Botulinum toxins for the prevention of migraine in adults
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, sites unclear 2 treatments, 12 weeks FU per treatment Fixed injections of Botox vs placebo Assessments carried out at baseline and at week 12, week 22, week 24 | |
| Participants | Inclusion criteria: history of CM for at least 6 months prior to the screening visit; ≥ 15 headache d during the 4‐week screening period; ≥ 4 headache episodes lasting ≥ 4 h and ≥ 50% of headache days were migraine Exclusion criteria: conditions causing chronic facial pain such as temporomandibular disorder and fibromyalgia; use of headache prophylaxis medication within 4 weeks of the screening visit; diagnosis of myasthenia gravis, Eaton‐Lambert Syndrome, amyotrophic lateral sclerosis; previous use of any BTX of any sero‐type for any reason; skin infections or acne that would interfere with the injection sites; acupuncture, TENS, cranial traction, dental splints for headache, nociceptive trigeminal inhibition, occipital nerve block treatments, or injection of anaesthetics/steroids within 4 weeks of screening N = 52, mean age 43, M 13/F 39 CM only eligible, MOH eligible Y/N not reported Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox 155 U, number and location of injection sites not reported Control: matched placebo injections | |
| Outcomes | Assessment of CM impacts questionnaire Assessment of CM symptoms questionnaire HIT‐6 Migraine‐specific questionnaire | |
| Notes | Protocol NCT01833130 Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) No diary data recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: subject, caregiver and investigator blind, participant blinded with matched placebo injections; no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | High risk | Dropouts at 56% |
| Selective reporting (reporting bias) | Unclear risk | Not yet published, information and data from trial registry only |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 3 months FU Fixed injections of Botox vs placebo injections Assessments at baseline and 1 month and 3 months post‐treatment | |
| Participants | Inclusion criteria: aged 18‐50 years; history of severe or moderately severe migraine with/without aura (criteria used IHS 1988); 4‐8 attacks per month or stable headache frequency and severity for the past 1 year; users of prophylactic medication such as beta‐blockers and flunarizine were not excluded. Exclusion criteria: cardiovascular disease, peripheral venous disease and seizures; pregnancy or lactation; abused drugs or alcohol in the preceding 2 years; > 15 days headache per month or history of complicated migraine N = 32, mean age not stated, M 8/F 24 CM excluded, MOH excluded Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox 50 U, 10 injections (3 pericranial muscle regions) Comparator: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine days Number of migraine attacks Proportion of responders Use of rescue medication Severity of migraine Overall treatment effect Quality‐of‐life questionnaire Adverse events | |
| Notes | Funding source unclear Diary period 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Low risk | Appears to be no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Data reported in unusual format |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 3 treatments, 90 days FU per treatment 'Follow the pain' (fixed for occipitalis) injections of Botox vs placebo injections Assessments carried out at baseline and 30 days, 60 days and 90 days post‐treatment for each treatment | |
| Participants | Inclusion criteria: aged 18‐65 years; average of at least 4 moderate‐to‐severe migraine episodes but ≤ 15 headache d/month (criteria used ICHD‐I); migraine episodes for at least 1 year prior to enrolment and first diagnosed before age 50 years; stable medical condition; chronic medication regimens, if any, stable including migraine prophylactic medications ≥ 3 months prior to baseline period; willing and able to stay on current medications during the course of the study Exclusion criteria: any medical condition or used any agent that may have put them at risk with exposure to this formulation of Botox; infection or skin problem at any of the injection sites or a known allergy or sensitivity to the trial medication or its components; history of “complicated” migraine; inadequate response (in the investigator’s opinion) to ≥ 2 prophylactic treatments after an adequate trial; psychiatric problems severe enough to interfere with trial implementation; previous therapy with BTX of any sero‐type, or injection of anaesthetics or steroids into the trial‐targeted muscles during the 30 d immediately prior to initiation baseline period; overusing or abusing symptomatic medication, alcohol, or drugs; concurrent chronic use or chronic use in the 3 months prior to the screening period of muscle relaxants; concurrently participating in another investigational trial or participated in such a trial in the 30 d immediately prior to baseline; condition that in the investigator’s opinion might have put person at significant risk, might have confounded the trial or might have interfered with participation in the trial; pregnancy, breastfeeding, or planning a pregnancy during the trial; unable or unwilling to use a reliable form of contraception during the trial CM excluded, MOH excluded Years since diagnosis of migraine 23 years, number migraine attacks during baseline diary period 6.5 | |
| Interventions | Intervention: Botox 110‐260 U, 23‐58 injections (frontal/glabellar 25‐40 U, occipitalis 20 U, temporalis 20‐50 U, masseter optional; 0‐50 U, trapezius 20‐60 U, semispinalis 10‐20 U, splenius capitis 10‐20 U) Comparator: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine attacks Proportion of responders Use of rescue medication Severity of migraine Patient global assessment Adverse events | |
| Notes | Trial includes 30‐day, single‐blind, placebo run‐in phase to identify placebo responders and placebo non‐responders Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used |
| Allocation concealment (selection bias) | Low risk | Neither the investigator nor the participant knew the treatment stratum or random block size. An individual with no other trial involvement reconstituted 3 vials of trial medication and drew the trial drug into the syringes for administration. The syringes were then given to the investigator for injection |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded with matched placebo injections, an individual with no other trial involvement reconstituted 3 vials of trial medication and drew the trial drug into the syringes for administration. The syringes were then given to the investigator for injection |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: an individual with no other trial involvement reconstituted 3 vials of trial medication and drew the trial drug into the syringes for administration. The syringes were then given to the investigator for injection |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout rate per group not given |
| Selective reporting (reporting bias) | Unclear risk | Data not provided for secondary outcomes ‐ narrative description of results only |
| Study size | Unclear risk | 50‐199 participants per arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 2 treatments, 12 weeks FU per treatment Fixed plus optional 'follow the pain' injections of Botox vs placebo Assessments carried out at baseline and 4 weeks, 8 weeks and 12 weeks after each treatment out to 24 weeks, (further 3 treatments and 32 weeks of assessments in OL phase) | |
| Participants | Inclusion criteria: aged 18‐65 years; migraine meeting the diagnostic criteria listed in ICHD‐II (2004), migraine, with the exception of ‘‘complicated migraine’’ (i.e. hemiplegic migraine, basilar‐type migraine, ophthalmoplegic migraine, migrainous infarction); provided diary data on ≥ 20 of 28 days during baseline; ≥15 headache days with each day consisting of ≥ 4 h of continuous headache and with ≥ 50% of days being migraine or probable migraine days during baseline period; ≥ 4 distinct headache episodes, each lasting ≥ 4 h Exclusion criteria: any medical condition that might put participants at increased risk if exposed to onabotulinumtoxinA (e.g. neuromuscular diseases); other primary or secondary headache disorders; use of any headache prophylactic medication within 28 days before start of baseline; Beck Depression Inventory score of > 24 at baseline; fibromyalgia; psychiatric disorders that could have interfered with trial participation; previous exposure to any botulinum neurotoxin sero‐type; women of childbearing potential must have had a negative urine pregnancy test prior to each injection and have been using a reliable means of contraception; investigators were trained not to enrol participants who frequently used opioids as acute pain medication N = 679, mean age 42 years, M 85/F 594 CM only eligible, MOH eligible Y/N 462/217 Years since onset of CM 20, number of migraine days during baseline diary period 19 | |
| Interventions | Intervention: Botox 31 fixed injections, total 155 U, followed by 8 optional follow the pain injections, total 40 U. PREEMPT paradigm: frontalis 20 U in 4 sites; corrugator 10 U in 2 sites; procerus 5 U in 1 site; occipitalis 30 U in 6 sites up to 40 U in 8 sites; temporalis 40 U in 8 sites up to 50 U in 10 sites; trapezius 30 U in 6 sites; cervical paraspinal 20 U in 4 sites Control: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine days Number of migraine attacks Proportion of responders (≥ 50% reduction in migraine) Use of rescue medication HIT‐6 Headache impact score Migraine specific quality of life questionnaire HRQoL Adverse events QALYs Incremental cost‐effectiveness ratio | |
| Notes | Protocol NCT00156910 Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Upon randomisation subject number was linked to next randomisation number grouped within strata for that site, site was then notified of medication kit assigned to that number |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: placebo arm, prepacked medication kits with number for assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: placebo arm, prepacked medication kits with number for assignment |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals balanced across groups and adjusted LOCF method used |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Study size | Low risk | ≥ 200 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, sites unclear Single treatment , 90‐day FU Fixed injections of Botox vs placebo injections Assessments carried out at baseline and 30, 60 and 90 days post‐treatment | |
| Participants | Inclusion criteria: history ≥ 1 year of EM, with/without aura (criteria used ICHD‐I), aged between 19 and 65 years Exclusion criteria: > 15 headaches per month; a history of complicated migraine (with aura); previous treatment with BTX of any sero‐type; substance abuse, including overuse of analgesics and migraine treatments; current prophylactic treatment for migraine, and caffeine. Pregnancy or breastfeeding; any condition, which, in the opinion of the investigator, could compromise the results of the trial N = 30, mean age 41, M 6/F 24 CM excluded, MOH excluded Years since diagnosis of migraine 16 years, number of migraine attacks 5 during baseline diary period | |
| Interventions | Intervention: Botox 50 U, 15 injections (temporalis, 10 U total at 2 sites; frontalis, 10 U total at 4 sites; glabellar, 8 U total at 4 sites; procerus, 2 U at 1 site; trapezius, 10 U total at 2 sites; splenius capitis, 10 U total at 2 sites) Comparator: matched placebo injections | |
| Outcomes | Number of migraine attacks Duration of migraine Use of rescue medication Participant‐ and investigator‐related 6‐point global effectiveness evaluation scale Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Uncontactable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of assessors |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed |
| Selective reporting (reporting bias) | Unclear risk | Group means reported without standard errors or standard deviations |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, active control, parallel‐group, single‐site 2 rounds of treatment, 3 months FU for first treatment and 6 months for second Follow the pain injections of Botox plus placebo vs sodium valproate tablets plus placebo injections Assessments carried out at baseline and 1 month, 3 months, 6 months and 9 months post‐treatment | |
| Participants | Inclusion criteria: EM or CM (criteria used for diagnosis not clear, ICHD‐II cited in Background); aged between 18‐65 years with EM (defined for this trial as ≥ 3 migrainous headaches but < 15 d/month) or CM (defined for this trial as migrainous headaches on ≥ 15 d/month); stable headache severity and pattern Exclusion Criteria: any medical condition or use of any agent that might expose them to risk if they received Botox; prior exposure, allergy, or sensitivity to any component of BTX of any sero‐type or to divalproex; skin problems, infections, profound atrophy, or excessive weakness in the target areas of the injection sites; concomitant prophylactic migraine therapy or a history of overusing symptomatic medication; headache disorders outside of the classification strata outlined; pregnancy, breastfeeding, or planning a pregnancy during the trial N = 59, mean age 42, M 9/F 50 EM/CM 45/14, MOH excluded Number of headache days 11.7 during baseline diary period, headache severity 3.5 on 5‐point scale where 5 is most severe | |
| Interventions | Intervention: Botox ≤ 100 U, number of injections unclear (procerus 2.5‐5 U, corrugators 2.5‐5U, frontalis 25 U, temporalis 7.5‐20 U, splenius capitis 2.5‐10 U, sternocleidomastoid 7.5‐15 U, trapezius 2.5‐5 U, occipitalis 2.5‐5 U, cervical paraspinalis 7.5‐15 U, semispinalis capitis 5‐10 U, masseter 5‐15 U Comparator: sodium valproate tablets, 250 mg twice daily | |
| Outcomes | Number of headache days Proportion of responders (≥ 50% reduction in migraine) Headache severity Headache index MIDAS HIT‐6 24 hr migraine QoL Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox). Diary period 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: double dummy, participant blinded with matched placebo injections or placebo tablets, no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: double dummy, no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | High risk | Dropouts at 44% |
