pieces were counted. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
ACTRN, Australian Clinical Trials Registration Number; ANOVA, one‐way Analysis Of Variance; ASA I or II, ASA Physical Status Classification System: ASA I represents a normal healthy patient, ASA II represents a patient with mild systemic disease; BMI, Body Mass Index; cc, cubic centimetres; CHOs, community health officers; cm, centimetres; CTRI, Clinical Trials Registry of India; DIC, Disseminated Intravascular Coagulopathy; dL, decilitres; EudraCT, European Clinical Trials database; fL, femtolitres (measurement of mean corpuscular volume); g, grams; Hb, Haemoglobin; HELLP syndrome, Hemolysis (destruction of red blood cells), Elevated Liver enzymes (which indicate liver damage), and Low Platelet count; HIV, Human Immunodeficiency Virus; Hong Kong SAR, Hong Kong Special Adminstrative Region; IU, International Units; kg, kilograms; km, kilometres; L, litres; mcg, micrograms; mg, milligrams; min, minutes; mL, millilitres; mmHG, millimetres of mercury (unit of pressure); mmol, millimoles; NCT, National Clinical Trial (number); NEPU, National Perinatal Epidemiology Unit; NHS, National Health Service; nm, nanometres; NNU, Neonatal Unit; PACTR, Pan African Clinical Trials Registry; PPH, Postpartum Haemorrhage; PROM, Premature Rupture Of Membranes; RCOG, Royal College of Obstetricians and Gynaecologists; UK, United Kingdom; UNDP/UNFPA, United Nations Development Programme/United Nations Population Fund; USA, United States of America; WHO, World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of misoprostol administration | |
| Same drug intervention both arms and only different dose of carbetocin administration | |
| Not eligible intervention | |
| Intervention given after the third stage of labour | |
| Not eligible intervention | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible uterotonic | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible uterotonic | |
| Not eligible uterotonic | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible uterotonic | |
| Not eligible uterotonic | |
| Intervention for treatment of PPH | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Inappropriate population (excluded women who had PPH) | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Same drug intervention both arms and only different dose of carbetocin administration | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Inappropriate population | |
| Intervention for treatment of PPH | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Not eligible population (terminations 2nd trimester) | |
| Not randomised | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different route of misoprostol administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Treatment (not prevention) of PPH. | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not randomised | |
| Not eligible intervention | |
| Not appropriate intervention (comparing timing of oxytocin) | |
| Intervention for treating PPH | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Not randomised | |
| Inappropriate population (excluded women who had PPH) | |
| Not appropriate intervention (comparing timing of oxytocin) | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention (given oral ergometrine for 6 days) | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Ineligible population (excluded women with PPH) | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of misoprostol administration | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different route of misoprostol administration | |
| Not eligible intervention | |
| Innapropriate population | |
| Innapropriate population | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention (carboprost) | |
| Not eligible intervention (carboprost) | |
| Not eligible intervention (carboprost) | |
| Not eligible intervention | |
| Not eligible population (2nd trimester) | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of misoprostol administration | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different type of the same drug | |
| Not eligible intervention | |
| Not randomised | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Same drug intervention both arms and only different route of oxytocin administration | |
| Not eligible intervention | |
| Study withdrawn | |
| Not eligible intervention | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different dose of carbetocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different route and timing of oxytocin administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Inappropriate population (excluded women who had PPH) | |
| Not eligible intervention | |
| Same drug intervention both arms and only different dose of oxytocin administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not randomised | |
| Not eligible intervention | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different doses of drug administration | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Not eligible intervention | |
| Inappropriate population (measured blood loss after the delivery of the placenta) | |
| Not eligible intervention | |
| Not eligible population (2nd stage) | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible intervention | |
| Qausi‐randomised | |
| Not eligible intervention | |
| Not randomised | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different timings of drug administration | |
| Same drug intervention both arms and only different timings of drug administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not randomised | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Quasi‐randomised | |
| Innapropriate population | |
| Innapropriate population | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Administered for treatment of PPH | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Quasi‐randomised | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible intervention | |
| Not randomised | |
| Not eligible intervention | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible uterotonic | |
