Vitamina D para el tratamiento del asma
Appendices
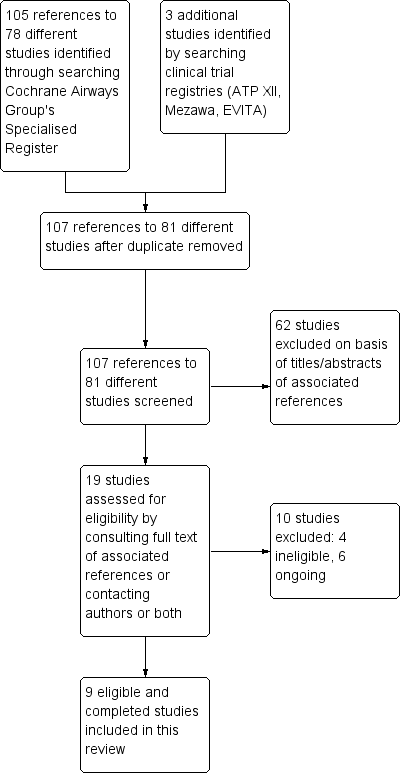
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to retrieve trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Vitamin D Explode All
#6 MeSH DESCRIPTOR Vitamin D Deficiency Explode All
#7 "vitamin d"
#8 #5 or #6 or #7
#9 #4 and #8
(in search line #1, MISC1 refers to the field in the record where the reference has been coded for condition, in this case, asthma)
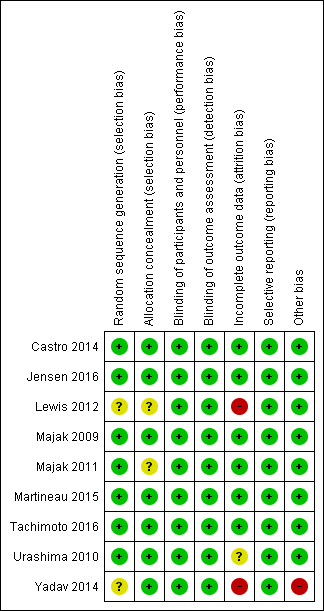
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

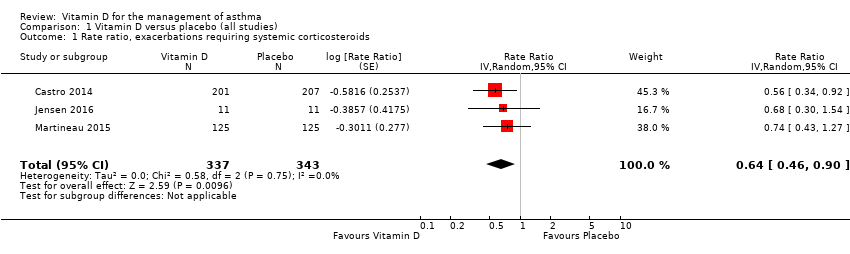
Forest plot of comparison: 1 Vitamin D versus placebo (all studies), outcome: 1.1 Rate ratio, exacerbations requiring systemic corticosteroids.

Forest plot of comparison: 1 Vitamin D versus placebo (all studies), outcome: 1.5 People with one or more exacerbations requiring ED visit or hospitalisation or both.
In the control group 6 out of 100 people had a visit to ED or hospitalisation over 8 months, compared to 3 (95% CI 1 to 5) out of 100 on vitamin D.

Forest plot of comparison: 1 Vitamin D versus placebo (low risk of bias), outcome: 1.10 People with one or more study‐defined exacerbations.

In the control group 29 out of 100 people had a study‐defined exacerbation over 7 months, compared to 18 (95% CI 10 to 29) out of 100 on Vitamin D.
Comparison 1 Vitamin D versus placebo (all studies), Outcome 1 Rate ratio, exacerbations requiring systemic corticosteroids.
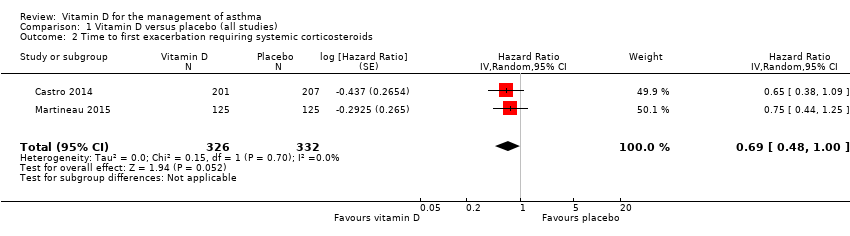
Comparison 1 Vitamin D versus placebo (all studies), Outcome 2 Time to first exacerbation requiring systemic corticosteroids.
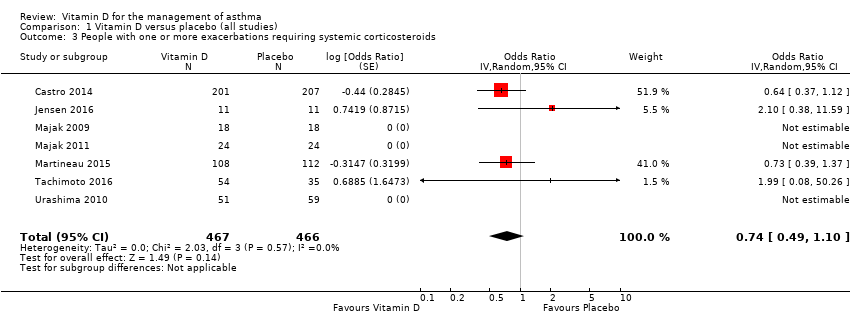
Comparison 1 Vitamin D versus placebo (all studies), Outcome 3 People with one or more exacerbations requiring systemic corticosteroids.

Comparison 1 Vitamin D versus placebo (all studies), Outcome 4 People with one or more exacerbations requiring systemic corticosteroids (risk difference).
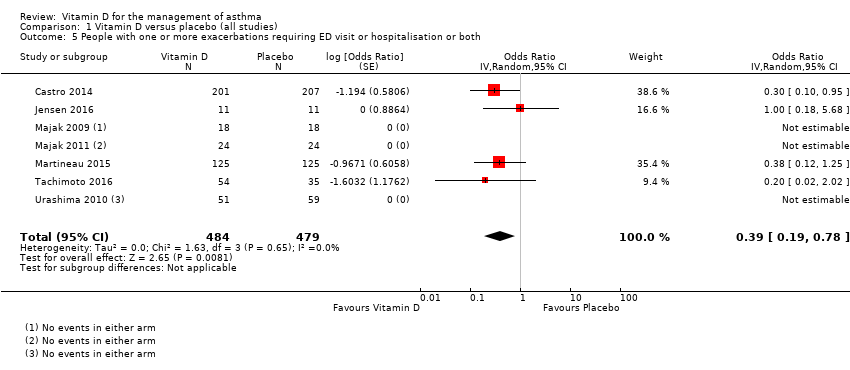
Comparison 1 Vitamin D versus placebo (all studies), Outcome 5 People with one or more exacerbations requiring ED visit or hospitalisation or both.

Comparison 1 Vitamin D versus placebo (all studies), Outcome 6 ACT/C‐ACT score.

Comparison 1 Vitamin D versus placebo (all studies), Outcome 7 People with fatal asthma exacerbation.
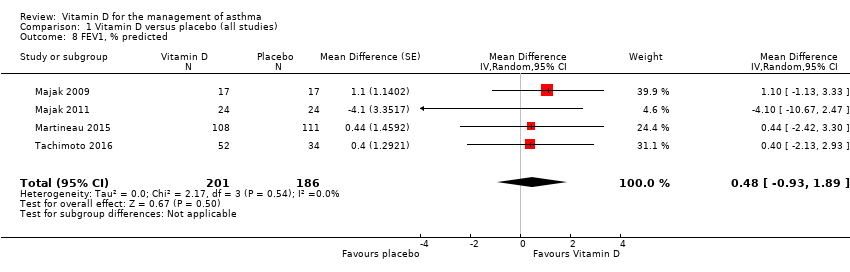
Comparison 1 Vitamin D versus placebo (all studies), Outcome 8 FEV1, % predicted.
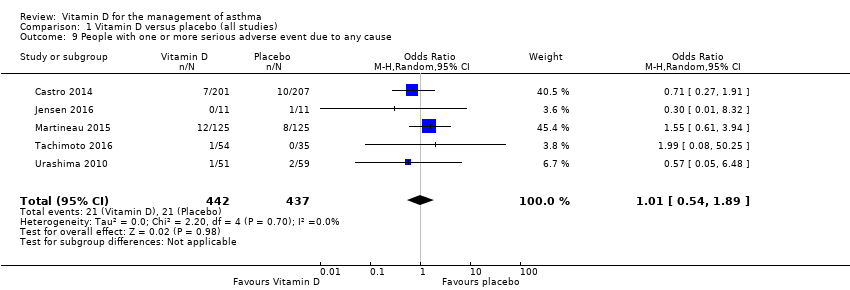
Comparison 1 Vitamin D versus placebo (all studies), Outcome 9 People with one or more serious adverse event due to any cause.
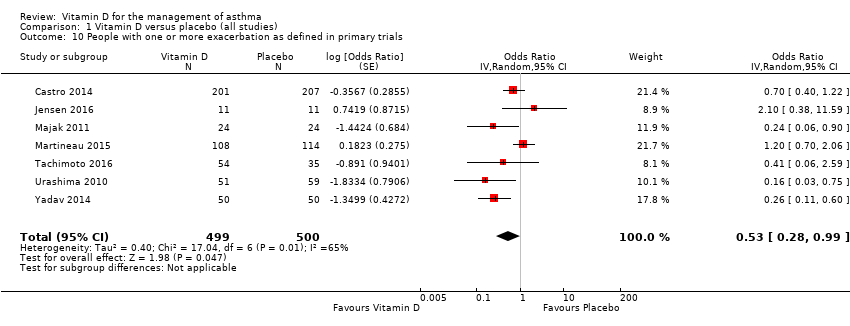
Comparison 1 Vitamin D versus placebo (all studies), Outcome 10 People with one or more exacerbation as defined in primary trials.
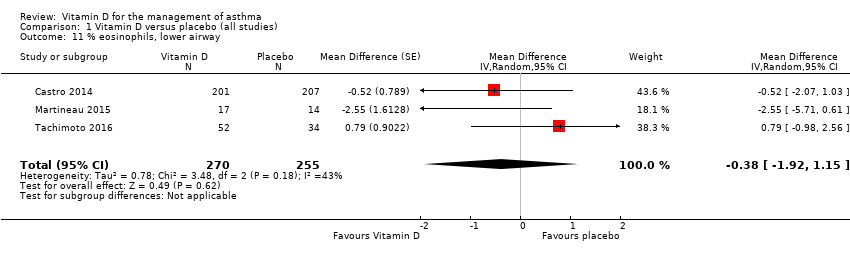
Comparison 1 Vitamin D versus placebo (all studies), Outcome 11 % eosinophils, lower airway.

Comparison 1 Vitamin D versus placebo (all studies), Outcome 12 Peak expiratory flow rate.

Comparison 1 Vitamin D versus placebo (all studies), Outcome 13 People with one or more adverse reactions attributed to vitamin D.
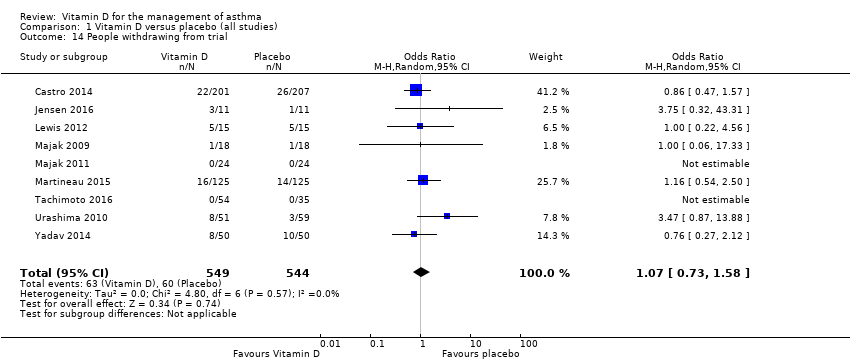
Comparison 1 Vitamin D versus placebo (all studies), Outcome 14 People withdrawing from trial.

Comparison 2 Vitamin D versus placebo (sensitivity analysis excluding studies at high risk of bias), Outcome 1 People with one or more study‐defined exacerbation.

Comparison 2 Vitamin D versus placebo (sensitivity analysis excluding studies at high risk of bias), Outcome 2 People withdrawing from trial.
| Vitamin D versus placebo for the management of asthma (all studies) | ||||||
| Patient or population: children and adults with predominantly mild to moderate asthma Setting: primary and secondary care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin D | |||||
| Rate ratio, exacerbations requiring systemic corticosteroids | Study population | RR 0.64 | 680 | ⊕⊕⊕⊕ | Evidence based primarily on adults with mild to moderate asthma | |
| 0.44 events per person per year1 | 0.28 events per person per year (0.20 to 0.40) | |||||
| People with 1 or more exacerbations requiring ED visit or hospitalisation or both. Follow‐up: 6 to 12 months | Study population | OR 0.39 | 963 | ⊕⊕⊕⊕ | Evidence based primarily on children and adults with mild to moderate asthma | |
| 63 per 1000 | 25 per 1000 | |||||
| FEV1, % predicted. Follow‐up: 6 to 12 months | The mean FEV1, % predicted was 85.62% | The mean FEV1, % predicted in the intervention group was 0.48% more (0.93 fewer to 1.89 more) | ‐ | 387 | ⊕⊕⊕⊕2 | Evidence based primarily on children and adults with mild to moderate asthma |
| ACT/C‐ACT score. Follow‐up: 6 to 12 months | The mean ACT/C‐ACT score was 20 points | The mean ACT/C‐ACT score in the intervention group was 0.08 points fewer (0.7 fewer to 0.54 more) | ‐ | 713 | ⊕⊕⊕⊕2 | Evidence based primarily on adults with mild to moderate asthma |
| People with fatal asthma exacerbation. Follow‐up: 6 to 12 months | Study population | Not estimable | 963 | ⊕⊕◯◯3 LOW | No fatal asthma exacerbations occurred in included studies | |
| 0 per 1000 | 0 per 1000 | |||||
| People with 1 or more serious adverse event due to any cause. Follow‐up: 6 to 12 months | Study population | OR 1.01 | 879 | ⊕⊕⊕◯4 MODERATE | Evidence based primarily on children and adults with mild to moderate asthma | |
| 48 per 1000 | 49 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The event rate in participants randomised to placebo was estimated by calculating the weighted mean of event rates reported in placebo arms of included studies. | ||||||
| Analysis | Random‐effects model | Fixed‐effect model |
| People with 1 or more exacerbations requiring systemic corticosteroids (risk difference) | (RD ‐0.01, 95% CI ‐0.04 to 0.02) | (RD ‐0.03, 95% CI ‐0.07 to 0.01) |
| ACT/C‐ACT score | (MD ‐0.08, 95% CI ‐0.70 to 0.54) | (MD ‐0.09, 95% CI ‐0.64 to 0.46) |
| People with 1 or more serious adverse event due to any cause | (OR 1.01, 95% CI 0.54 to 1.89) | (OR 1.00, 95% CI 0.54 to 1.85) |
| People with 1 or more study‐defined exacerbation | (OR 0.53, 95% CI 0.28 to 0.99) | (OR 0.66, 95% CI 0.48 to 0.91) |
| % eosinophils, lower airway | (MD ‐0.38, 95% CI ‐1.92 to 1.15) | (MD ‐0.26, 95% CI ‐1.35 to 0.83) |
| Peak expiratory flow rate | (MD 3.16, 95% CI ‐13.40 to 19.72) | (MD 4.09, 95% CI ‐1.34 to 9.52) |
| People withdrawing from the trial | (OR 1.07, 95% CI 0.73 to 1.58) | (OR 1.09, 95% CI 0.74 to 1.59) |
| Sensitivity analyses are presented only for those outcomes where results of analyses using random‐effects versus fixed‐effect models are non‐identical. Abbreviations: CI, confidence interval; MD, mean difference; OR, odds ratio; RD, risk difference. | ||
