Terlipresina versus otros fármacos vasoactivos para el síndrome hepatorrenal
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 9 participants included. Type 2 hepatorenal syndrome = 13 participants included. Demographics: Terlipressin group: mean age 55 years, 75% men, alcoholic cirrhosis 33%. Other vasoactive drug group: mean age 56 years, 70% men, alcohol‐related cirrhosis 20%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 1 mg/4 hours. With no response, dose increased to 2 mg/4 hours. Response defined as reduction in serum creatinine ≥ 25% from baseline after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.1 μg/kg/minute. Dose increased in steps of 0.05 μg/kg/minute every 4 hours until the mean arterial pressure was increased to at least 10 mmHg compared to baseline. Maximum dose 0.7 μg/kg/minute. Cointervention: Both arms treated with albumin to maintain a central venous pressure between 10 cmH2O and 15 cmH2O. Mean dose of albumin in terlipressin group. 46 g/day (range 35 to 65). Mean dose of albumin in noradrenaline group 56 g/day (range 40 to 75). During follow‐up, participants with ascites were treated with diuretics and large volume paracentesis followed by albumin infusions as needed. | |
| Outcomes | No predefined outcome (pilot study). Survival and reversal of hepatorenal syndrome reported. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum 2 weeks. Follow‐up: 90 days. | |
| Country of origin | Italy. | |
| Inclusion period | Data not available. | |
| Notes | Full paper. All survivors underwent liver transplantation at end of follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. All participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | No funding or other support from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label multicentre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 51 participants included. Demographics: Terlipressin group: mean age 43 years, 67% men, aetiology mostly viral hepatitis. Other vasoactive drug group: mean age 46 years, 71% men, aetiology mostly viral hepatitis. | |
| Interventions | Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 3 mg/24 hours. With no response, dose primarily increased to 6 mg/24 hours and secondarily to 12 mg/24 hours. Response defined as a reduction of serum creatinine ≥ 25% compared to baseline after every 48 hours of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours guided by a mean arterial pressure around 85 mmHg to 90 mmHg. Maximum dose 3 mg/hour. Cointervention: Both arms treated with albumin to maintain a central venous pressure between 10 cmH2O and 15 cmH2O. Dose not reported. | |
| Outcomes | Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival and treatment costs. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. | |
| Country of origin | Egypt. | |
| Inclusion period | January 2009 to April 2012. | |
| Notes | Full paper. Participants who died within 72 hours after randomisation excluded from study. We contacted the authors, but were unable gather any further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes (text did not explain if envelopes were serially numbered). |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | High risk | No missing outcome data described. Participants who died within 72 hours excluded from analyses, but number allocated to 2 intervention groups not given. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open label, multicentre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 44 participants included. Type 2 hepatorenal syndrome = 4 participants included. Demographics: Terlipressin group: mean age 60 years, men 78%, viral aetiology 37%. Other vasoactive drug group: mean age 65 years, men 52%, viral aetiology 38%. | |
| Interventions | Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 3 mg/24 hours. With no response, dose primarily increased to 6 mg/24 hours and then to 12 mg/24 hours. Response defined as a reduction of serum creatinine of ≥ 25% compared to baseline after every 48 hours of treatment. Other vasoactive drugs:midodrine and octreotide Midodrine Administration form: oral tablet. Dose: dose titration regimen. Initial dose 7.5 mg/8 hours. With no response, dose increased to 12.5 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 25% from baseline after 3 days of treatment. Octreotide Administration form: subcutaneous bolus injection. Dose: dose titration regimen. Initial dose 100 mg/8 hours. With no response, dose increased to 200 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 25% from baseline after 3 days of treatment. Cointervention: both arms treated with albumin; 1 g/kg bodyweight at day 1, followed by 20 g/day to 40 g/day. | |
| Outcomes | Primary: reversal of hepatorenal syndrome. Secondary: 3 months survival. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 14 days. Follow‐up: 3 months. | |
| Country of origin | Italy. | |
| Inclusion period | 2008 to 2012. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and the trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | Low risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome: not reported. Type 1 hepatorenal syndrome = 36 participants included. Type 2 hepatorenal syndrome = 4 participants included. Demographics: Terlipressin group: not available. Other vasoactive drug group: not available. | |
| Interventions | Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 4 mg/24 hours. With no response, dose increased stepwise to 12 mg/24 hours. Response defined as a reduction in serum creatinine of ≥ 50% from baseline or reversal of hepatorenal syndrome. Other vasoactive drug:octreotide. Administration form: subcutaneously bolus injection. Dose: dose titration regimen. Initial dose 100 mg/8 hours. With no response, dose increased to 200 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 50% from baseline or reversal of hepatorenal syndrome. Cointervention: both arms treated with albumin; 1 g/kg bodyweight at day 1, followed by 20 g/day to 40 g/day. | |
| Outcomes | No description. Data on reversal of hepatorenal syndrome and mortality available. | |
| Treatment duration | Treatment duration: data not available. Follow‐up: 30 days. | |
| Country of origin | Romania. | |
| Inclusion period | Data not available. | |
| Notes | Abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 2 hepatorenal syndrome = 46 participants included. Demographics: Terlipressin group: mean age 46 years, 87% men, alcohol‐related cirrhosis 65%. Other vasoactive drug group: mean age 48 years, 70% men, alcohol‐related cirrhosis 70%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased primarily to 1 mg/6 hours and then to 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stop if central venous pressure exceeded 18 cmH2O. | |
| Outcomes | Primary: reversal of hepatorenal syndrome. Secondary: 3 months mortality. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 3 months. | |
| Country of origin | India. | |
| Inclusion period | January 2009 to December 2011. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | High risk | Investigators excluded 12 participants from analyses after randomisation. Reasons for exclusions/withdrawals included sepsis (7), severe coronary artery disease (1), hepatocellular carcinoma (1), diabetic nephropathy (1), and refusal to participate (2). Authors did not provide information about allocation group. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 41 participants included. Demographics: Terlipressin group: mean age 56.9 years, 85% men, alcohol‐related cirrhosis 75%. Other vasoactive drug group: mean age 54.7 years, 95.2% men, alcohol‐related cirrhosis 61.9%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to maximum of 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline (+furosemide). Noradrenaline Administration form: continuous intravenous infusion Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Furosemide Administration form: continuous intravenous infusion. Dose: dose titration regimen. Furosemides added, if urine output < 200 mL/4 hours despite reaching an increase in mean arterial pressure of ≥ 10 mmHg. Initial dose 0.001 mg/kg/minute and adjusted to maintain a urine output of > 40 mL/hour. Cointervention: both arms treated with intravenous third‐generation cephalosporins and albumin 20 g/day to 40 g/day. Administration stopped temporarily if central venous pressure increased > 12 cm/H2O or if serum albumin > 4 g/L. | |