| Selective reporting (reporting bias) | Unclear risk | Some time points not reported for some outcomes |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, unclear sites Single treatment, appears to be 3 months FU Fixed injections of Botox 100 U vs matched placebo injections Assessments carried out at baseline and 1 month, 2 months and 3 months post‐treatment | |
| Participants | Inclusion criteria: age > 18 years; migraine (IHS 1988); previous history of conventional treatment failure and functional disability Exclusion criteria: non adherence to the protocol and non‐acceptance by participant; pregnancy; lactation; treatment with aminoglycosides; acne conglobata; Lambert‐Eaton myasthenic syndrome; history of hypersensitivity to the components of the BTX and secondary headache N = 30, mean age not stated, M 5/F 25 EM/CM unclear, MOH eligibility unclear Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox 100 U from 25 injections (4 front points total 10 U, 1 application in procerus muscle and 1 application in each corrugator muscle total 10 U, 3 points each temporalis muscle total 30 U, 5 points in each trapezius muscle total 50 U) Comparator: matched placebo injections | |
| Outcomes | MIDAS MIGSEV Quality‐of‐life measure Adverse events | |
| Notes | Funding source unclear No diary data Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No protocol and no migraine frequency data |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, single‐site Single treatment, 3‐month FU (optional OL treatment at end of trial for placebo arm) Fixed injections of Botox versus placebo injections Assessments carried out at baseline and 1 month, 2 months and 3 months post‐treatment | |
| Participants | Inclusion criteria: age > 18 years; history (≥ 6 months) of headache (ICHD‐II diagnosis 1.1 (migraine without aura), 1.2 (migraine with aura), 1.7 (migrainous disorder not fulfilling above criteria), or 2.2 (chronic tension‐type headache)); onset before the age of 50 years; HIT‐6 score > 56; episodic chronic headache; stable headache severity and pattern; failed at least 1 attempt with preventive medications because of compliance, adherence, or AE issues; women of childbearing potential taking approved birth control measures and having a negative urine pregnancy test prior to administration of trial medications Exclusion criteria: any medical condition that might put them at risk with Botox exposure (e.g. myasthenia gravis, Eaton‐Lambert Syndrome, amyotrophic lateral sclerosis); any disease that might interfere with neuromuscular function; uncontrolled systemic disease; abnormal pathology contributing to headaches; concurrent infection at proposed injection sites; pregnant or breast‐feeding; currently using aminoglycoside antibiotics, curare‐like agents, or other agents that might interfere with neuromuscular function; undergone injection of anaesthetics or steroids, within 1 month immediately prior to enrolment, into the muscles to be injected in the trial; previous BTX treatment with any sero‐type; currently participating in another drug or device trial or had done so within the 30 days before the baseline period; suspected hypersensitivity to Botox or any of the ingredients in the proprietary formulation; known or suspected drug or alcohol abuse N = 61, mean age 42 years, M 9/F 52 EM/CM unclear, MOH eligibility unclear Number of headache days 8 during baseline diary period, severity of migraine (max recorded during baseline period) 2.2 (scale unclear) | |
| Interventions | Intervention: Botox 139 U, 17 injections (corrugator 2 applications of 6 U, splenius capitis 2 applications of 10 U, trapezius 4 applications of 10 U, temporalis 4 applications of 10 U, procerus 1 application of 3 U, frontalis 4 applications of 6 U) Comparator: matched placebo injections | |
| Outcomes | Number of headache episodes Number of headache days Number of headache‐free days Percentage of headache episodes with aura Severity of headaches HIT‐6 (mean in 30 days compared to baseline) MIQ (MIDAS & QoL) | |
| Notes | The trial received enrolment of subjects with diagnostic criteria 1.1 and 1.2 only Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Randomisation of participants was performed by a supervisory individual not associated with the trial |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded with matched placebo injections, research co‐ordinator, who was not involved with the participants prepared the injections |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: research co‐ordinator, who was not involved with the participants prepared the injections |
| Incomplete outcome data (attrition bias) | Unclear risk | Treatment of missing data not described |
| Selective reporting (reporting bias) | Unclear risk | Full data not given (P values only, no AE data given) |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, active controlled, parallel‐group multi‐site Single treatment, 12‐week FU (additional treatment for all participants in OL phase) Fixed plus follow the pain injections of Botox plus placebo tablets vs placebo injections and topiramate tablets Assessments carried out at baseline and 4 weeks and 12 weeks post‐treatment | |
| Participants | Inclusion criteria: CM (criteria used ICHD‐II); aged 18‐65 years; female participants of child‐bearing potential with a negative urine pregnancy test who practiced reliable contraception throughout the trial period Exclusion criteria: pregnancy, breastfeeding, or planning pregnancy during the time frame of the trial; headache disorders other than CM; medical disorders that increase risk with exposure to Botox; significant liver or renal impairment including kidney stones; ketogenic diets; previous use of BTX or topiramate; recent alcohol/drug abuse or overuse of acute medication N = 59, mean age 40 years, M 5/F 54 CM only eligible, MOH excluded Years since diagnosis of migraine 16 years, severity of migraine 2.8 on 3‐point scale | |
| Interventions | Intervention: Botox up to 200 U, number and location of injections not reported (100 U fixed locations + up to 100 U in follow the pain), average dose 109 U Comparator: topiramate 25 mg escalated to max 200 mg (average 136 mg) | |
| Outcomes | Number headache days Number migraine attacks Proportion of responders (≥ 50% reduction in migraine) Money spent on rescue medication Physician's global assessment HIT‐6 MIDAS MIQ Severity of migraine | |
| Notes | Funding source unclear Diary period 28 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | A sealed card marked with participant's trial number was delivered via FedEx to the trial site. A research co‐ordinator, not involved with the trial, would open the card, note the treatment assignment, prepare treatment, and hand to trial co‐ordinator, who assisted the investigator with the injections |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded with matched placebo injections, research co‐ordinator, who was not involved with the trial, prepared the injections |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: research co‐ordinator, who was not involved with the trial, prepared the injections |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear reporting of attrition |
| Selective reporting (reporting bias) | High risk | Some outcomes missing from results (severity/MIQ/AEs) |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over, multi‐site Single treatment, 3‐month FU (additional treatment given post cross‐over) Fixed plus follow the pain injections of OnabotulinumtoxinA vs placebo injections Assessments carried out at baseline and 1 month, 2 months, and 3 months post‐treatment | |
| Participants | Inclusion criteria: CM (criteria used ICHD‐II); aged 18‐65; history of CM according to the criteria proposed as an appendix diagnosis in 2006 by the Headache Classification Committee; preventive medications were of a stable dose for at least 6 weeks prior to screening and maintained throughout trial period; women of childbearing potential agreed to urine pregnancy test at screening and a medically acceptable form of contraception Exclusion criteria: pregnancy, planning pregnancy during the trial period, breastfeeding, or of childbearing potential and not practicing a reliable form of birth control (see inclusion criteria); headache disorders outside IHS‐defined CM; evidence of underlying pathology contributing to headaches; pathology of the salivary glands such as sialadenitis (e.g. Sjorgen’s syndrome, viral or bacterial sialadenitis) or condition or symptom that would alter the content of saliva; any medical condition that may increase their risk with exposure to BTX‐A including diagnosed myasthenia gravis, Eaton–Lambert syndrome, amyotrophic lateral sclerosis, or any other significant disease that might interfere with neuromuscular function; profound atrophy or weakness of muscles in the target areas of injection; skin conditions or infections at any of the injection sites; allergy or sensitivities to any component of test medications; active major psychiatric or depressive disorders including alcohol/drug abuse; met IHS criteria for Medication Overuse with opioid‐ or butalbital‐containing products; planning or requiring surgery during the trial; history of poor compliance with medical treatment as determined by the investigator; currently participating in an investigational drug trial or had participated in an investigational drug trial within the previous 30 days of the screening visit N = 20, mean age 49, M 5/F 15 CM only eligible, MOH eligibility unclear Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox ≥ 155 U, 31 injections following PREEMPT paradigm with optional follow the pain injections in occipitalis, temporalis, and trapezius Comparator: matched placebo injections | |
| Outcomes | Number of headache days | |
| Notes | Protocol NCT01071096 Funding source unclear Diary period 1 month Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Third party responsible for generation of number and preparation of medications Quote: "Randomisation of subjects was performed by a supervisory individual not associated with the trial, who numbered trial medications in a manner which was blinded to subject, coordinator, and investigator" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded with matched placebo injections, trial medications were numbered by a supervisory individual not associated with the trial in a manner which was blind to subject, co‐ordinator and investigator |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: trial medications were numbered by a supervisory individual not associated with the trial in a manner which was blind to subject, co‐ordinator and investigator |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant dropped out |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 3‐month FU Fixed injections with Dysport vs placebo Assessments carried out at baseline and 4 weeks, 8 weeks and 12 weeks post‐treatment | |
| Participants | Inclusion criteria: aged 18‐65 years; experienced an average of 2‐8 migraine attacks per month over the 3 months prior to a screening period; 2‐8 migraine attacks occurred during the 4‐week screening period; medication for acute migraine and prophylactic treatment was permitted if doses were stable. Exclusion criteria: pure migraine with aura as defined by ICHD‐II; history of complicated migraine; pregnancy; lactation; not using adequate contraception; history of drug abuse; treatment with Botox within the past 6 months; previously experienced an adverse reaction to Botox; history of botulism or other neuromuscular disorders; current treatment with aminoglycoside antibiotics or other agents that could affect neuromuscular transmission; received unlicensed medication or investigational drugs within 6 months of the screening visit; any other clinically significant medical conditions that could influence trial results; liver transaminase levels had to be less than twice the upper normal values N = 128, mean age 39, M 7/F 120 (ITT population (127) used for baseline characteristics) EM/CM unclear, MOH eligibility unclear Years since diagnosis migraine 5, number of migraine attacks during baseline diary period 5 | |
| Interventions | Intervention (arm 1): Dysport 120 U, 6 injections ‐ 2 x frontal, 2 x temporal, 2 x occipital Intervention (arm 2): Dysport 240 U, 6 injections ‐ 2 x frontal, 2 x temporal, 2 x occipital Control: matched placebo injections | |
| Outcomes | Number of migraine attacks MIDAS Duration of migraine Adverse events | |
| Notes | Protocol NCT00258609 Funder: IPSEN Group (manufacturer of Dysport) Diary period 28 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with allocation, nurse performed reconstitution and had no further involvement with participants |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participants and all other personnel, apart from nurse who performed reconstitution of drug, were blind to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: participants and all other personnel, apart from nurse who performed reconstitution of drug, were blind to allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT with no description of method of imputation, stated dropouts 1/3/5 |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes discussed but data not always provided |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 2 treatments, 12 weeks FU per treatment Fixed plus optional follow the pain injections of Botox vs placebo Assessments carried out at baseline and 4 weeks, 8 weeks and 12 weeks after each treatment out to 24 weeks, (further 3 treatments and 32 weeks of assessments in OL phase) | |
| Participants | Inclusion criteria: aged 18‐65 years; migraine meeting the diagnostic criteria listed in ICHD‐II (2004) section 1, migraine (1), with the exception of ‘‘complicated migraine’’ (i.e., hemiplegic migraine, basilar‐type migraine, ophthalmoplegic migraine, migrainous infarction); provided diary data on ≥ 20 of 28 days during baseline; ≥ 15 headache days with each day consisting of ≥ 4 h of continuous headache and with ≥ 50% of days being migraine or probable migraine days during baseline period; ≥ 4 distinct headache episodes, each lasting ≥ 4 h Exclusion criteria: any medical condition that might put participants at increased risk if exposed to Botox (e.g. neuromuscular diseases); other primary or secondary headache disorders; use of any headache prophylactic medication within 28 days before start of baseline; Beck Depression Inventory score of > 24 at baseline; fibromyalgia; psychiatric disorders that could have interfered with trial participation; previous exposure to any botulinum neurotoxin sero‐type; Women of childbearing potential must have had a negative urine pregnancy test prior to each injection and have been using a reliable means of contraception; investigators were trained not to enrol participants who frequently used opioids as acute pain medication N = 705, mean age 41 years, M 103/F 602 CM only eligible, MOH eligible Y/N 444/261 Years since onset of CM 18, number of migraine days during baseline diary period 19 | |
