Tasas de respuesta y de remisión placebo en los ensayos aleatorios de tratamiento de inducción y mantenimiento para la colitis ulcerosa
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled trial (N = 165) | |
| Participants | Patients with active, mild‐to‐moderate UC | |
| Interventions | Group 1: budesonide foam (2 mg/25 mL) once daily Group 2: budesonide foam (2 mg/25 mL) twice daily Group 3: placebo | |
| Outcomes | Primary outcome: remission at week 6 (rectal bleeding subscore = 0, endoscopic subscore < 1 and stool frequency subscore = 0 or decrease > 1) | |
| Notes | Reported in abstract form only (unclear how many patients randomised to each group); not included in quantitative synthesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Reported in abstract form only |
| Methods | 6 week, randomised, double‐blind, placebo‐controlled, multi‐centre trial (N = 30) | |
| Participants | 30 subjects with mild‐to‐moderate disease Patients were grouped according to disease extent (14 in the distal (< 60 cm) group; 16 in the more extensive (> 60 cm) group) | |
| Interventions | Group 1: 4‐ASA 6 g (n = 17) Group 2: placebo (n = 13) 6 capsules administered twice daily to each group | |
| Outcomes | Primary outcomes: clinical improvement, adverse events and abnormalities in laboratory tests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'matched placebo' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across treatment groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | A prospective, controlled, randomised, double‐blind trial (N = 111) | |
| Participants | Patients with steroid‐dependent, active or inactive UC receiving prednisone at a daily dose of 10 to 40 mg at inclusion | |
| Interventions | Group 1: intra‐muscular or SC methotrexate 25 mg/week Group 2: placebo | |
| Outcomes | Primary outcome: success at week 16 (Mayo score < or = 2 with no item >1, complete steroid withdrawal with a forced tapering regimen, and no need for other immunosuppressant, tumour necrosis factor‐alpha (TNF‐α) antagonist or colectomy) Secondary outcomes: success at week 24, success at week 16 and 24, mucosal healing, clinical remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs not reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, phase IIa, parallel‐group, multicentre trial conducted at 30 sites in 6 countries (N = 111) | |
| Participants | Non‐hospitalised adults with UC (total Mayo score < 6) All enrolled patients had been treated with medication containing 5‐ASA at a stable dose for at least 2 weeks prior to randomisation, with the exception of individuals who had been treated with 5‐ASA medications at the maximum dose without significant improvement/those who had to discontinue Concomitatant therapy with purine analogues (AZA or 6‐MP) was permitted if unchanged for at least 12 weeks prior to randomisation | |
| Interventions | Patients received SC tralokinumab 300 mg (n = 56) or placebo (n = 55) every 2 weeks in a 1:1 ratio 12 week treatment period and 12 week follow‐up period | |
| Outcomes | Primary outcome: clinical response at week 8 Secondary outcomes: clinical remission and mucosal healing at week 8 and changes in total Mayo score, total modified Riley score, partial Mayo score and disease activity markers (CRP, albumin, faecal calprotectin) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Randomisation took place via an interactive voice or web response system at the end of the enrolment period |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial with identical placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal (13/56 discontinued from treatment group, 18/55 from placebo) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, controlled, double blind, escalating dose study (N = 40) | |
| Participants | Patients > 18 years with active distal UC extending 5–50 cm from the anal verge with a UCDAI score of 3–10 points | |
| Interventions | Cohort 1: 0.1 mg/ml alicaforsen enema (n = 8) Cohort 2: 0.5 mg/ml alicaforsen enema (n = 8) Cohort 3: 2 mg/ml alicaforsen enema (n = 8) Cohort 4: 4 mg/ml alicaforsen enema (n = 8) Each cohort contained 2 patients who received placebo enema (n = 8) | |
| Outcomes | Primary outcome: clinical response measured by the UCDAI and the CAI Seconary outcomes: individual components of the UCDAI, alicaforsen drug concentration and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were sequentially randomised to 4 cohorts of 10 patients each (8 to study drug, 2 to placebo) to receive study drug or placebo |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Each enema bottle was labelled with a unique reference number and a scratch off code to blind the investigators, study monitors, and patients to treatment assignment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal 39/40 and 24/40 patients completed the study through to months 2 and 6, respectively 16 patients did not complete the study (15 due to worsening disease and 1 patient for an adverse event) The ITT population was used for analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | A randomised, placebo‐controlled, double‐blind, two‐dose ranging multi‐center study (N = 112) | |
| Participants | Adult patients > 18 years with active distal UC and a left‐sided disease flare (mucosal involvement 5‐50 cm for the anal verge) Disease activity index (DAI) score score between 4‐10 that included an abnormal endoscopic score, and were receiving, alone or in combination, stable doses of oral mesalazine (> 30 days), AZA (> 60 days), or 6‐MP (> 60 days) prior to the study | |
| Interventions | Group 1: 120 mg alicaforsen daily for 10 days and then every other day thereafter (n = 22) Group 2: 240 mg alicaforsen every other day (n = 23) Group 3: 240 mg alicaforsen daily for 10 days and then every other day (n = 23) Group 4: 240 mg alicaforsen daily (n = 22) Group 5: placebo (n = 22) | |
| Outcomes | Primary outcome: UCDAI at week 6 Secondary outcomes: clinical improvement, relapse rates and durability of response | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | A double‐blind, placebo‐controlled, ascending dose trial of LDP‐02 (N = 29) | |
| Participants | Patients with active UC and a minimum MCS of 5, > 3 bowel movements daily compared with baseline, and endoscopic evidence of active disease | |
| Interventions | Group 1: LDP‐02 0.15 mg/kg SC (n = 5) Group 2: LDP‐02 0.15 mg/kg intravenously (IV) (n = 5) Group 3: LDP‐02 0.5 mg/kg IV (n = 5) Group 4: LDP‐02 2.0 mg/kg IV (n = 5) Group 5: placebo (n = 8) | |
| Outcomes | Primary outcome: meaningful endoscopic response (2 grade improvement) Secondary outcomes: endoscopic remission, clinical remission, adverse events | |
| Notes | Reported in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | The study states that 29 patients were evaluated, but endoscopic response was only reported for 28 patients in the results section |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Reported in abstract form only |
| Methods | Randomised, double‐blind, placebo‐controlled, 8 week induction trial involving 20 centres (N = 81) | |
| Participants | Patients with moderately active UC clinical activity index (CAI) 5‐9, with either stool frequency or rectal bleeding score > 1, and a modified Baron score of > 2, with disease minimum 25 cm from anal verge) | |
| Interventions | Group 1: MLN02 0.5 mg/kg (n = 58) Group 2: MLN02 2 mg/kg (n = 60) Group 3: placebo (n = 63) | |
| Outcomes | Primary outcome: Clinical remission at week 6 (defined as an UC clinical score of 0 or 1 and a modified Baron score of 0 or 1 with no evidence of rectal bleeding) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Neither the investigators nor the patients were aware of treatment assignment Placebo was identical to MLN02 |
| Blinding of outcome assessment (detection bias) | Low risk | The study was designed and implemented by the steering committee in collaboration with Millennium Pharmaceuticals, which analysed the data |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates were balanced across the groups with similar reasons for withdrawal (2%, 8% and 5% for the MLN02 0.5 mg/kg, MLN02 2.0 mg/kg and placebo groups, respectively) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, multicenter, phase III study (N = 281) | |
| Participants | Adult patients (> 18 years) with mild‐to‐moderate UC were eligible to participate if they had: disease extending at least 15 cm from the anal verge; and, mild‐to‐moderately active UC defined by a modified UCDAI score between 4‐10 with a sigmoidoscopy component score 2 and a rectal bleeding component score 1 | |
| Interventions | Group 1: mesalamine 4.8 g/day (n = 141) Group 2: placebo (n =140) Three tablets were given twice daily | |
| Outcomes | Primary outcome: clinical remission (UCDAI, stool frequency and bleeding scores of 0, and no fecal urgency) at week 6 Secondary outcomes: clinical remission at week 10, clinical remission at both weeks 6 and 10, endoscopic remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated in permutated blocks by a computer |
| Allocation concealment (selection bias) | Low risk | An interactive voice/web response system was used to manage the randomisation procedure and dispense study drug |
| Blinding of participants and personnel (performance bias) | Low risk | Study was double‐blind and patients received an identical placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Endoscopic images were reviewed by a single expert central reader who was blind to treatment assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | All of the efficacy outcomes were analysed according to the ITT principle 213 patients completed the study (84.3% in the mesalamine group and 67.4% in the placebo group) Adverse events were the most frequent cause of early withdrawal, and worsening of UC was the most common reason for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial with a 6 week induction (N = 374) and a 6 week open‐label phase (N = 521) followed by a 46 week maintenance phase (N = 373) | |
| Participants | Patients 18‐80 years with Mayo scores of > 6 and an endoscopic subscore of > 2 despite treatment with corticosteroids, purine antimetabolites and/or TNF‐α antagonists | |
| Interventions | Induction Cohort 1: IV vedolizumab 300 mg (n = 225) or placebo (n = 149) Cohort 2: open‐label IV vedolizumab 300 mg (n = 521) Maintenance IV vedolizumab 300 mg (n = 122) every 8 weeks, every 4 weeks (n = 125) or placebo (n = 126) | |
| Outcomes | Induction Primary outcome: clinical response at week 6 Maintenance Primary outcome: clinical remission at week 52 Secondary outcomes: durable clinical response at weeks 6 and 52, durable clinical remission at weeks 6 and 52, mucosal healing at week 52, and glucocorticoid‐free remission at week 52 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 3:2 ratio using computer‐generated randomisation schedules |
| Allocation concealment (selection bias) | Low risk | Centralised allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study; both the participant and physician were blinded to the treatment administered |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | The number of subjects who withdrew during the induction phase were 14 and 7 in the placebo and vedolizumab groups respectively Analyses were conducted according to the ITT principle |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicenter randomised double‐blind placebo‐controlled trial (N = 65) | |
| Participants | Patients > 18 years with UC who were in clinical and endoscopic remission Patients had a history of UC limited to rectum (15 cm) by previous endoscopic examination, evidence of clinical and endoscopic remission at entry Use of concomitant medication was prohibited during the trial | |
| Interventions | Group 1: 5‐ASA rectal suppository 0.5 g once daily (n = 31) Group 2: matched placebo (n = 34) Groups received treatment for 24 months | |
| Outcomes | Primary outcome: time to relapse (Relapse was defined as symptoms of rectal bleeding or increase in stool frequency for > 1 week and endoscopic evidence of inflammation on the individual DAI scales) Secondary outcomes: adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Not described beyond 'placebo identical to study medication' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, and single‐centre study (N = 123) | |
| Participants | Patients with moderate to severe, treatment refractory, active UC | |
| Interventions | Group 1: IV infliximab 3.5 mg/kg (n = 41) Group 2: IV infliximab 5 mg/kg (n = 41) Group 3: placebo (n = 41) Treatment administered at weeks 0, 2, and 6 and then every 8 weeks through week 22 Patients were followed up for 30 weeks | |
| Outcomes | Primary outcome: clinical response Secondary outcomes: clinical remission, mucosal healing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation performed |
| Allocation concealment (selection bias) | Low risk | Dynamic allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Randomised, phase III, double‐blind, double‐dummy, parallel‐group, placebo‐controlled, multicenter study (N = 343) | |
| Participants | Adult patients (> 18 years) with active, mild‐to‐moderate UC who had recently been diagnosed or relapsed During the screening period, patients could continue taking a stable dose of mesalamine (52.0 g/day), but mesalamine was withdrawn at baseline if the patient was eligible for inclusion | |