| Outcomes | Primary: reversal of hepatorenal syndrome. Secondary: 14 days mortality. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 14 days. Follow‐up: 14 days. | |
| Country of origin | India. | |
| Inclusion period | 3 years. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome: no description. Type 1 hepatorenal syndrome = 60 participants included. Demographics: no description. | |
| Interventions | Terlipressin: Administration form: intravenous. Other vasoactive drug:noradrenaline. Administration form: intravenous. Cointervention: both arms treated with albumin. Dose not reported. | |
| Outcomes | No description. Data on reversal of hepatorenal syndrome and mortality available. | |
| Treatment duration | Treatment duration: no description. Follow‐up: 90 days or death. | |
| Country of origin | India. | |
| Inclusion period | No description. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No description. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes described and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 40 participants included. Demographics: Terlipressin group: mean age 48 years, 85% men, alcohol‐related cirrhosis 60%. Other vasoactive drug group: mean age 48 years, 85% men, alcohol‐related cirrhosis 70%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to a maximum 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stop if central venous pressure exceeded 18 cmH2O. Participants with tense ascites had 3 L to 5 L paracentesis combined with infusions of 8 g of albumin for each litre of ascitic fluid removed. | |
| Outcomes | Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. | |
| Country of origin | India. | |
| Inclusion period | August 2005 to December 2006. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. All participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 46 participants included. Demographics: Terlipressin group: mean age 51 years, 83% men, alcohol‐related cirrhosis 43%. Other vasoactive drug group: mean age 48 years, 83% men, alcohol‐related cirrhosis 52%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to maximum 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to > 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stopped if central venous pressure exceeded 18 cmH2O. Participants with tense ascites had 3 L to 5 L paracentesis combined with infusions of 8 g of albumin for each litre of ascitic fluid removed. | |
| Outcomes | Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival. | |
| Treatment duration | Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. | |
| Country of origin | India. | |
| Inclusion period | January 2009 to 2011 October. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation list. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | Low risk | |
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants | Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 40 participants included. Type 2 hepatorenal syndrome = 40 participants included. Demographics: type 1 hepatorenal syndrome. Terlipressin group: mean age 46 years, 83% men, alcohol‐related cirrhosis 50%. Other vasoactive drug group: mean age 39 years, 83% men, alcohol‐related cirrhosis 50%. Demographics: type 2 hepatorenal syndrome. Terlipressin group: mean age 45 years, 83% men, alcohol‐related cirrhosis 53%. Other vasoactive drug group: mean age 43 years, 83% men, alcohol‐related cirrhosis 55%. | |
| Interventions | Terlipressin: Administration form: intravenous bolus injection. Dose: fixed dose 0.5 mg/6 hours. Other vasoactive drug:dopamine and furosemide. Administration form: continuous intravenous infusion. Dose: fixed doses of dopamine 2 μg/kg/minute and furosemide 0.01 mg/kg/hour. Cointervention: both arms treated with albumin 20 g/day. | |