| Interventions | Intervention: Botox 31 fixed injections (total 155 U) followed by 8 optional follow the pain injections (total 40 U). PREEMPT paradigm: frontalis 20 U in 4 sites; corrugator 10 U in 2 sites; procerus 5 U in 1 site; occipitalis 30 U in 6 sites up to 40 U in 8 sites; temporalis 40 U in 8 sites up to 50 U in 10 sites; trapezius 30 U in 6 sites; cervical paraspinal 20 U in 4 sites Control: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine days Number of headache episodes Monthly cumulation of headache hours Proportion of responders (≥ 50% reduction in migraine) Use of rescue medication HIT‐6 Headache impact score Migraine specific quality of life questionnaire HRQoL Adverse events QALYs Incremental cost‐effectiveness ratio | |
| Notes | Protocol NCT00156910 Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Upon randomisation subject number was linked to next randomisation number grouped within strata for that site, site was then notified of medication kit assigned to that number |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: placebo arm, prepacked medication kits with number for assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: placebo arm, prepacked medication kits with number for assignment |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals balanced across groups and adjusted LOCF method used |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Study size | Low risk | ≥ 200 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 4‐arm trial. 2 arms received 1 treatment before being re‐randomised, 2 arms received 3 treatments before being re‐randomised, 120 days FU per treatment This paper comprised 3 trials of which this is the first. (Elkind II 2006 ‐ included, Elkind Study III 2006 (Elkind 2006)‐ excluded) 3 arms with varying doses of fixed injections of Botox vs placebo injections Assessments carried out at baseline and 30 days, 60 days, 90 days and 120 days post‐treatment for each treatment cycle | |
| Participants | Inclusion criteria: aged 18‐65 years; IHS–defined migraines with or without aura; average of 4‐8 moderate‐severe migraines per month that occurred with a stable frequency and severity and had begun at least 1 year prior to the trial; first diagnosed with migraine before age 50 years; able to distinguish between migraine and non migraine headaches; stable medical condition; if taking chronic medications (including prophylactic migraine medications), on stable doses and regimens for at least 3 months prior to enrolment, to be continued throughout the trial Exclusion criteria: > 15 headache d/month; history of complicated migraine or typical migraine pain localised predominantly to the occipital or suboccipital region; consistently refractory to multiple acute therapies or had never tried any acute therapies; overuse of symptomatic medications; excessive use of caffeine; alcohol/drug abuse; any medical condition or use of any agent that might have put the participant at increased risk with exposure to Botox or interfered with trial participation or the results; pregnancy, breastfeeding, or planning a pregnancy during the trial period; previous injection with any BTX or allergy to any of the components of the trial medication; prior injection of anaesthetic or steroid into the muscles to be injected in the month immediately prior to enrolment; participation in another investigational drug or device clinical trial either concurrently or in the month immediately prior to enrolment; infection or skin problems at the injection site Participants with migraine headache at the time of treatment may have been randomised/injected at a later date within 2 weeks of the scheduled visit N = 418, mean age 44 years, M 64/F 354 Some CM inadvertently included against protocol EM/CM 409/9, MOH excluded Years since onset of migraine 21, number of migraine attacks in baseline diary period 6 | |
| Interventions | Intervention (arm 1): 50 U Botox total of 11 injections ‐ frontal 4 sites, temporal 2 sites, glabellar 5 sites Intervention (arm 2): 25 U Botox total of 11 injections ‐ frontal 4 sites, temporal 2 sites, glabellar 5 sites Intervention (arm 3): 7.5 U Botox total of 11 injections ‐ frontal 4 sites, temporal 2 sites, glabellar 5 sites Control: matched placebo injections | |
| Outcomes | Number of migraine attacks | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Low risk | Missing data low and balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes discussed but data not always provided |
| Study size | Unclear risk | 50‐199 participants per arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 2 treatment cycles, 120 days FU per treatment. Follows on from Elkind I 2006 Fixed injections of Botox dosing trial Assessments carried out at baseline and 30 days, 60 days, 90 days and 120 days post‐treatment for each treatment cycle | |
| Participants | Inclusion criteria: aged 18‐65 years; IHS–defined migraines with or without aura; average of 4‐8 moderate‐severe migraines per month that occurred with a stable frequency and severity and had begun at least 1 year prior to the trial; first diagnosed with migraine before age 50 years; able to distinguish between migraine and non migraine headaches; stable medical condition; if taking chronic medications (including prophylactic migraine medications), on stable doses and regimens for at least 3 months prior to enrolment, to be continued throughout the trial Exclusion criteria: > 15 headache d/month; history of complicated migraine or typical migraine pain localised predominantly to the occipital or suboccipital region; consistently refractory to multiple acute therapies or had never tried any acute therapies; overuse of symptomatic medications; excessive use of caffeine; alcohol/drugs abuse; any medical condition or use of any agent that might have put the participant at increased risk with exposure to Botox or interfered with trial participation or the results; pregnancy, breastfeeding, or planning a pregnancy during the trial period; previous injection with any BTX or allergy to any of the components of the trial medication; prior injection of anaesthetic or steroid into the muscles to be injected in the month immediately prior to enrolment; participation in another investigational drug or device clinical trial either concurrently or in the month immediately prior to enrolment; infection or skin problems at the injection site participants with migraine headache at the time of treatment may have been randomised/injected at a later date within 2 weeks of the scheduled visit N = 183, baseline data given for Elkind I 2006 participant set only. Some attrition of participants between end of study I and start of study II not described in detail Some CM inadvertently included against protocol EM/CM unclear, MOH excluded | |
| Interventions | Intervention (arm 1): 50 U Botox total of 11 injections ‐ frontal 4 sites, temporal 2 sites, glabellar 5 sites Intervention (arm 2): 25 U Botox total of 11 injections ‐ frontal 4 sites, temporal 2 sites, glabellar 5 sites | |
| Outcomes | Number of migraine attacks | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Low risk | Missing data at 22% vs 16% of total participant numbers balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes discussed but data not always provided |
| Study size | Unclear risk | 50‐199 participants per arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 16‐week FU Fixed injections of Botox vs placebo Assessments carried out at baseline and at 4 weeks, 8 weeks, 12 weeks and 16 weeks post‐treatment | |
| Participants | Inclusion criteria: 6‐month history, prior to baseline, of CM; migraine episodes meeting the criteria 1.1 or 1.2 of the ICHD‐I; 15 headache days during the prospective baseline phase; anyone taking preventive medications must have been on stable dosages for 60 days prior to trial entry and willing to remain at same doses for the duration of the trial; women were required to be practicing an acceptable method of contraception and have a pregnancy test or to be incapable of pregnancy Exclusion criteria: previous treatment with BTX of any sero‐type for any therapeutic reason; history of myasthenia gravis, Eaton‐Lambert syndrome, amyotrophic lateral sclerosis or other disorder of neuromuscular function; concomitant use of aminoglycoside antibiotics, curare‐like agents or other agents that might interfere with neuromuscular function; diagnoses of migraine beginning for the first time after age 50 years; cluster headaches or basilar, ophthalmoplegic, hemiplegic migraine, or exclusively having migraine aura without headache; more painful condition than their migraine pain, progressive neurological disorders, or a structural disorder of the brain from birth, trauma, or past infection; received injections or oral corticosteroids within 30 days prior to the baseline diary initiation visit; significant major psychiatric disorder; receiving antipsychotic medication; Beck Inventory of Depression Scores > 24; received an investigational drug or used an investigational device within 30 days of trial entry; taking triptans > 3 days per week, ergotamine > 2 days per week, or dihydroergotamine > 2 days per week or any combination of the above medications > 3 days per week; consuming caffeine from dietary and medicinal sources > 500 mg/d on a daily basis > 28 days prior to trial enrolment; taking opioids > 2 days per week; taking simple analgesics on average > 2 tablets/d ≥ 5 days per week for at least 28 days; taking combination analgesics on average > 3 tablets per day and ≥ 3 days per week for at least 28 days; using a combination of any of the previous on ≥ 4 days per week for at least 28 days N = 41, mean age 42 years, M 11/F 30 CM only eligible, MOH excluded Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox 100 U, 22 injections (glabella 20 U, 4 sites; temporal 20 U, 4 sites; frontal 10 U, 4 sites; suboccipital 30 U, 6 sites; trapezius 20 U, 4 sites) Control: matched placebo injections | |
| Outcomes | Number of migraine episodes Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded, with matched placebo injections, research nurse who was not involved with the trial participants prepared the injections, trial medication was delivered to treating physician in identical looking syringes |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: research nurse, who was not involved with the trial participants prepared the injections, trial medication was delivered to treating physician in identical looking syringes |
| Incomplete outcome data (attrition bias) | Low risk | Missing data low and balanced across groups at 10% vs 14% of total participant numbers |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported but standard errors or equivalent not reported and some interim time points missing |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, single‐site 3 cycles of treatment at 4‐week intervals (some uncertainty as to whether participants were treated at each visit or just at first visit), trial length 12 weeks in total Follow the pain injections of Prosigne vs placebo Assessments carried out at baseline, 4 weeks, 8 weeks and 12 weeks | |
| Participants | Inclusion criteria: aged 18‐85 years; diagnosis of CM associated with cutaneous allodynia, according to the ICHD (edition revised, 2006) Exclusion criteria: diagnosis of neuromuscular diseases (myasthenia gravis, Eaton‐Lambert syndrome and amyotrophic lateral sclerosis); conditions that could affect pericranial muscles; other primary or secondary headaches; complicated migraine; CM associated with analgesic overuse; severe systemic diseases; other neurologic or psychiatric disorders; fibromyalgia; myofascial syndrome; temporomandibular disorder N = 38, mean age 45, M 9/F 29 CM only eligible, MOH excluded Severity of migraine 7.3 cm on 10 cm VAS | |
| Interventions | Intervention: Prosigne 12‐24 injections, maximum dose 96 U (frontal 2‐4 sites on each side 3 U per site; temporal 2‐4 sites on each side 4 U per site; occipital 2‐4 sites on each side 5 U per site) Control: matched placebo injections | |
| Outcomes | Number of migraine episodes with cutaneous allodynia Severity of headache pain (VAS) Use of rescue medication Adverse events | |
| Notes | Funder: Brazilian National Institutes of Science Diary period 30 days Protocol NCT01357798 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded, with matched placebo injections, 1 designated investigator who had access to randomisation sequence provided the trial medication to the principal investigator according to the allocation list generated |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: 1 designated investigator who had access to randomisation sequence provided the trial medication to the principal investigator according to the allocation list generated |
| Incomplete outcome data (attrition bias) | Low risk | Appear to be no dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol and methods reported |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, single‐site Single treatment with 4‐month FU 3‐arm trial, HengLi fixed muscle site injections vs HengLi acupoint site injections vs placebo Assessments carried out at baseline and 1 month, 2 months, 3 months and 4 months postbaseline | |
| Participants | Inclusion criteria: EM and CM, criteria used ICHD‐I; aged 18‐57 years; history of migraines with or without aura for 1‐16 years; experienced headache ≥ 15 d/month were diagnosed as CM; women of childbearing potential were required to be taking approved birth control measures and to have a negative urine pregnancy test prior to administration of trial medications Exclusion criteria: any medical or neurological conditions that might put them at risk with Botox exposure, such as amyotrophic lateral sclerosis, myasthenia gravis, Eaton‐Lambert Syndrome; any disease that might interfere with neuromuscular function; abnormal pathology contributing to migraine; uncontrolled systemic disease; concurrent infection at proposed injection sites; pregnant or breast‐feeding; currently using aminoglycoside antibiotics, curare‐like agents, or other agents that might interfere with neuromuscular function; undergone injection of anaesthetics or steroids, within 1 month immediately prior to enrolment, into the muscles to be injected in the trial; previously received Botox treatment with any sero‐type; currently participating in another drug or device trial or had done so within the 30 days before the baseline period; suspected hypersensitivity to Botox or any of the ingredients in the proprietary formulation; known or suspected drug or alcohol abuse N = 102, mean age 41, M 21/F 81 EM/CM 66/36, MOH eligibility unclear Years since diagnosis of migraine 6, number of migraine attacks during baseline diary period 7, severity of migraine 7.4 cm on 10 cm VAS | |