| Quasi‐randomised | |
| Innapropriate population | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible outcomes | |
| Same drug intervention both arms and only different regimen of oxytocin administration | |
| Not eligible uterotonic (oral ergometrine) | |
| Not eligible intervention | |
| Self‐administered drug | |
| Not eligible intervention | |
| Not eligible outcomes | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different doses of drug administration | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Not eligible intervention | |
| Same drug intervention both arms and only different doses of drug administration |
PPH: postpartum haemorrhage
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 100 women with singleton term pregnancy undergoing caesarean with spinal anaesthesia |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion or 400 mcg of misoprostol administered sublingually |
| Outcomes | Additional uterotonics, blood loss (mL), change in Hb level, fever,.shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | Randomised trial. |
| Participants | 542 nulliparous pregnant women |
| Interventions | 20 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered sublingually |
| Outcomes | PPH (not defined), third‐stage duration (min), headache, shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Written in Persian and awaiting translation. |
| Methods | Randomised trial. |
| Participants | 120 women |
| Interventions | 10 IU of oxytocin administered intramuscularly or 400 mcg of misoprostol administered sublingually |
| Outcomes | PPH at 500. Blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Cannot obtain full text. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Cluster‐randomised trial. |
| Participants | 1200 women from 30 health centres |
| Interventions | 600 mcg of Methylergometrine administered intramuscularly or 600 mcg of misoprostol administered orally |
| Outcomes | PPH at 500, third‐stage duration (min).,additional uterotonics,transfusion, blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Require intracluster correlation coefficient. |
| Methods | Randomised trial. |
| Participants | 200 women |
| Interventions | Not known dose of ergometrine administered intravenously or not known dose of misoprostol administered orally |
| Outcomes | Additional uterotonics, PPH (not defined), third‐stage duration (min), transfusion. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 152 women with no clear inclusion criteria |
| Interventions | Not known dose of carbetocin of unknown route or not known dose of oxytocin of unknown route |
| Outcomes | PPH (not defined). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 1750 women |
| Interventions | 5 IU of oxytocin administered intramuscularly then increased to 10 IU or ergometrine 500 mcg plus 5 IU of oxytocin administered intramuscularly |
| Outcomes | PPH at 500, blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Open‐label randomised trial. |
| Participants | 143 women with term singleton pregnancies |
| Interventions | Not known dose of oxytocin of unknown route or Not known dose of oxytocin of unknown route plus 600 mcg of misoprostol administered rectally. |
| Outcomes | Change in Hb level. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Double‐blinded randomised trial. |
| Participants | Not known how many women randomised |
| Interventions | Not known dose of oxytocin administered intravenously or 400 mcg of misoprostol administered orally. |
| Outcomes | PPH at 500, PPH at 1000, additional uterotonics, transfusion, change in Hb level, blood loss (mL), fever, shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | Not known how many women randomised |
| Interventions | 200 mcg of Methylergometrine of unknown route or 400 mcg of misoprostol administered sublingually. |
| Outcomes | PPH (not defined), additional uterotonics, change in HB level, third‐stage duration (min), blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hb, haemoglobin; IU, international unit;mcg, microgram; mL,: milliltre; PPH, postpartum haemorrhage
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Buccal misoprostol during cesarean section for preventing postpartum hemorrhage |
| Methods | Placebo‐controlled randomised trial. |
| Participants | 120 women undergoing an elective or emergency caesarean birth at 24 weeks of gestation or later with risk factors for PPH. |
| Interventions | 400 mcg of misoprostol administered buccally or placebo |
| Outcomes | PPH at 1000, additional uterotonics, transfusion. |
| Starting date | February 2008, Updated 2012 |
| Contact information | Dr. Jose E. Gonzalez |
| Notes | This study is shown as currently recruiting participants. |
| Trial name or title | Comparing misoprostol and oxytocin in UnijectTM for Postpartum Hemorrhage (PPH) Prevention in Mali |
| Methods | Double‐blinded randomised trial. |
| Participants | 140 women with a pregnancy over 34 weeks and a risk factor for PPH. |
| Interventions | 100 mcg of carbetocin administered intravenously or 10 IU of oxytocin administered intravenously. |
| Outcomes | Change in Hb level, nausea, vomiting, fever, shivering. |
| Starting date | Start date not known. |
| Contact information | Milton Cesar Gomez Gomez |
| Notes | This study is shown as not yet recruiting. |
| Trial name or title | Comparing misoprostol and oxytocin in Uniject for postpartum hemorrhage (PPH) prevention in Senegal |
| Methods | Open‐label cluster‐randomised trial. |
| Participants | 1365 women giving birth in community health centres with a trained study provider. |
| Interventions | 600 mcg of misoprostol administered orally or 10 IU of oxytocin administered intramuscularly. |
| Outcomes | Change in Hb level, nausea, vomiting, fever, shivering. |
| Starting date | June 2012 |
| Contact information | Gynuity health projects |
| Notes | This study is shown as completed. |
| Trial name or title | Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox) |
| Methods | Randomised trial |
| Participants | Women delivering vaginally, singleton pregnancy |