| Interventions | Group 1: MMX mesalamine 2.4 g/day (n = 86) Group 2: MMX mesalamine 4.8 g/day (n = 85) Group 3: Asacol 2.4 g/day (n = 86) Group 4: Placebo (n = 86) Treatment administered for 8 weeks All patients received 4 tablets and 2 capsules in the morning, 2 capsules at lunchtime, and 2 capsules in the evening | |
| Outcomes | Primary outcome: proportion of patients in clinical and endoscopic remission Secondary outcomes: clinical remission, clinical improvement, changes in modified UC‐DAI score, changes in sigmoidoscopic (mucosal) appearance (baseline to week 8), changes in rectal bleeding and stool frequency (from baseline to any study visit), treatment failure rate, and time to withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were randomised centrally via an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Asacol tablets contained 400 mg mesalamine and were enclosed in a capsule for blinding purposes Double‐dummy design: all patients received 4 tablets and 2 capsules in the morning, 2 capsules at lunchtime, and 2 capsules in the evening |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across intervention groups with similar reasons for withdrawal 52/86 patients in the placebo group, 70/86 patients in the MMX 2.4 g group, 72/85 patients in the MMX 4.8 g group, and 70/86 patients in the Asacol group completed the study All analyses were performed according to the ITT principle |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial (N = 24) | |
| Participants | Patients > 18 years of age with active steroid‐resistant UC (MCS: 6‐12 points, failure to respond to at least 2 weeks of 40 mg/day of prednisolone) | |
| Interventions | Patients received either an infusion of 1 g rituximab or placebo on day 1 and at 2 weeks | |
| Outcomes | Primary outcome: clinical remission at week 4 | |
| Notes | This drug was not shown to be an effective therapy for active steroid‐resistant UC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in a 2:1 (treatment:placebo) ratio in blocks of 5 by the hospital pharmacy department; the pharmacists had no other involvement in the trial |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from patients and investigators |
| Blinding of participants and personnel (performance bias) | Low risk | Allocation was not revealed until the last patient completed the trial |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment of response or remission was made before unblinding |
| Incomplete outcome data (attrition bias) | High risk | There was a high drop‐out rate in both groups 6/16 patients in the rituximab group and 2/8 patients in the placebo group completed the 12 week study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, multicenter clinical trial comparing rosiglitazone to placebo (N = 105) | |
| Participants | Adult patients with mild‐to‐moderately active UC (as defined by a modified Mayo score between 4‐10) Concomitant treatment with corticosteroids was permitted if the dose was stable for a minimum of 4 weeks prior to randomisation and did not exceed prednisone 20 mg/day, budesonide 9 mg/day, or equivalent | |
| Interventions | Group 1: rosiglitazone 4 mg (n = 52) Group 2: placebo (n = 53) Treatment taken orally twice daily for 12 weeks | |
| Outcomes | Primary outcome: clinical response (> 2 point decrease in the Mayo score) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted block randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by Data Coordinating Center at the University of Pennsylvania Each site was provided with a randomisation list and treatment packs; treatment packs were assigned sequentially at each site according to the list |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 20 patients in the placebo group and 10 patients in the treatment group dropped out before week 12 |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Phase III, multicenter, double‐blind, parallel‐group study in patients with mild‐to‐moderately active UC (N = 280) | |
| Participants | Patients > 18 years with newly diagnosed or relapsing (relapsed 6 weeks before baseline), mild‐to‐moderately active UC (UCDAI score of 4–10) with a sigmoidoscopy score > 1 and a PGA score > 2 with compatible histology | |
| Interventions | Placebo (n = 93), MMX mesalamine 2.4 g/day (n = 93) (1.2 g given twice daily), or MMX mesalamine 4.8 g/day (n = 94) given once daily (1:1:1) | |
| Outcomes | Primary outcome: clinical and endoscopic remission (defined as a modified UCDAI score of 1, with a score of 0 for rectal bleeding and stool frequency, no mucosal friability, and > 1 point reduction from baseline for sigmoidoscopic score) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised centrally via an interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | To ensure that the study was blinded, allocation of active drug and placebo was concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were highest in the placebo group, primarily due to lack of efficacy (41/93 in the placebo group, 17/93 in the 2.4 g/day group and 21/94 in the 4.8 g/day group) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled trial (N = 305) | |
| Participants | Adult patients > 18 years with UC in remission (defined as rectal bleeding = 0 and mucosal appearance < 2 using the revised Sutherland Disease Activity Index) | |
| Interventions | Mesalamine granules (Apriso) 1.5 g/day dosed once daily (n = 209) or placebo (n = 96) for 6 months | |
| Outcomes | Primary outcome: percentage of patients who were relapse free at 6 months Secondary outcomes: percentages of patients with a level of change from baseline in rectal bleeding score, mucosal appearance score, PGA and stool frequency at months 1, 3, and 6 and end of treatment; percentage of patients classified as treatment success, relapse‐free duration, and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned a unique treatment ID number via a randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | The study was double‐blind with a matched placebo |
| Blinding of outcome assessment (detection bias) | Low risk | The investigators, the subjects and the research staff (including project biostatisticians) were blinded to study medication assignment until after database lock at the end of the study |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, multinational, randomised, parallel‐group, placebo‐controlled study (N = 127) | |
| Participants | Adult patients > 18 years with previously diagnosed mild‐to‐moderate UC (UCDAI score 3‐8) | |
| Interventions | Group 1: oral mesalazine 4 g/day + mesalazine 1 g enema (n = 71) Group 2: oral mesalazine 4 g/day + placebo enema (n = 56) | |
| Outcomes | Primary outcome: remission rates at week 4 based on UCDAI score Secondary outcomes: remission rates at week 8, improvement rates at weeks 4 and 8, time to cessation of rectal bleeding, adverse events, laboratory tests at weeks 4 and 8 (serum creatinine, liver enzymes, platelets, white blood count, red blood count, and urinary tests for protein and haemoglobin) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across groups with similar reasons for withdrawal (58/71 patients in the mesalazine enema group and 40/56 patients in the placebo group completed week 8) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | 8‐week, phase II, double‐blind, placebo‐controlled, randomised, multi‐centre study (N = 109) | |
| Participants | Adult patients > 18 years with an active UC disease flare (defined as a MCS 6‐10 with a endoscopic subscore of > 2) | |
| Interventions | Group 1: IV BMS‐936557 10 mg/kg (n = 55) Group 2: placebo (n =54) Treatment administered at weeks 0, 2, 4 and 6 Oral 5‐ASA, prednisolone 20 mg/day, AZA and 6‐MP were continued at stable doses during the study. | |
| Outcomes | Primary outcome: rate of clinical response at day 57 Secondary outcomes: clinical remission and mucosal healing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally using dynamic treatment allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment assignment was blinded for personnel at the study sites and for patients; the study site pharmacist/designated nurse was unblinded for study drug preparation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, intra‐individual, dose escalating study (N = 17) | |
| Participants | Adult patients >18 years with moderately active UC (defined by a UCDAI score 6‐10, with a proctosigmoidoscopy score of 2) | |
| Interventions | Group 1: IFN‐βb‐1a SC injection 3 times a week at variable doses for a variable duration of treatment (n = 10) If improvement was observed after six injections at any dose, the patient entered a maintenance treatment phase of 6‐12 injections at that dose If no improvement after six injections or if remission occurred at any point, treatment was stopped | |
| Outcomes | Primary outcomes: response (decrease of at least 3 points from baseline in the UCDAI symptoms score and PGA (without the proctosigmoidoscopic score)); and remission (complete resolution of clinical symptoms (all clinical UCSS subscores = 0) with a proctosigmoidoscopy score of 0 or 1 at any time during treatment Secondary outcomes: overall treatment and endpoint responses, clinical endpoint responses, safety data | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer generated list and stratified by centre with block size of 3 (2:1 IFN‐β‐1a:placebo) |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by Corporate Biometrics Department of Serono International SA |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | One patient was excluded a priori due to mis‐allocation of study drug 6/10 (60%) of patients in the IFN‐β‐1a group and 2/7 (28.6%) of patients in the control group stopped treatment early |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Double‐blind, randomised, placebo‐controlled trial (N = 63) | |
| Participants | Adult patients > 18 years with refractory, moderate to severely active UC | |
| Interventions | Group 1: low trough concentration (5‐10 ng/ml) oral tacrolimus (n = 22) | |
| Outcomes | Primary outcome: proportion of patients with improvement (combination of partial and complete response) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Doses in the placebo group were pseudo‐adjusted to preserve study blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All 65 patients completed the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Double‐blind, randomised, placebo‐controlled, multicenter trial (N = 62) | |
| Participants | Hospitalised, adult patients with steroid‐refractory, moderate‐to‐severe UC | |
| Interventions | Group 1: oral tacrolimus (initial oral dose 1‐2.5 mg twice daily depending on patient’s weight. Blood was taken at 12 and at 24 hours to assess trough concentrations after initial dose, and subsequent doses were adjusted to maintain concentrations within target) (n = 32) Group 2: placebo (n = 30) | |
| Outcomes | Primary outcome: clinical response at 2 weeks (defined by an improvement in all DAI subscores and a reduction in total DAI score by at least 4 points) Secondary outcomes: mucosal healing and clinical remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation performed by the Control Center (Bellsystem24, a third‐party organization independent of study physicians and sponsor) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | To preserve blinding, blood trough levels were measured by SRL (a third‐party organization independent of study physicians and sponsor) and relayed to the Control Center (Bellsystem24) Dosages were calculated at the Control Center based on the trough levels |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind controlled trial (N = 67) | |
| Participants | Patients with chronic (steroid therapy at > 7.5 mg/day for at least 4 months of the proceeding year), active UC (Mayo clinic score of > 7 at entry) | |
| Interventions | Group 1: oral methotrexate 2.5 mg/wk ‐ 2.5 mg/day (n = 30) Group 2: identical placebo (n = 37) | |
| Outcomes | Primary outcome: clinical remission (MCS < 3 and steroid‐free) Secondary outcomes: time to first remission, clinical relapse (increase in the MCS > 3 and/or reintroduction of steroids at a dose of > 300 mg/month) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralised pharmacy randomisation Prepackaged coded sets (equal number of methotrexate or placebo tablets) were delivered to each centre |
| Blinding of participants and personnel (performance bias) | Low risk | The centralized pharmacist and an unblinded observer were the only individuals with access to the allocation code |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 2/30 patients in the methotrexate group dropped out; 9/37 patients in the placebo group dropped out ITT principle was used for analyses |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Double‐blind, randomised, placebo controlled trial (N = 43) | |
| Participants | Adult patients > 18 years with UC who had failed to respond to glucocorticoid treatment (at least 30 mg prednisolone a week, or equivalent) and were not in need of urgent colectomy At screening, all patients were required to have UCDAI > 6 and a sigmoidoscopy score > 2 on the Baron scale | |