| Interventions | Intervention (arm 1): HengLi fixed muscle site, 10 injections of 2.5 U each, total dose 25 U (1 on each side for frontal and occipital belly of occipitofrontalis, corrugator supercilii, temporalis and superior part of trapeziuem) Intervention (arm 2): HengLi acupoint site, 10 injections of 2.5 U each, total dose 25 U (Yintang EX‐HN3, at the midpoint of the line connecting the 2 medial ends of eyebrows; Taiyang EX‐HN5, at the point of intersection of the continuations of the eyebrow and the lower eyelid in the lateral direction, on the lateral border of the orbit; Baihui GV20, at the middle of the vertex, on the line connecting the apexes of the 2 ears; Shuaigu GB8, directly above the ear apex, 1.5 inches above the hairline; Fengchi GB20, at the posterior lateral aspect of the neck, in the fossa between the superior margins of the trapezius and sternocleidomastoid muscles; and Tianzhu BL10, 1.3 inches lateral to the point 0.5 inches directly above the midpoint of the posterior hairline, in the depression lateral to the border of the trapezius muscle Control: placebo injections matched to fixed muscle site arm | |
| Outcomes | Number of migraine attacks Duration of migraine Severity of migraine Frequency of migraine symptoms Adverse events | |
| Notes | Funder: Scientific and Technique Support Project of Gansu Province (090NKCA112), China Diary period 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind Quote: "participant blind with matched placebo injections, a research coordinator who was not directly involved with the subjects managed the randomisation and preparation of injections." Since dummy injections are not mentioned it cannot be the case that personnel carrying out injections are blind to active assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: a research co‐ordinator who was not directly involved with the participants managed the randomisation and preparation of injections, analysis of the diary data was conducted by investigators who did not know the exact group of each participant |
| Incomplete outcome data (attrition bias) | Low risk | Appear to be no dropouts |
| Selective reporting (reporting bias) | High risk | Use of rescue medications recorded but not reported |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group unclear sites Single treatment 4 weeks FU Fixed injections of Botox vs placebo Assessments carried out at baseline and 4 weeks post‐treatment | |
| Participants | Inclusion criteria: aged ≥ 18 years; migraine for > 3 months that fail to respond to ≥ 2 major anti‐migraine drugs; meeting criteria of CM Exclusion criteria: pregnant or may become pregnant; disease of neuromuscular junction or drugs that affect neuromuscular junction; allergy to Botox; previous use of Botox for migraine by similar methodology N = 25, mean age 60, M 13/F 12 CM only eligible, MOH eligibility unclear Severity of migraine 8.2 on 10 cm VAS | |
| Interventions | Intervention: Botox 200‐300 U depending on neck and body size, 22‐24 injections (frontalis 20 U, 5 sites/side; temporalis 30 U, 2 sites/side; occipitalis 5 U, 1 site/side); cervical region: splenius cervicis, semispinalis capitis, trapezius muscles 45‐60 U 3‐5 sites/side) Control: matched placebo injections | |
| Outcomes | Severity of migraine Global assessment score Pain impact questionnaire HIT‐6 Migraine‐specific quality of life Adverse events | |
| Notes | Protocol NCT00660192 Funding source unclear Diary period 28 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant and investigator blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: participant and investigator blind |
| Incomplete outcome data (attrition bias) | High risk | 20% dropped out N = 5 |
| Selective reporting (reporting bias) | Unclear risk | Not yet published, information and data from trial registry only |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over, unclear sites Single treatment with 4 months FU precross‐over Unclear method of injections of Botox vs placebo Assessments carried out at baseline and 6 weeks and 4 months post‐treatment | |
| Participants | Inclusion criteria: aged 18‐70 years; clinically confirmed diagnosis of migraine without aura, criteria used ICHD, edition not stated; duration of illness, so far refractory to conservative treatment (physiotherapy, massages, stretching exercises, peroral medication) at least 2 years Exclusion criteria: participation in another clinical trial within the past 3 months; specific pain in neck/shoulder region in need of different specific treatment (i.e. acute nerve irritation with disc prolapse, manifest inflammatory processes etc.); contraindication of treatment with BTX (ascertained sensitivity to clostridial toxin or to 1 of the other ingredients and generalised disorders of muscular activity, e.g. myasthenia gravis or Lambert‐Eaton syndrome); pregnancy or breastfeeding or inadequate or no contraception in women of childbearing age; serious concomitant illnesses involving the internal organs in particular; systemic diseases; serious neurologic disorders; abuse of alcohol, drugs and narcotics; medication with anticoagulants and heparin preparation (topically applied heparin unguents excluded), thrombocyte aggregation inhibitors, amino‐glycoside antibiotics, spectinomycin or muscle relaxants of the tubocurarine type N = 22, mean age 45, M 5/F 17 EM/CM unclear, MOH eligibility unclear Baseline disease characteristics not stated | |
| Interventions | Intervention: Botox 10 U 2 injections in total into corrugator muscle and occipitalis muscle of the side affected Control: matched placebo injections | |
| Outcomes | Pain intensity (severity) VAS Number of headache attacks Duration of headache attacks Use of rescue medication Short form‐McGill Pain Questionnaire Northwick Park Neck Pain Questionnaire International quality of life assessment SF 36 Scale of attitudes toward disabled persons Oswestry low back pain disability questionnaire Adverse events | |
| Notes | Funder: German society of Neurology (statistical analysis only). States no funds came from manufacturers of BTX Diary period unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated from which group dropouts (3) occurred. Participants lost to FU not analysed |
| Selective reporting (reporting bias) | High risk | AEs recorded but not reported or commented upon |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, single‐site Single treatment with 12 weeks FU 4‐arm trial fixed injections Botox vs Prosigne (2 dosing arms) vs saline Assessments carried out at baseline and 4 weeks, 8 weeks and 12 weeks post‐treatment | |
| Participants | Inclusion criteria: aged 21‐60 years; chronic daily unilateral headache characterised as CM > 12 months' duration and not responding to standard treatment with antidepressants, nonsteroidal anti‐inflammatory agents or anticonvulsants or beta‐blockers Exclusion criteria: presence of an infectious process at the site of application; associated immunological diseases; previous history of allergy to Botox; application of Botox during the last year; diabetes mellitus N = 40, mean age 46 years, M 14/F 26 CM only eligible, MOH eligibility unclear Years since diagnosis of CM 16, severity of migraine 10.0 on 10 cm VAS | |
| Interventions | Intervention (arm 1): Botox 10 injections, total dose 25 U (8 different sites in the frontal region and 2 sites in the temporal region, 1 site on each side) Intervention (arm 2): Prosigne 10 injections, total dose 25 U (8 different sites in the frontal region and 2 sites in the temporal region, 1 site on each side) Intervention (arm 3): Prosigne 10 injections, total dose 33.3 U (8 different sites in the frontal region and 2 sites in the temporal region, 1 site on each side) Control: matched placebo injections | |
| Outcomes | Number of headache attacks Severity of headache Time to 50% pain relief | |
| Notes | Funding source unclear Diary period 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blind with matched placebo injections, 1 of the trial authors prepared injections and a second trial author, who was unaware of the content of the previously prepared syringes delivered the treatment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: 1 of the trial authors prepared injections and a second trial author, who was unaware of the content of the previously prepared syringes delivered the treatment, data were evaluated by a third trial author |
| Incomplete outcome data (attrition bias) | Low risk | Missing data balanced across groups (1/1/0/1) |
| Selective reporting (reporting bias) | Unclear risk | AEs not mentioned at all in report |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, active control, parallel‐group, single site 2 treatments with 3 months between treatments and 6 months FU after second treatment Fixed plus follow the pain injections of Botox 200 U plus placebo tablets vs TOPAMAX (topiramate) tablets plus placebo injections Assessments carried out at baseline and 1 month, 3 months, 6 months and 9 months after first treatment | |
| Participants | Inclusion criteria: outpatient; aged 18‐65 years; diagnosed CM not attributable to another cause; CM headache was defined as migraine headache with or without aura occurring on ≥ 15 d/month for > 3 months in the absence of medication overuse with at least 2 of the following characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, and/or aggravation by or causing avoidance of routine physical activity (e.g. walking or climbing stairs); at least 1 of the following occurred during headache: nausea and/or vomiting or photophobia and/or phonophobia; stable headache severity and pattern; headache data for at least 6 months prior to trial drug administration Exclusion criteria: pregnancy or planning to become pregnant during the trial period; breastfeeding; of childbearing potential and not practicing a reliable method of birth control; people with chronic tension‐type headaches based on recognised criteria; evidence of underlying conditions judged to preclude treatment with either test medication; previously used trial medications for any reason; unable to discontinue any prohibited medication(s), including carbonic anhydrase inhibitors (e.g. acetazolamide, dichlorphenamide), digoxin, metformin, central nervous system depressants (including alcohol), non‐trial migraine prophylaxis medications (e.g. propranolol, amitriptyline, sodium valproate), non‐trial anticonvulsant or antiepileptic medications, agents that might interfere with neuromuscular function (e.g. aminoglycoside antibiotics, curare‐like agents),or hormonal contraceptives; evidence of recent alcohol/drug abuse or acute medication overuse N = 60, mean age 37, M 6/F 54 CM only eligible, MOH eligibility unclear Number of headache/migraine days during baseline period 16, migraine severity 2.9 on 5‐point scale | |
| Interventions | Intervention: Botox 200 U, 100 U fixed site and 100 U follow the pain, number and location of injection sites not reported plus placebo tablets Control: TOPAMAX (topiramate) 100‐200 mg/d, plus matched placebo injections | |
| Outcomes | Number of migraine attacks Proportion of 50% responders Severity of migraine Use of rescue medication MIDAS MIQ Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 1 month Uncontactable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: double dummy, participant blinded with matched placebo injections or placebo tablets, no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: double dummy, no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | High risk | Numbers analysed and numbers imputed unclear. Dropouts excluded from analysis higher than 10% for all outcomes |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled,parallel‐group, unclear sites 2 treatments, 90‐day FU, unclear at what point second treatment occurred Follow the pain injections of BTX‐A vs placebo Assessments carried out at baseline, 30 days, 60 days and 90 days | |
| Participants | Inclusion criteria: CM with no response to intramuscular injections of BTX‐A Exclusion criteria not stated N = 94, mean age and M/F ratio not stated Duration of disease 18 years | |
| Interventions | Intervention: subcutaneous injection of BTX‐A, up to 200 U into trigeminal or occipital area Control: matched placebo injections | |
| Outcomes | Number of migraine days Proportion of 50% responders Other outcomes not yet reported | |
| Notes | Publication due in 2017, only skeletal information available. Unpublished information provided through correspondence with trial author Funding source unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: the research assistant, who had access to the randomisation list, delivered verum or placebo injections to the investigator. The investigator who injected the headache sufferers, the participants, and the investigator who assessed outcome were all blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: the research assistant, who had access to the randomisation list, delivered verum or placebo injections to the investigator. The investigator who injected the headache sufferers, the participants, and the investigator who assessed outcome were all blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of dropouts not stated |
| Selective reporting (reporting bias) | Unclear risk | Not yet published, information and data from abstract and brief trial author correspondence only |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, active‐control, parallel‐group, multi‐site Single treatment, 12‐week FU Fixed injections of Botox vs histamine. Double dummy Assessments carried out at baseline and every 30 days out to 12 weeks post‐treatment | |