| Interventions | One dose of 100 mcg intramuscular Carbetocin given for active management of the third stage of labour, immediately after the birth of the baby. One dose of 10 IU intramuscular Syntocinon given for active management of the third stage of labour, immediately after the birth of the baby. One dose of 500 mcg/5 IU intramuscular Syntometrine given for active management of the third stage of labour, immediately after the birth of the baby. |
| Outcomes | Requirement for additional uterotonic drugs |
| Starting date | February 2015 |
| Contact information | Tim Draycott, North Bristol NHS Trust/University of Bristol |
| Notes | Study Chair: |
| Trial name or title | Efficiency of carbetocin in the prevention of the postpartum haemorrhage: a clinical double‐blinded randomised study |
| Methods | Open‐label randomised trial. |
| Participants | Women undergoing a vaginal birth at home with a trained study provider. |
| Interventions | 600 mcg of misoprostol administered orally or 10 IU of oxytocin administered intramuscularly. |
| Outcomes | PPH at 1000, additional uterotonics, transfusion, nausea, headache, abdominal pain. |
| Starting date | 15/07/2010 |
| Contact information | Milton Cesar Gomez Gomez |
| Notes | This study is shown as not yet recruiting. |
| Trial name or title | Comparison of the effect of rectal misoprostol and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 600 mcg of misoprostol administered rectally. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/4/2010 |
| Contact information | Dr. Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Comparison effect of carbetocine and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 100 mcg of carbetocin administered intramuscularly. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/1/2010 |
| Contact information | Dr. Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage |
| Methods | Randomised trial. |
| Participants | 300 women with singleton, term pregnancies. |
| Interventions | 10 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered orally. |
| Outcomes | Change in haemoglobin. |
| Starting date | 22/12/2009 |
| Contact information | Simindokht Moradi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Misoprostol versus oxytocin for prevention of post partum hemorrhage |
| Methods | Double‐dummy randomised trial. |
| Participants | 400 women undergoing vaginal birth with a singleton pregnancy. |
| Interventions | 400 mcg misoprostol administered orally or 20 IU of oxytocin administered through an intravenous infusion. |
| Outcomes | Change in Hb level, vomiting, fever, shivering. |
| Starting date | May 2013 |
| Contact information | Dr Minoo Rajaei |
| Notes | This study is shown as ongoing, but not recruiting participants. |
| Trial name or title | Comparison between rectal & sublingual misoprostol before caesarian section to reduce intra & post‐operative blood loss |
| Methods | Placebo‐controlled randomised trial. |
| Participants | 635 women undergoing elective caesarean with a singleton term pregnancy and only 1 previous caesarean. |
| Interventions | 400 mcg of misoprostol administered rectally or 400 mcg of misoprostol administered sublingually or placebo. |
| Outcomes | Change in Hb level, blood loss. |
| Starting date | February 2013 |
| Contact information | Mohamed S Sweed, |
| Notes | This study is shown as completed. |
| Trial name or title | Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally. |
| Methods | Randomized, non‐inferiority trial at 22 centres in 10 countries. |
| Participants | Women delivering vaginally, cervical dilatation equal to or less than 6cm, singleton pregnancy. |
| Interventions | Carbetocin RTS 100 micrograms solution for intramuscular (IM) injection to be administered once during the third stage of labour. Oxytocin 10 IU solution for intramuscular (IM) injection to be administered once during the third stage of labour. |
| Outcomes | The proportion of women with blood loss of 500 mL or more or the use of additional uterotonics at one hour and up to two hours for women who continue to bleed after one hour. (composite primary outcome). The blood loss will be measured with a plastic drape placed under the woman's buttocks. |
| Starting date | July 2015 |
| Contact information | Mariana Widmer ([email protected]) |
| Notes | ACTRN12614000870651 |
Hb, haemoglobin; IU, international unit;mcg, microgram; PPH, postpartum haemorrhage; RTS, room temperature stable
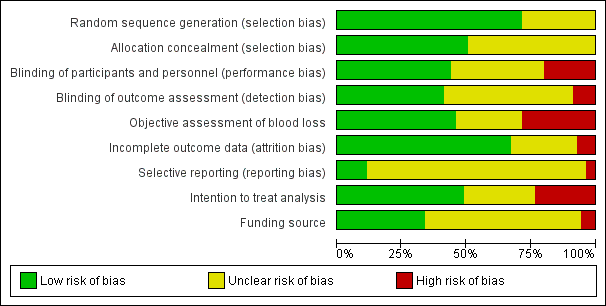
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
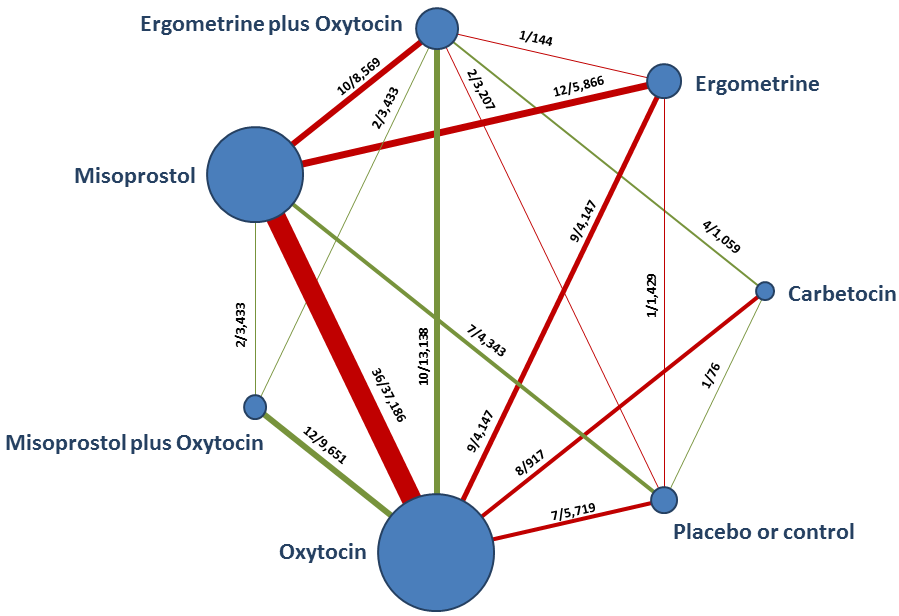
Network diagram for PPH ≥ 500 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green when more than 50% of the trials involved in the specific direct comparison are judged to be at “low risk of bias” if they were double‐blinded, and had allocation concealment with little loss to follow‐up (less than 10%). The colour is red when less than 50% of the trials are at “low risk of bias”. Multi‐arm trials contribute to more than one comparison.
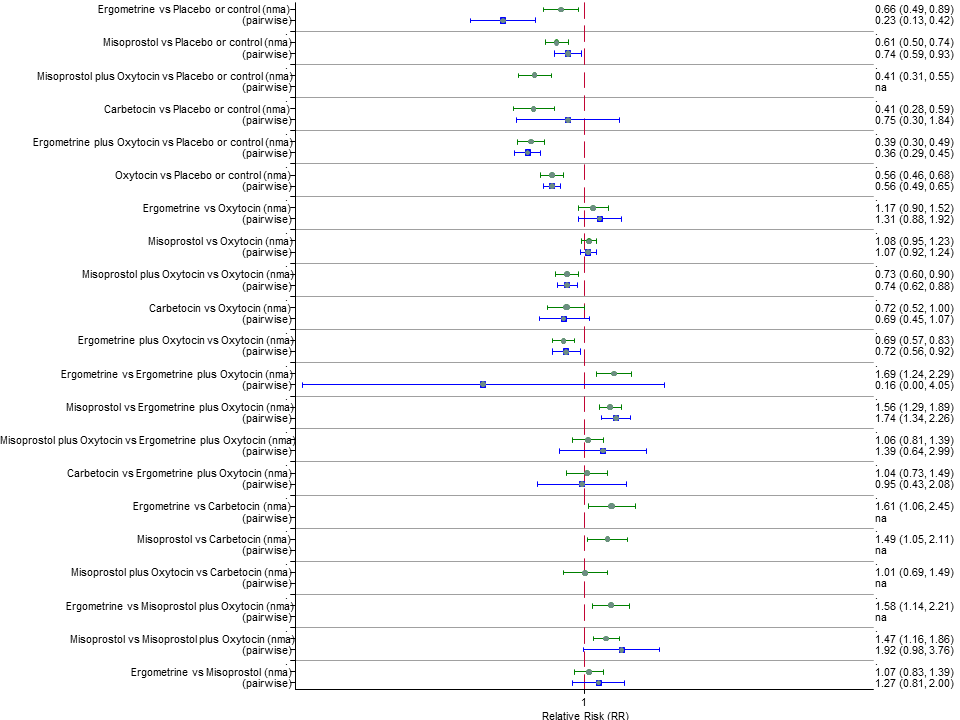
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL. Ranking indicates the cumulative probability of being the best drug, the second best, the third best, etc. The x‐axis shows the relative ranking and the y‐axis the cumulative probability of each ranking. We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available drug options.
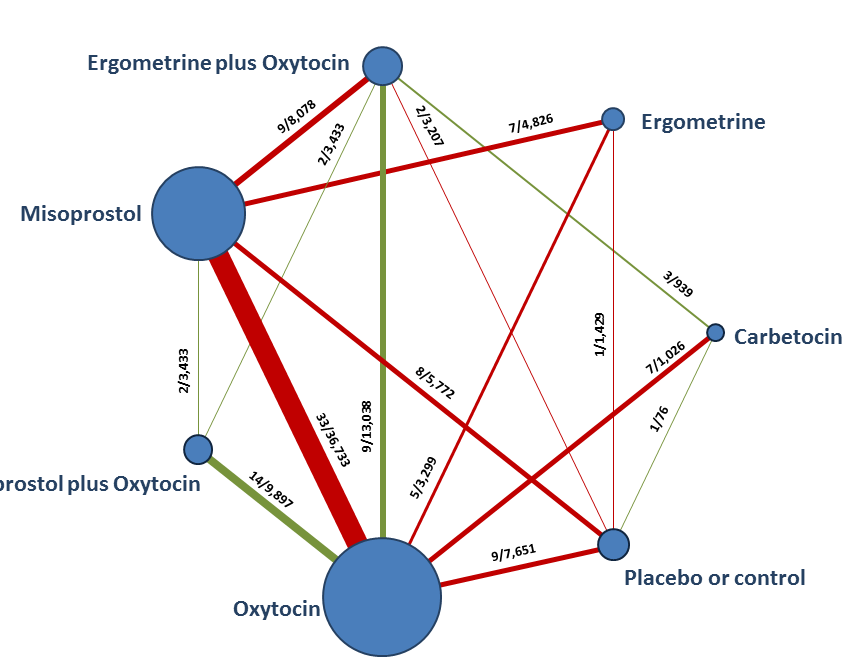
Network diagram for PPH ≥ 1000 mL.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 1000 mL.
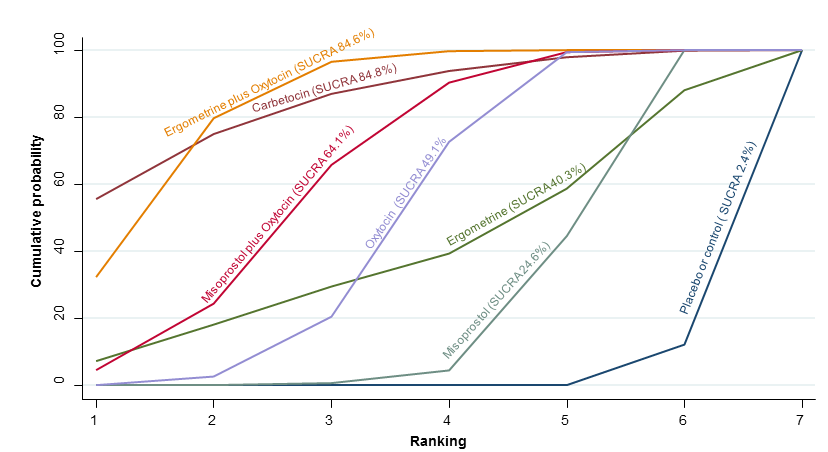
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 1000 mL.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for maternal death.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of maternal death.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for maternal death or severe morbidity.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of maternal deaths or severe morbidity events.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of additional uterotonics.

Cumulative rankograms comparing each of the uterotonic drugs for the requirement of additional uterotonics.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of blood transfusion.

Cumulative rankograms comparing each of the uterotonic drugs for the requirement of blood transfusion.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of manual removal of placenta.

Cumulative rankograms comparing each of the uterotonic drugs for the requirement of manual removal of placenta.
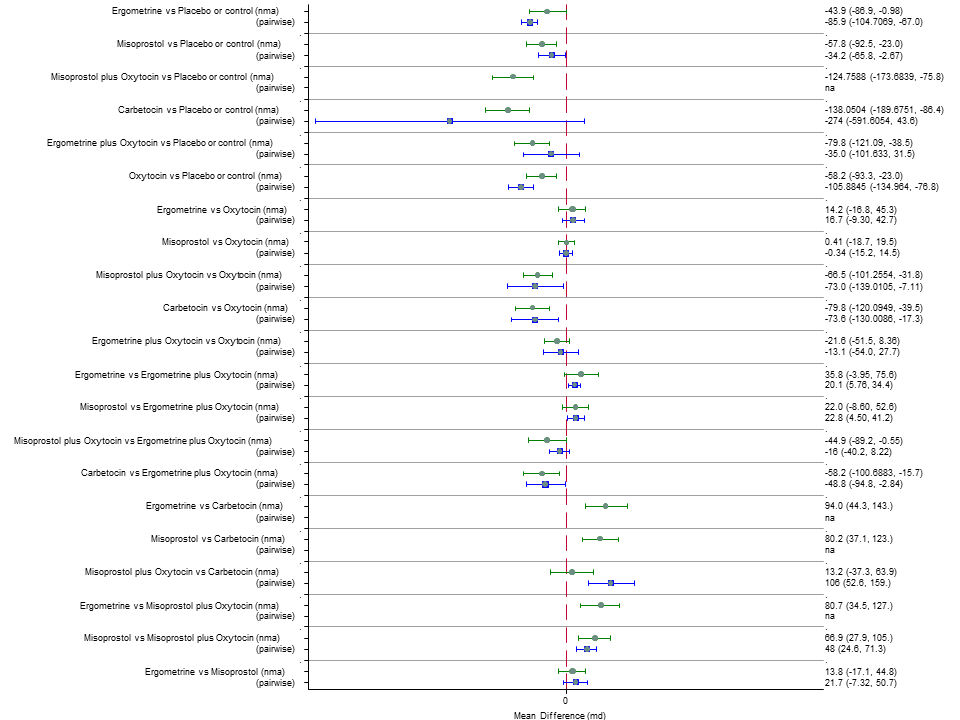
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for blood loss (mL).

Cumulative rankograms comparing each of the uterotonic drugs for blood loss (mL).
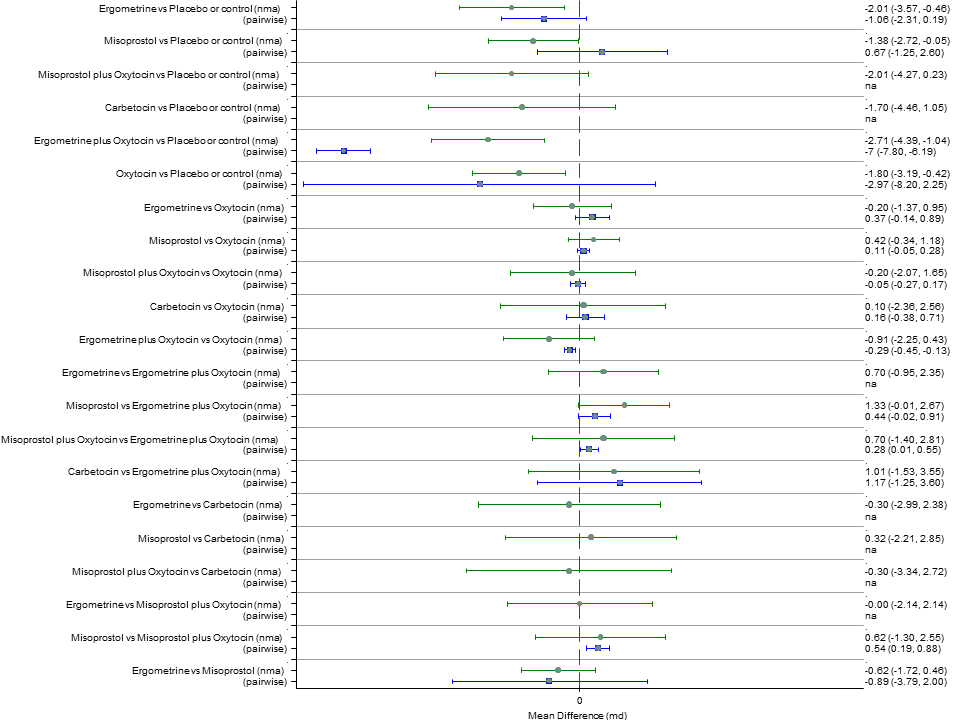
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for duration of third stage (minutes).

Cumulative rankograms comparing each of the uterotonic drugs for duration of third stage (minutes).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for change in haemoglobin measurements before and after birth (g/L).

