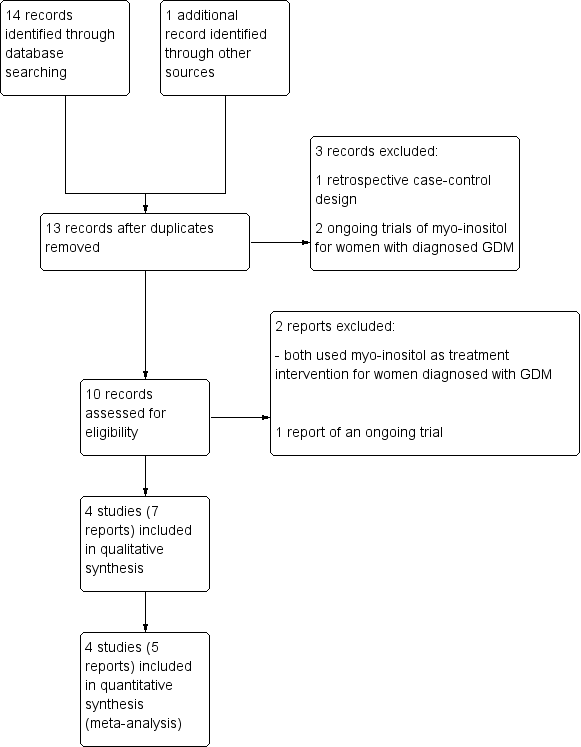
Administración prenatal de suplementos dietéticos con mioinositol en pacientes embarazadas para prevenir la diabetes gestacional
Appendices
Appendix 1. Search terms
ClinicalTrials.gov and WHO ICTRP
gestational diabetes AND myoinositol
gestational diabetes AND myo‐inositol
gestational diabetes AND myo inositol
gestational diabetes AND inositol)
gdm AND myoinositol
gdm AND myo‐inositol
gdm AND myo inositol
gdm AND inositol
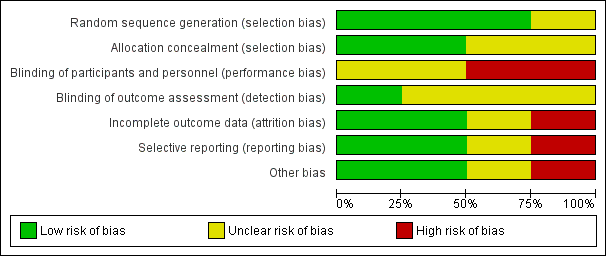
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
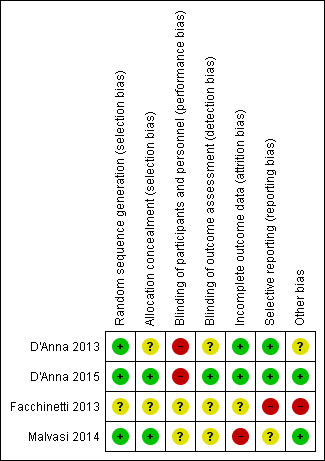
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
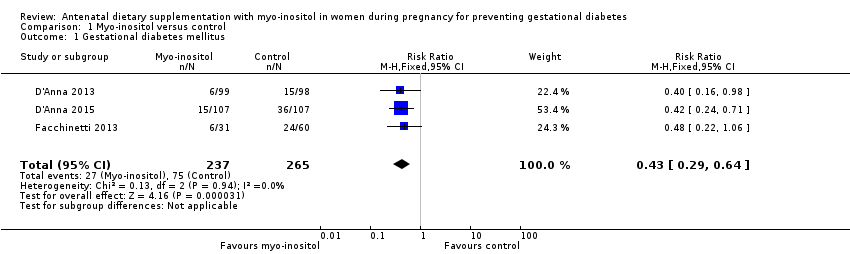
Comparison 1 Myo‐inositol versus control, Outcome 1 Gestational diabetes mellitus.
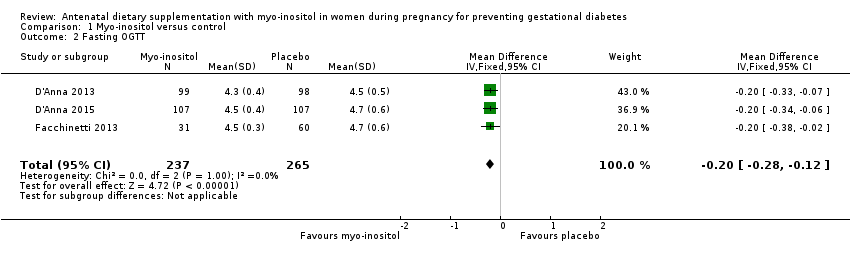
Comparison 1 Myo‐inositol versus control, Outcome 2 Fasting OGTT.
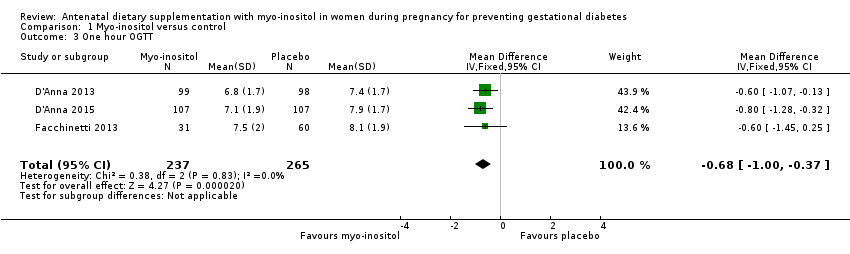
Comparison 1 Myo‐inositol versus control, Outcome 3 One hour OGTT.

Comparison 1 Myo‐inositol versus control, Outcome 4 Two hour OGTT.

Comparison 1 Myo‐inositol versus control, Outcome 5 Hypertensive disorders of pregnancy.

Comparison 1 Myo‐inositol versus control, Outcome 6 Caesarean section.
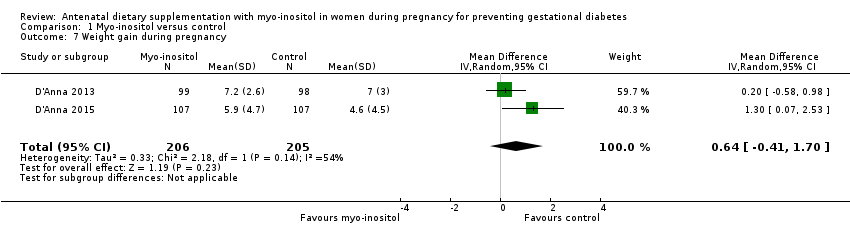
Comparison 1 Myo‐inositol versus control, Outcome 7 Weight gain during pregnancy.
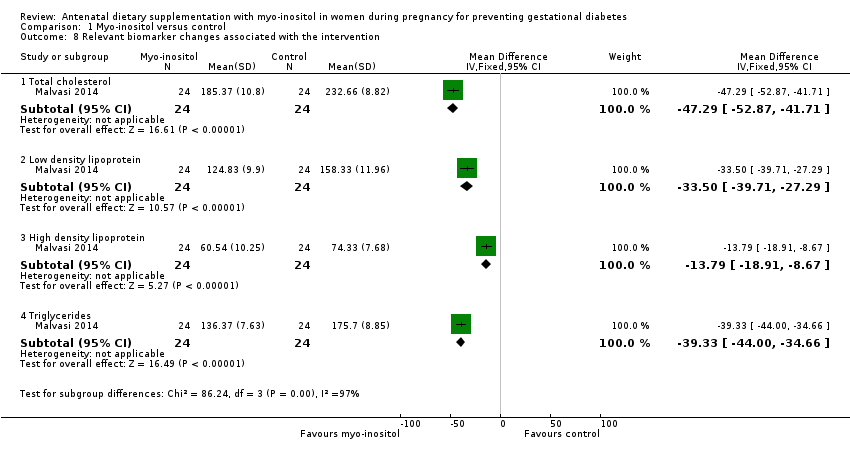
Comparison 1 Myo‐inositol versus control, Outcome 8 Relevant biomarker changes associated with the intervention.

Comparison 1 Myo‐inositol versus control, Outcome 9 Adverse effects of intervention.
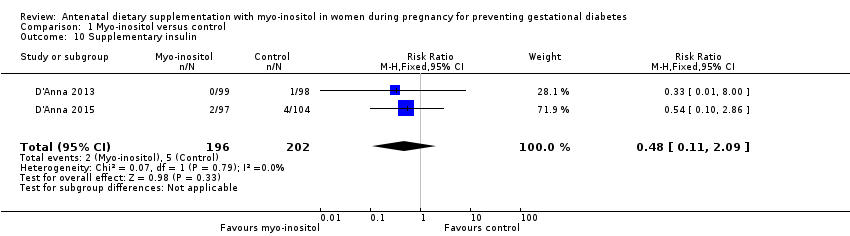
Comparison 1 Myo‐inositol versus control, Outcome 10 Supplementary insulin.
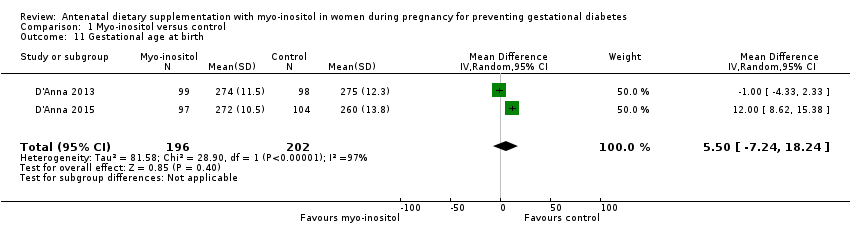
Comparison 1 Myo‐inositol versus control, Outcome 11 Gestational age at birth.
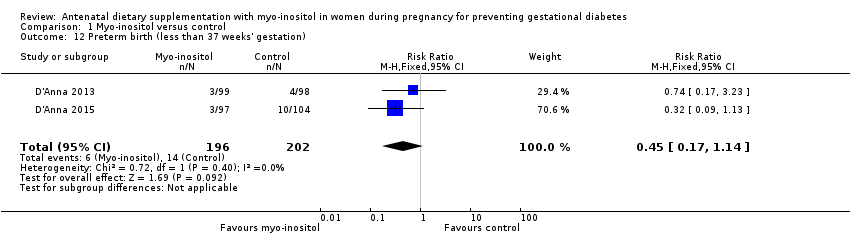
Comparison 1 Myo‐inositol versus control, Outcome 12 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Myo‐inositol versus control, Outcome 13 Macrosomia.

Comparison 1 Myo‐inositol versus control, Outcome 14 Birthweight.

Comparison 1 Myo‐inositol versus control, Outcome 15 Shoulder dystocia.

Comparison 1 Myo‐inositol versus control, Outcome 16 Respiratory distress syndrome.
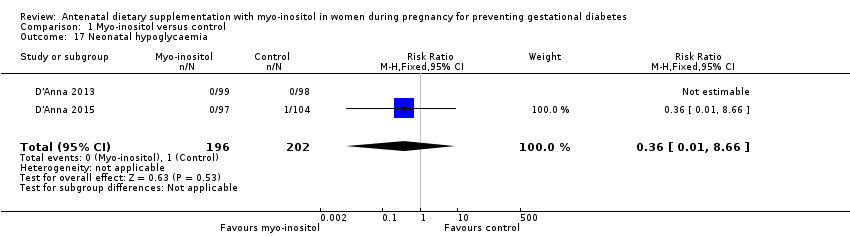
Comparison 1 Myo‐inositol versus control, Outcome 17 Neonatal hypoglycaemia.
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included) Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.43 | 502 | ⊕⊕⊝⊝ | GDM diagnosed using IADPSG 2010 criteria | |
| 28 per 100 | 12 per 100 | |||||
| Weight gain during pregnancy | The mean weight gain during pregnancy was 0 | The mean weight gain during pregnancy in the intervention group was 0.64 more (0.41 fewer to 1.7 more) | ‐ | 411 | ⊕⊝⊝⊝ | D'Anna 2015 included obese pregnant women and D'Anna 2013 included non‐obese women with a family history of type 2 diabetes Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.43 | 398 | ⊕⊝⊝⊝ | Random‐effects model | |
| 4 per 100 | 2 per 100 | |||||
| Caesarean section | Study population | RR 0.95 | 398 | ⊕⊕⊝⊝ | ||
| 45 per 100 | 43 per 100 | |||||
| Perineal trauma | Not estimable | (0 studies) | No data reported for perineal trauma in any of the included studies | |||
| Postnatal depression | Not estimable | (0 studies) | No data reported for postnatal depression in any of the included studies | |||
| Type 2 diabetes | Not estimable | (0 studies) | No data reported for type 2 diabetes in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded (‐1) due to unclear risk of bias for allocation concealment in two of the included trials (one trial did not provide sufficient detail to determine allocation concealment and one trial (reported as a conference abstract) had no details of random sequence generation, allocation concealment or blinding) and for high risk of performance bias for lack of blinding (two trials were open‐label trials with no blinding of participants or researchers, however one trial explicitly described blinding of outcome assessors and was assessed as low risk of detection bias). 2 Studies were conducted in Italy with Caucasian women and generalisability of findings is limited, downgraded (‐1). 3 Evidence of imprecision with wide confidence intervals crossing the line of no effect, downgraded (‐1). 4 Heterogeneity high with I2 = 54% (indirectness) probably due to different study populations, downgraded (‐1). 5 Wide confidence intervals with very low event rates and a small sample size suggest evidence of imprecision, downgraded (‐1). 6 Downgraded (‐1) due to insufficient evidence to judge allocation concealment in one trial and subsequent judgement of unclear risk of bias. The other trial had a low risk of bias for allocation concealment. Both trials were open‐label with no blinding of participants or researchers, although one trial explicitly stated that outcome assessors were blinded to treatment allocation. | ||||||
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
| Patient or population: pregnant women who were at risk of GDM Setting: Italy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Myo‐inositol | |||||
| Large‐for‐gestational age | not estimable | (0 studies) | No data reported for large‐for‐gestational age in any of the included studies | |||
| Perinatal mortality | not estimable | (0 studies) | No data reported for perinatal mortality in any of the included studies | |||
| Composite of serious neonatal outcomes | not estimable | (0 studies) | No data reported for composite of serious neonatal outcomes in any of the included studies | |||
| Neonatal hypoglycaemia | Study population | RR 0.36 | 398 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Adiposity | not estimable | (0 studies) | No data reported for adiposity in any of the included studies | |||
| Diabetes | not estimable | (0 studies) | No data reported for diabetes in any of the included studies | |||
| Neurosensory disability | not estimable | (0 studies) | No data reported for neurosensory disability in any of the included studies | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No blinding in either study and reporting of allocation concealment was unclear in one of the studies, downgraded (‐1). 2 Both studies were conducted in Italy with Caucasian women and may not be generalisable to other settings, downgraded (‐1). 3 Wide confidence intervals with very low event rates suggest evidence of imprecision, downgraded (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational diabetes mellitus Show forest plot | 3 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| 2 Fasting OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.12] |
| 3 One hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [1.00, ‐0.37] |
| 4 Two hour OGTT Show forest plot | 3 | 502 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.07, ‐0.43] |
| 5 Hypertensive disorders of pregnancy Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 8.41] |
| 6 Caesarean section Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 7 Weight gain during pregnancy Show forest plot | 2 | 411 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.41, 1.70] |
| 8 Relevant biomarker changes associated with the intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Total cholesterol | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐47.29 [‐52.87, ‐41.71] |
| 8.2 Low density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐33.50 [‐39.71, ‐27.29] |
| 8.3 High density lipoprotein | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐13.79 [‐18.91, ‐8.67] |
| 8.4 Triglycerides | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐39.33 [‐44.00, ‐34.66] |
| 9 Adverse effects of intervention Show forest plot | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Supplementary insulin Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.11, 2.09] |
| 11 Gestational age at birth Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | 5.50 [‐7.24, 18.24] |
| 12 Preterm birth (less than 37 weeks' gestation) Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.14] |
| 13 Macrosomia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 6.37] |
| 14 Birthweight Show forest plot | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐60.47 [‐265.21, 144.26] |
| 15 Shoulder dystocia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.12, 44.30] |
| 16 Respiratory distress syndrome Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.60] |
| 17 Neonatal hypoglycaemia Show forest plot | 2 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.66] |