| Participants | Inclusion criteria: aged 18‐65 years; history of migraine for several years; diagnosed migrainous using criteria ICHD‐II; unresponsive to available abortive (acetaminophen (paracetamol), ergotamine, dexamethasone, sumatriptan) and/or prophylactic agents (beta blocker, amitriptyline, sodium valproate, topiramate) without sustained pain‐free response; attack frequency of 4‐6 per month; severity of 2–3; overuse of acute pharmacotherapy Exclusion criteria: pregnant women; daily headaches; radiological tests revealing any pathology, including computer‐assisted tomography N = 100, mean age 35, M 9/F 92 EM/CM unclear, MOH Y only eligible Years since diagnosis of migraine 15, number of headache attacks during baseline diary period 4, severity of migraine 2.9 on 3‐point scale | |
| Interventions | Intervention: Botox, 50 U, 10 injections into fixed sites including procerus, corrugator, frontalis, temporalis and occipitalis plus placebo injections into upper arm Control: histamine, 1‐10 µg injected into upper arm twice a week for 12 weeks plus placebo injections into head and neck sites | |
| Outcomes | Number of migraine attacks Duration of migraine Severity of migraine se of rescue medication MIDAS Adverse events | |
| Notes | Funding source unclear Diary period 4 weeks Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double dummy, research collaborator prepared injections and placebos for both histamine and BTX in numbered matching vials so that neither participants nor physicians were able to identify drug |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind, double dummy, research collaborator prepared injections and placebos for both histamine and BTX in numbered matching vials so that neither participants nor physicians were able to identify drug |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts uneven 10% vs 20% of total participant numbers and imputation method not stated |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Study size | Unclear risk | 50‐199 participants per arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 12‐week FU Fixed injections of Dysport (2 dosing arms) vs placebo Assessments carried out at baseline and 4 weeks, 8 weeks and 12 weeks post‐treatment | |
| Participants | Inclusion criteria: aged 18‐65 years; at least a 1‐year history of migraine with or without aura; first manifestation under 50 years of age; stable frequency of 3–6 attacks per month ICHD‐I; not previously received Botox; no concomitant prophylactic migraine treatment was allowed during the trial; acute medication for migraine allowed for a maximum of 10 d/month; participants restricted to only 1 type of escape medication that they preferably used (either analgesic or triptan) Exclusion criteria: non‐migraine headaches for > 10 d/month before, but not after, injection; women who were pregnant or not using adequate contraception; history of alcohol or other drug abuse; previously experienced an adverse reaction to Botox; treated with aminoglycoside antibiotics (or other medication affecting neuromuscular transmission), antidepressants, neuroleptics, antiepileptics or anticoagulants; severe psychiatric disturbance, a skin disorder at the injection site, a predisposition to bleeding, or an anticipated lack of compliance and cooperation N = 127, mean age 46, M 20/F 102 (characteristics given for analysed population ‐ 122) CM excluded, MOH excluded Years since onset of migraine 27, number of migraine attacks during baseline diary period 5 | |
| Interventions | Intervention (arm 1): Dysport 210 U from 18 injections, trapezius 45 U, splenius capitis 20 U, temporalis 20 U, frontalis 10 U, corrugator 10 U Intervention (arm 2): Dysport 80 U from 18 injections, trapezius 45 U, splenius capitis 20 U, temporalis 20 U, frontalis 10 U, corrugator 10 U Control: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine attacks Duration of migraine Severity of migraine Use of rescue medication Migraine related disability Becks depression inventory Total tenderness score Patient global evaluation of treatment efficacy Adverse events | |
| Notes | Funder: Ipsen Pharma, Ettlingen, Germany (manufacturer of Dysport) Diary period 28 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, drug prepared by third person |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded with matched placebo injections, third person, who was not involved with the trial prepared the injections in a separate room |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: third person, who was not involved with the trial prepared the injections in a separate room |
| Incomplete outcome data (attrition bias) | Unclear risk | LOCF but unclear how often this had to be used |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes analysed descriptively |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 3 treatment cycles, 3 months FU per treatment Fixed injections of Botox (3 dosing arms) vs placebo Assessments carried out at baseline and 30 days, 60 days, 90 days post‐treatment per cycle | |
| Participants | Inclusion criteria: aged 18‐65 years; average of at least 3 moderate‐severe untreated migraine episodes per month (ICHD‐I); or at least 3 treated migraine episodes of any severity per month; ≤ 15 headache d/month as confirmed by a headache diary during the baseline period; migraine episodes must have occurred for at least 1 year prior to enrolment and be first diagnosed before age 50 years; stable medical condition and acceptable blood haematology and chemistry results; willing to discontinue headache prophylactic medications for at least 3 months immediately prior to the initiation of the baseline period and had to be willing and able to stay on current medications (other than headache prophylaxis) during the course of the trial Exclusion criteria: any medical condition or used any agent that may have put them at risk with exposure to Botox; an infection or skin problem at any of the injection sites or a known allergy or sensitivity to the trial medication or its components; inadequate response to ≥ 3 prophylactic treatments after an adequate trial; Beck Depression Inventory score of > 24; psychiatric problems severe enough to interfere with trial participation or results; previous therapy with BTX of any sero‐type; been injected with anaesthetics or steroids into the trial‐targeted muscles during the 30 days immediately prior to initiation of the baseline period; overusing or abusing symptomatic medication, alcohol or drugs; concurrent chronic use or chronic use in the 3 months prior to the screening period of muscle relaxants; concurrently participating in another investigational trial or who had participated in such a trial in the 30 days immediately prior to baseline period; uncontrolled systemic disease or any condition that might have put the participant at significant risk, might have confounded the trial, or might have interfered significantly with participation in the trial; women who were pregnant, nursing, or planning a pregnancy during the trial or who were unable or unwilling to use a reliable form of contraception during the trial N = 495, mean age 43, M 60/F 435 CM excluded, MOH excluded Years since onset of migraine 23 | |
| Interventions | Intervention (arm 1): Botox 255 U, 20 injections, frontalis 30 U, 4 sites; corrugator 15 U, 2 sites; temporalis 30 U, 4 sites; splenius capitis 30 U, 2 sites; trapezius 60 U, 4 sites; semispinalis capitis 30 U, 2 sites; suboccipital region 30 U, 2 sites Intervention (arm 2): Botox 150 U, 20 injections, frontalis 20 U, 4 sites; corrugator 10 U, 2 sites; temporalis 20 U, 4 sites; splenius capitis 20 U, 2 sites; trapezius 40 U, 4 sites; semispinalis capitis 20 U, 2 sites; suboccipital region 20 U, 2 sites Intervention (arm 3): Botox 75 U, 20 injections, frontalis 10 U, 4 sites; corrugator 5 U, 2 sites; temporalis 10 U, 4 sites; splenius capitis 10 U, 2 sites; trapezius 20 U, 4 sites; semispinalis capitis 10 U, 2 sites; suboccipital region 10 U, 2 sites Control: matched placebo injections | |
| Outcomes | Number of headache days Number of migraine days Number of migraine attacks Proportion of 50% responders Severity of migraine Use of rescue medication MIDAS Headache pain specific quality of life measure Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: participant blinded, with matched placebo injections, an individual with no other trial involvement reconstituted vials of trial medication with all dilutions carried out so that total volume in each vial was the same, vials numbered according to randomisation and reconstituted according to volume of diluent assigned to each randomisation number |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind: an individual with no other trial involvement reconstituted vials of trial medication with all dilutions carried out so that total volume in each vial was the same, vials numbered according to randomisation and reconstituted according to volume of diluent assigned to each randomisation number |
| Incomplete outcome data (attrition bias) | Unclear risk | Method of imputation not stated and group split of dropouts not given |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported, primary outcome data given but secondary data showing no significant between‐group differences not fully reported |
| Study size | Unclear risk | 50‐199 participants per arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 90‐day FU Fixed injections of Botox, 4 arms with different doses and sites vs placebo in all 3 muscle sites Assessment at baseline and at 30 days, 60 days and 90 days post‐treatment | |
| Participants | Inclusion criteria: stable medical condition with migraine headaches as defined by the ICHD ‐ edition not stated; with or without aura; average of 4‐8 moderate‐severe migraine headaches per month; aged 18‐65 years; first diagnosed before age 50; able to distinguish migraine from non migraine headaches; acute migraine therapy must have typically relieved their migraines to an acceptable level; headache frequency/severity must have been stable; chronic medications (if needed) must have been stable for ≥ 3 months prior to enrolment; prophylactic medications had been discontinued must have been at least 3 months before enrolment; previous medications, including any migraine prophylaxis treatments, stay on the same dose and regimen during the course of the trial Exclusion criteria: typical migraine pain was localised predominantly to the occipital or suboccipital regions; > 15 headache d/month (any type); history of complicated migraine; migraine at the time of treatment were ineligible to be treated that day but could return to be enrolled on a subsequent day; infection or skin problem at the injection site; other medical condition that might have put a person at increased risk with exposure to Botox or that was severe enough to interfere with trial participation or results; using agents that interfered with neuromuscular function; overusing symptomatic medications; abusing alcohol or drugs; previously received BTX therapy; known allergy or sensitivity to the trial medication or its components; women who were pregnant, breastfeeding, or planning a pregnancy during the trial period; received an injection of anaesthetic or steroid into the muscles to be injected in the month prior to enrolment; participated in another investigational drug or device clinical trial within the past month N = 232, mean age 44, M 33/F 199 CM excluded, MOH excluded Years since onset of migraine 24, number of migraine attacks during baseline diary period 6 | |
| Interventions | Intervention (arm 1): Botox 25 U in all 3 muscle areas, 11 sites Intervention (arm 2): Botox 10 U in frontal muscle area, 4 sites, plus matched placebo injections into temporal and glabellar muscles Intervention (arm 3): Botox 6 U in temporal muscle area, 2 sites, plus matched placebo injections into frontal and glabellar muscles Intervention (arm 4): Botox 9 U in glabellar muscle, 5 sites, plus matched placebo injections into frontal and temporal muscles Control: placebo in all 3 muscle sites | |
| Outcomes | Number of migraine attacks Severity of migraine Duration of migraine Number of nonmigraine headaches Use of rescue medications Presence or absence of aura Presence or absence of associated symptoms Patient global assessment of response to treatment Headache pain‐specific quality‐of‐life questionnaire Migraine‐specific measure of quality of life Migraine impact questionnaire SF‐36 Treatment assessment questionnaire Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections, no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated from which group dropouts (7) occurred. ITT analysis stated |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported, primary outcome data given but secondary data with none statistically significant results were reported narratively only |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment, 3‐month FU Fixed injections of Botox (2 dosing arms) vs placebo Assessments carried out at baseline and at 1 month, 2 months and 3 months post‐treatment | |
| Participants | Inclusion criteria: aged 18‐65 years; history of ICHD‐1 defined migraine, with or without aura; average of 2‐8 moderate‐severe migraines per month over the previous 3 months; 2‐8 such migraines during the 1‐month baseline period; first diagnosis of migraine before the age of 50 years; able to distinguish migraine from non‐migraine headaches; stable frequency and severity of migraines; no unstable medical conditions; migraines relieved to an acceptable level by acute migraine therapy; doses of concurrent prophylactic medications for migraine stable for at least 3 months immediately prior to enrolment Exclusion criteria: any medical condition or the use of any agent that may have put the participant at risk with exposure to Botox; history of complicated migraine; typical migraine pain localised predominantly to the occipital or suboccipital region of the cranium; planned or actual pregnancy or lactation; known allergy or sensitivity to the trial medication or its components; injection of anaesthetic or steroid into the muscles to be injected in the month immediately prior to enrolment; > 15 headache d/month; symptomatic medication overuse N = 123, mean age 44, M 18/F 105 CM excluded, MOH excluded Years since onset of migraine 23, number of migraine attacks during baseline diary period 4 | |
| Interventions | Intervention (arm 1): Botox 75 U in 11 injection sites (frontalis 30 U, 4 sites; temporalis 18 U, 2 sites; glabellar 27 U, 5 sites) Intervention (arm 2): Botox 25 U in 11 injection sites (frontalis 10 U, 4 sites; temporalis 6 U, 2 sites; glabellar 9 U, 5 sites) Control: matched placebo injections | |
| Outcomes | Number of migraine attacks Proportion of 50% responders Severity of migraine Use of rescue medication Patient global assessment of response to treatment Adverse events | |
| Notes | Funder: Allergan, Inc., Irvine, California (manufacturer of Botox) Diary period 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant dropped out |
| Selective reporting (reporting bias) | Unclear risk | Incomplete reporting of outcomes |
| Study size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, single‐site Single treatment, 3‐month FU Fixed injections of BTX‐A (brand not reported) vs placebo Assessments carried out at baseline and 1 month, 2 months and 3 months postbaseline | |
| Participants | Inclusion criteria: aged 18‐65; migraine headaches occurring > 5 times/month and meeting ICHD‐I criteria for migraine headache with or without aura Exclusion criteria: none stated N = 49, (analysed population used for baseline characteristics N = 32) mean age 42, M 5/F 27 EM/CM unclear, MOH eligibility unclear Years with migraine 20, number of migraine days during baseline diary period 19, severity of migraine 5.2 on 10 cm VAS | |
| Interventions | Intervention: BTX‐A (brand not reported), weight‐based dosing, < 65 kg: 135 U, ≥ 65 kg: 210 U, 22 injection sites (corrugator 2 sites 5 U/5 U, frontalis 4 sites 20 U/20 U, temporalis 4 sites 20 U/40 U, posterior neck 6 sites 60 U/90 U, occipitalis 2 sites 10 U/10 U, sternocleidomastoid 4 sites 20 U/40 U) Control: matched placebo injections | |
| Outcomes | Number of migraine attacks Severity of migraine Duration of migraine Use of rescue medication Migraine‐specific questionnaire | |
| Notes | Funder: Comprehensive Neuroscience Program and the Uniformed Services University of the Health Sciences award Diary period 30 days Contacted without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind: participant blinded with matched placebo injections no methods given for blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind: no methods given for blinding of personnel |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers randomised to each group and dropouts per group not stated |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported, primary outcome data given but some secondary data with no significant between group differences were not fully reported |
| Study size | High risk | < 50 participants per treatment arm |
AE: adverse event; BTX: botulinum toxin; BTX‐A: botulinum toxin type A; CM: chronic migraine; EM: episodic migraine; F: female; FU: follow‐up; LOCF: last observation carried forward; HIT‐6: Headache Impact Test ‐ 6 question version; HRQoL: Health‐Related Quality‐of‐Life score; ICHD: International Classification of Headache Disorders; IHS: International Headache Society ITT: intention‐to‐treat; M: male; MIDAS: Migraine Disability Assessment Score; MIGSEV: migraine severity scale; MIQ: Migraine Impact Questionnaire; MOH: medication overuse headache; OL: open‐label; QUALY: quality adjusted life years; SF‐36: 36‐Item Short Form Health Survey; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No relevant comparison, BTX used in both arms | |
| Clinical assessments carried out at baseline and after all participants had crossed over to receive BTX, no precross‐over recording of clinical outcomes took place | |
| Some included participants had tension‐type headache and migraine; contact with trial author confirmed that data for migraine‐only participants were not available | |
| Surgical removal of trigger sites in addition to BTX treatment | |
| Trial of people with chronic daily headache; contact with trial author confirmed that data for migraine‐only participants were not available | |
| Health economics paper, data sourced from trial publications | |
| Report of placebo‐treated participants only |
BTX: botulinum toxin
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Single treatment with 12‐week FU 4 arms of injections of Botox vs placebo Assessments carried out at baseline, 2, 4, 8, 12 and 16 weeks |
| Participants | Inclusion criteria not stated Exclusion criteria not stated N = 48 CM/EM eligibility unclear, MOH eligibility unclear |
| Interventions | Intervention (arm 1): injections of Botox (dose and number of injections unclear) into frontal and temporal sites Intervention (arm 2): matched injections of Botox into frontal sites and injections of placebo into temporal sites Intervention (arm 3): matched injections of placebo into frontal sites and injections of Botox into temporal sites Control: matched injections of placebo into frontal and temporal sites |
| Outcomes | Frequency of migraine Duration of migraine Severity of migraine |
| Notes | Abstract only Funder: Allergan Inc Diary period not stated |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site Number of treatment sessions not stated, 3‐month time frame Injections of Dysport (fixed or follow the pain not stated) vs placebo Assessment time points not stated |
| Participants | Inclusion criteria: migraine without aura or with typical aura as defined by IHS criteria, migraine attacks persisting for > 1 year; 2‐6 migraine attacks/month of at least moderate severity over the 3 months preceding the pre‐inclusion visit; 2‐6 migraine attacks of at least moderate severity during the screening period Exclusion criteria: non‐migraine headaches such as tension‐type headaches; migraine with prolonged aura, familial hemiplegic migraine, basilar migraine, migraine aura without headache, migraine with acute onset aura, ophthalmoplegic migraine, retinal migraine, complications of migraine; onset of migraine is after age of 50; overuse of acute migraine medications (individuals who take medications for acute migraine > 10 d/month) or have a history of drug or alcohol abuse Target N = 150 CM/EM included, MOH excluded |
| Interventions | Intervention: Dysport injections into pericranial muscles, number and dose not stated Control: placebo |
| Outcomes | Number of migraine attacks Intensity of the migraine attacks Duration of the migraine attacks Use of rescue medication Quality‐of‐life measure (instrument unspecified) |
| Notes | Protocol (NCT00301665) only, enrolment completed 2005 Funder Ipsen Diary period not stated |
| Methods | Not stated if randomised but is stated as double‐blind, placebo‐controlled, parallel‐group, number of sites unclear 2 treatment cycles, 90 days between treatments Fixed injections of Botox vs placebo Assessments carried out at 3 months after each treatment session |
| Participants | Inclusion criteria: aged 18‐65 years; 3‐15 days migraine per month; suffered migraines for at least 1 year Exclusion criteria not stated Target N = 90 CM excluded, MOH eligibility unclear |
| Interventions | Intervention: Botox (25 U) in fixed muscle injection sites (temporalis, frontalis, procerus, corrugator) Control: matched placebo injections |
| Outcomes | Frequency of migraine (unit not stated) Use of rescue medication Quality‐of‐life measure (instrument unspecified) |
| Notes | Trial design publication only Funder: German Federal Ministry of Education and Research Diary period not stated |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 3 treatment cycles, 90 days between treatments Follow the pain injections of Botox vs placebo following a single‐blind placebo run‐in phase to classify participants as placebo responders or nonresponders Assessments carried out at baseline, and every 30 days out to day 270. An additional assessment for days ‐60 to ‐30 was carried out to make the placebo response classification |
| Participants | Inclusion criteria: aged 18‐65 years; headaches on > 15 days of the 30‐day baseline period; any combination of migraines, with/without aura, migrainous/probable migraine, and/or episodic/chronic tension‐type headaches defined by ICHD‐I criteria; stable medical condition; chronic medication regimens, including prophylactic medications, had to be stable for at least 3 months prior to baseline period; willing and able to stay on current medications during the course of the trial Exclusion criteria: any medical condition or used any agent that may have put them at risk with exposure to Botox; infection or skin problem at any of the injection sites; known allergy or sensitivity to the trial medication or its components; history of “complicated” migraine; Beck Depression inventory score > 24; previous therapy with BTX of any serotype, an injection of anaesthetics or steroids into the trial‐targeted muscles during the 30 days prior to baseline period; overusing or abusing symptomatic medication, alcohol, or drugs; concurrent chronic use or chronic use in the 3 months prior to the screening period of muscle relaxants; women who were pregnant, nursing, or planning a pregnancy during the trial, or who were unable or unwilling to use a reliable form of contraception during the trial N = 355, mean age 43.5 years, M 55/F 300 (inclusive of all headache types) Baseline characteristics for migraine‐only participants not stated |
| Interventions | Intervention: Botox 105‐200 U, number of injections per site determined by treating physician (frontal/glabellar 25‐40 U, occipitalis 20 U, temporalis 20‐50 U, masseter optional; 0‐50 U, trapezius 20‐60 U, semispinalis 10‐20 U, splenius capitis 10‐20 U) Control: matched placebo injections |
| Outcomes | Number of headache‐free days Proportion of responders Number of headache attacks Use of rescue medication MIDAS Headache Pain‐Specific Quality of life questionnaire Adverse events |
| Notes | Data for migraine‐only participants required for inclusion Funder: Allergan Inc Diary period 30 days |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group, multi‐site 3 treatment cycles, 90 days between treatments Fixed injections of Botox (3 dosing arms) vs placebo following a single‐blind placebo run‐in phase to classify participants as placebo responders or nonresponders Assessments carried out at baseline, and every 30 days out to day 270. An additional assessment for days ‐60 to ‐30 was carried out to make the placebo response classification |
| Participants | Inclusion criteria: aged 18‐65 years; headaches on > 15 days during a 30‐day baseline period; headaches could include any combination of migraines: those with/without aura, migrainous headache, probable migraine, and/or episodic or chronic tension‐type headaches according to ICHD‐I criteria; medically stable; long‐term medication (including long‐term prophylactic headache medications) had to be stable for at least 3 months immediately before the baseline period; acute headache pain medication could be taken as needed; willing to continue taking current medications during the course of the trial Exclusion criteria: any medical condition (e.g. neuromuscular disorders) or used any agent that might expose them to risk if they received Botox; infection or skin problem at any of the injection sites, known allergy or sensitivity to the trial medication or to its components; cluster headache or chronic paroxysmal hemicrania, analgesic rebound headache, headache secondary to head trauma or whiplash injury, a history of “complicated” migraine (e.g. migrainous infarction, hemiplegic migraine, ophthalmoplegic migraine, or basilar migraine), a Beck Depression Inventory score > 24, previous therapy with BTX of any serotype, an injection of anaesthetics or corticosteroids into the trial‐targeted muscles during the 30 days immediately before the baseline period; abuse of symptomatic medication, alcohol, or drugs; concurrent or long‐term use of muscle relaxants (e.g. cyclobenzaprine, carisoprodol, or benzodiazepines) during the 3 months before the screening period was prohibited; women who were pregnant or nursing or unable or unwilling to use a reliable form of contraception during the trial N = 702, mean age 43.4 years, M 120/F 582 (inclusive of all headache types) Baseline characteristics for migraine‐only participants not stated |
| Interventions | Intervention (arm 1): Botox 255 U, 20 injections, frontalis 30 U, 4 sites; corrugator 15 U, 2 sites; temporalis 30 U, 4 sites; splenius capitis 30 U, 2 sites; trapezius 60 U, 4 sites; semispinalis capitis 30 U, 2 sites; suboccipital region 30 U, 2 sites Intervention (arm 2): Botox 150 U, 20 injections, frontalis 20 U, 4 sites; corrugator 10 U, 2 sites; temporalis 20 U, 4 sites; splenius capitis 20 U, 2 sites; trapezius 40 U, 4 sites; semispinalis capitis 20 U, 2 sites; suboccipital region 20 U, 2 sites Intervention (arm 3): Botox 75 U, 20 injections, frontalis 10 U, 4 sites; corrugator 5 U, 2 sites; temporalis 10 U, 4 sites; splenius capitis 10 U, 2 sites; trapezius 20 U, 4 sites; semispinalis capitis 10 U, 2 sites; suboccipital region 10 U, 2 sites Control: matched placebo injections |
| Outcomes | Number of headache‐free days Proportion of responders Number of headache attacks Number of moderate‐severe migraines Use of rescue medication MIDAS Headache Pain‐Specific Quality of life questionnaire AE |
| Notes | Data for migraine‐only participants required for inclusion Funder: Allergan Inc Diary period 30 days |
AE: adverse event; BTX: botulinum toxin; CM: chronic migraine; EM: episodic migraine; F: female; FU: follow‐up; ICHD: International Classification of Headache Disorders; IHS: International Headache Society M: male; MIDAS: Migraine Disability Assessment Score; MOH: medication overuse headache
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ASIS for Botox in chronic migraine (ASISinCM) |
| Methods | Randomised, double‐blind, active control, parallel‐group Aim 2 only relevant to this review: guided delivery of Botox into subdermal bloodless space, between the skin and muscle vs Botox injected intramuscularly |
| Participants | Inclusion criteria: aged 18‐65 years; history of chronic migraine (with/without aura) according to the criteria proposed by the Headache Classification Committee of the IHS for at least 3 months prior to enrolment; preventive medication, if taking, must be stable for at least 3 months Exclusion criteria: headache disorders outside IHS‐defined chronic migraine definition; evidence of underlying pathology contributing to their headaches; any pathology of the salivary glands such as sialadenitis or condition or symptom that would alter the content of saliva; any medical condition that may increase their risk with exposure to Botox including diagnosed myasthenia gravis, Eaton‐Lambert syndrome, amyotrophic lateral sclerosis, or any other significant disease that might interfere with neuromuscular function; profound atrophy or weakness of muscles in the target areas of injection Target N = 60 |