Cumulative rankograms comparing each of the uterotonic drugs for change in haemoglobin measurements before and after birth (g/L).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for neonatal unit admissions.
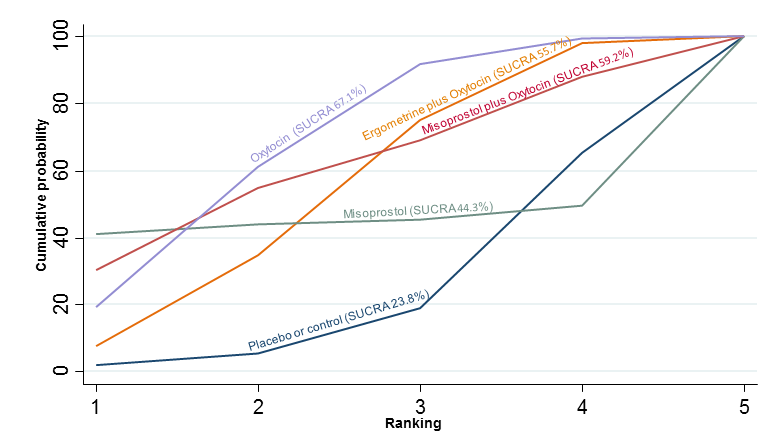
Cumulative rankograms comparing each of the uterotonic drugs for neonatal unit admissions.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for breastfeeding at discharge.

Cumulative rankograms comparing each of the uterotonic drugs for breastfeeding at discharge.
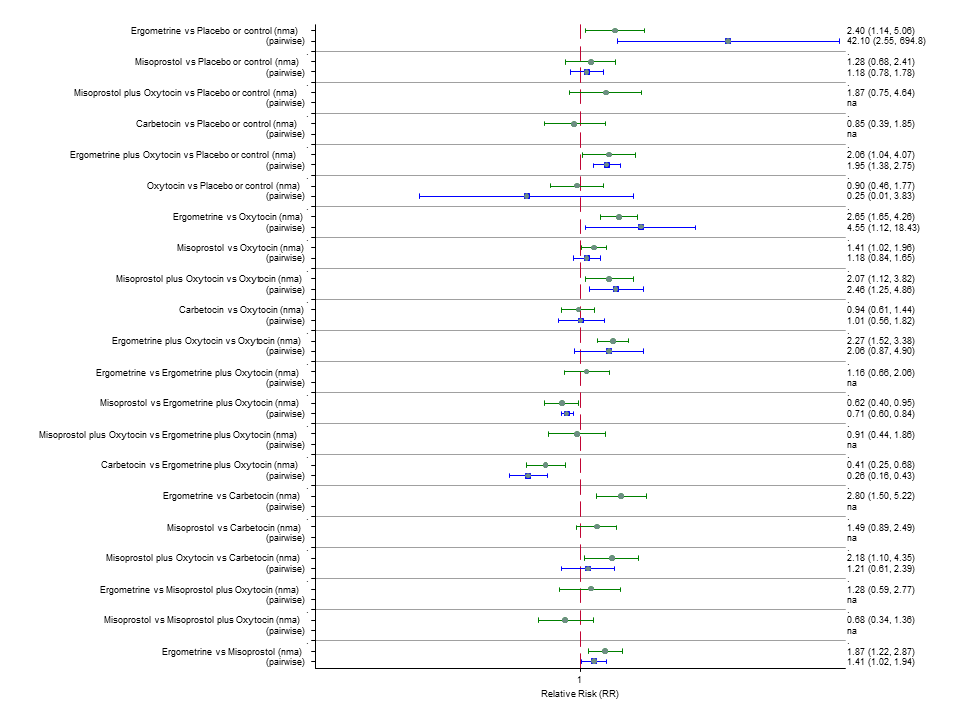
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for nausea.
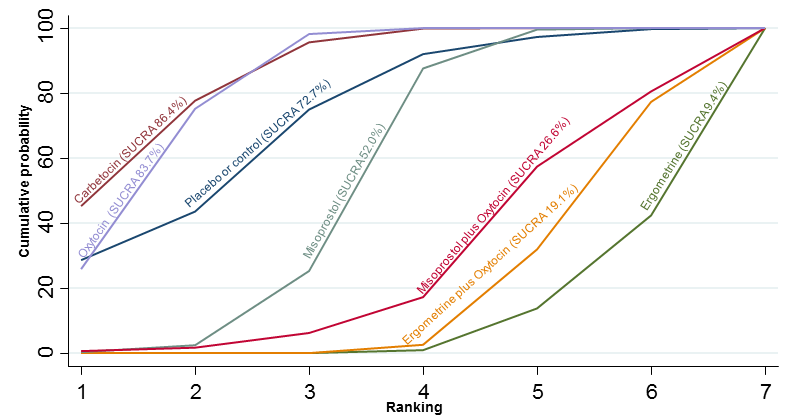
Cumulative rankograms comparing each of the uterotonic drugs for nausea.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for vomiting.

Cumulative rankograms comparing each of the uterotonic drugs for vomiting.
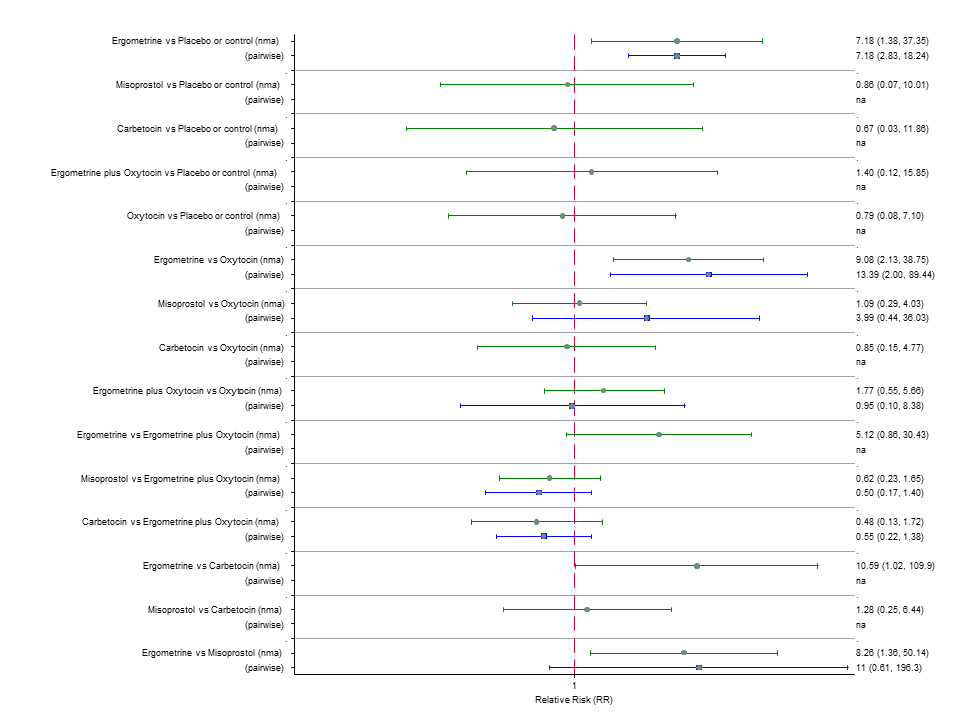
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for hypertension.
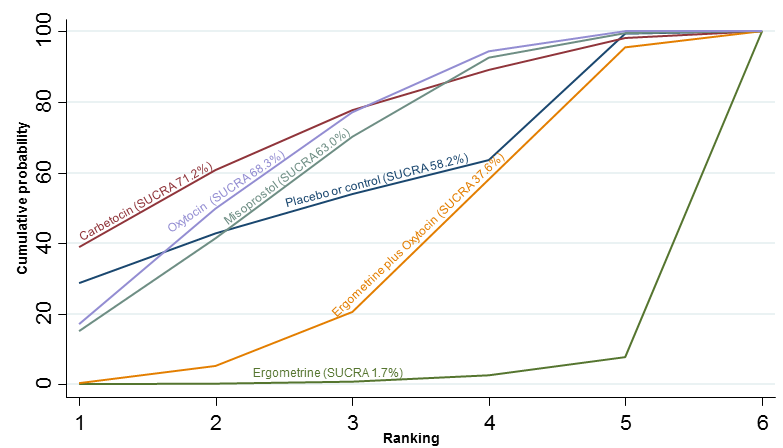
Cumulative rankograms comparing each of the uterotonic drugs for hypertension.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for headache.
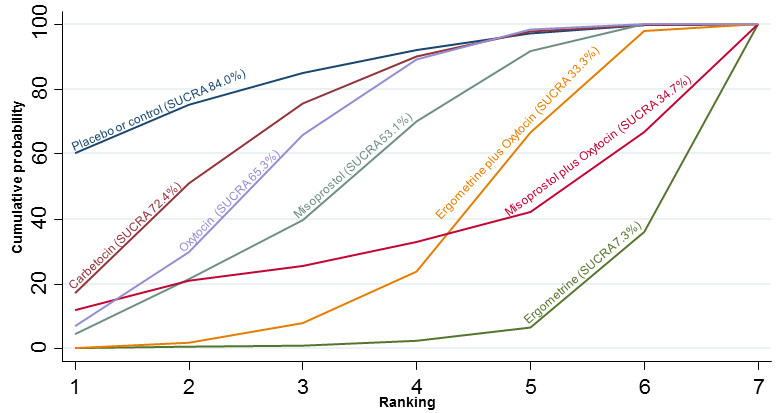
Cumulative rankograms comparing each of the uterotonic drugs for headache.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for fever.

Cumulative rankograms comparing each of the uterotonic drugs for fever.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for shivering.

Cumulative rankograms comparing each of the uterotonic drugs for shivering.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for tachycardia.

Cumulative rankograms comparing each of the uterotonic drugs for tachycardia.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for hypotension.

Cumulative rankograms comparing each of the uterotonic drugs for hypotension.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for abdominal pain.

Cumulative rankograms comparing each of the uterotonic drugs for abdominal pain.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by mode of birth (vaginal birth).
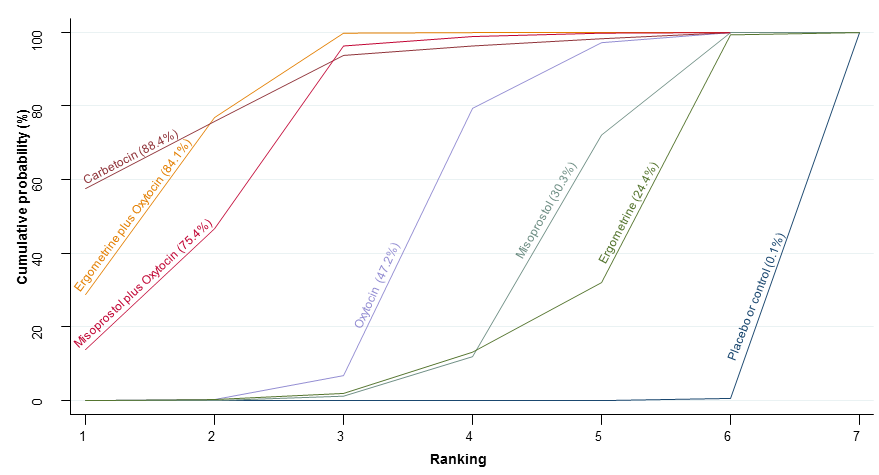
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by mode of birth (vaginal birth).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by mode of birth (caesarean).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by mode of birth (caesarean).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by prior risk for PPH (low risk).
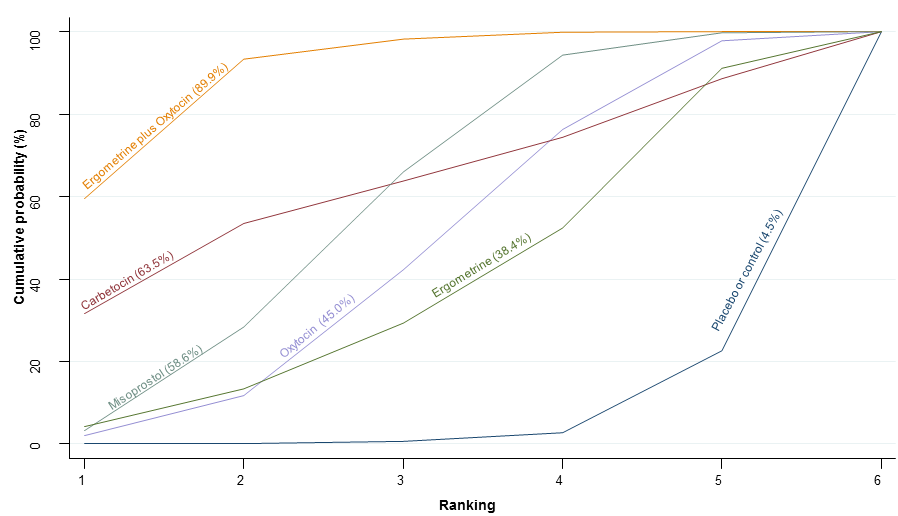
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by prior risk for PPH (low risk).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by prior risk for PPH (high risk).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by prior risk for PPH (high risk).
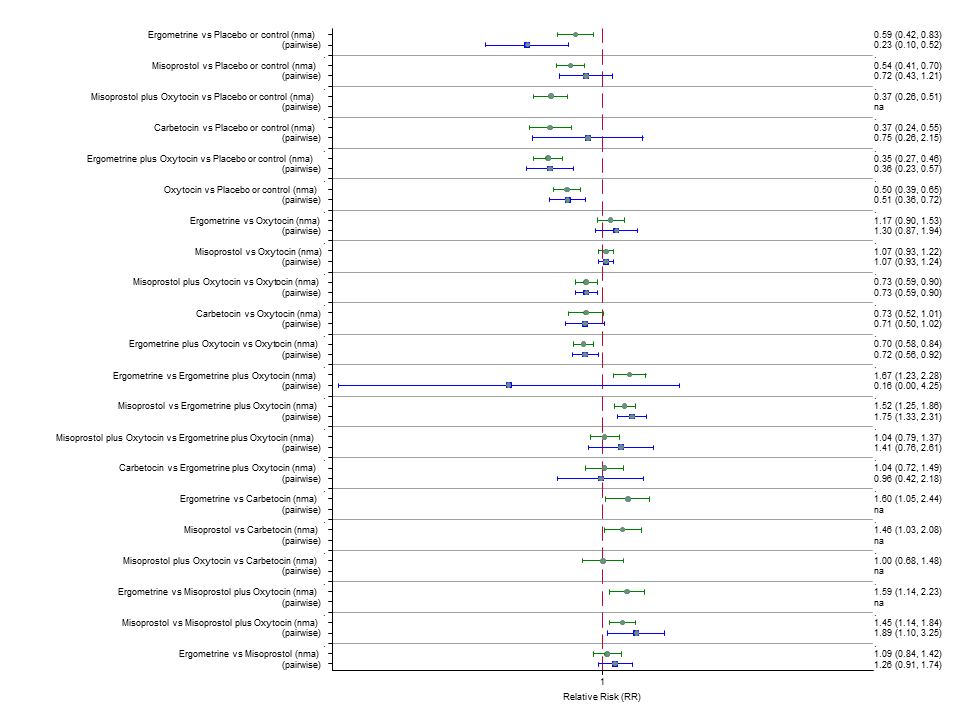
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by healthcare setting (hospital setting).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by healthcare setting (hospital setting).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by healthcare setting (community setting).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by healthcare setting (community setting).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a low dose (less or equal to 500 mcg).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a low dose (less or equal to 500 mcg).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a high dose (600 mcg or more).
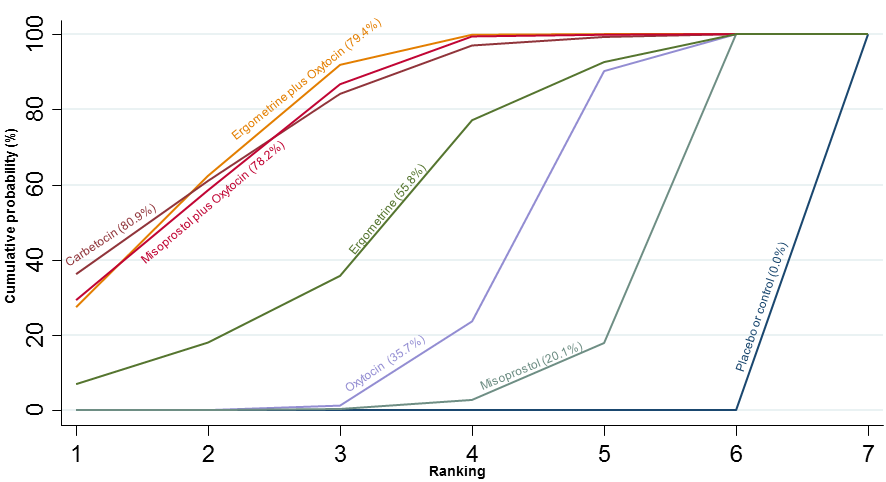
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 1000 mL restricted to misoprostol studies that use a high dose (600 mcg or more).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.
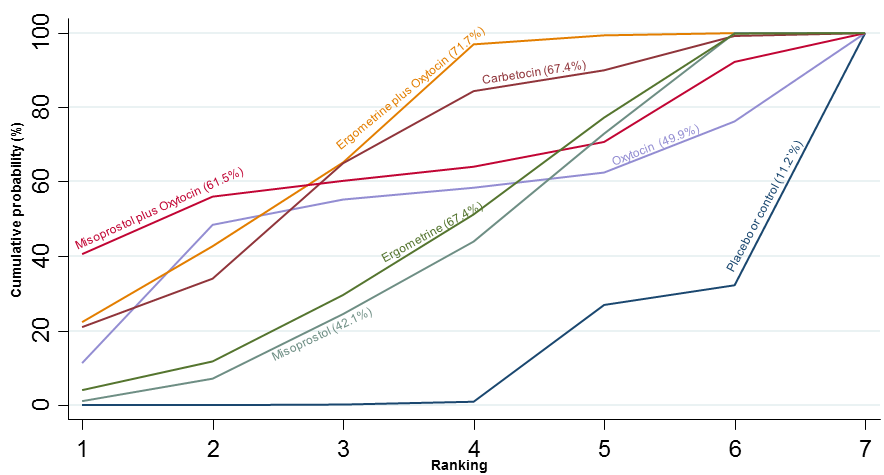
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous infusion only of any dose.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous infusion only of any dose.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to low risk of bias studies only.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to low risk of bias studies only.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to studies with funding source at low risk of bias (public or no funding).

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to studies with funding source at low risk of bias (public or no funding).

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to studies with an objective method of measuring blood loss.

Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to studies with an objective method of measuring blood loss.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to large studies (> 400 participants).
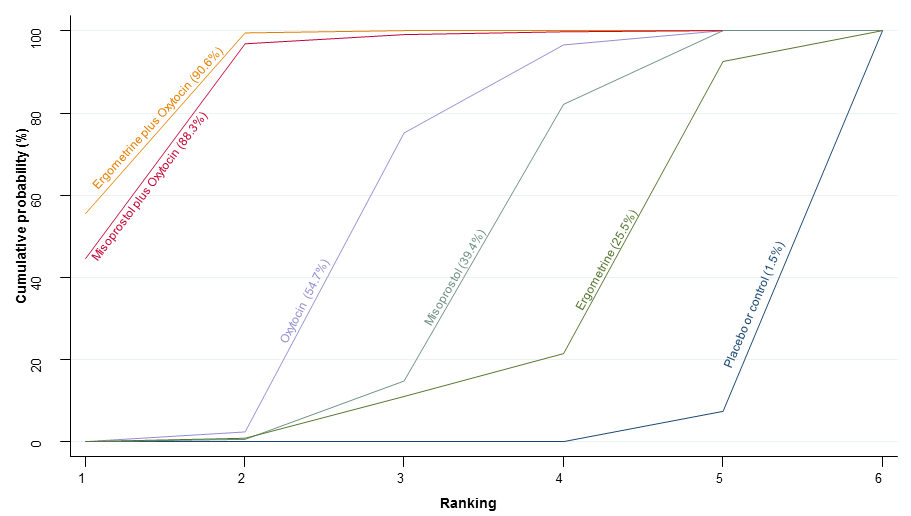
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to large studies (> 400 participants).
| Effects of uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis | |||||
| Patient or population: Women giving birth and at the third stage of labour Settings: Hospital setting Intervention: Ergometrine plus oxytocin, Carbetocin, Misoprostol plus oxytocin Comparison: Oxytocin | |||||
| Outcomes | Effects and 95% confidence intervals in the effects. Main comparator is oxytocin. | Comments | |||
| Risk with ergometrine plus oxytocin* | Risk with carbetocin* | Risk with misoprostol plus oxytocin* | Risk with oxytocin** | ||
| PPH ≥500 mL | 7.2% (6 to 8.7) for vaginal births 51.7% (42.7 to 62.2) for caesareans | 7.6% (5.5 to 10.5) for vaginal births 53.9% (38.9 to 74.9) for caesareans | 7.7% (6.3 to 9.5) for vaginal births 54.7% (44.9 to 67.4) for caesareans | 10.5% (9.8 to 11.3) for vaginal births 74.9% (65.7 to 85.4) for caesareans | There was evidence of global inconsistency in this analysis ( P = 0.046). However, the comparisons in this table were consistent except for the comparison of ergometrine versus no treatment not included in this table‐based on a single study. |
| RR 0.69 (0.57 to 0.83) (NMA) RR 0.72 (0.56 to 0.92) (Pairwise) | RR 0.72 (0.52 to 1.00) (NMA) RR 0.69 (0.45 to 1.07) (Pairwise) | RR 0.73 (0.60 to 0.90) (NMA) RR 0.74 (0.62 to 0.88) (Pairwise) | 1 | ||
| ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 10 studies (13,138 women, I2=57.4%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, imprecision and inconsistency based on 8 studies (917 women, I2 = 49.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 12 studies (9651 women, I2 = 60.5%) | |||
| PPH ≥1000 mL | 2.8% (2.2 to 3.4) for vaginal births 10.7% (8.5 to 13.2) for caesareans | 2.5% (1.4 to 4.6) for vaginal births 9.7% (5.3 to 17.8) for caesareans | 3.2% (2.6 to 4.1) for vaginal births 12.5% (10 to 15.8) for caesareans | 3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans | There was no evidence of global inconsistency (P = 0.345) in this analysis. |
| RR 0.77 (0.61 to 0.95) (NMA) RR 0.73 (0.57 to 0.93) (Pairwise) | RR 0.70 (0.38 to 1.28) (NMA) RR 0.71 (0.38 to 1.35) (Pairwise) | RR 0.90 (0.72 to 1.14) (NMA) RR 0.89 (0.71 to 1.12) (Pairwise) | 1 | ||
| ⊕⊕⊕⊕ high confidence in estimate based on 9 studies (13,038 women, I2 = 0%) | ⊕⊕⊝⊝ low confidence in estimate due to risk of bias and imprecision based on 7 studies (1026 women, I2 = 0%) | ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 14 studies (9897 women, I2 = 0%) | |||
| Vomiting | 1.9% (1.3 to 2.7) for vaginal births 16.1% (11 to 23.7) for caesareans | 0.5% (0.3 to 0.9) for vaginal births 4.6% (2.9 to 7.4) for caesareans | 1.3% (0.8 to 2) for vaginal births 11.2% (7.1 to 17.6) for caesareans | 0.6% (0.5 to 0.6) for vaginal births 5.2% (4.9 to 5.5) for caesareans | There was no evidence of global inconsistency (P = 0.06) in this analysis. |
| RR 3.10 (2.11 to 4.56) (NMA) RR 3.15 (1.72 to 5.78) (Pairwise) | RR 0.89 (0.55 to 1.42) (NMA) RR 0.88 (0.39 to 1.99) (Pairwise) | RR 2.16 (1.37 to 3.39) (NMA) RR 2.25 (1.45 to 3.48) (Pairwise) | 1 | ||
| ⊕⊕⊕⊕ high confidence in estimate based on 8 studies (9811 women, I2 = 48.1%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 10 studies (1939 women, I2 = 59.2%) | ⊕⊕⊕⊕ high confidence in estimate due to imprecision based on 9 studies (5015 women, I2 = 30.1%) | |||
| Hypertension | 1.2% (0.4 to 4) for vaginal births 29.6% ( to ) for caesareans | 0.6% (0.1 to 3.3) for vaginal births 14.2% (2.5 to 79.7) for caesareans | Risks not available as no studies report this outcome | 0.7% (0.7 to 0.8) for vaginal births 16.7% (11.2 to 24.9) for caesareans | There was no evidence of global inconsistency (P = 0.481) in this analysis. |
| RR 1.77 (0.55 to 5.66) (NMA) RR 0.95 (0.10 to 8.38) (Pairwise) | RR 0.85 (0.15 to 4.77) (NMA) | RR not available as no studies reported this outcome | 1 | ||
| ⊕⊕⊝⊝ low confidence in estimate due to inconsistency and imprecision based on 2 studies (1039 women, I2 = 73.2%) | ⊕⊕⊝⊝ low confidence in estimate due to imprecision and based only on indirect evidence | Quality of the evidence cannot be assessed as no studies report this outcome | |||
| Fever | 3% (1.5 to 6) for vaginal births 11.7% (6.5 to 23.2) for caesareans | 3.1% (0.8 to 12.1) for vaginal births 12% (3.1 to 46.6) for caesareans | 11.4% (8 to 16.4) for vaginal births 44.2% (30.9 to 63.2) for caesareans | 3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans | There was no evidence of global inconsistency (P = 0.352) in this analysis. |
| RR 0.84 (0.42 to 1.67) (NMA) RR 1.07 (0.47 to 2.43) (Pairwise) | RR 0.86 (0.22 to 3.35) (NMA) RR 2.11 (0.18 to 24.40) (Pairwise) | RR 3.18 (2.22 to 4.55) (NMA) RR 2.96 (1.95 to 4.51) (Pairwise) | 1 | ||
| ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 2 studies (1591 women, I2 = 0%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 3 studies (292 women, I2 = 40.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 15 studies (8209 women, I2 = 77.8%) | |||
| *The risks in the ergometrine plus oxytocin, carbetocin, misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effects of the interventions (and its 95% CI). **The risk in the oxytocin group (and its 95% confidence interval) is based on a meta‐analysis of proportions from the studies included in this review for this group. | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect | |||||