| Interventions | Group 1: IV infliximab (5 mg/kg) at weeks 0 and 2 (n = 23) Group 2: placebo at weeks 0 and 2 (n = 20) | |
| Outcomes | Primary outcome: clinical remission (defined as UCCS < 2) at 6 weeks | |
| Notes | Author provided further verbal information on allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation performed by Schering‐Plough Author confirmed adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Pharmacists, investigators and participants were blinded to the treatment administered |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the 6 week study and all results reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, placebo‐controlled, double‐blind study (N = 390) | |
| Participants | Non‐hosptialized, adult patients with moderately to severely active UC (Mayo score > 6 points and endoscopic subscore > 2 points) despite treatment with corticosteroids and/or immunosuppressants | |
| Interventions | Group 1: adalimumab 160 mg at week 0, 80 mg at week 2, 40 mg at weeks 4 and 6 (n = 130) Group 2: adalimumab 80 mg at week 0, 40 mg at weeks 2, 4 and 6 (n = 130) Group 3: placebo (n = 130) | |
| Outcomes | Primary outcome: clinical remission (MCS < 2 with no individual subscore > 1) at week 8 Secondary outcomes: clinical response (> 3 point decrease in MCS and greater than or equal to 30% from baseline plus a decrease in rectal bleeding subscore > 1 or an absolute rectal bleeding subscore of 0 or 1), mucosal healing, adverse events | |
| Notes | The original study protocol described SC adalimumab 160 mg at week 0, 80 mg at week 2, 40 mg at weeks 4 and 6 or placebo The protocol was amended at the request of the European regulatory authorities | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation performed by the study sponsor |
| Blinding of participants and personnel (performance bias) | Low risk | Patients, study site personnel, study investigators, and the study sponsor were blinded to treatment assignment throughout the study; patients in the placebo group received the same number of injections as patients in the adalimumab treatment group(s) |
| Blinding of outcome assessment (detection bias) | Low risk | Study site personnel, and study investigators were blinded to treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | The study reports primary outcome data for the amended protocol group only Patients enrolled before the amendment were not included in the primary analysis data set |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, multi‐center placebo‐controlled study (N = 84) | |
| Participants | Male and female patients aged 18–65 with UC as confirmed by histopathology as well as active disease defined by a Mayo score ≥ 4 and < 10 with an endoscopic subscore of ≥2 points and fecal calprotectin ≥ 100 mg/kg | |
| Interventions | Group 1: IV Anrukinzumab 200 mg (n = 21) Group 2: IV Anrukinzumab 400 mg (n = 21) Group 3: IV Anrukinzumab 600 mg (n = 21) Group 4: Placebo (n = 21) | |
| Outcomes | Primary outcome: Fold change from baseline in fecal calprotectin at week 14 Secondary outcomes: endpoints included fold change from baseline in fecal calprotectin at weeks 2, 4, 8 and 12, pharmacokinetics, total IL‐13, antidrug and neutralising antibodies, as well as safety and tolerability of anrukinzumab | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were balanced across groups, with 10/21 patients in the placebo group, 13/21 patients in the 200 mg group, 15/21 patients in the 400 mg group and 7/21 patients in the 600 mg group completing treatment |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial (N = 510) | |
| Participants | Patients with mild‐to‐moderately active UC inadequately controlled with oral 5‐ASAs | |
| Interventions | Group 1: Budesonide MMX 9 mg Group 2: placebo Patients received treatment for 8 weeks in addition to their existing 5‐ASA medication | |
| Outcomes | Primary outcome: combined clinical and endoscopic remission at week 8 Secondary outcomes: clinical remission, endoscopic remission and histological healing | |
| Notes | Reported in abstract form only; not included in quantitative synthesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Two of the secondary outcomes (clinical remission and endoscopic remission) not reported on in abstract |
| Other bias | Unclear risk | Study reported in abstract form only |
| Methods | Randomised, double‐blind placebo controlled trial (N = 364) (ACT‐1) | |
| Participants | Adult ambulatory patients with moderately to severely active UC despite concurrent and stable treatment with oral corticosteroids and/or immunosuppressives were included Diagnosis of disease was confirmed by colonoscopy with biopsy | |
| Interventions | Group 1: 10 mg/kg infliximab (n = 122) Group 2: 5 mg/kg infliximab (n = 121) Group 3: placebo (n = 121) Patients received treatment at at weeks 0, 2, 6, 14, 22, 30, 38, and 46 | |
| Outcomes | Primary outcome: clinical response at week 8 Secondary outcomes: clinical response or remission with discontinuation of corticosteroids at week 30 in both studies and at week 54 in ACT‐1; clinical remission and mucosal healing at weeks 8 and 30 in both studies and at | |
| Notes | Author provided further information on method of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation with a dynamic treatment allocation stratified according to the investigational site and whether patients had corticosteroid refractory disease |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind placebo controlled trial (N = 364) (ACT‐2) | |
| Participants | Adult ambulatory patients with moderately to severely active UC despite concurrent and stable treatment with oral corticosteroids and/or immunosuppressives were included Diagnosis of disease was confirmed by colonoscopy with biopsy | |
| Interventions | Group 1: 10 mg/kg infliximab (n = 120) Group 2: 5 mg/kg infliximab (n = 121) Group 3: placebo (n = 123) Patients received treatment at weeks 0, 2, 6, 14, and 22 | |
| Outcomes | Primary outcome: clinical response at week 8 Secondary outcomes: clinical response or remission with discontinuation of corticosteroids at week 30 in both studies and at week 54 in ACT‐1; clinical remission and mucosal healing at weeks 8 and 30 in both studies and at week 54 in ACT‐1; and a clinical response at week 8 in patients with a history of disease refractory to corticosteroids | |
| Notes | Author provided further information on method of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation with a dynamic treatment allocation stratified according to the investigational site and whether patients had corticosteroid refractory disease |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, placebo‐controlled, double‐blind within‐cohort study (N = 48); single ascending dose stage (N = 25) | |
| Participants | Adult patients (18‐70 years) with a diagnosis of UC for > 12 weeks and a MCS of > 5 points at screening | |
| Interventions | In the single ascending dose, 5 groups of patients received etrolizumab or placebo: Group 1: IV etrolizumab 0.3 mg/kg (n = 4) or placebo Group 2: IV etrolizumab 1.0 mg/kg (n = 4) or placebo Group 3: IV etrolizumab 3.0 mg/kg (n = 4) or placebo Group 4: IV etrolizumab 10.0 mg/kg (n = 4) or placebo Group 5: SC etrolizumab 3.0 mg/kg (n = 4) or placebo Group 6: Placebo (n = 5) | |
| Outcomes | Primary outcomes: adverse events, serious adverse events, dose limiting toxicity, maximum tolerated dose | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation conducted by a biostatistician |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind with matched placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were similar across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
| Methods | Randomised, placebo‐controlled, double‐blind within‐cohort study (N = 48); multiple dose stage (N = 23) | |
| Participants | Adult patients (18‐70 years) with a diagnosis of UC for > 12 weeks and a MCS of > 5 points at screening | |
| Interventions | During the multiple dose stage 5 cohorts of patients received etrolizumab or placebo: Group 7: SC etrolizumab 0.5 mg/kg (n = 4) Group 8: SC etrolizumab 1.5 mg/kg (n = 5) Group 9: SC etrolizumab 3.0 mg/kg (n = 4) Group 10: IV etrolizumab 4.0 mg/kg (n = 5) placebo: placebo (n = 5) | |
| Outcomes | Primary outcomes: adverse events, serious adverse events, dose limiting toxicity, maximum tolerated dose | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by a biostatistician |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind with matched placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, integrated phase 2/3 dose‐finding/dose‐confirming study (N = 291) (PURSUIT‐IV) | |
| Participants | Patients had confirmed diagnoses of UC and moderate‐to‐severe disease activity (MCS 6–12, including an endoscopic subscore ≥2), and failed to tolerate or had an inadequate response to ≥1 conventional therapy, or were corticosteroid‐dependent (i.e. unable to taper corticosteroids without UC symptom recurrence) Patients who had previously received anti‐TNF‐α therapy were excluded | |
| Interventions | Group 1: golimumab 1 mg/kg (n = 62) Group 2: golimumab 2 mg/kg (n = 75) Group 3: golimumab 4 mg/kg (n = 77) Group 4: placebo (n = 77) | |
| Outcomes | Primary outcome: clinical response at week 6 Secondary outcomes: clinical remission, mucosal healing, MCS change, PMCS change, IBDQ change at week 6; CRP change at weeks 2 and 4; and adverse events | |
| Notes | See Sandborn 2014a and Sandborn 2014b for PURSUIT‐M and PURSUIT‐SC, respectively Following review of data from both SC and IV induction studies enrolment in the phase III portion of PURSUIT‐IV was stopped because efficacy was lower than expected; there were no safety concerns | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Conducted by a central randomisation centre |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Mucosal healing was defined by a Mayo endoscopy subscore of 0 or 1 as assessed by a local endoscopist Methods used to blind other outcome assessors were not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups (5, 3, 3 and 2 patients from the placebo, 1 mg/kg, 2 mg/kg and 4 mg/kg groups discontinued before week 6, respectively) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo controlled trial comparing cyclosporine to placebo for the treatment of mild‐to‐moderate, active, left‐sided UC (N = 40) | |
| Participants | Adult patients with active (diagnosed according to symptomatic, radiographic and endoscopic criteria) left‐sided disease receiving no concomitant therapy, oral steroids, oral salicylates or oral steroids combined with salicylates | |
| Interventions | Group 1: once daily enema with cyclosporine 350 mg (n = 20) Gruop 2: placebo enema (n = 20) | |
| Outcomes | Patients were evaluated 4 weeks after treatment Outcomes: clinical improvement, clinical remission, adverse events, histological disease activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified according to concomitant treatment (no treatment, oral steroids, oral salicylates or oral steroids and oral salicylates); the randomisation sequence was developed by the Section of Biostatistics |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | All patients were instructed to add 3.5 mL of blinded‐study medication to the enema |
| Blinding of outcome assessment (detection bias) | Unclear risk | Histological assessments were blinded Methods used to blind other outcome assessors were not described |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, dose‐escalation trial comparing repifermin (keratinocyte growth factor‐2) to placebo (N = 88) | |
| Participants | Adult patients 18 years or older with mildly to moderately active UC (MCS 3‐10) despite treatment with oral 5‐ASA, corticosteroids, AZA and/or 6‐MP | |
| Interventions | Group 1: placebo (n = 28) Group 2: repifermin 1 lg/kg (n = 11) Group 3: repifermin 5 lg/kg (n = 11) Group 4: repifermin 1 lg/kg (n = 12) Group 5: repifermin 25 lg/kg (n = 12) Group 6: repifermin 50 lg/kg (n = 14) | |
| Outcomes | Primary outcomes (safety): adverse events at each visit; laboratory abnormalities; and the frequency of anti‐repifermin antibodies at baseline and week 6 (and at month 6 in patients positive for antirepifermin antibody at week 6) Primary outcome (efficacy): clinical remission Secondary outcomes (efficacy): (i) clinical response (improvement in MCS > 3 points); (ii) clinical response (improvement in MCS > 2 points) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was generated by a statistician at Human Genome Sciences Inc. (Rockville, MD, USA) |
| Allocation concealment (selection bias) | Low risk | Sealed randomisation envelopes were provided by the study statistician and maintained in the pharmacy or a secure drug storage facility at each site; treatment allocation was available to the study pharmacist or nurse responsible for preparing the drug, but not to other study personnel |
| Blinding of participants and personnel (performance bias) | Low risk | Repifermin and placebo had a similar clear and colourless appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across treatment groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | ULTRA2 was a randomised, double‐blind, placebo‐controlled trial comparing adalimumab to placebo (N = 494) | |
| Participants | Non‐hospitalized, adult patients with moderate to severely active UC who received concomitant therapy with oral corticosteroids or immunosuppressants Patients were stratified based on prior exposure to TNF‐α antagonists | |
| Interventions | Group 1: SC adalimumab 160 mg at week 0, 80 mg at week 2, and then 40 mg every other week (n = 248) Group 2: placebo (n = 246) | |
| Outcomes | Primary outcomes: remission (MCS < 2 with no subscore > 1) at weeks 8 and 52 Secondary outcomes: clinical response, mucosal healing, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised, computer‐generated randomisation (stratified by prior anti‐TNF‐α exposure) |
| Allocation concealment (selection bias) | Unclear risk | Centralised, computer‐generated randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Matched placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Prospective, multicenter, double‐blind, double‐dummy, randomised, placebo‐controlled trial (N = 509) | |
| Participants | Adult patients (18‐75 years) with mild‐to‐moderate UC (defined by UCDAI ≥ 4 and ≤ 10) A ≥ 2‐day wash out period for oral mesalamine or other 5‐ASA product was required Patients were excluded if there was a history of oral or rectal corticosteroid, immunosuppressant or biologic use within the preceding 4 weeks, 8 weeks and 3 months, respectively | |
| Interventions | Participants were randomised to one of 4 groups: Group 1: Budesonide‐MMX 9 mg (n = 123) Group 2: Budesonide‐MMX 6 mg (n = 121) Group 3: placebo (n = 121) Group 4: Asacol 2.4g/day (mesalamine 800 mg 3 times daily) (n = 124) | |
| Outcomes | Primary outcome: combined clinical and endoscopic remission at 8 weeks Secondary outcomes: clinical improvement (≥3 point reduction in UCDAI), endoscopic improvement, symptom resolution, histologic healing, adverse events/potential glucocorticoid adverse effects | |
| Notes | A modified ITT analysis was used by the authors Details on the reasons for the use of the modified ITT analysis are available in the FDA Review document produced by Dr. Marjorie Dennis, available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203634_uceris_toc.cfm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in blocks |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally using an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | All patients were accounted for in the final analysis which was a modified ITT analysis 349/489 (71.4%) patients in the modified ITT group completed the study Proportions of patients who did not complete the study and reasons for discontinuation were similar across treatment groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled trial (N = 194) | |
| Participants | Adult patients > 18 years with a confirmed diagnosis of UC for > 3 months Patients were required to have a MCS between 6‐12 | |
| Interventions | Group 1: tofacitinib (CP‐690, 550) 0.5 mg (n = 31) Group 2: tofacitinib (CP‐690, 550) 3.0 mg (n = 33) Group 3: tofacitinib (CP‐690, 550) 10.0 mg (n = 49) Group 4: tofacitinib (CP‐690, 550) 15.0 mg (n = 48) Group 5: placebo (n = 48) Treatment administered twice daily for 8 weeks, and followed until week 12 | |
| Outcomes | Primary outcome: clinical response at 8 weeks Secondary outcomes: clinical remission at 8 weeks; endoscopic response at 8 weeks; endoscopic remission at 8 weeks; change from baseline in the PMCS at 2, 4, and 8 weeks; change from baseline in MCS at 8 weeks; change from baseline in the CRP concentration at 4 and 8 weeks; change from baseline in fecal calprotectin concentration at 2, 4, and 8 weeks; changes from baseline in low‐density lipoprotein and high‐density lipoprotein cholesterol concentrations and serum creatinine concentrations at 8 and 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally, according to a computer‐generated randomisation schedule, with the use of permuted blocks |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally, according to a computer‐generated randomisation schedule, with the use of permuted blocks |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled study (N = 490) | |
| Participants | Adult patients > 18 years with a confirmed diagnosis of UC for at least 3 months Patients had a MCS of 6‐12, and a current/previous inadequate response to (or did not tolerate): oral 5‐aminosalicylates for 6 weeks, prednisone 40 mg/day for 2 weeks or intravenous hydrocortisone 400 mg/day for 1 week Concurrent therapies, including stable doses of oral 5‐ASA, prednisolone (30 mg/day), budesonide (9 mg/day; Crohn's disease), AZA, 6‐MP, methotrexate (Crohn's disease), and antibiotics (Cron's disease) were permitted | |
| Interventions | Group 1: abatacept 30 mg/kg (n = 141) Group 2: abatacept 10 mg/kg (n = 139) Group 3: abatacept 3 mg/kg (n = 70) Group 4: placebo (n = 140) Patients were dosed at weeks 0, 2, 4, and 8 | |
| Outcomes | Primary outcome: response at week 12 Secondary outcomes: remission and mucosal healing at week 12 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally using dynamic treatment allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Colon biopsies were analyzed by a central pathologist in a blinded fashion Methods for blinding other outcome assessors were not described |
| Incomplete outcome data (attrition bias) | Low risk | Patients who discontinued were considered not to have a response/remission Discontinuation was balanced across groups with similar reasons for withdrawal (4/141 in the 30 mg/kg group; 6/139 in the 10 mg/kg group ; 2/70 in the 3 mg/kg group; 5/140 in the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Phase III, multi‐centre, randomised, double‐blind, placebo‐controlled trial (N = 265) | |
| Participants | Adult subjects with mild‐to‐moderately active (defined as baseline MMDAI between 5‐10 and a score > 2 for endoscopic and rectal bleeding subscore) ulcerative proctitis or ulcerative proctosigmoiditis | |
| Interventions | Patients were randomised 1:1 to receive rectally administered budesonide foam 2 mg/25 mL twice daily for 2 weeks followed by 2 mg/25 mL once daily for 4 weeks, or placebo | |
| Outcomes | Primary outcome: proportion of patients achieving remission at week 6 | |
| Notes | Reported in abstract form only Identical in design to BUCF3002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions rather than final counts reported in abstract |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | Abstract publication; insufficient detail provided |
| Methods | Phase III, multi‐centre, randomised, double‐blind, placebo‐controlled trial (N = 281) | |
| Participants | Adult subjects with mild‐to‐moderately active (defined as baseline MMDAI between 5‐10 and a score > 2 for endoscopic and rectal bleeding subscore ulcerative proctitis or ulcerative proctosigmoiditis | |
| Interventions | Patients were randomised 1:1 to receive budesonide foam 2 mg/25 mL twice daily for 2 weeks followed by 2 mg/25 mL once daily for 4 weeks, or placebo | |
| Outcomes | Primary outcome: proportion of patients achieving remission at week 6 | |
| Notes | Reported in abstract form only Identical in design to BUCF3001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Proportions rather than final counts reported in abstract |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Abstract publication; insufficient detail provided |
| Methods | Phase III, multicenter, placebo‐controlled, double‐blind, randomised‐withdrawal study (N = 464) | |
| Participants | Participants in Program of Ulcerative Colitis Research Studies Utilizing an Invetigational Treatment (PURSUIT)‐ M had completed 1 of 2 golimumab induction studies | |
| Interventions | Patients received the following every 4 weeks through week 52: Group 1: placebo (n = 156) | |
| Outcomes | Primary outcome: maintenance of clinical response through week 54 among golimumab‐induction responders (assessed by Mayo scores calculated at weeks 0, 30, and 54) Secondary outcomes: clinical remission at weeks 30 and 54; mucosal healing at weeks 30 and 54; clinical remission at weeks 30 and 54 among patients who had clinical remission at PURSUIT‐M baseline; and corticosteroid‐free clinical remission at week 54 among patients receiving concomitant corticosteroids at PURSUIT‐M baseline | |
| Notes | See Rutgeerts 2015 and Sandborn 2014b for PURSUIT IV and PURSUIT‐SC, respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment was performed using a central randomisation centre |
| Blinding of participants and personnel (performance bias) | Unclear risk | double‐blind; not adequately described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not adequately described |
| Incomplete outcome data (attrition bias) | Low risk | Patients with missing data for a dichotomous end point were considered failures For continuous outcomes the last observation in PURSUIT‐M was carried forward when data was missing |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | A phase II dose‐finding study and a phase III dose‐confirming study (multi‐centre) (PURSUIT‐SC) | |
| Participants | Patients had moderate‐to‐severe UC and had an inadequate response or failed to tolerate 1 or more of the following conventional therapies: oral 5‐ASA, oral corticosteroids, AZA, and 6‐MP | |
| Interventions | Phase II (N = 169) Group 1: SC golimumab 100/50 mg (n = 41) Group 2: SC golimumab 200/100 mg (n = 42) Group 3: SC golimumab 400/200 mg (n = 43) Group 4: placebo (n = 42) Phase III (N = 774) Group 1: SC golimumab 200/100 mg (n = 258) Group 2: SC golimumab 400/200 mg (n = 258) Group 3: placebo (n = 258) Patients received treatment at weeks 0 and 2 | |
| Outcomes | Primary outcome: clinical response at week 6 Secondary outcomes: clinical remission at week 6, mucosal healing, and IBDQ score change | |
| Notes | See Rutgeerts 2015 and Sandborn 2014a for PURSUIT‐IV and PURSUIT‐M, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation to treatment was performed using a central randomisation centre |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind; not described in detail |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described in detail |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Phase IIa randomised, double‐blind, placebo‐controlled,8‐week study (N = 252) | |
| Participants | Patients ≥ 18 years of age with moderately to severely active UC (confirmed by endoscopic evidence; MCS ≥ 6 and a Mayo endoscopic subscore ≥ 2 within the 2 weeks prior | |
| Interventions | Group 1: IV eldelumab 15 mg/kg (n = 84) Group 2: IV eldelumab 25 mg/kg (n = 85) Group 3: placebo (n = 83) Patients treated on days 1 and 8 and every other week thereafter | |
| Outcomes | Primary outcome: clinical remission (MCS ≤ 2; no individual subscale score > 1) at week 11 Secondary outcomes: MCS, clinical response and mucosal healing at week 11 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation numbers were assigned in the order in which patients qualified for treatment |
| Allocation concealment (selection bias) | Low risk | Sponsor‐owned central randomisation system allocated treatment |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment assignment was blinded for patients and study site personnel and maintained throughout the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Endoscopy subscores were determined by the local investigator who was |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled phase II trial (N = 149) | |
| Participants | Patients 18‐75 years with moderate to severe UC (extending beyond the rectum) despite treatment for at least 14 days with oral prednisone (40–50 mg/day) | |
| Interventions | Group 1: basiliximab 20 mg (n = 46) Group 2: basiliximab 40 mg (n = 52) Group 3: placebo (n = 51) | |
| Outcomes | Primary outcome: clinical remission at week 8 Secondary outcomes: clinical remission at week 4, clinical response at weeks 4 and 8, mucosal healing at weeks 4 and 8, clinical relapse after week 4 (for subjects in clinical remission at week 4), and concomitant corticosteroid use (median daily dose over time and cumulative dose) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using an interactive web response system |
| Blinding of participants and personnel (performance bias) | Low risk | All sponsor and study site personnel, including the endoscopist and pathologist, were blinded to subject treatment assignment Identically packaged placebo |
| Blinding of outcome assessment (detection bias) | Low risk | All sponsor and study site personnel, including the endoscopist and pathologist, were blinded to subject treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Phase III, randomised, prospective, double‐blind, placebo‐controlled study (N = 249) | |
| Participants | Patients with symptoms of acute UC, a baseline MMDAI 6‐10 (a subscale rating of ≥ 2 for both rectal bleeding and mucosal appearance of mild‐to‐moderate active UC) and disease extending at least 20 cm from the rectum Patients had not taken ≥ 6.75 g/day of balsalazide, or > 2.4 g/day of mesalamine or equivalent dose of a 5‐ASA product 14 days before receiving study medication | |
| Interventions | Group 1: balsalazide 1.1 g (administered as three tablets twice daily for 8 weeks) Group 2: matched placebo Patients were instructed to return unused study drug and used or partially used packaging at weeks 1, 2, 4, and 8 to determine compliance with therapy | |