| Interventions | Intervention: Botox injection guided by device to delivery into subdermal bloodless space between skin and muscle at 6 muscle group sites (glabella, frontal, temporal, occipital, paraspinal, and trapezius) Control: conventional intramuscular injections of Botox in same muscle groups (Gglabella, frontal, temporal, occipital, paraspinal, and trapezius) |
| Outcomes | Number of headache days Total cumulative hours of headache on headache days AE |
| Starting date | January 2016 |
| Contact information | Li Nguyen, MD (714)‐453‐7857 dr.li.nguyen@asis‐inc.com Thanh Phung, MD 714‐893‐1915 thanhphung@idit‐inc.com |
| Notes | The recruitment status of this trial is unknown. The completion date has passed and the status has not been verified in more than two years Protocol: NCT:02074163 Funder: ASIS Corporation listed as sponsors and collaborators |
| Trial name or title | A study to evaluate botulinum toxin type A for injection HengLi for prophylactic treatment of chronic migraine |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Hypothesis relating to this review: that Botox for injection (HengLi) for prophylactic treatment with chronic migraine in adults is safe and efficacious |
| Participants | Inclusion criteria: age ≥ 18 and ≤ 65, male or female; complying with the ICHD‐3(β) diagnostic criteria for chronic migraine Exclusion criteria: pregnancy, nursing, or planning a pregnancy during the trial period, or women of childbearing potential, not using a reliable means of contraception; known allergy or sensitivity to trial medication or its component; accepted prophylactic treatments of migraine within the 4 weeks before screening; cardiac functional insufficiency; renal insufficiency (serum creatinine > 1.5 times upper limit of normal); hepatic diseases (alanine aminotransferase or aspartate aminotransferase > twice upper limit of normal); systemic myoneural junction diseases (e.g. myasthenia, Eaton‐Lambert syndrome, amyotrophic lateral sclerosis, etc.); history of facial palsy; infection or dermatological condition at the injection sites; other types of migraine that do not comply with the diagnostic criteria for chronic migraine; BTX therapy in the past 6 months; used aminoglycoside antibiotics in the recent week or need to use aminoglycoside antibiotics during conduct of the clinical trial; severe cognitive disorder or mental illness, alcohol or drug abuse; involved in other clinical trials over the 3 months prior to this trial Target N = 288 |
| Interventions | Intervention: Botox for injection in these trials, minimum intramuscular dose of 155 U of Botox (HengLi) administered to 31 injection sites across 7 head and neck muscles using a fixed‐site, fixed‐dose injection paradigm (each injection was 5 U in 0.1 mL). In addition, up to 40 U Botox, administered intramuscularly to 8 additional injection sites across 3 head and neck muscles, was allowed, using a follow‐the‐pain approach. Thus, the minimum dose was 155 U and the maximum dose was 195 U Control: matched placebo injections |
| Outcomes | Number of headache days Number of headache attacks Number of migraine attacks Proportion of responders (≥ 50% reduction in migraine) Use of rescue medications Severity of migraine Duration of migraine MIDAS HIT‐6 |
| Starting date | September 2014 |
| Contact information | Lanzhou Institute of Biological Products Co., Ltd |
| Notes | Protocol NCT02291380 Funder: Lanzhou Institute of Biological Products Co., Ltd listed as Sponsors and Collaborators |
| Trial name or title | Chronification and reversibility of migraine (CHARM) |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group Hypothesis relating to this review: treatment with BTX‐A injections (at the start of the therapy) will increase the success rate of withdrawal therapy or will improve quality of life during the withdrawal period |
| Participants | Inclusion criteria: suffering from chronic migraine according to the ICHD‐II criteria for chronic migraine with medication overuse according to the ICHD‐II criteria Exclusion criteria: age under 18 years; other neurological conditions that may interfere with the trial; any oncological or psychiatric disease, any cognitive disorders and/or behavioural problems which may interfere with the trial; substance abuse; use of non‐triptan or non‐analgesic acute anti‐headache medication; pregnancy, planned pregnancy, current nursing; specific risk factors for BTX and magnetic resonance imaging Target N = 180 |
| Interventions | Intervention: withdrawal therapy with 1‐time concomitant BTX‐A injections in 31 locations according to the injection protocol by Allergan (total 155U) |
| Outcomes | Number of headache days Number of migraine days Number of migraine attacks Proportion of responders (≥ 50% reduction in migraine) HIT‐6 Depression scales SF‐36 questionnaire |
| Starting date | 1 September 2012 |
| Contact information | |
| Notes | Full publication due in 2017 Protocol NTR3440 Funder: NWO (VIDI grant) |
AE: adverse events; BTX: botulinum toxin; BTX‐A: botulinum toxin type A HIT‐6: Headache Impact Test‐6 question version; ICHD: International Classification of Headache Disorders; IHS: International Headache Society; MIDAS: Migraine Disability Assessment
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 5 | 1915 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.76] |
| Analysis 1.1  Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Number of migraine days. | ||||
| 1.1 Chronic migraine | 4 | 1497 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.73, ‐1.41] |
| 1.2 Episodic migraine | 1 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.77, 0.37] |
| 2 Number of headache days Show forest plot | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| Analysis 1.2 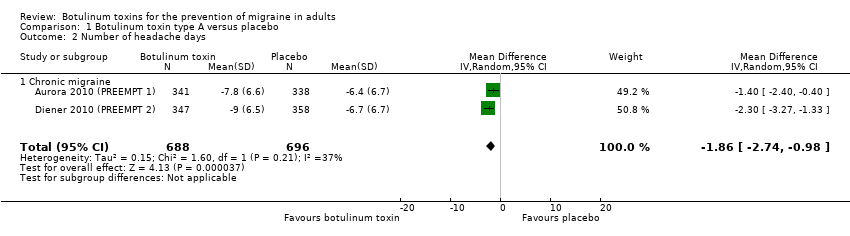 Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Number of headache days. | ||||
| 2.1 Chronic migraine | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| 3 Number of migraine attacks Show forest plot | 6 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.34, 0.41] |
| Analysis 1.3  Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Number of migraine attacks. | ||||
| 3.1 Chronic migraine | 1 | 679 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.71, 0.91] |
| 3.2 Episodic migraine | 3 | 1096 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.43] |
| 3.3 Mixed | 2 | 229 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐6.78, 2.63] |
| 4 Severity of migraine (Visual Analogue Score 0‐10) Show forest plot | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐4.16, ‐2.45] |
| Analysis 1.4  Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Severity of migraine (Visual Analogue Score 0‐10). | ||||
| 4.1 Chronic migraine | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐3.31, ‐2.09] |
| 4.2 Episodic migraine | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐4.9 [‐6.56, ‐3.24] |
| 4.3 Mixed | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐4.52, ‐2.48] |
| 5 Use of rescue medication Show forest plot | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| Analysis 1.5  Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Use of rescue medication. | ||||
| 5.1 Chronic migraine | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| 6 Total adverse events Show forest plot | 13 | 3325 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.47] |
| Analysis 1.6  Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Total adverse events. | ||||
| 6.1 Chronic migraine | 5 | 1494 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.40] |
| 6.2 Episodic migraine | 6 | 1673 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 6.3 Mixed | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.57, 3.76] |
| 7 Adverse event ‐ blepharoptosis Show forest plot | 7 | 1867 | Risk Ratio (M‐H, Random, 95% CI) | 7.29 [3.18, 16.73] |
| Analysis 1.7 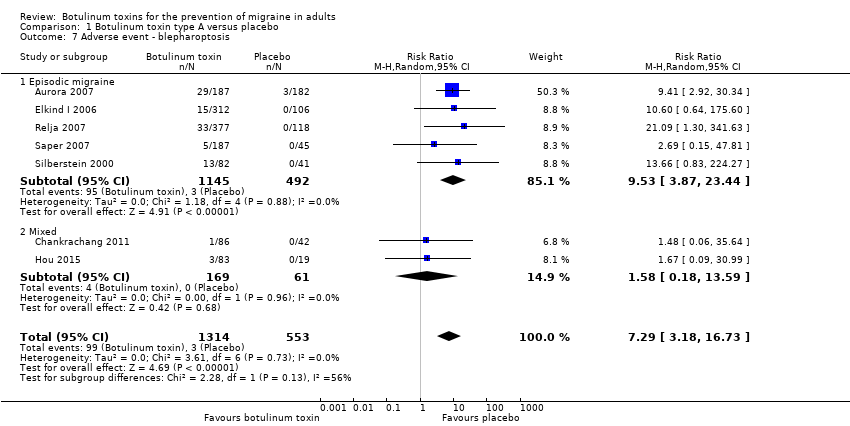 Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Adverse event ‐ blepharoptosis. | ||||
| 7.1 Episodic migraine | 5 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 9.53 [3.87, 23.44] |
| 7.2 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.18, 13.59] |
| 8 Adverse event ‐ muscle weakness Show forest plot | 6 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 13.67 [6.73, 27.75] |
| Analysis 1.8  Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse event ‐ muscle weakness. | ||||
| 8.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 12.68 [3.49, 46.05] |
| 8.2 Episodic migraine | 4 | 1223 | Risk Ratio (M‐H, Random, 95% CI) | 14.12 [6.05, 32.94] |
| 9 Adverse event ‐ neck pain Show forest plot | 6 | 2424 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.06, 4.32] |
| Analysis 1.9  Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse event ‐ neck pain. | ||||
| 9.1 Chronic migraine | 3 | 1432 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.48, 4.12] |
| 9.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 [2.27, 6.79] |
| 9.3 Mixed | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.09, 10.47] |
| 10 Adverse event ‐ injection site pain Show forest plot | 8 | 1332 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.02, 4.33] |
| Analysis 1.10  Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse event ‐ injection site pain. | ||||
| 10.1 Chronic migraine | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.81, 8.15] |
| 10.2 Episodic migraine | 3 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [1.14, 9.13] |
| 10.3 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.58] |
| 11 Total treatment related adverse events Show forest plot | 6 | 2893 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.73, 2.75] |
| Analysis 1.11 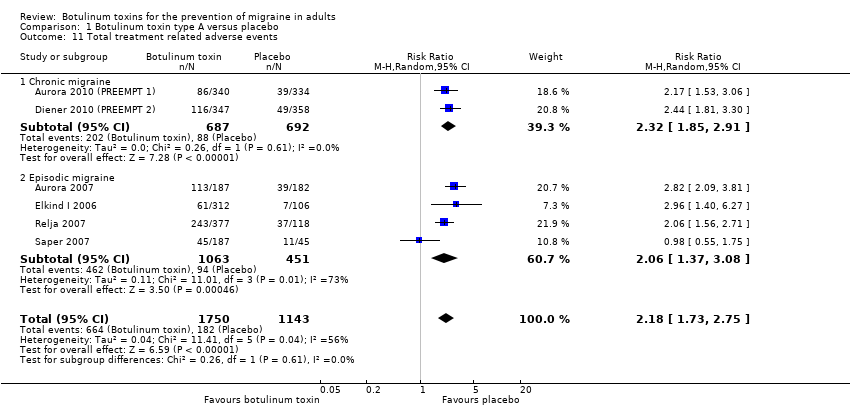 Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Total treatment related adverse events. | ||||
| 11.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.85, 2.91] |
| 11.2 Episodic migraine | 4 | 1514 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.37, 3.08] |
| 12 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 4 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [1.52, 7.07] |
| Analysis 1.12  Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Withdrawals due to adverse events in trials with multiple rounds of treatment.. | ||||
| 12.1 Chronic migraine | 2 | 1384 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [1.38, 9.98] |
| 12.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.81, 9.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Migraine impact and disability assessment scores Show forest plot | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 4.27 [‐28.15, 36.69] |
| Analysis 2.1 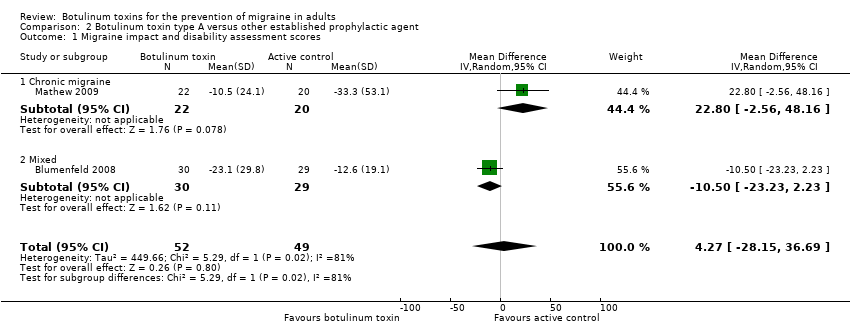 Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 1 Migraine impact and disability assessment scores. | ||||
| 1.1 Chronic migraine | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 22.8 [‐2.56, 48.16] |
| 1.2 Mixed | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐10.50 [‐23.23, 2.23] |
| 2 Total adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| Analysis 2.2 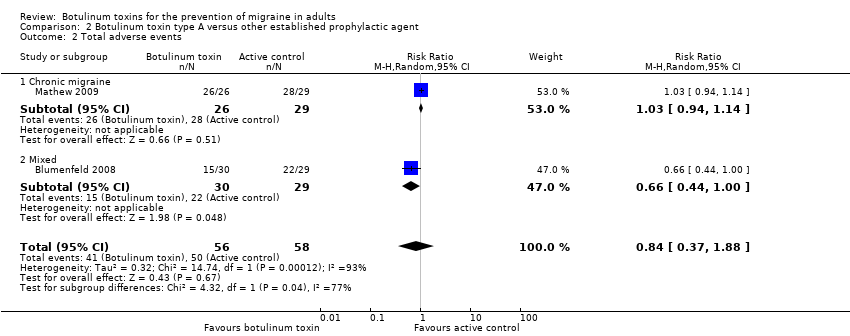 Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 2 Total adverse events. | ||||
| 2.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.14] |
| 2.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 1.00] |
| 3 Total treatment related adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| Analysis 2.3 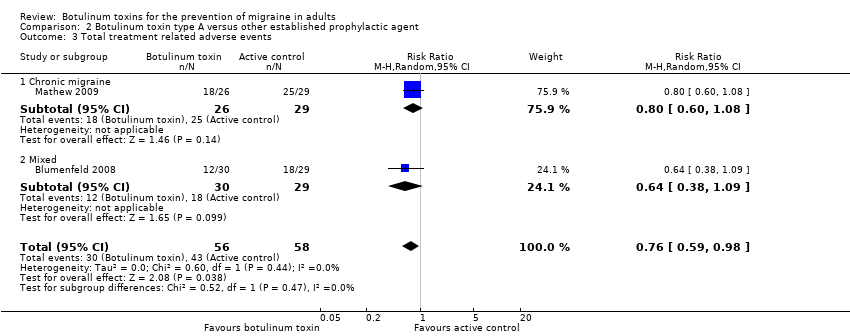 Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 3 Total treatment related adverse events. | ||||
| 3.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.08] |
| 3.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.09] |
| 4 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.79] |
| Analysis 2.4  Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 4 Withdrawals due to adverse events in trials with multiple rounds of treatment.. | ||||
| 4.1 Chronic migraine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.11, 1.28] |
| 4.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total adverse events Show forest plot | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.47, 5.32] |
| Analysis 3.1 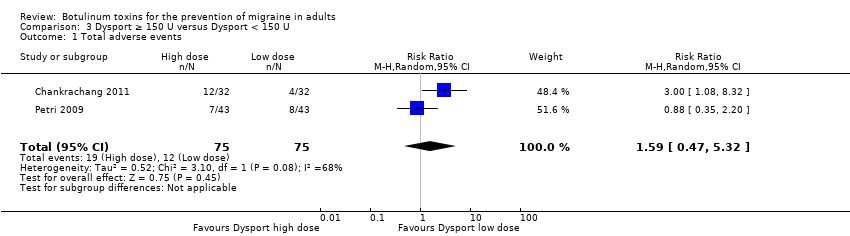 Comparison 3 Dysport ≥ 150 U versus Dysport < 150 U, Outcome 1 Total adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| Analysis 4.1  Comparison 4 Botox dosing studies, Outcome 1 Number of migraine days. | ||||
| 1.1 Episodic migraine Botox ≥ 50 U vs Botox < 50 | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 2 Adverse event ‐ muscle weakness Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.39] |
| Analysis 4.2  Comparison 4 Botox dosing studies, Outcome 2 Adverse event ‐ muscle weakness. | ||||
| 2.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 2.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.64] |
| 3 Adverse event ‐ blepharoptosis Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Botox dosing studies, Outcome 3 Adverse event ‐ blepharoptosis. | ||||
| 3.1 ≥ 50 U versus < 50 U in frontalis and/or corrugator | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.20, 4.43] |
| 3.2 ≥ 30 U versus < 30 U in frontalis and/or corrugator | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.58, 9.65] |
| 3.3 ≥ 10 U versus < 10 U in frontalis and/or corrugator | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.99, 5.94] |
| 4 Adverse event ‐ neck pain Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.65] |
| Analysis 4.4  Comparison 4 Botox dosing studies, Outcome 4 Adverse event ‐ neck pain. | ||||
| 4.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.83, 1.89] |
| 4.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.86] |
| 5 Adverse event ‐ injection site pain Show forest plot | 2 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.52, 2.51] |
| Analysis 4.5  Comparison 4 Botox dosing studies, Outcome 5 Adverse event ‐ injection site pain. | ||||
| 5.1 Botox ≥ 200 U versus Botox < 200 U | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.13, 11.97] |
| 5.2 Botox ≥150 U versus Botox <150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.48, 4.41] |
| 5.3 Botox ≥ 50 U versus Botox < 50 U | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.34, 4.12] |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.1 Number of migraine days. Mazza 2016 and Cady 2014 removed for sensitivity analysis of small trial effect. Data for Mazza 2016 is endpoint data.