| Study | Definition |
| Meeting criteria for treatment failure and 1 or more of the following:
| |
| Exacerbation requiring rescue oral corticosteroids, documented in medical or pharmacy records or both | |
| Exacerbation not defined or reported in study manuscript | |
| Exacerbation not defined or reported in study manuscript; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study | |
| Reported but not defined in study manuscript; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study | |
| Deterioration in asthma resulting in (A) treatment with oral corticosteroids, or (B) hospital admission or emergency department treatment, or (C) decrease in the morning PEFR to more than 25% below the mean run‐in value on 2 or more consecutive days | |
| Worsening of asthma symptoms prompting a need for a change in asthma treatment (from authors) | |
| Asthma attack that included wheezing, improved by inhalation of a beta‐stimulant in participants who already had a diagnosis of asthma; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study | |
| Reported but not defined in study manuscript | |
| FEV1, forced expiratory volume in one second; PEFR, peak expiratory flow rate. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate ratio, exacerbations requiring systemic corticosteroids Show forest plot | 3 | 680 | Rate Ratio (Random, 95% CI) | 0.64 [0.46, 0.90] |
| 2 Time to first exacerbation requiring systemic corticosteroids Show forest plot | 2 | 658 | Hazard Ratio (Random, 95% CI) | 0.69 [0.48, 1.00] |
| 3 People with one or more exacerbations requiring systemic corticosteroids Show forest plot | 7 | 933 | Odds Ratio (Random, 95% CI) | 0.74 [0.49, 1.10] |
| 4 People with one or more exacerbations requiring systemic corticosteroids (risk difference) Show forest plot | 7 | 933 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 5 People with one or more exacerbations requiring ED visit or hospitalisation or both Show forest plot | 7 | 963 | Odds Ratio (Random, 95% CI) | 0.39 [0.19, 0.78] |
| 6 ACT/C‐ACT score Show forest plot | 3 | 713 | Mean Difference (Random, 95% CI) | ‐0.08 [‐0.70, 0.54] |
| 7 People with fatal asthma exacerbation Show forest plot | 7 | 963 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 FEV1, % predicted Show forest plot | 4 | 387 | Mean Difference (Random, 95% CI) | 0.48 [‐0.93, 1.89] |
| 9 People with one or more serious adverse event due to any cause Show forest plot | 5 | 879 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.89] |
| 10 People with one or more exacerbation as defined in primary trials Show forest plot | 7 | 999 | Odds Ratio (Random, 95% CI) | 0.53 [0.28, 0.99] |
| 11 % eosinophils, lower airway Show forest plot | 3 | 525 | Mean Difference (Random, 95% CI) | ‐0.38 [‐1.92, 1.15] |
| 12 Peak expiratory flow rate Show forest plot | 2 | 302 | Mean Difference (Random, 95% CI) | 3.16 [‐13.40, 19.72] |
| 13 People with one or more adverse reactions attributed to vitamin D Show forest plot | 5 | 879 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 14 People withdrawing from trial Show forest plot | 9 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 People with one or more study‐defined exacerbation Show forest plot | 6 | 899 | Odds Ratio (Random, 95% CI) | 0.64 [0.34, 1.21] |
| 2 People withdrawing from trial Show forest plot | 7 | 963 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.88] |