| Outcomes | Primary outcome: proportion of patients achieving clinical improvement ( ≥ 3 point improvement in MMDAI) and improvement in rectal bleeding ( ≥ 1 point improvement) at 8 weeks Secondary outcomes: proportion of patients in clinical remission, proportion of patients with mucosal healing, proportion of patients with complete remission and mean change from baseline in MMDAI score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were randomised (2:1), using a centralized, automated, validated interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Both the investigator and patient were blinded to assigned treatment throughout the study All tablets were identical in appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled study (N = 186) | |
| Participants | Outpatients (male or female) 18–80 years of age with a clinical diagnosis of mild‐to‐moderately active UC involving the colon proximal to 15 cm above the anal verge and with a baseline UCDAI score of 4‐11 | |
| Interventions | Group 1: tetomilast 25 mg (n = 62) Group 2: tetomilast 50 mg (n = 62) Group 3: placebo (n = 62) | |
| Outcomes | Primary outcome: improvement at week 8 (defined as a reduction of 3 points in the total UCDAI score compared to baseline) Secondary outcomes: proportion of patients in remission (UCDAI score, 0–1), clinical improvement at week 4, change from baseline in total UCDAI score and UCDAI component scores, change from baseline in quality of life, proportion of patients with improvement in the Feagan Score, time to clinical improvement (number of days from randomisation to the first visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | With the exception of the programmer and project statistician performing the interim analyses, all persons involved in the conduct and management of the study were blinded to the individual patient treatment assignments until after the database was locked The blind was not broken for any patient during this study Matching placebo tablets |
| Blinding of outcome assessment (detection bias) | Low risk | With the exception of the programmer and project statistician performing the interim analyses, all persons involved in the conduct and management of the study were blinded to the individual patient treatment assignments until after the database was locked The blind was not broken for any patient during this study |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Placebo‐controlled, double‐blind, and randomised study (N = 87) | |
| Participants | Patients, age 15‐70 years, with mild‐to‐moderate UC (defined by symptomatic, radiographic, endoscopic criteria) Patients receiving corticosteroids or SASP were required to stop such therapy at least 1 week prior to start of study Pre‐entry evaluations included history, physical, blood count, chemistry screening, urinalysis, stool sample (had to be negative for ova, parasites, enteric pathogens) | |
| Interventions | Group 1: 4.8 g/day Asacol (400 mg of 5‐ASA, coated with pH‐sensitive polymer Eudragit‐S which dissolves at pH 7 or higher) (n = 38) Group 2: 1.6 g/day Asacol (400 mg of 5‐ASA, coated with pH‐sensitive polymer Eudragit‐S which dissolves at pH 7 or higher) (n = 11) Group 3: matched placebo (500 mg microcellulose with identical pH‐sensitive coating (n = 38) Patients received 12 tablets daily for 6 weeks | |
| Outcomes | Primary outcome: clinical response, described as 'complete', 'partial', or 'no response', was determined on the basis of stool frequency, amount of rectal bleeding, and physician's global assessment (which included sigmoidoscopic appearance) on 4‐point scales, compared to baseline data Secondary outcomes: complete response' indicated resolution of all symptoms, adverse events | |
| Notes | Early termination of treatment for any reason was deemed to constitute treatment failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was developed by the Section of Medical Research Statistics, Rochester Methodist Hospital |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation by pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | More placebo patients (n= 16) did not complete the study than 5‐ASA patients (n = 5) Placebo patients were more likely to drop out do to flare of UC or no improvement |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Multicenter, double‐blind, placebo‐controlled, computer‐randomised study (N = 158) | |
| Participants | Patients, age 18‐75 years, with mild‐to‐moderately active UC as diagnosed by symptomatic, radiographic, and endoscopic criteria Cases of both newly and previously diagnosed disease showing continued active signs, despite SASP therapy were included Steroid therapy had to be stopped at least one month before start of study SASP and topical rectal therapies were discontinued at least 1 week before start of study Concomitant use of other investigational drugs was not permitted | |
| Interventions | Group 1: 1.6 g/day oral mesalamine (Asacol) in 400 mg tablets coated with pH‐sensitive polymer (Eudragit‐S) (n = 53) Group 2: 2.4 g/day oral mesalamine (Asacol) in 400 mg tablets coated with pH‐sensitive polymer (Eudragit‐S) (n = 53) Group 3: placebo tablets (n = 52) | |
| Outcomes | Primary outcome: Clinical grading was based on stool frequency, rectal bleeding, sigmoidoscopic findings, and patient's functional assessment, each on 4‐point scale, which together gave the 'physician's global assessment', also on a 4‐point scale. The change in this clinical grade was indicated by classifying each patient as being 'in remission', 'improved', 'maintained', or 'worsened' Secondary outcomes: withdrawals and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | A 6‐week, randomised, double‐blind trial (N = 38) | |
| Participants | 43 patients were initially randomised; 5 patients were excluded due to protocol violations Patients were diagnosed with ulcerative proctosigmoiditis and had endoscopic evidence of inflammation occurring between 5‐60 cm from the anal verge | |
| Interventions | Nightly butyrate enema (n = 19) or placebo (saline) enema (n = 19) Maximum treatment duration was 6 weeks Concomitant oral medications were held constant Topical rectal therapies were discontinued | |
| Outcomes | Primary outcome: clinical improvement (a decrease in UCDAI > 2 or a score < 3 at week 6) Secondary outcomes: complete response (remission or complete response, as defined by a UCDAI score < 3), UCDAI score, endoscopic mucosal appearance, histological grade, adverse events, compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | The concentration, dose and frequency of the enemas were identical |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal 28/38 patients completed the 6 week study (14 placebo, 14 experimental) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Multicentre double‐blind randomised placebo‐controlled trial (N = 153) | |
| Participants | Patients with active ulcerative colitis extending no more than 50 cm from the anal verge | |
| Interventions | Group 1: 5‐ASA enema 4 g/day (n = 76) Group 2: placebo (n = 77) Patinets received treatment once daily for 6 weeks | |
| Outcomes | Primary outcome: clinical response Secondary outcomes: adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | The medication and placebo were identical in colour, consistency and packaging |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 6 patients dropped out of the 5‐ASA group for worsening disease or unsatisfactory response compared to 14 placebo patients |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | 6‐week, randomised, double‐blind placebo‐controlled design (N = 59) | |
| Participants | Patients were > 18 years who had UC involving 5‐50 cm of colon continuously from the anus, confirmed by sigmoidoscopy with biopsies taken from an area of active disease Patients had to have a minimum score of 3 on a 12‐point DAI | |
| Interventions | Group 1: 4 g 5‐ASA enema (60 mL) (n = 29) Group 2: placebo enema (n = 30) Patients were instructed to use one enema daily at bedtime | |
| Outcomes | Primary outcome: physician's global assessment of the patient at the end of the study period, mean DAI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind medication was prepackaged to ensure that an equal and random assignment within each centre occurred |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐outs balanced across groups with similar reasons for withdrawal There were 12 dropouts (five in the active and seven in the placebo group) during the study because of insufficient efficacy |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
| Methods | Double‐blind, placebo‐controlled, multicenter, parallel trial (N = 136) | |
| Participants | Adults > 18 years with ulcerative colitis extending at least 20 cm proximal to the anus Patients had to have a minimum score of 4 measured by DAI (four subgroups for each of bowel frequency, presence of blood, sigmoidoscopic appearance, and physician's assessment of severity for a maximum score of 12) | |
| Interventions | Group 1: Rowasa (250 mg tablets) taken as four tablets, four times per day, 4 g/day (n = 47) Group 2: Rowasa (250 mg tablets) taken as four tablets, four times per day, 2 g/day (n = 45) Group 3: Identical‐appearing placebo (n = 44) Treatment duration was 6 weeks | |
| Outcomes | Primary outcome: changes in the disease activity index and PGA The change in PGA was described as 'much or somewhat improved', 'unchanged', or 'somewhat worse or much worse' The change in the disease activity index score was evaluated in terms of end of study score minus 'baseline' Secondary outcome: adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: identical placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 34% drop‐out rate, however drop‐outs appear to be balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | 52‐week, phase 2/3, randomised, double‐blind, placebo‐controlled study (N = 274) | |
| Participants | Japanese patients > 15 years of age with biopsy‐confirmed, moderately to severely active UC (defined as MCS of 6–12 points and an endoscopy subscore of > 2) despite concurrent treatment with stable doses of oral corticosteroids | |
| Interventions | Group 1: adalimumab 80/40 mg (n = 87) Group 2: adalimumab 160/80 mg (n = 90) Group 3: placebo (n = 96) | |
| Outcomes | Primary outcomes: clinical response, clinical remission and mucosal healing at weeks 8, 32 and 52 Secondary outcomes: rectal bleeding subscore, physician global assessment, stool frequency indicative of mild disease; IBDQ response; response per partial MCS; rates of steroid‐free status and steroid‐free remission at week 32 and 52 in patients taking corticosteroids at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was based on a centrally designed randomisation table |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across treatment groups: Week 8: placebo 8/96; 80/40 mg 4/87; 160/80 8/90 |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial (N = 208) | |
| Participants | Patients with moderate‐to‐severely active UC | |
| Interventions | Group 1: 5 mg/kg infliximab (n = 104) Group 2: placebo (n = 104) Patients received treatment at weeks 0, 2 and 6 Patients with a lower MCS at week 8 than at baseline were further treated with infliximab at weeks 14 and 22 | |
| Outcomes | Primary outcome: clinical response Secondary outcomes: clinical remission, mucosal healing, serum infliximab levels, adverse events | |
| Notes | Reported in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described beyond 'double‐blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Reported in abstract form only |
| Methods | Prospective, multicenter, double‐blind, double‐dummy, randomised, placebo‐controlled trial (N = 410) | |
| Participants | Adult patients (18‐75 years) with mild‐to‐moderate UC as defined by UCDAI score of ≥ 4 and ≤ 10 | |
| Interventions | Budesonide‐MMX 9 mg (n = 127) Budesonide‐MMX 6 mg (n = 128) Placebo (n = 128) Entocort (budesonide controlled ileal release) 9 mg daily (n = 126) Placebo formulations were available for the Entocort® capsules and the Budesonide‐MMX® tablets | |
| Outcomes | Primary outcome: combined clinical and endoscopic remission at 8 weeks (UCDAI score ≤ 1, with subscores of zero for rectal bleeding and stool frequency, no mucosal friability at colonoscopy and a reduction of ≥ 1 point in the endoscopic index score) Secondary outcomes: clinical improvement (≥ 3 point reduction in UCDAI), endoscopic improvement, symptom resolution, histologic healing and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised in blocks of 4 to each of the treatment arms |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using an interactive voice response system |
| Blinding of participants and personnel (performance bias) | Low risk | Physicians, patients and outcome assessors were blinded to the treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | The proportions of patients who did not complete the study as well as reasons for study discontinuation were similar across different treatment groups. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | A randomised, double‐blind, placebo‐controlled, multi‐centre trial (N = 159) | |
| Participants | Patients (> 12 years of age) diagnosed with active UC (as defined as a Mayo score 5‐10 points, inclusive) for at least 4 months Concurrent medication permitted: 5‐ASA drugs, methylprednisolone, AZA, and 6‐MP Concurrent medication not permitted: methotrexate, cyclosporine, tacrolimus, antibiotics, and rectally administered corticosteroids | |
| Interventions | Daclizumab 1 mg/kg at weeks 0 and 4 (IV): (n = 56); Daclizumab 2 mg/kg at weeks 0, 2, 4, and 6 (IV): (n = 47) Placebo: (n = 56) | |
| Outcomes | Primary outcome: induction of remission at week 8 (remission defined as a MCS of 0 on the endoscopic and rectal bleeding subscores and a score of 0 or 1 on the stool frequency and physician's global assessment subscores) Secondary outcomes: response at week 8; clinical response at week 8; endoscopic response at week 8; and MCS and total histopathology disease severity scores at weeks 0 and 8 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Patients and investigative staff (except for the study pharmacist at each site) were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rates were high, but balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind placebo‐controlled study (N =80) | |
| Participants | Patients 18‐70 years with a histologically confirmed diagnosis of ulcerative colitis for at least 3 months prior to study entry Patients were required to have active UC (MCS > 6, endoscopic subscore > 2), despite being on stable doses of 5‐ASA or SASP for 3 weeks; orazathioprine or 6‐MP for 3 months, which were to be continued throughout the study; or oral steroids (up to 40 mg/day prednisolone or equivalent) for 2 weeks, which could be tapered at the investigator’s discretion | |
| Interventions | Single Dose Phase Group 1: IV PF‐00547,659 0.03 mg/kg (n = 4) Group 7: placebo (n = 6) Multiple Dose Phase Group 1: IV PF‐00547,659 0.1 mg/kg (n = 4) Group 6: placebo (n = 14) | |
| Outcomes | Primary outcome: safety and tolerability (adverse events, laboratory tests, and immunogenicity) Secondary outcomes: clinical/endoscopic response or remission rates, and biomarkers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted using a sequential numbering system based on the order of patient enrolment |
| Blinding of participants and personnel (performance bias) | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, phase II study comparing SC etrolizumab to matched placebo (N = 124) | |
| Participants | Adult patients (18‐75 years) with a diagnosis of UC for > 12 weeks and a MCS of > 5 points at screening (> 6 points at US sites) and a centrally read MCS score of > 2, a rectal bleeding subscore > 1, and disease extension > 25 cm from the anal verge | |
| Interventions | Group 1: etrolizumab 100 mg (n = 41) Patients received 100 mg at weeks 0, 4 and 8, with placebo administered at week 2 Group 2: etrolizumab 300 mg (n =40) Patients received a 420 mg loading dose at week 0, followed by 300 mg at weeks 2, 4 and 8 | |
| Outcomes | Primary outcome: clinical remission at week 10 | |
| Notes | 124 patients were randomly assigned to placebo (n = 43), etrolizumab 100 mg (n = 41) or etrolizumab 300 mg (n = 40) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted with an interactive voice and web response system |
| Blinding of participants and personnel (performance bias) | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients Both etrolizumab and placebo appeared as a transparent fluid within the syringes to maintain masking |
| Blinding of outcome assessment (detection bias) | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐outs were balanced across groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Phase III multicentre, randomised, double‐blind, placebo‐con‐trolled, parallel‐group study (N = 129) | |
| Participants | Patients 15‐74 years old with mild‐to‐moderate UC and rectal inflammation Additional inclusion criteria were rectal mucosal score of 2 or higher in the colonoscopic observation of the entire colon at the time of registration, UCDAI score of 4‐8, and disease status of first attack or relapsing/remitting pattern | |
| Interventions | Group 1: mesalazine 1 g (n = 65) Group 2: placebo suppository (n = 64) | |
| Outcomes | Primary outcome: endoscopic remission at week 4 Secondary outcomes: clinical remission rate after 4 weeks of treatment (percentage of patients with UCDAI scores of 2 or less and a bleeding score of 0), the change in the UCDAI score and the change in each item score and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to receive mesalazine or placebo suppositories at the start of study drug administration, according to a computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients dropped out of the mesalazine group; 10 patients dropped out of the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Single centre double‐blind placebo‐controlled trial (N = 27) | |
| Participants | Patients > 18 years with endoscopically confirmed UC extending < 15cm from the anal verge | |
| Interventions | Group 1: 0.5 g 5‐ASA suppository (n = 14) Group 2: placebo suppository (n = 13) Patients received treatment three times daily for 6 weeks | |
| Outcomes | Primary outcome: clinical remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Identical placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | There were 2 drop‐outs in the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, phase IIa study (N = 102) | |
| Participants | Patients were 20‐65 years of age with a diagnosis of moderately active UC (MCS 6‐10, a rectal bleeding subscore of 1 or higher, and an endoscopic subscore of 2 or higher) Patients had inadequately responded or had an intolerance to 5‐ASA and/or corticosteroids | |
| Interventions | Group 1: 960 mg AJM 300 (n = 51) Group 2: placebo (n = 51) Patients received treatment 3 times daily for 8 weeks | |
| Outcomes | Primary outcome: clinical response (decrease in MCS > 3 points and a decrease of > 30% from the baseline score, with a decrease > 1 point on the rectal bleeding subscore or an absolute rectal bleeding subscore of 0 or 1) Secondary outcomes: clinical remission (MCS of < 2 and no subscore > 1), mucosal healing (endoscopic subscore of 0 or 1), PMCS and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dynamic balancing allocation with minimization method |
| Allocation concealment (selection bias) | Low risk | Randomisation performed centrally |
| Blinding of participants and personnel (performance bias) | Low risk | Patients, assessing physicians, and the funder were blinded to the assignment of treatment throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | Patients, assessing physicians, and the funder were blinded to the assignment of treatment throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | 10 patients discontinued from the placebo group; 4 patients discontinued from the AMJ 300 group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
UC: ulcerative colitis
ASA: aminosalicylic acid
SC: subcutaneous
TNF‐α: Tumour necrosis factor‐alpha
AZA: azathioprine
6‐MP: 6‐mercaptopurine
CRP: C‐reactive protein
DAI: Disease Activity Index
UCDAI: Ulcerative Colitis Disease Activity Index
CAI: Clinical Activity Index
ITT: intention‐to‐treat
LDP‐02: vedolizumab ‐ a humanised a4b7 antibody
MCS: Mayo Clinic Score
MLN02: vedolizumab ‐ a humanised a4b7 antibody
IV: intraveneous
IBDQ: Inflammatory Bowel Disease Questionnaire
UCCS: Ulcerative Colitis Clinical Score
PGA: physician's global assessment
MMX: Multi Matrix System
BMS‐936557: anti‐IP‐10 antibody
IFN‐βb‐1a: interferon beta‐1a
ACT‐1: Active Ulcerative Colitis Trial 1
ACT‐2: Active Ulcerative Colitis Trial 2
PMCS: Partial Mayo Clinic Score
MMDAI: Modified Mayo Disease Activity Index
SASP: sulfasalazine
AJM 300: an oral alpha4 integrin antagonist
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| UCDAI not used | |
| UCDAI not used | |
| Not RCT | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Unclear scoring | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| UCDAI not used | |
| Not RCT | |
| UCDAI not used | |
| UCDAI not used | |
| Hospitalised patients | |
| UCDAI not used | |
| UCDAI not used | |
| Not RCT | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| No placebo arm | |
| Pooled analysis | |
| No placebo arm | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| No data reported | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Hospitalised patients | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| UCDAI not used | |
| Unable to obtain | |
| UCDAI not used | |
| UCDAI not used | |
| Unable to obtain | |
| Pooled analysis | |
| Unable to obtain | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Unable to obtain | |
| UCDAI not used | |
| No placebo arm | |
| Pooled analysis | |
| UCDAI not used | |
| No placebo arm | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| Unclear scoring | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| Pooled analysis | |
| Not RCT | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Drug not of interest | |
| UCDAI not used | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| Drug not of interest | |
| UCDAI not used | |
| UCDAI not used | |
| Not RCT | |
| Pooled analysis | |
| No placebo arm | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| Pooled analysis | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| Drug not of interest | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| UCDAI not used | |
| Pooled analysis | |
| UCDAI not used |
UCDAI: Ulcerative Colitis Disease Activity Index
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 131 patients with active, moderate‐to‐severe UC |
| Interventions | DIMS0150 Placebo |
| Outcomes | Primary: clinical remission Secondary: mucosal healing, symptomatic remission |
| Notes |
| Methods | Double‐blind, placebo‐controlled, first‐in‐human trial |
| Participants | 37 patients with active UC |
| Interventions | AVX‐470 Placebo |
| Outcomes | Primary: Adverse events Secondary: pharmacokinetics, immunogenicity Exploratory: clinical and endoscopic response and remission |
| Notes |
| Methods | Multicenter, randomised, double‐blind, phase IIa trial |
| Participants | 120 patients with mildly‐to‐moderate active UC |
| Interventions | K(D)PT Placebo |
| Outcomes | Primary: sustained clinical improvement Secondary: remission rates and clinical response |
| Notes |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial |
| Participants | 165 patients with active, mild to moderate distal UC |
| Interventions | Once‐daily budesonide 2 mg/25 ml foam Twice‐daily budesonide 2 mg/25 ml foam Placebo |
| Outcomes | Primary: complete mucosal healing, adverse events |
| Notes |
| Methods | Phase IIb, randomised, placebo‐controlled trial |
| Participants | 252 adults with UC |
| Interventions | Eldelumab 15 mg/kg Eldelumab 25 mg/kg Placebo |
| Outcomes | Primary endpoint was clinical remission (Mayo score ≤ 2; no individual subscale score > 1) at week 11 Key secondary endpoints included Mayo score clinical response and mucosal healing at week 11 |
| Notes |
| Methods | Double‐blind, placebo‐controlled phase II trial |
| Participants | 197 adults with moderate‐to‐severe UC |
| Interventions | Ozanimod 0.5 mg Ozanimod 1.0 mg Placebo |
| Outcomes | Primary: clinical remission Secondary: clinical response, change in Mayo Clinic Score, mucosal healing |
| Notes |
| Methods | Two identical phase III studies |
| Participants | Patients had moderately to severely active UC |
| Interventions | Tofacitinib Placebo |
| Outcomes | Primary: clinical remission Secondary: mucosal healing |
| Notes |
| Methods | Single or multiple ascending dose trial |
| Participants | 74 patients with UC |
| Interventions | GS‐5745 (0.3, 1.0, 2.5 or 5.0 mg/kg; 3 total IV infusions) GS‐5745 (150 mg; 5 weekly SC injections) Placebo |
| Outcomes | The primary outcomes were the safety, tolerability and pharmacokinetics of escalating single and multiple doses of GS‐5745 |
| Notes |
| Methods | Exploratory, 2‐centre (neoplastic lesions [NL] and BE), randomised, placebo‐controlled, observer‐blind phase IIa study |
| Participants | 18 patients aged 22–63 years with moderate‐to‐severe active UC |
| Interventions | Nanocort Placebo |
| Outcomes | Primary: adverse events Secondary: pharmacokinetics, efficacy |
| Notes |
UC: ulcerative colitis
DIMS0150: An experimental drug ‐ a toll‐like receptor 9 agonist
AVX‐470: An experimental drug ‐ an orally delivered tumour necrosis factor‐alpha antagonist
K(D)PT: An experimental drug ‐ a novel tripeptide
GS‐5745: An experimental drug ‐ an anti‐matrix metalloproteinase‐9 monoclonal antibody
IV: intravenous
SC: subcutaneous
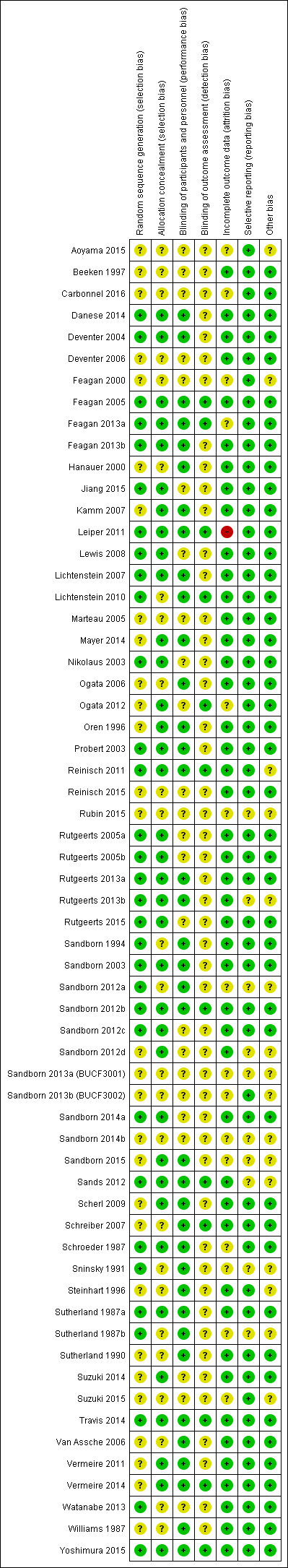
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
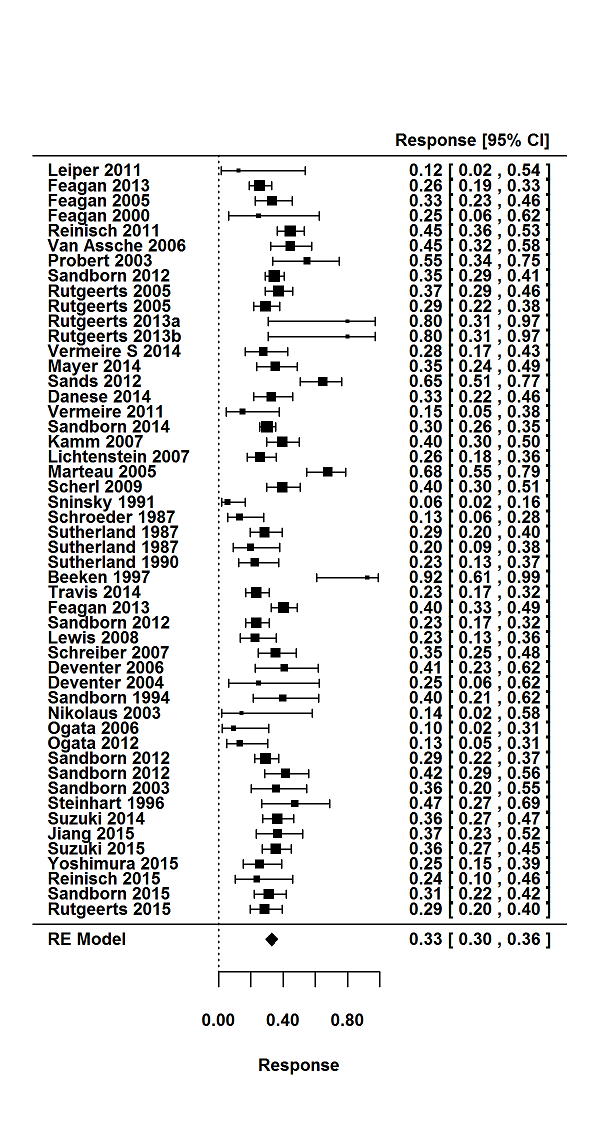
Response rates in induction phases.
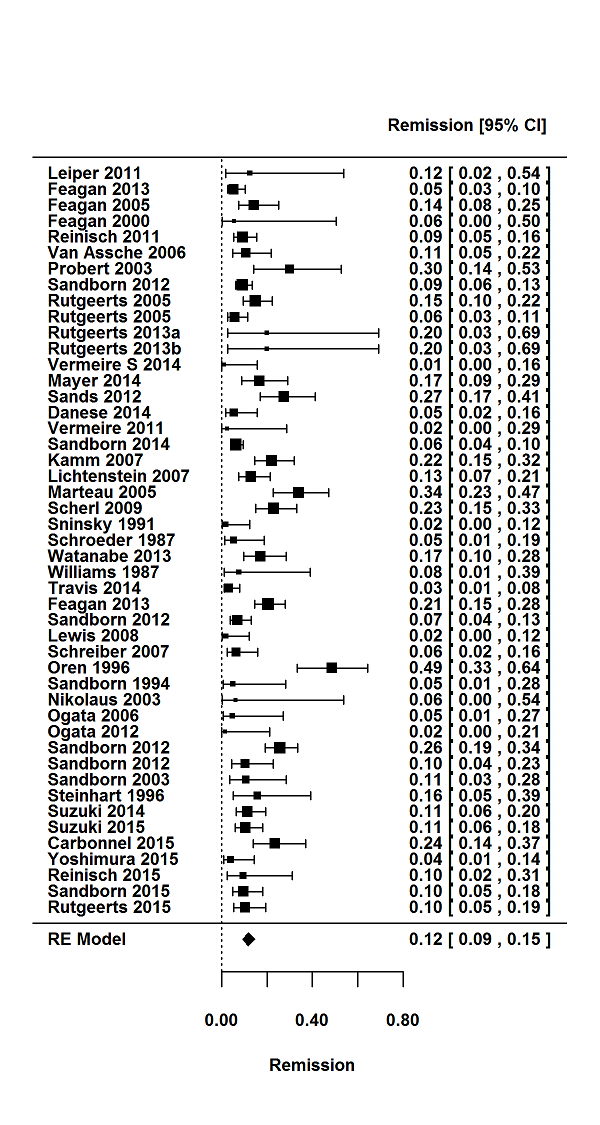
Remission rates in induction phases.
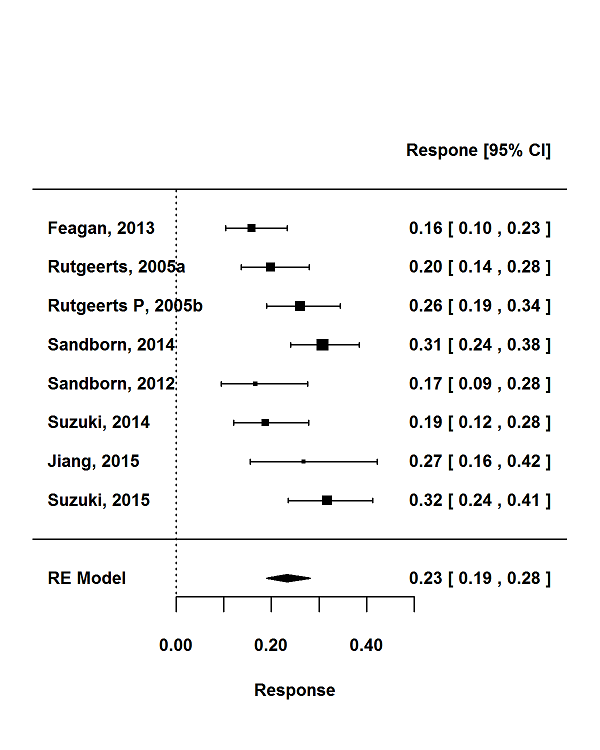
Response rates in maintenance phases.
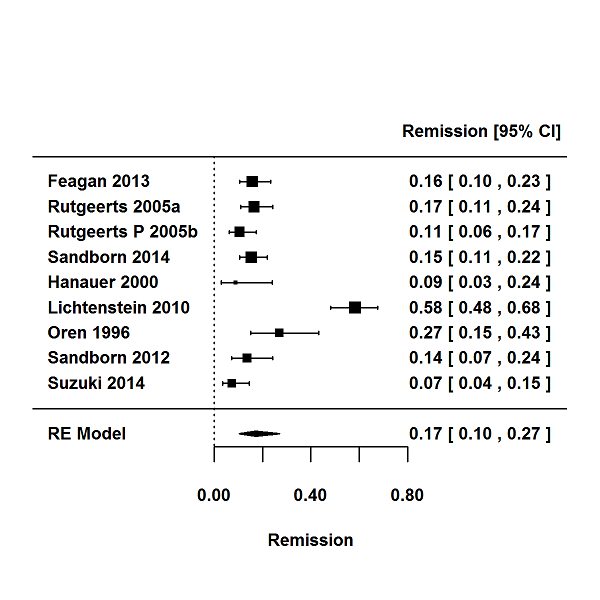
Remission rates in maintenance phases.
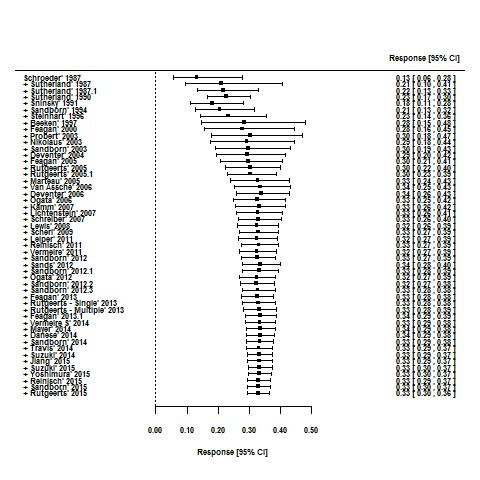
Cumulative placebo response rates 1987‐2015.
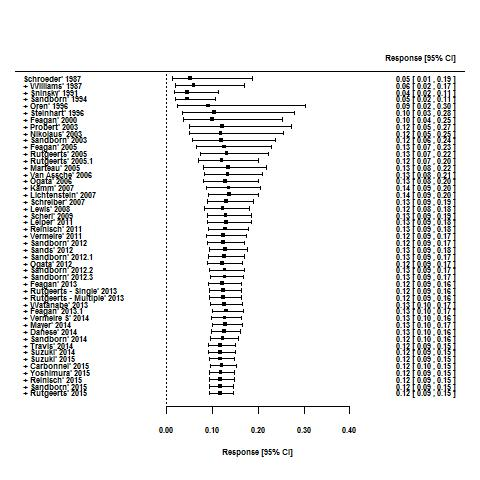
Cumulative placebo remission rates 1987‐2015.