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.4 Severity of migraine (Visual Analogue Score 0‐10)

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.6 Total adverse events

Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Number of migraine days.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Number of headache days.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Number of migraine attacks.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Severity of migraine (Visual Analogue Score 0‐10).

Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Use of rescue medication.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Total adverse events.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Adverse event ‐ blepharoptosis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse event ‐ muscle weakness.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse event ‐ neck pain.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse event ‐ injection site pain.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Total treatment related adverse events.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Withdrawals due to adverse events in trials with multiple rounds of treatment..

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 1 Migraine impact and disability assessment scores.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 2 Total adverse events.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 3 Total treatment related adverse events.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 4 Withdrawals due to adverse events in trials with multiple rounds of treatment..

Comparison 3 Dysport ≥ 150 U versus Dysport < 150 U, Outcome 1 Total adverse events.

Comparison 4 Botox dosing studies, Outcome 1 Number of migraine days.

Comparison 4 Botox dosing studies, Outcome 2 Adverse event ‐ muscle weakness.

Comparison 4 Botox dosing studies, Outcome 3 Adverse event ‐ blepharoptosis.

Comparison 4 Botox dosing studies, Outcome 4 Adverse event ‐ neck pain.

Comparison 4 Botox dosing studies, Outcome 5 Adverse event ‐ injection site pain.
| Botulinum toxin type A compared to placebo for the prevention of migraine in adults | |||||
| Patient or population: adults with migraine | |||||
| Outcomes | Result with placebo | Result with botulinum toxin type A | Relative effect | № of participants | Quality of the evidence |
| Number of migraine days per month: chronic migraine only | The mean number of migraine days (chronic migraine only) ranged from 12 to 20 days | MD 3.1 days lower | ‐ | 1497 | ⊕⊕⊝⊝ |
| Number of migraine days per month | The mean number of migraine days ranged from 4 to 20 days | MD 2.4 days lower | ‐ | 1915 | ⊕⊝⊝⊝ |
| Number of headache days per month: chronic migraine only | The mean number of headache days (chronic migraine only) ranged from 13 to 13.4 days | MD 1.9 days lower | ‐ | 1384 | ⊕⊕⊕⊕ |
| Number of migraine attacks | The mean number of migraine attacks ranged from 1.9 to 7.8 attacks | MD 0.5 attacks lower | ‐ | 2004 | ⊕⊕⊝⊝ |
| Headache intensity measure (Visual Analogue Score 0‐10) | The mean severity of migraine (Visual Analogue Score 0‐10) ranged from 6.2 to 9.2 cm | MD 3.3 cm lower | ‐ | 209 | ⊕⊝⊝⊝ |
| Global impression scale | The mean global impression scale was 58.6 points | MD 1.6 points higher | ‐ | 45 | ⊕⊝⊝⊝ |
| Total number of participants experiencing an adverse event | Trial population | RR 1.28 | 3325 | ⊕⊕⊕⊝ | |
| 471 per 1000 | 603 per 1000 | ||||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded once due to inconsistency: statistical heterogeneity observed despite similarities in populations and doses. | |||||
| Botulinum toxin type A compared to other established prophylactic agent for the prevention of migraine in adults | |||||
| Patient or population: adults with migraine | |||||
| Outcomes | Result with other established prophylactic agent | Result with botulinum toxin type A | Relative effect | № of participants | Quality of the evidence |
| Number of migraine days per month: chronic migraine only | One trial using topiramate in its comparison arm reported narratively on this outcome stating that there was no significant difference between groups. | ‐ | 43 | ⊕⊝⊝⊝ | |
| Number of headache days per month | The mean number of headache days was 6.6 days | MD 1 day lower | ‐ | 59 | ⊕⊝⊝⊝ |
| Number of migraine attacks per month | ‐ | ‐ | ‐ | ‐ | ‐ |
| Headache intensity measure | The mean severity of migraine was 2.3 points | MD 0.4 points lower | ‐ | 46 | ⊕⊝⊝⊝ |
| Global impression of disease | The mean global impression of disease ranged from 9.8 to 16.5 points | MD 4.3 points higher | ‐ | 101 | ⊕⊝⊝⊝ |
| Total number of participants experiencing an adverse event | Trial population | RR 0.76 | 114 | ⊕⊝⊝⊝ | |
| 862 per 1000 | 724 per 1000 | ||||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded once due to risk of bias: unclear or high risk for selection, performance, detection and attrition bias. | |||||
| Term | Definition |
| Chronic migraine (IHS 1988) | Not defined |
| Chronic migraine (IHS 2004) | Description: migraine headache occurring on ≥ 15 days per month for > 3 months in the absence of medication overuse Diagnostic criteria |
| Chronic migraine (IHS 2013) | Description: headache occurring on ≥ 15 days per month for > 3 months, which has the features of migraine headache on at least 8 days per month Diagnostic criteria A. Headache (tension‐type‐like and/or migraine‐like on ≥ 15 days/month for > 3 months and fulfilling criteria B and C B. Occurring in a patient who has had at least 5 attacks fulfilling criteria B‐D for migraine without aura and/or criteria B and C for migraine with aura C. On 8 days per month for > 3 months, fulfilling any of the following:
D. Not better accounted for by another ICHD‐III diagnosis |
| Medication overuse headache (IHS 1988) | Not defined |
| Medication overuse headache (IHS 2004) | Diagnostic criteria |
| Medication overuse headache (IHS 2013) | Description: headache occurring on ≥ 15 days/month developing as a consequence of regular overuse of acute or symptomatic headache medication (on ≥ 10, or ≥ 15 days/month, depending on the medication) for > 3 months. It usually, but not invariably, resolves after the overuse is stopped. Diagnostic criteria |
| Migraine (IHS 2013) | Migraine has 2 major subtypes. Migraine without aura is a clinical syndrome characterised by headache with specific features and associated symptoms. Migraine with aura is primarily characterised by the transient focal neurological symptoms that usually precede or sometimes accompany the headache. |
| Migraine with aura (IHS 2013) | Description: recurrent attacks, lasting minutes, of unilateral fully reversible visual, sensory or other central nervous system symptoms that usually develop gradually and are usually followed by headache and associated migraine symptoms Diagnostic criteria C. At least 2 of the following 4 characteristics:
D. Not better accounted for by another ICHD‐3 diagnosis, and transient ischaemic attack has been excluded. |
| Migraine without aura (IHS 2013) | Description: recurrent headache disorder manifesting in attacks lasting 4‐72 h. Typical characteristics of the headache are unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity and association with nausea and/or photophobia and phonophobia. Diagnostic criteria A. At least 5 attacks1 fulfilling criteria B–D
D. During headache at least one of the following: 1. nausea and/or vomiting, 2. photophobia and phonophobia E. Not better accounted for by another ICHD‐III diagnosis. |
| SNARE complex (Goodsell 2013) | Soluble NSF‐attachment protein receptor (NSF: N‐ethylmaleimide‐sensitive factor) |
| SNAP‐25 (Goodsell 2013) | Synaptosomal‐associated protein‐25 |
| Trade name | Manufacturer | FDA‐issued name | Sero‐type |
| Botox | Allergan | OnabotulinumtoxinA | Botulinum toxin type A |
| Botox cosmetic | Allergan | OnabotulinumtoxinA | Botulinum toxin type A |
| Dysport | Ipsen | AbobotulinumtoxinA | Botulinum toxin type A |
| HengLi | Lanzhou Institute of biological products | Not issued | Botulinum toxin type A |
| Myobloc | Solstice | RimabotulinumtoxinB | Botulinum toxin type B |
| Prosigne | Lanzhou Institute of biological products | Not issued | Botulinum toxin type A |
| Xeomin | Merz | IncobotulinumtoxinA | Botulinum toxin type A |
| Allergan, Ipsen and Galderma all responded but were unable to provide additional eligible data. Merz, Solstice, and Lanzhou Institute of biological products were contacted without response. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 5 | 1915 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.76] |
| 1.1 Chronic migraine | 4 | 1497 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.73, ‐1.41] |
| 1.2 Episodic migraine | 1 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.77, 0.37] |
| 2 Number of headache days Show forest plot | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| 2.1 Chronic migraine | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| 3 Number of migraine attacks Show forest plot | 6 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.34, 0.41] |
| 3.1 Chronic migraine | 1 | 679 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.71, 0.91] |
| 3.2 Episodic migraine | 3 | 1096 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.43] |
| 3.3 Mixed | 2 | 229 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐6.78, 2.63] |
| 4 Severity of migraine (Visual Analogue Score 0‐10) Show forest plot | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐4.16, ‐2.45] |
| 4.1 Chronic migraine | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐3.31, ‐2.09] |
| 4.2 Episodic migraine | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐4.9 [‐6.56, ‐3.24] |
| 4.3 Mixed | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐4.52, ‐2.48] |
| 5 Use of rescue medication Show forest plot | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| 5.1 Chronic migraine | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| 6 Total adverse events Show forest plot | 13 | 3325 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.47] |
| 6.1 Chronic migraine | 5 | 1494 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.40] |
| 6.2 Episodic migraine | 6 | 1673 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 6.3 Mixed | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.57, 3.76] |
| 7 Adverse event ‐ blepharoptosis Show forest plot | 7 | 1867 | Risk Ratio (M‐H, Random, 95% CI) | 7.29 [3.18, 16.73] |
| 7.1 Episodic migraine | 5 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 9.53 [3.87, 23.44] |
| 7.2 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.18, 13.59] |
| 8 Adverse event ‐ muscle weakness Show forest plot | 6 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 13.67 [6.73, 27.75] |
| 8.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 12.68 [3.49, 46.05] |
| 8.2 Episodic migraine | 4 | 1223 | Risk Ratio (M‐H, Random, 95% CI) | 14.12 [6.05, 32.94] |
| 9 Adverse event ‐ neck pain Show forest plot | 6 | 2424 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.06, 4.32] |
| 9.1 Chronic migraine | 3 | 1432 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.48, 4.12] |
| 9.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 [2.27, 6.79] |
| 9.3 Mixed | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.09, 10.47] |
| 10 Adverse event ‐ injection site pain Show forest plot | 8 | 1332 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.02, 4.33] |
| 10.1 Chronic migraine | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.81, 8.15] |
| 10.2 Episodic migraine | 3 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [1.14, 9.13] |
| 10.3 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.58] |
| 11 Total treatment related adverse events Show forest plot | 6 | 2893 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.73, 2.75] |
| 11.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.85, 2.91] |
| 11.2 Episodic migraine | 4 | 1514 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.37, 3.08] |
| 12 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 4 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [1.52, 7.07] |
| 12.1 Chronic migraine | 2 | 1384 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [1.38, 9.98] |
| 12.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.81, 9.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Migraine impact and disability assessment scores Show forest plot | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 4.27 [‐28.15, 36.69] |
| 1.1 Chronic migraine | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 22.8 [‐2.56, 48.16] |
| 1.2 Mixed | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐10.50 [‐23.23, 2.23] |
| 2 Total adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| 2.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.14] |
| 2.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 1.00] |
| 3 Total treatment related adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| 3.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.08] |
| 3.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.09] |
| 4 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.79] |
| 4.1 Chronic migraine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.11, 1.28] |
| 4.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total adverse events Show forest plot | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.47, 5.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 1.1 Episodic migraine Botox ≥ 50 U vs Botox < 50 | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 2 Adverse event ‐ muscle weakness Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.39] |
| 2.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 2.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.64] |
| 3 Adverse event ‐ blepharoptosis Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 ≥ 50 U versus < 50 U in frontalis and/or corrugator | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.20, 4.43] |
| 3.2 ≥ 30 U versus < 30 U in frontalis and/or corrugator | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.58, 9.65] |
| 3.3 ≥ 10 U versus < 10 U in frontalis and/or corrugator | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.99, 5.94] |
| 4 Adverse event ‐ neck pain Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.65] |
| 4.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.83, 1.89] |
| 4.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.86] |
| 5 Adverse event ‐ injection site pain Show forest plot | 2 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.52, 2.51] |
| 5.1 Botox ≥ 200 U versus Botox < 200 U | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.13, 11.97] |
| 5.2 Botox ≥150 U versus Botox <150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.48, 4.41] |
| 5.3 Botox ≥ 50 U versus Botox < 50 U | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.34, 4.12] |