| Outcomes | Primary outcomes: reversal of hepatorenal syndrome, 15 and 30 days' survival. Secondary outcomes: cost of treatment. | |
| Treatment duration | Treatment duration: 5 days. Follow‐up: 30 days. | |
| Country of origin | India. | |
| Inclusion period | February 2005 to June 2010. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | High risk | Number of participants with (or without) reversal of hepatorenal syndrome not reported. |
| For‐profit bias | Low risk | Authors declared no conflict of interests. The randomised clinical trial received financial support from the Indian Council of Medical Research and did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Comparing terlipressin with placebo. | |
| Compared bolus injections of terlipressin with continuous infusions of terlipressin. | |
| Compared terlipressin with placebo. | |
| Compared terlipressin with placebo. | |
| Compared terlipressin with placebo. | |
| Observational study. No information about harms. | |
| Compared terlipressin with placebo. | |
| Compared terlipressin with placebo. | |
| Quasi‐randomised trial. No information about harms. | |
| Compared terlipressin with placebo. | |
| Compared noradrenaline with midodrine and octreotide. | |
| Compared high dose of terlipressin with low dose of terlipressin. | |
| Compared terlipressin with placebo. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality: bias control Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| Analysis 1.1  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control. | ||||
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| Analysis 1.2  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug. | ||||
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| Analysis 1.3  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome. | ||||
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 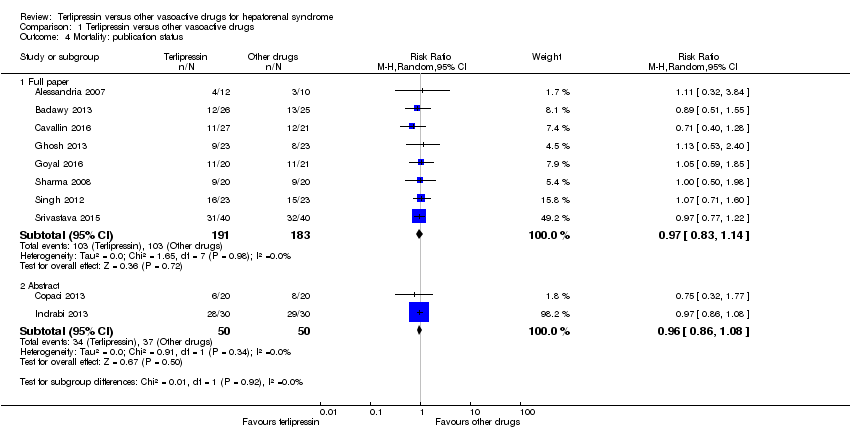 Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status. | ||||
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| Analysis 1.5  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug. | ||||
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| Analysis 1.6 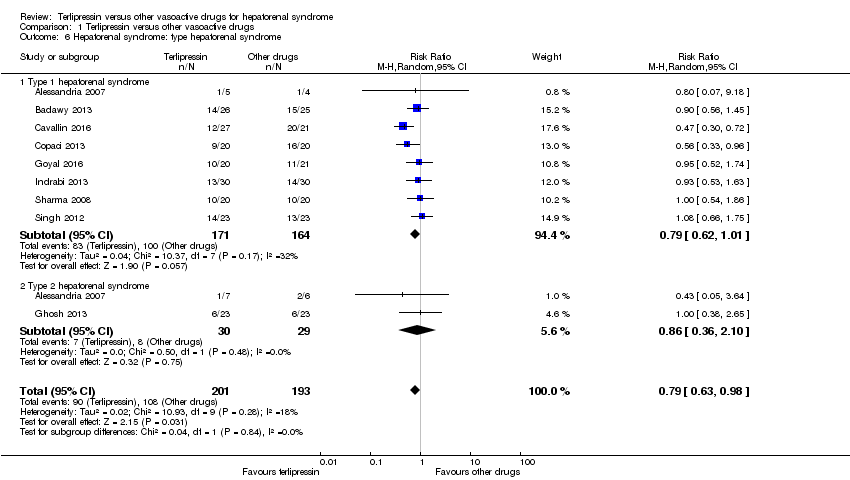 Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome. | ||||
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| Analysis 1.7 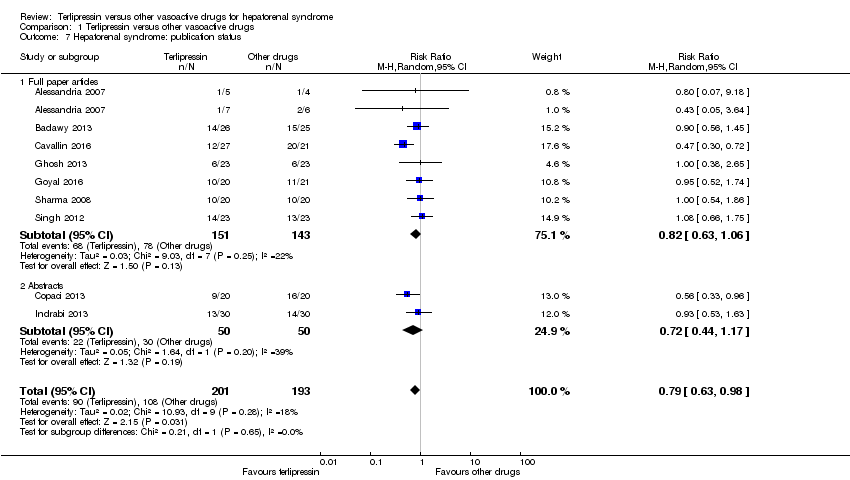 Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status. | ||||
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| Analysis 1.8  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug. | ||||
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event. | ||||
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events Show forest plot | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| Analysis 1.10  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events. | ||||
| 11 Non‐serious adverse event: types Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types. | ||||
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.

Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
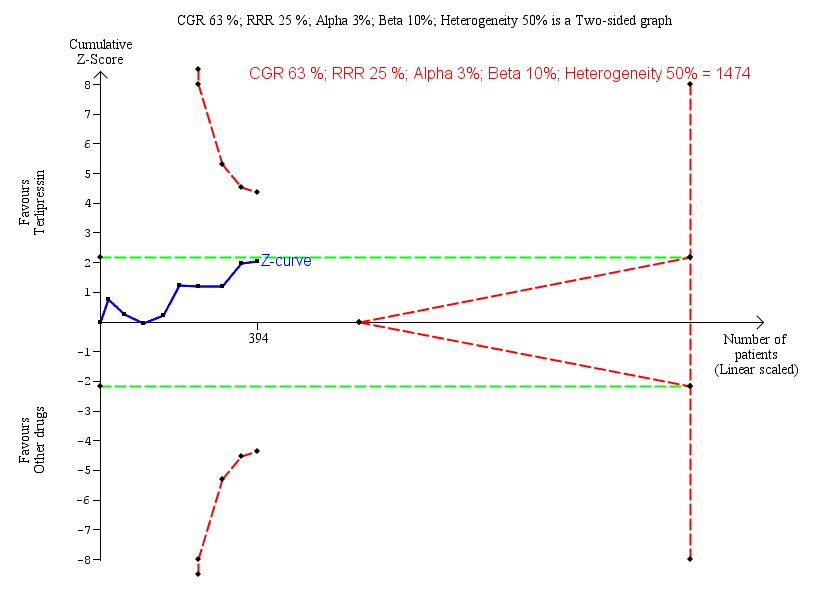
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.

Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types.
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 | |||||
| Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) | Study population | RR 0.79 | 394 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 | |||||
| Serious adverse events | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 | 221 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias. bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis. cClinical heterogeneity. dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality: bias control Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events Show forest plot | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| 11 Non‐serious adverse event: types Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |