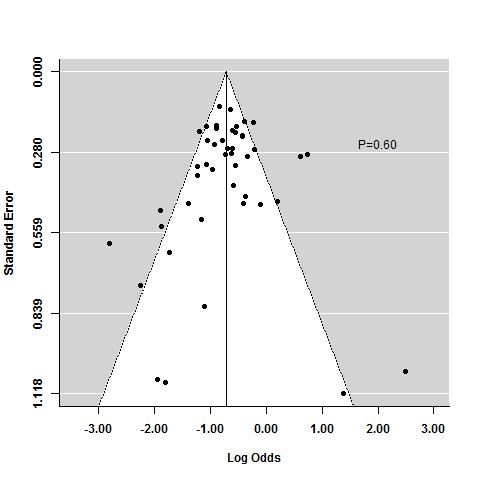
Funnel plot test for asymmetry: response
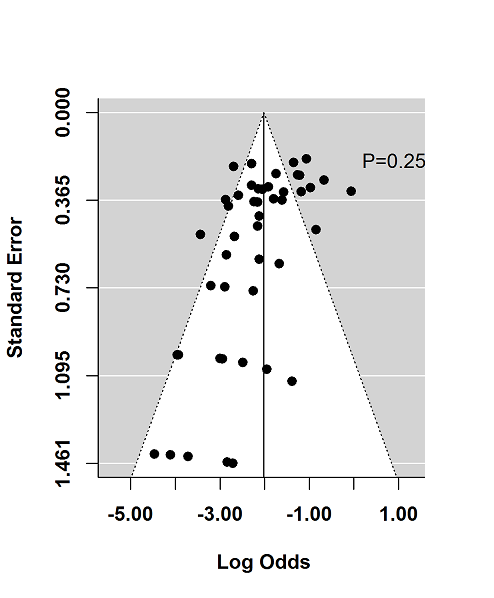
Funnel plot test for asymmetry: remission
| Traditional design features | Novel design features | Other quality measures | |
| Increase in placebo response | Follow up > 12 months | Use of PROs | |
| Decrease in placebo response | Using treatment naive patients | Induction phases to identify drug non‐responders Adaptive group allocation Stepped wedge trial | Using biomarkers instead of PROs Enrolling patients with more severe disease Controlling for centre effects |
| Table constructed from information presented in Enck 2013. PRO: patient reported outcome | |||
| Increase in placebo response and remission rate | Longer study duration |
| Decrease in placebo response and remission rate | Defining response as UCDAI ≥ 3 |
| Table constructed from information presented in Su 2007 UCDAI: Ulcerative Colitis Disease Activity Index | |
| Trial | Phase | Setting (number of centres) | Comparator | Placebo patients | Mean age | Follow‐up (weeks) | Mean entry UCDAI score | Response definition | Remission definition | |
| 1 | induction (1) | Multicenter, single country (NS) | Budesonide foam | NS | NS | 6 | NS | NS | RBS = 0, endoscopic sub score < 1 and stool frequency sub score = 0 or decrease > 1 | |
| 2 | induction (2) | Multicenter, multinational (4) | Aminosalicylate | 13 | 48 | 6 | 7.8 | Mean/median score improvement | NS | |
| 3 | induction (3) | Multicenter, multinational (26) | Methotrexate | 51 | NS | 24 | NS | NS | Mayo Clinic subscore < 2 with no item >1 | |
| 4 | induction (4) | Multicenter, multinational (30) | Tralokinumab | 55 | 41 | 24 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| 5 | induction (5) | Multicenter, multinational (30) | Alicaforsen | 22 | 50 | 6 | 6.5 | Decrease in RBS of 0‐1or more from baseline | NS | |
| 6 | induction (6) | Multicenter (NS) | Alicaforsen | 8 | 4 | 7.5 | Percent reduction in DAI | NS | ||
| 7 | induction (7) | Multicenter, single country (NS) | Vedolizumab | 8 | NS | 4 | 8 | Improvement in Baron ≥ 2 points | Mayo 0; Modified Baron 0 | |
| 8 | induction (8) | Multicenter, single country (20) | Vedolizumab | 63 | 38.9 | 6 | 6.7 | Improvement in UCCS ≥ 3 points | UCCS ≤ 1 and a modified Baron ≤ 1 | |
| 9 | induction (9) | Multicenter, multinational (26) | Mesalamine | 141 | 40.4 | 10 | NS | UCDAI decrease by ≥3 points | UCDAI, SFS and RBS scores of 0, and no fecal urgency | |
| 10 | induction (10) | Multicenter, multinational (211) | Vedolizumab | 149 | 41.2 | 6 | 8.6 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤ 2 points; no individual sub score > 1 point | |
| maintenance (1) | Multicenter, multinational (211) | Vedolizumab | 126 | 40.3 | 52 | 8.4 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤ 2 points; no individual sub score > 1 point | ||
| 11 | maintenance (2) | Multicenter (9) | Mesalamine | 34 | 37.3 | 96 | NS | NS | UCDAI score = 0 was the definition of clinical and endoscopic remission Relapse defined as symptoms of rectal bleeding or increase in stool frequency for > 1 wk and endoscopic evidence of inflammation | |
| 12 | induction (11) | Single centre | Infliximab | 41 | 34.5 | 8 | NS | Decrease in total MCS > 3 points or > 30% from baseline, with a decrease in RBS > 1 point or an absolute RBS of 0 or 1 | Total Mayo score = 2 points with no individual sub score > 1 point | |
| maintenance (3) | Single centre | Infliximab | 41 | 34.5 | 30 | NS | Decrease in total MCS > 3 points or > 30% from baseline, with a decrease in RBS > 1 point or an absolute RBS of 0 or 1 | Total Mayo score of < 2 points with no individual sub score > 1 point | ||
| 13 | induction (12) | Multicenter. multinational (49) | MMX mesalamine | 86 | 43.2 | 8 | NS | UCDAI decrease by ≥3 points | UCDAI ≤1+ RBS=0 + SFS=0 ; and ≥1 point reduction in sigmoidoscopy score | |
| 14 | induction (13) | Single country (1) | Rituximab | 8 | 50 | 24 | 7.6 | Decrease in Mayo ≥ 3 points | Decrease in Mayo to ≤ 2 | |
| 15 | induction (14) | Multicenter, single country (15) | Rosiglitazone | 53 | 12 | NS | Decrease in Mayo ≥2 points | Mayo score ≤ 2 | ||
| 16 | induction (15) | Multicenter, multinational (52) | MMX mesalamine | 93 | 42.6 | 8 | NS | UCDAI decrease by ≥3 points | UCDAI ≤1+ RBS=0 + SFS=0 ; and ≥1 point reduction in sigmoidoscopy score | |
| 17 | maintenance (4) | Multicenter, multinational (48) | Mesalamine | 96 | 46 | 24 | NS | NS | Relapse free at 6 months | |
| 18 | induction (16) | Multicenter, multinational (43) | Mesalazine enema | 56 | NS | 8 | NS | UCDAI decrease by ≥2 points | UCDAI ≤1 | |
| 19 | induction (17) | Multicenter, multinational (54) | BMS‐936557 | 54 | 41.8 | 8 | 7.9 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| 20 | induction (18) | Multicenter, multinational (6) | rIFN‐β‐1a | 7 | 6 | NS | Reduction of ≥3 points in the UCSS symptoms score and PGA | All clinical UCSS sub scores equal to 0, with a proctosigmoidoscopy score of 0 or 1 | ||
| 21 | induction (19) | Multicenter, single country (17) | Tacrolimus | 21 | 30 | 2 | 9.4 | Reduction in DAI of more than 4 points with improvement of all categories | Complete resolution of all symptoms (all assessment scores were zero) | |
| 22 | induction (20) | Multicenter, single country (NS) | Tacrolimus | 30 | NS | 2 | 9.1 | Reduction in DAI of more than 4 points with improvement of all categories | Total DAI score 2 with all individual sub scores of 0 or 1 | |
| 23 | induction (21) | Multicenter, single country (12) | Methotrexate | 37 | 38.9 | 36 | 6.8 | NS | MCS (including the endoscopic sub score) of < 3 with no steroid use, and without a score of < 2 without sigmoidoscopy results | |
| maintenance (5) | Multicenter, single country (12) | Methotrexate | 37 | 38.9 | 36 | 6.8 | NS | Relapse was an increase in the MCS of > 3 (not including sigmoidoscopy) and/or reintroduction of steroids at a dose of > 300 mg/month | ||
| 25 | induction (22) | Multicenter, multinational (4) | Infliximab | 20 | NS | 6 | 8.5 | Decrease in Baron of ≥ 1 | UCCS ≤ 2 AND/OR Baron score = 0 | |
| 25 | induction (23) | Multicenter, multinational (94) | Adalimumab | 130 | NS | 8 | 8.7 | Decrease in Mayo > 3 points and decrease in the RBS >1/absolute RBS of 0 or 1 | Mayo score < 2 with no individual sub score > 1 | |
| 26 | induction (24) | Multicenter, multinational (38) | Anrukinzumab | 21 | 36.6 | 32 | 6.6 | Decrease from baseline of ≥3 points in total Mayo score, with at least a 30% change, accompanied by ≥1 point decrease or absolute score of 0 or 1 in RBS | Defined as proportion of subjects with a total Mayo score ≤ 2, with no individual sub score > 1 | |
| 27 | induction (25) | NS | Budesonide MMX® | NS | NS | NS | rectal bleeding and stool frequency sub scores = 0 | |||
| 28 | induction (26) | Multicenter, multinational (62) | Infliximab | 121 | 41.4 | 8 | 8.4 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| maintenance (6) | ||||||||||
| 29 | induction (27) | Multicenter, multinational (55) | Infliximab | 123 | 39.3 | 8 | 8.5 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| maintenance (7) | ||||||||||
| 30 | induction (28) | Multicenter, multinational (15) | Etrolizumab | 5 | 30.2 | 4 | 9 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| 31 | induction (29) | Multicenter, multinational (15) | Etrolizumab | 5 | 39 | 5 | 10 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| 32 | induction (30) | |||||||||
| 33 | induction (31) | Single centre | Cyclosporin | 20 | 4 | NS | Reduction of ≥3 points in DAI | UCDAI=0 | ||
| 34 | induction (32) | Multicenter, single country (15) | Repifermin | 28 | NS | 6 | Decrease in Mayo ≥3 points compared with baseline at week 4 | A score of zero on the sigmoidoscopy all sub scores = 0 (SFS, PGA, RBS) | ||
| 35 | induction (33) | Multicenter, multinational (103) | Adalimumab | 260 | 41.3 | 8 | 8.9 | Decrease in Mayo ≥ 3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point | |
| maintenance (8) | ||||||||||
| 36 | induction (34) | Multicenter, multinational (108) | Budesonide MMX | 128 | 8 | NS | ≥3‐point decrease in UCDAI, and ≥1‐point reduction in the endoscopy sub score | UCDAI ≤1+ RBS=0 + SFS=0; no mucosal on colonoscopy ; and ≥1 point reduction in sigmoidoscopy score | ||
| 37 | induction (35) | Multicenter, multinational (51) | Tofacitinib | 48 | 42.5 | 8 | 8.2 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | MCS = 2 with no individual sub score> 1 | |
| 38 | induction (36) | Multicenter, multinational (142) | Abatacept | 140 | 40.9 | 12 | 8.8 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | MCS = 2 with no individual sub score> 1 | |
| maintenance (9) | Multicenter, multinational (142) | Abatacept | 66 | NS | 52 | NS | NS | NS | ||
| 39 | induction (37) | Multicenter, multinational (NS) | Budesonide Foam | NS | NS | 7.9 | NS | Endoscopy score ≤ 1, RBS = 0 and improvement or no change from baseline in stool frequency subscales of MMDAI** | ||
| 40 | induction (38) | Multicenter, multinational (NS) | Budesonide Foam | NS | NS | NS | 8 | NS | Endoscopy score ≤ 1, RBS = 0 and improvement or no change from baseline in stool frequency subscales of MMDAI | |
| 41 | maintenance (10) | Multicenter, multinational (217) | Golimumab | 331 | 39 | 8 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point | |
| 42 | induction (39) | Multicenter, multinational (251) | Golimumab | 156 | 40.2 | 54 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point | |
| 43 | induction (40) | Multicenter, multinational (75) | Eldelumab | 83 | 42.7 | 11 | 8.6 | Mayo score < 2 points | Reduction | |
| 44 | induction (41) | Multicenter, multinational (46) | Basiliximab | 51 | 38 | 8 | NS | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point | |
| 45 | induction (42) | Multicenter, single country (55) | Balsalazide | 83 | 45.4 | 8 | 8 | ≥3 point improvement in modified Mayo, ≥1 point improvement in RBS | 0 for RBS and combined score of ≤2 for SFS and PGA using the Modified Mayo subscales | |
| 46 | induction (43) | Multicenter, single country (35) | Tetomilast | 62 | 45.5 | 8 | 7.5 | Reduction of ≥3 points in DAI | UCDAI ≤1 | |
| 47 | induction (44) | Single center | Mesalamine | 38 | 42.7 | 6 | NS | 'substantial' improvement in scores | Complete resolution of symptoms (total score 0) | |
| 48 | induction (45) | Multicenter, single country (9) | Mesalamine | 52 | 39.2 | 6 | NS | Reduction in the PGA score and in at least one other component score | Complete resolution of all symptoms with all assessment scores 0 | |
| 49 | induction (46) | Multicenter, single country (2) | Butyrate | 19 | 38.6 | 6 | 7.8 | Reduction of ≥2 points in UCDAI | UCDAI ≤1 | |
| 50 | induction (47) | Multicenter, multinational (8) | Aminosalicylate | 77 | 36 | 6 | NS | PGA, % drop in DAI from baseline (total and subscores) | NS§ | |
| 51 | induction (48) | Multicenter, single country (2) | Aminosalicylate | 30 | 36 | 6 | NS | PGA, mean DAI | NS | |
| 52 | induction (49) | Multicenter, multinational (7) | Aminosalicylate | 44 | 37.8 | 6 | 8.2 | PGA, mean DAI | NS | |
| 53 | maintenance (11) | Multicenter, single country (65) | Adalimumab | 96 | 41.3 | 52 | 8.5 | Decrease of > 3 points and > 30 % from | Full Mayo | |
| 54 | induction (51) | Multicenter, single country (NS) | Infliximab | 104 | NS | 8 | NS | NS | NS | |
| maintenance (12) | Multicenter, single country (NS) | Infliximab | 104 | NS | 30 | NS | NS | NS | ||
| 55 | induction (52) | Multicenter, multinational (69) | Budesonide MMX | 128 | 39.9 | 8 | 6.2 | ≥3‐point decrease in UCDAI, and ≥1‐point reduction in the endoscopy subscore | UCDAI ≤1+ RBS=0 + SFS=0; no mucosal on colonoscopy; and ≥1 point reduction in sigmoidoscopy score | |
| 56 | induction (53) | Multicenter, multinational (40) | Daclizumab | 56 | 40.7 | 20 | 8 | Decrease in Mayo ≥ 3 points | Mayo 0 for endoscopy and RBS; Mayo 0/1 for SFS† and PGA‡ | |
| 57 | induction (54) | Multicenter, multinational (17) | PF‐00547,659 | 20 | 47.9 | 4 | 7.5 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point | |
| 58 | induction (55) | Multicenter. Multinational (40) | Etrolizumab | 43 | 37.5 | 10 | 9.1 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point | |
| 59 | induction (56) | Multicenter, single country (45) | Aminosalicylate | 64 | 41.3 | 4 | 5.5 | NS | Rectal mucosal score of 0 or 1 | |
| 60 | induction (57) | Multicenter, single country (2) | NS | 13 | 42.7 | 6 | 7.4 | NS | DAI score of 0 | |
| 61 | induction (58) | Multicenter, single country (42) | AJM300 | 51 | 42.6 | 8 | 7.7 | Decrease in MCS of at least 3 points and | MCS of 2 or lower and no subscore higher than 1 | |
| NS: not stated RBS: rectal bleeding score DAI: Disease Activity Index UCCS: Ulcerative Colitis Clinical Score UCDAI: Ulcerative Colitis Disease Activity Index SFS: stool frequency score PGA: physician's global assessment | ||||||||||
| Response | Remission | |||||||
| Trials | Pooled rate % (95% CI) | I2 % | 12 P value | Trials | Pooled rate % (95% CI) | I2 % | I2 P value | |
| All trials | 50 | 33 (30‐36) | 73 | < 0.001 | 47 | 12 (9‐15) | 75 | < 0.001 |
| Trial setting | ||||||||
| Multi‐centre, single‐country | 14 | 29 (23‐35) | 64 | 0.003 | 16 | 11 (7‐17) | 75 | < 0.001 |
| Multi‐centre, multi‐national | 31 | 35 (31‐40) | 78 | < 0.001 | 27 | 12 (10‐16) | 79 | < 0.001 |
| Single‐centre | 4 | 26 (14‐44) | 62 | 0.06 | 3 | 6(2‐16) | 0 | 0.74 |
| Design | ||||||||
| Stand‐alone induction | 38 | 34 (29‐39) | 76 | < 0.001 | 35 | 11 (9‐14) | 68 | < 0.001 |
| Induction and maintenance | 12 | 32 (29‐35) | 28 | 0.04 | 12 | 13 (8‐20) | 87 | < 0.001 |
| First author country | ||||||||
| North America | 26 | 32 (27‐36) | 73 | < 0.001 | 23 | 11 (9‐15) | 72 | < 0.001 |
| Europe | 18 | 37 (30‐44) | 73 | < 0.001 | 17 | 12 (8‐18) | 74 | < 0.001 |
| Other | 6 | 29 (22‐38) | 55 | < 0.05 | 7 | 12 (5‐25) | 86 | < 0.001 |
| Drug class | ||||||||
| Corticosteroid | 2 | 23 (19‐29) | 0 | 1.0 | 2 | 5 (2‐11) | 48 | < 0.17 |
| Amicosalicylate | 11 | 32 (20‐47) | 92 | < 0.001 | 9 | 18 (12‐24) | 67 | < 0.005 |
| Immunosuppressant | 3 | 19 (7‐43) | 68 | 0.04 | 5 | 13 (3‐38) | 86 | < 0.001 |
| Biological | 29 | 35 (31‐38) | 52 | < 0.001 | 28 | 11 (9‐14) | 61 | < 0.001 |
| Other | 5 | 34 (25‐44) | 29 | 0.26 | 3 | 7 (3‐18) | 47 | 0.14 |
| Route of administration | ||||||||
| Topical | 7 | 39 (27‐53) | 73 | < 0.001 | 5 | 18 (9‐31) | 59 | 0.04 |
| Oral | 17 | 28 (22‐34) | 77 | < 0.001 | 16 | 10 (6‐17) | 88 | < 0.001 |
| Intravenous | 17 | 35 (30‐41) | 63 | < 0.001 | 17 | 13 (10‐17) | 57 | 0.003 |
| Subcutaneous | 8 | 35 (30‐40) | 42 | 0.05 | 8 | 8 (7‐10) | 4 | 0.44 |
| Disease severity on entry | ||||||||
| Mild‐moderate | 21 | 32 (25‐39) | 80 | < 0.001 | 18 | 12 (8‐17) | 77 | < 0.001 |
| Moderate‐severe | 29 | 34 (30‐38) | 59 | < 0.001 | 29 | 12 (9‐15) | 75 | < 0.001 |
| Disease duration on entry | ||||||||
| < 5 years | 5 | 47 (37‐57) | 53 | 0.06 | 9 | 21 (17‐25) | 0.0 | 0.4 |
| > 5 years | 29 | 33 (28‐38) | 81 | < 0.001 | 28 | 11 (8‐15) | 82 | < 0.001 |
| Inclusion criteria | ||||||||
| Minimum total score > 6 | 21 | 34 (30‐39) | 67 | < 0.001 | 21 | 12 (9‐17) | 83 | < 0.001 |
| Minimum total score < 6 | 24 | 34 (29‐40) | 69 | < 0.001 | 21 | 13 (9‐17) | 70 | < 0.001 |
| Endoscopy subscore for inclusion | ||||||||
| > 2 | 27 | 34 (30‐37) | 59 | < 0.001 | 27 | 12 (9‐15) | 71 | < 0.001 |
| < 2 | 4 | 46 (31‐61) | 79 | 0.002 | 4 | 25 (11‐48) | 90 | < 0.001 |
| Not stated | 17 | 29 (21‐39) | 79 | < 0.001 | 14 | 8 (5‐13) | 49 | 0.015 |
| Bleeding score for inclusion | ||||||||
| Yes | 9 | 37 (29‐45) | 77 | < 0.001 | 9 | 16 (10‐23) | 79 | < 0.001 |
| No/not stated | 41 | 32 (28‐36) | 70 | < 0.001 | 38 | 11 (8‐14) | 73 | < 0.001 |
| Duration of follow‐up visits | ||||||||
| < 8 weeks | 37 | 33 (29‐34) | 81 | < 0.001 | 32 | 11 (9‐14) | 71 | < 0.001 |
| > 8 weeks | 9 | 32 (27‐37) | 42 | < 0.001 | 11 | 14 (8‐23) | 85 | < 0.001 |
| Number of follow up visits | ||||||||
| < 3 | 16 | 32 (23‐44) | 81 | < 0.001 | 13 | 11 (7‐19) | 63 | 0.001 |
| > 3 | 24 | 34 (30‐38) | 69 | < 0.001 | 24 | 12 (9‐16) | 84 | < 0.001 |
| Publication date | ||||||||
| Before (and including) 2007 | 23 | 33 (26‐40) | 78 | < 0.001 | 19 | 13 (9‐19) | 75 | < 0.001 |
| After 2008 | 27 | 33 (29‐36) | 66 | < 0.001 | 28 | 11 (8‐14) | 4 | < 0.001 |
| Time point to measure remission | ||||||||
| < 6 weeks | 17 | 31 (23‐41) | 86 | < 0.001 | 19 | 11 (8‐17) | 70 | < 0.001 |
| > 6 weeks | 26 | 34 (31‐38) | 61 | < 0.001 | 26 | 12 (9‐15) | 71 | < 0.001 |
| Improvement in endoscopy subscore required for definition | ||||||||
| Yes | 21 | 31 (27‐36) | 77 | < 0.001 | 22 | 10 (7‐13) | 76 | < 0.001 |
| No | 29 | 35 (29‐40) | 69 | < 0.001 | 25 | 14 (10‐19) | 71 | < 0.001 |
| Improvement in bleeding subscore required for definition | ||||||||
| Yes | 13 | 31 (26‐37) | 66 | < 0.001 | 12 | 12 (9‐17) | 65 | 0.001 |
| No | 37 | 34 (30‐39) | 75 | < 0.001 | 35 | 12 (9‐15) | 77 | < 0.001 |
| Response | Remission | |||
| Study characteristic | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value |
| Trial setting | ||||
| Multi‐centre, single‐country | 1.0 | 0.16 | 1.0 | 0.59 |
| Multi‐centre, multi‐national | 1.39 (0.96‐2.03) | 1.11 (0.64‐1.94) | ||
| Single‐centre | 0.95 (0.45‐1.99) | 0.56 (0.14‐2.22) | ||
| Design | ||||
| Stand‐alone induction vs. induction and maintenance | 0.86 (0.61‐1.22) | 0.40 | 1.21 (0.70‐2.07) | 0.50 |
| First author country | ||||
| North America | 1.0 | 0.24 | 1.0 | 0.80 |
| Europe | 1.28 (0.90‐1.81) | 1.15 (0.66‐2.01) | ||
| Other | 0.86 (0.52‐1.42) | 1.24 (0.59‐2.61) | ||
| Drug class | ||||
| Corticosteroid | 1.0 | 0.30 | 1.0 | |
| Amicosalicylate | 1.59 (0.75‐3.36) | 3.95 (1.37‐11.40) | 0.02 | |
| Immunosuppressant | 0.86 (0.30‐2.44) | 4.95 (1.47‐16.73) | ||
| Biological | 1.74 (0.86‐3.50) | 2.36 (0.83‐6.40) | ||
| Other | 1.69 (0.71‐3.98) | 1.48 (0.37‐5.88) | ||
| Route of administration | ||||
| Topical | 1.0 | 0.12 | 1.0 | |
| Oral | 0.58 (0.35‐0.98) | 0.62 (0.25‐1.53) | 0.34 | |
| Intravenous | 0.82 (0.49‐1.39) | 0.70 (0.29‐1.70) | ||
| Subcutaneous | 0.82 (0.45‐1.47) | 0.41 (0.15‐1.13) | ||
| Disease severity on entry | ||||
| Mild‐moderate vs. moderate‐severe | 1.10 (0.80‐1.51) | 0.57 | 0.94 (0.56‐1.56) | 0.80 |
| Disease duration on entry | ||||
| < 5 years vs > 5 years | 0.54 (0.32‐0.92) | 0.02 | 0.57 (0.30‐1.11) | 0.10 |
| Inclusion criteria | ||||
| Minimum total score > 6 vs. minimum total score < 6 | 1.00 (0.73‐1.35) | 0.98 | 1.00 (0.59‐1.68) | 0.99 |
| Endoscopy subscore for inclusion | ||||
| > 2 | 1.0 | 0.02 | 1.0 | 0.01 |
| > 1 | 1.70 (1.02‐2.82) | 2.60 (1.25‐5.42) | ||
| Not stated | 0.78 (0.56‐1.10) | 0.68 (0.39‐1.20) | ||
| Bleeding score for inclusion | ||||
| Yes vs. no/not stated | 1.70 (1.02‐2.82) | 0.02 | 0.67 (0.38‐1.20) | 0.18 |
| Duration of follow‐up visits | ||||
| < 8 weeks vs. > 8 weeks | 0.88 (0.57‐1.37) | 0.59 | 1.41 (0.77‐2.58) | 0.26 |
| Number of follow‐up visits | ||||
| < 3 weeks vs. > 3 weeks | 1.05 (0.70‐1.57) | 0.83 | 1.08 (0.55‐2.12) | 0.82 |
| Publication date | ||||
| Before (and including) 2007 vs. after 2007 | 0.96 (0.70‐1.33) | 0.81 | 0.77 (0.47‐1.29) | 0.32 |
| Improvement in endoscopy subscore required for definition | ||||
| Yes vs. no | 1.16 (0.85‐1.59) | 0.35 | 1.54 (0.95‐2.48) | 0.08 |
| Improvement in bleeding subscore required for definition | ||||
| Yes vs. no | 1.18 (0.83‐1.67) | 0.36 | 1.0 (0.58‐1.74) | 0.99 |
| Timepoint to measure response response/remission | ||||
| < 6 weeks vs. > 6 weeks | 1.08 (0.76‐1.53) | 0.68 | 0.97 (0.60‐1.59) | 0.92 |
| Number of follow‐up visits | ||||
| < 3 visits vs. > 3 visits | 1.05 (0.70‐1.57) | 0.83 | 1.08 (0.55‐2.12) | 0.82 |
| Duration of follow‐up | ||||
| < 8 weeks vs. > 8 weeks | 0.88 (0.57‐1.37) | 0.59 | 1.41 (0.77‐2.58) | 0.26 |
| Screening visits | ||||
| Yes vs. no | 1.12 (0.75‐0.66) | 0.6 | 0.95 (0.53‐1.72) | 0.9 |
| Number of trial centres | ||||
| per 1‐centre increment | 1.00 (1.00‐1.03) | 0.728 | 1.00 (0.99‐1.00) | 0.304 |
| Publication year | ||||
| Per 1 = year increment | 1.01 (0.99‐1.03) | 0.24 | 0.99 (0.95‐1.03) | 0.65 |
| Extensive disease/pancolitis | ||||
| > 30% vs. < 30% | 1.01 (0.69‐1.47) | 0.969 | 1.23 (0.64‐2.36) | 0.532 |
| Concurrent steroids | ||||
| Yes vs. no | 0.88 (0.59‐1.32) | 0.539 | 1.13(0.63‐2.05) | 0.68 |
| Concurrent immunosuppressive | ||||
| Yes vs. no | 0.76 (0.53‐1.16) | 0.727 | 0.18 (0.66‐2.10) | 0.575 |
| Ratio of active drug | ||||
| Placebo > 1 vs. < 1 | 1.01 (0.68‐1.50) | 0.972 | 0.91 (0.49‐1.67) | 0.757 |
| Primary time point to measure endpoint | ||||
| per 1‐week increment | 1.00 (0.93‐1.07) | 0.97 | 1.06 (1.02‐1.10) | 0.01 |