Intervenciones para la hemocromatosis hereditaria
References
Referencias de los estudios incluidos en esta revisión
Jump to:
Referencias de los estudios en curso
Jump to:
Referencias adicionales
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 38. Post‐randomisation dropouts: 0 (0%). Revised sample size: 38. Mean age: 52 years. Number of women: 10 (26.3%). Symptomatic: not stated. Asymptomatic: not stated. Mean follow‐up period: 8 months. Target used for iron reduction: serum ferritin ≤ 50 μg/L. Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: therapeutic erythrocytapheresis (n = 19). Further details: 350 mL to 800 mL of red blood cells once every 2 weeks. Group 2: phlebotomy (n = 19). Further details: 500 mL of whole blood once weekly. Treatment duration: variable depending upon the iron to be removed. | |
| Outcomes | Mortality, adverse events, and health‐related quality of life. | |
| Notes | We attempted to contact the corresponding author to obtain additional information on risk of bias and outcomes in June 2015. We did not receive any reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned in a 1:1 ratio by an independent person working as quality assurance manager". |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly assigned in a 1:1 ratio by an independent person working as quality assurance manager". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "single‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "single‐blind". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "This work was performed with the support of the Sanquin Blood Bank grants 03‐006". |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 53. Post‐randomisation dropouts: 7 (13.2%). Revised sample size: 46. Mean age: 55 years. Number of women: not stated. Symptomatic: not stated. Asymptomatic: not stated. Mean follow‐up period: 1 year (after this there was cross‐over). Target used for iron reduction: serum ferritin ≤ 50 μg/L. Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: therapeutic erythrocytapheresis (n = 20). Further details: 350 mL to 800 mL red blood cells; variable frequency depending on serum ferritin level. Group 2: phlebotomy (n = 26). Further details: 500 mL per single treatment; variable frequency depending on serum ferritin level. Treatment duration: 1 year. | |
| Outcomes | None of our outcomes of interest were reported at the end of the first treatment. | |
| Notes | Reasons for post‐randomisation dropouts: not stated clearly. We attempted to contact the corresponding author in June 2015 to obtain additional information on risk of bias and outcomes. We did not receive any reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: probably not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: probably not blinded. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: important outcomes such as mortality and complications were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Norway. Number randomised: 62. Post‐randomisation dropouts: 0 (0%). Revised sample size: 62. Mean age: 42 years. Number of women: 6 (9.7%). Symptomatic: not stated. Asymptomatic: not stated. Mean follow‐up period (for all groups): 3 months. Target used for iron reduction: serum ferritin ≤ 50 μg/L. Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: therapeutic erythrocytapheresis (n = 30). Further details: 400 mL per single treatment bi‐weekly*. Group 2: phlebotomy (n = 32). Further details: 450 mL per single treatment weekly. Treatment duration: 12 weeks. | |
| Outcomes | Adverse events. | |
| Notes | We attempted to contact the corresponding author in June 2015 to obtain additional information on risk of bias and outcomes. We did not receive any reply. * It was unclear whether the authors meant 'bi‐weekly' to be once every two weeks or twice weekly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure was centralised to one of the participating centres, using a randomly generated sequence". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "different treatment". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were 6 dropouts/withdrawal but data regarding tolerance reported. |
| Selective reporting (reporting bias) | High risk | Comment: no data about mortality but just tolerability. |
| For‐profit bias | Low risk | Quote: "Grants from Helse Vest RHF and Helse Fonna HF (public hospital trusts)". |
| Other bias | Low risk | Comment: no other risk of bias. |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Mi‐iron |
| Methods | Randomised clinical trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Erythrocytapheresis versus plasmapheresis |
| Outcomes | Modified Fatigue Impact Scale Medical Outcomes Study 36‐item short form V.2 Hospital Anxiety and Depression Scale Arthritis Impact Measurement Scales 2 short form |
| Starting date | June 2012 |
| Contact information | Martin Delatycki ([email protected]) |
| Notes |
HFE = human haemochromatosis protein.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events (proportion) Show forest plot | 2 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.43] |
| Analysis 1.1 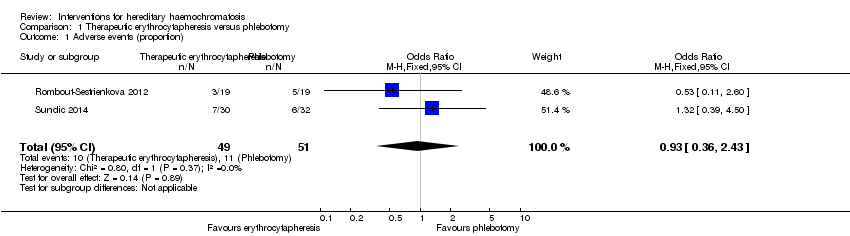 Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 1 Adverse events (proportion). | ||||
| 2 Adverse events (number) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 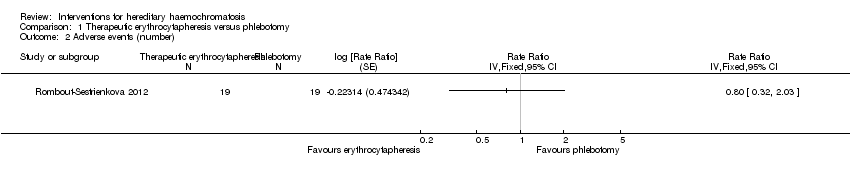 Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 2 Adverse events (number). | ||||
| 3 Health‐related quality of life (EQ‐VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 3 Health‐related quality of life (EQ‐VAS). | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
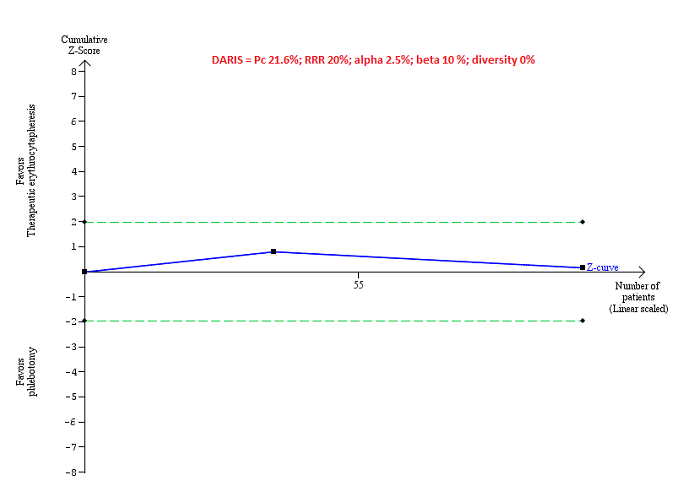
Trial Sequential Analysis of adverse events (proportion) performed using an alpha error of 2.5%, power of 90% (beta error of 10%), relative risk reduction of 20%, control group proportion (Pc) observed in trials (21.6% for proportion of people with adverse events), and observed diversity (0%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS) that the boundaries could not be drawn. The Z‐curve (blue line) does not cross the conventional boundaries (dotted green line). There was a high risk of random errors.

Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 1 Adverse events (proportion).

Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 2 Adverse events (number).

Comparison 1 Therapeutic erythrocytapheresis versus phlebotomy, Outcome 3 Health‐related quality of life (EQ‐VAS).
| Erythrocytapheresis versus phlebotomy for hereditary haemochromatosis | |||||
| Patient or population: people with hereditary haemochromatosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Phlebotomy | Therapeutic erythrocytapheresis | ||||
| Long‐term mortality | None of the included trials reported mortality beyond 1 year. | ||||
| Mortality Follow‐up period: 8 months | There was no mortality in either group in the short‐term in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Serious adverse events Follow‐up period: 8 months | There were no serious adverse events in either group in the 1 trial that reported this information. | 38 | ⊕⊝⊝⊝ | ||
| Health‐related quality of life Follow‐up period: 8 months | The mean health‐related quality of life in the control groups was | The mean health‐related quality of life in the intervention groups was | ‐ | 38 | ⊕⊝⊝⊝ |
| Health‐related quality of life beyond one year | None of the included trials reported health‐related quality of life beyond one year | ||||
| *The basis for the assumed risk is the mean control group proportion or control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level for risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events (proportion) Show forest plot | 2 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.43] |
| 2 Adverse events (number) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 Health‐related quality of life (EQ‐VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |