Denervación renal para la hipertensión resistente
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods |
| |
| Participants |
Exclusion criteria: secondary hypertension, eGFR < 40 mL/min/1.73 m², history of severe cardiovascular disease or stroke in the previous three months, history of contraindication or intolerance to the study drugs, type 1 diabetes mellitus, brachial circumference > 42 cm, atrial fibrillation, unsuitable renal artery anatomy (accessory renal arteries > 3 mm in diameter, main renal artery < 4 mm in diameter or < 20 mm in length, renal artery stenosis > 30%, prior renal artery intervention or kidney length < 90 mm) ruled out by computed tomography angiogram, magnetic resonance angiogram or renal angiogram | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Modified intention‐to‐treat and per‐protocol analyses performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "The randomisation sequence was generated by computer and stratified by centres using randomised blocks of small size and permutation of treatments within each block" |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | Low risk | blinded outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | 5/48 (10%) drop‐outs in treatment group (three lost to follow‐up and two with missing ABPM). A modified intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | all the pre‐specified outcomes have been reported |
| Other bias | Low risk | The funder of the study (French Ministry of Health) had no role in study design, data collection, data analysis, data interpretation, or writing of the report |
| Methods |
| |
| Participants |
Exclusion criteria: mean day‐time systolic BP on 24‐hour ABPM < 135 and > 149 mmHg or mean day‐time diastolic BP < 90 and > 94 mmHg, unsuitable anatomy for renal denervation, severe renal artery stenosis, eGFR < 45 mL/min/1.73 m², change in BP medication in the 4 weeks preceding randomisation, unwillingness to adhere to unchanging BP medication during the study period of 6 months, unstable angina pectoris, myocardial infarction within 6 months prior to randomisation, planned surgery or cardiovascular intervention within 6 months after randomisation, severe heart valve disease, pregnancy, and severe comorbidities with limited life expectancy | |
| Interventions |
| |
| Outcomes |
| |
| Notes | Intention‐to‐treat and per‐protocol analyses performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "Patients were assigned to the treatment groups by simple randomisation, in a 1:1 ratio, via an internet‐based system using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | Low risk | single blind |
| Blinding of outcome assessment (detection bias) | Low risk | all investigators (including personnel responsible for BP assessment) were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | 8/71 (11%) drop‐outs (6 in RD and 2 in sham procedure); intention‐to‐treat and per‐protocol analyses performed |
| Selective reporting (reporting bias) | Low risk | all the pre‐specified outcomes have been reported |
| Other bias | Unclear risk | no apparent other sources of bias |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | study in abstract version only. Unclear if patients were truly randomised (quote: "21 patients were randomised to PRD. 6 patients served as controls") | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | not specified |
| Selective reporting (reporting bias) | Unclear risk | not specified |
| Other bias | Unclear risk | not specified |
| Methods |
| |
| Participants |
Exclusion criteria: Main renal arteries < 4 mm in diameter or < 20 mm treatable length, multiple renal arteries, renal artery stenosis > 50% or renal artery aneurysm in either renal artery, history of prior renal artery intervention including balloon angioplasty or stenting and unilateral (functional or morphological) kidney, > 1 inpatient hospitalisation for hypertensive crisis not related to non‐adherence to medication within the previous year, type 1 diabetes mellitus and ≥ 1 episodes of orthostatic hypotension not related to medication changes, secondary hypertension | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | not specified |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs. Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | all the pre‐specified outcomes have been reported |
| Other bias | High risk | Honoraria from Medtronic. Involvement of Medtronic in data collection and statistical analyses |
| Methods |
| |
| Participants |
Exclusion criteria: secondary and spurious hypertension, known primary hyperaldosteronism not adequately treated, eGFR < 45 mL/min/1.73 m², urine albumin/creatinine ratio > 50 mg/mmol, type 1 diabetes mellitus, stenotic valvular heart disease, myocardial infarction, unstable angina, or CVA in the prior 6 months, haemodynamically or anatomically significant renal artery abnormalities or stenosis > 50% or prior renal artery intervention, known primary pulmonary hypertension, known pheochromocytoma, Cushing's disease, coarctation of the aorta, hyperthyroidism or hyperparathyroidism | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "randomisation performed using a permuted block randomisation list" |
| Allocation concealment (selection bias) | Low risk | quote: "A hospital employee opened a sealed envelope arranged in a fixed order" |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | High risk | open label |
| Incomplete outcome data (attrition bias) | Low risk | no drop‐outs |
| Selective reporting (reporting bias) | High risk | some pre‐specified outcomes were not reported |
| Other bias | Unclear risk | Honoraria from Medtronic and Hemo Sapiens. Involvement of industry in data collection and analyses not specified |
| Methods |
| |
| Participants |
Exclusion criteria: secondary hypertension, non‐compliance with medical treatment, presence of any chronic renal disease (serum creatinine > 200 µmol/L), pregnancy, history of myocardial infarction or stroke in the previous 6 months, presence of severe valvular stenotic disease, anatomical abnormality or a variant structure of either renal artery, including aneurysm, stenosis, a reference diameter < 4 mm and a length < 20 mm, an increased bleeding risk (thrombocytopenia < 50.000 platelets/µL and an INR > 1.5) | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | 38/101 (37%) drop‐outs (7 in RD and 31 in PHAR group); intention‐to‐treat and per‐protocol analyses performed |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes have been reported |
| Other bias | Unclear risk | No apparent other sources of bias |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Single‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | Not specified |
| Selective reporting (reporting bias) | High risk | Some pre‐specified outcomes were not reported |
| Other bias | Unclear risk | No apparent other sources of bias |
| Methods |
| |
| Participants |
Exclusion criteria: pregnancy, no compliance, heart failure (NYHA 3 to 4), left ventricular ejection fraction < 50%. Unstable coronary heart disease, coronary intervention within 6 months, myocardial infarction within 6 months. Claudication. Orthostatic syncope within 6 months, secondary hypertension, permanent atrial fibrillation. significant heart valve disease. Clinically significant abnormal electrolytes, haemoglobin, liver enzymes and TSH. Second and third degree heart block, macroscopic haematuria, proximal significant coronary stenosis, renal artery anatomy not suitable for renal artery ablation (stenosis, diameter < 4 mm, length < 20 mm, multiple renal arteries, severe calcifications) | |
| Interventions |
| |
| Outcomes |
| |
| Notes | study in abstract version only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | not specified |
| Selective reporting (reporting bias) | High risk | Some pre‐specified outcomes were not reported |
| Other bias | Unclear risk | not specified |
| Methods |
| |
| Participants |
Exclusion criteria: eGFR < 45 mL/min/1.73 m², type 1 diabetes mellitus, contraindications to MRI, substantial stenotic valvular heart disease, pregnancy or planned pregnancy during the study, history of myocardial infarction, unstable angina or cerebrovascular accident in the previous 6 months, haemodynamically significant renal artery stenosis, previous renal artery intervention or renal artery anatomy ineligible for treatment (< 4 mm diameter, < 20 mm length or more than one main renal arteries) | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | quote: "Randomisation was done with sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Data analysers were not masked to treatment assignment |
| Incomplete outcome data (attrition bias) | Low risk | 6/100 (6%) drop‐outs (3 in RD and 3 in control group); quote: "all analyses were done with data for all patients at randomisation minus those lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes have been reported |
| Other bias | High risk | Data were monitored, collected, and managed by the sponsor (Ardian) |
| Methods |
| |
| Participants |
Exclusion criteria: secondary causes of hypertension or more than one hospitalisation for hypertensive emergency in the previous year, primary pulmonary hypertension, 24‐h ABPM average SBP < 135 mmHg, eGFR < 45 mL/min/1.73 m², type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation other than nocturnal respiratory support for sleep apnoea, renal artery stenosis > 50%, renal artery aneurysm, prior renal artery intervention, multiple renal arteries, renal artery diameter < 4 mm or treatable segment < 20 mm in length, myocardial infarction, unstable angina pectoris, syncope or a cerebrovascular accident within 6 months of the screening period, history of pheochromocytoma, Cushing’s disease, coarctation of the aorta, hyperthyroidism or hyperparathyroidism, pregnancy, nursing or planning to be pregnant | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: "Randomization (2:1 ratio) is performed using an interactive voice response system" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Single‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | quote: "Outcome's assessors were blinded to the treatment. Blood pressure assessments were done by blinded, trained personnel". |
| Incomplete outcome data (attrition bias) | Low risk | 16/535 (3%) drop‐outs (14 in RD and 2 in sham procedure); ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes have been reported |
| Other bias | High risk | quote: "Data were collected and analysed by the sponsor (Medtronic, Minneapolis, Minnesota) and independently validated by Harvard Clinical Research Institute (Boston, Massachusetts)" |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | High risk | Open label |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | No apparent other sources of bias |
| Methods |
| |
| Participants |
Exclusion criteria: eGFR < 45 mL/min/1.73 m², type 1 diabetes mellitus, stenotic valvular heart disease and pregnancy. Renal artery anatomy ineligible for treatment (main renal arteries < 4 mm in diameter or < 20 mm in length, abnormality or stenosis in either renal artery, prior renal artery angioplasty or stenting or multiple main renal arteries) | |
| Interventions |
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "All the patients were randomly divided using a computer algorithm into two groups" |
| Allocation concealment (selection bias) | Unclear risk | not specified |
| Blinding of participants and personnel (performance bias) | High risk | open label |
| Blinding of outcome assessment (detection bias) | High risk | open label |
| Incomplete outcome data (attrition bias) | Low risk | no drop‐outs |
| Selective reporting (reporting bias) | Low risk | all the pre‐specified outcomes have been reported |
| Other bias | Unclear risk | no apparent other sources of bias |
Legend
ABPM: ambulatory blood pressure monitoring; ACEi: Angiotensin‐converting enzyme inhibitors; ARBs: Angiotensin receptor blockers; BP: blood pressure; CCBs: calcium channel blockers; CVA: cardiovascular; eGFR: estimated glomerular filtration rate; ITT: intention‐to‐treat; RCT: randomized controlled trial; RD: renal denervation; SBP: systolic blood pressure.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| not RCT | |
| wrong population | |
| not RCT | |
| not RCT | |
| not RCT | |
| wrong intervention | |
| not RCT | |
| not RCT | |
| wrong population | |
| not RCT | |
| not RCT | |
| not RCT | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong population | |
| not RCT | |
| not RCT | |
| wrong population | |
| wrong population | |
| not RCT | |
| not RCT | |
| wrong intervention | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong population | |
| wrong population | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong intervention | |
| wrong population | |
| wrong population | |
| wrong population | |
| wrong population | |
| not RCT | |
| wrong population | |
| wrong population | |
| not RCT | |
| not RCT | |
| wrong population | |
| wrong intervention | |
| wrong intervention | |
| wrong intervention | |
| wrong intervention | |
| wrong intervention | |
| not RCT | |
| wrong intervention | |
| wrong population | |
| not RCT | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong population | |
| wrong population | |
| not RCT | |
| wrong population | |
| not RCT | |
| wrong outcome | |
| wrong intervention | |
| wrong intervention | |
| not RCT | |
| wrong population | |
| not RCT |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Renal denervation by Allegro System in patients with resistant hypertension |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, secondary hypertension. ICD or pacemaker, myocardial infarction, unstable angina, syncope, cerebrovascular accident in the previous 6 months. Intravascular thrombosis or unstable atherosclerotic plaques, significant valvular heart disease. Renal artery stenosis (≥ 50%) or renal artery aneurysm in either renal artery, history of prior renal artery intervention including balloon angioplasty or stenting. Multiple renal arteries where the main renal artery is estimated to supply < 75% of the kidney. Main renal arteries with < 4 mm diameter or with < 20 mm treatable length (by visual estimation), renal artery abnormalities |
| Interventions |
|
| Outcomes |
|
| Starting date | May 2013 |
| Contact information | Xiongjing Jiang: [email protected] |
| Notes |
| Trial name or title | Sympathetic renal denervation versus increment of pharmacological treatment in resistant arterial hypertension |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, secondary hypertension, unsuitable anatomy of renal arteries (diameter < 4 mm and length < 20 mm) including significant (≥ 50%) renal arterial stenosis, renal artery stent, single functional kidney, previous nephrectomy, contrast agent allergy, hyperthyroidia, Treatment with an aldosterone receptor blocker (spironolactone, eplerenone), pre‐randomization serum potassium (K+) level ≥ 5.5 mmol/L, significant renal vascular anomalies, significant valvular heart disease, major vascular event (myocardial infarction, unstable angina or cerebrovascular disease) < 6 months prior to study enrolment |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2012 |
| Contact information | Anna OLiveras, PhD 0034932483162 87052 @parcdesalutmar.cat |
| Notes |
| Trial name or title | Study of catheter‐based renal denervation therapy in hypertension (DEPART) |
| Methods |
|
| Participants |
Exclusion criteria: unsuitable anatomy of renal arteries (diameter < 4 mm and length < 20 mm) including significant (≥ 50%) renal arterial stenosis, renal artery stent or single functional kidney. Secondary hypertension, previous nephrectomy, contrast agent allergy, Hyperthyroidia |
| Interventions |
|
| Outcomes |
|
| Starting date | January 2012 |
| Contact information | Contact: ARGACHA Jean Francois, MD [email protected] |
| Notes |
| Trial name or title | Multi‐center, randomized, single‐blind, sham controlled clinical investigation of renal denervation for uncontrolled hypertension (EnligHTN IV) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension. Previous renal denervation, secondary hypertension, significant renovascular abnormalities. Myocardial infarction, unstable angina pectoris, or cerebrovascular accident < 180 days prior to enrolment. Blood clotting abnormalities, life expectancy < 12 months. Renal arteries < 4 mm in diameter or < 20 mm in length or multiple renal arteries where the main renal arteries supply < 75% of the kidney, abdominal aortic aneurysm (AAA), pheochromocytoma, Cushing's disease, coarctation of the aorta, hyperthyroidism and hyperparathyroidism |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | NA |
| Notes |
| Trial name or title | Effect of renal denervation on arterial stiffness and haemodynamics in patients with uncontrolled hypertension (ENSURE) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension, ABPM 24 hour average SBP < 135 mmHg |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2014 |
| Contact information | Yawei Xu; [email protected] |
| Notes |
| Trial name or title | Investigatorsteered project on intravascular renal denervation for management of drugresistant hypertension (INSPiRED) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, secondary hypertension, unsuitable anatomy of renal arteries (diameter < 4 mm and length < 20 mm) including significant (≥ 50%) renal arterial stenosis, renal artery stent or single functional kidney, isolated systolic or isolated diastolic hypertension, body mass index ≥ 40 kg/m², unstable diabetes mellitus, major cardiovascular events within 6 months prior to enrolment, any serious medical condition, alcohol or substance abuse or psychiatric illnesses, patients on the waiting list of elective surgery |
| Interventions |
|
| Outcomes |
|
| Starting date | April 2014 |
| Contact information | Jan A. Staessen, MD, PhD; [email protected] |
| Notes |
| Trial name or title | Renal protection using sympathetic denervation in patients with chronic kidney disease (KPS) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, significant valvular disease, renovascular abnormalities, secondary hypertension, white coat hypertension |
| Interventions |
|
| Outcomes |
|
| Starting date | November 2013 |
| Contact information | Jean Claude Lubanda, Ass.Prof. MD; Jean‐[email protected] |
| Notes |
| Trial name or title | Full length versus proximal renal arteries ablation |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, significant valvular disease, ICD, renovascular abnormalities, secondary hypertension, white coat hypertension |
| Interventions |
|
| Outcomes |
|
| Starting date | March 2011 |
| Contact information | Yuehui Yin, MD; [email protected] |
| Notes |
| Trial name or title | Effects of renal denervation for resistant hypertension on exercise diastolic function and regression of atherosclerosis and the evaluation of new methods predicting a successful renal sympathetic denervation (RENEWAL‐EXERCISE, ‐REGRESS, and ‐PREDICT Trial From RENEWAL RDN Registry) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2013 |
| Contact information | Yangsoo Jang, MD 82‐2‐2228‐8460, [email protected] |
| Notes |
| Trial name or title | Renal denervation in patients with uncontrolled blood pressure |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension, previous renal denervation. Secondary hypertension, significant renovascular abnormalities, myocardial infarction, unstable angina pectoris, or cerebrovascular accident < 180 days prior to enrolment. Blood clotting abnormalities, life expectancy < 12 months, renal arteries < 4 mm in diameter or < 20 mm in length or multiple renal arteries where the main renal arteries supply < 75% of the kidney, abdominal aortic aneurysm (AAA). Pheochromocytoma, Cushing's disease, coarctation of the aorta, hyperthyroidism, hyperparathyroidism |
| Interventions |
|
| Outcomes |
|
| Starting date | August 2013 |
| Contact information | Ron Waksman, MD |
| Notes |
| Trial name or title | Renal denervation to improve outcomes in patients with end‐stage renal disease |
| Methods |
|
| Participants |
Exclusion criteria: myocardial infarction, unstable angina, cerebrovascular accident within 3 months of the screening visit |
| Interventions |
|
| Outcomes |
|
| Starting date | January 2014 |
| Contact information | Markus P Schlaich, MD Baker IDI Heart and Diabetes Institute |
| Notes |
| Trial name or title | Effect of renal denervation in end staged renal disease with resistant hypertension |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, secondary hypertension. ICD or pacemaker, myocardial infarction, unstable angina pectoris, syncope, cerebrovascular accident in the previous 6 months. Intravascular thrombosis or unstable atherosclerotic plaques, significant valvular heart disease, renal artery stenosis (≥ 50%) or renal artery aneurysm in either renal artery, history of prior renal artery intervention including balloon angioplasty or stenting, multiple renal arteries where the main renal artery is estimated to supply < 75% of the kidney. Main renal arteries with < 4 mm diameter or with < 20 mm treatable length (by visual estimation). Renal artery abnormalities. |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2014 |
| Contact information | Kiyuk Chang, MD, PhD; [email protected] |
| Notes |
| Trial name or title | The Australian SHAM controlled clinical trial of renal denervation in patients with resistant hypertension |
| Methods |
|
| Participants |
Exclusion criteria: renal artery anatomy ineligible for treatment, eGFR < 15mL/min/1.73m² (using MDRD calculation), myocardial infarction, unstable angina or cerebrovascular accident within 3 months of screening visit, life expectancy < 12 months, pregnancy |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2015 |
| Contact information | Markus P Schlaich, Professor +61 3 85321502, [email protected] Murray Esler, Professor +61 3 85321338, [email protected] |
| Notes |
| Trial name or title | High frequency guided renal artery denervation for improving outcome of renal ablation procedure |
| Methods |
|
| Participants |
Exclusion criteria: secondary hypertension, severe renal artery stenosis or dual renal arteries, congestive heart failure, left ventricular ejection fraction < 35%, previous renal artery stenting or angioplasty, type 1 diabetes mellitus |
| Interventions |
|
| Outcomes |
|
| Starting date | February 2013 |
| Contact information | NA |
| Notes |
| Trial name or title | Distal renal denervation |
| Methods |
|
| Participants |
Exclusion criteria: secondary hypertension, 24‐h mean systolic BP < 135 mmHg, eGFR < 30 mL/min/1.73 m², disease of renal artery, any clinically important disorders/comorbidities significantly increasing risk of endovascular intervention |
| Interventions |
|
| Outcomes |
|
| Starting date | January 2013 |
| Contact information | Stanislav Pekarskiy, MD, PhD |
| Notes |
| Trial name or title | Comparison of renal sympathetic denervation with spironolactone in patients with still a high blood pressure despite the use of 3 different antihypertensive agents |
| Methods |
|
| Participants |
Exclusion criteria: secondary hypertension, renal arteries inaccessible for endovascular denervation, suboptimal dosing of BP lowering medication, non compliant to treatment, white coat hypertension, pregnancy, eGFR < 45 mL/min/1.73 m², use of vitamin K antagonist that can not be discontinued for a short period, spironolactone intolerance, myocardial infarction or cerebrovascular accident 3 months prior to randomisation, life expectancy < 2 years |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2012 |
| Contact information | A van den Meiracker, MD, PhD +31‐10‐4639222, [email protected] |
| Notes |
| Trial name or title | A study of renal denervation in patients with treatment resistant hypertension (PaCE) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension, previous renal denervation, secondary hypertension, significant renovascular abnormalities. Myocardial infarction, unstable angina pectoris or cerebrovascular accident < 180 days prior to enrolment. Blood clotting abnormalities, life expectancy < 12 months, renal arteries < 4 mm in diameter or < 20 mm in length or multiple renal arteries where the main renal arteries supply < 75% of the kidney. Pheochromocytoma, Cushing's disease, coarctation of the aorta |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | Harindra C. Wijeysundera, MD |
| Notes |
| Trial name or title | Rapid renal sympathetic denervation for resistant hypertension II (RAPID II) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy,type 1 diabetes mellitus, renal anatomy unsuitable for treatment, significant valvular heart disease, scheduled or planned surgery within 6 months of study entry |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2013 |
| Contact information | Dierk Scheinert, MD Guiseppe Mancia, MD Universita Milano‐Bicocca, Ospedale San Gerardo di Monza |
| Notes |
| Trial name or title | Renal denervation for resistant hypertension (RDNP‐2012‐01) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, unsuitable anatomy of renal arteries (diameter < 4 mm and length < 20 mm) |
| Interventions |
|
| Outcomes |
|
| Starting date | February 2012 |
| Contact information | Markus Schlaich, MD Baker IDI Heart & Diabetes Institute |
| Notes |
| Trial name or title | Effect of renal denervation on no‐mediated sodium excretion and plasma levels of vasoactive hormones (RENO) |
| Methods |
|
| Participants |
Exclusion criteria: non‐compliance, pregnancy, radiocontrast allergy, malignancy, congestive heart failure, unstable angina pectoris, previous myocardial infarction or PCI (< 6 mdr), secondary hypertension, renal artery stenosis or multiple renal arteries on CT, claudication |
| Interventions |
|
| Outcomes |
|
| Starting date | March 2012 |
| Contact information | Esper N Bech, MD, Ph.D; [email protected] |
| Notes |
| Trial name or title | Renal sympathetic denervation and insulin sensitivity (RENSYMPIS Study) |
| Methods |
|
| Participants |
Exclusion criteria: secondary hypertension, pseudohypertension, pregnancy, significant stenotic valvular disease, oral anticoagulation, CCS III‐IV symptoms or CABG/PCI in the previous 6 months, prior stroke, contrast agent allergy, inappropriate renal artery anatomy (< 4mm diameter, < 20mm length) |
| Interventions |
|
| Outcomes |
|
| Starting date | January 2013 |
| Contact information | Tuomas Paana, M.D; [email protected] |
| Notes |
| Trial name or title | Renal denervation in treatment resistant hypertension (ReSET‐2) |
| Methods |
|
| Participants |
Exclusion criteria: pregnancy, non compliance, heart failure (NYHA 3‐4), Left ventricular ejection fraction < 50%, unstable coronary heart disease, coronary intervention within 6 months, myocardial infarction within 6 months, claudication, orthostatic syncope within 6 months, secondary hypertension, permanent atrial fibrillation, significant heart valve disease. Clinically significant abnormal electrolytes, haemoglobin, liver enzymes and TSH. Second and third degree heart block, macroscopic haematuria, proximal significant coronary stenosis, renal artery anatomy not suitable for renal ablation (stenosis, diameter < 4 mm, length < 20 mm, multiple renal arteries, severe calcifications). Moderate/severe obstructive sleep apnoea (AHI > 15) if on CPAP treatment |
| Interventions |
|
| Outcomes |
|
| Starting date | January 2013 |
| Contact information | Henrik Vase, MD, PhD [email protected] Ole Mathiassen, MD, PhD [email protected] |
| Notes |
| Trial name or title | Renal sympathetic denervation in patients with chronic kidney disease and resistant hypertension (RSD4CKD) |
| Methods |
|
| Participants |
Exclusion criteria: treatment with corticosteroids, nonsteroidal antiinflammatory or immunosuppressive drugs, connective‐tissue disease, obstructive uropathy, congestive heart failure (NYHA class III or IV), significant renovascular abnormalities (history of prior renal artery intervention, including balloon angioplasty or stenting; double renal artery on one side, distortion, and extension), measured by abdominal ultrasound or renal angiograms. History of myocardial infarction, unstable angina, cerebrovascular accident or alimentary tract haemorrhage in the previous 3 months, sick sinus syndrome, history of allergy to contrast media, psychiatric disorders, drug or alcohol abuse and pregnancy |
| Interventions |
|
| Outcomes |
|
| Starting date | November 2012 |
| Contact information | Shan Qi jun; [email protected] |
| Notes |
| Trial name or title | Renal sympathetic denervation in patients with drug‐resistant hypertension and symptomatic atrial fibrillation (RSDforAF) |
| Methods |
|
| Participants |
Exclusion Criteria: pregnancy, type 1 diabetes mellitus, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension, white‐coat hypertension, previous renal denervation, secondary hypertension, significant renovascular abnormalities, myocardial infarction, unstable angina pectoris or cerebrovascular accident < 180 days prior to enrolment. Blood clotting abnormalities, life expectancy < 12 months, renal arteries < 4 mm in diameter or < 20 mm in length or multiple renal arteries where the main renal arteries supply < 75% of the kidney. Pheochromocytoma, Cushing's disease, coarctation of the aorta, severely enlarged left atria ≥ 55 mm, sick sinus syndrome, reversible causes of AF |
| Interventions |
|
| Outcomes |
|
| Starting date | July 2012 |
| Contact information | Qijun Shan; [email protected] |
| Notes |
| Trial name or title | Renal sympathetic denervation as a new treatment for therapy resistant hypertension (SYMPATHY) |
| Methods |
|
| Participants |
Exclusion Criteria: Pregnancy, type 1 diabetes mellitus, eGFR (mL/min/1.73 m²) < 20, chronic oxygen support or mechanical ventilation, primary pulmonary hypertension, white‐coat hypertension, previous renal denervation, secondary hypertension, significant renovascular abnormalities. Myocardial infarction, unstable angina pectoris or cerebrovascular accident < 180 days prior to enrolment. Blood clotting abnormalities, life expectancy < 12 months, renal arteries < 4 mm in diameter or < 20 mm in length or multiple renal arteries where the main renal arteries supply < 75% of the kidney. Pheochromocytoma, Cushing's disease, coarctation of the aorta |
| Interventions |
|
| Outcomes |
|
| Starting date | May 2013 |
| Contact information | Peter J Blankestijn, MD, PhD; [email protected] |
| Notes |
| Trial name or title | Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN‐4) |
| Methods |
|
| Participants |
Exclusion Criteria: pregnancy, inappropriate renal artery anatomy, type 1 diabetes mellitus, one or more episodes of orthostatic hypotension, chronic oxygen other than nocturnal respiratory support for sleep apnoea, primary pulmonary hypertension, previous organ transplant |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | David Kandzari, MD Piedmont Heart Institute |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 4 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.45, 3.84] |
| Analysis 1.1 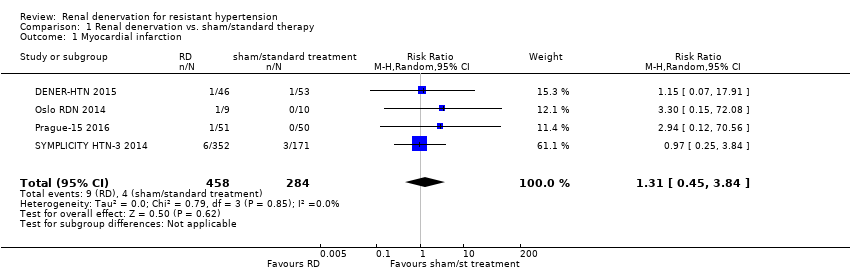 Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 1 Myocardial infarction. | ||||
| 2 ischaemic stroke Show forest plot | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.72] |
| Analysis 1.2  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 2 ischaemic stroke. | ||||
| 3 unstable angina Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.06] |
| Analysis 1.3  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 3 unstable angina. | ||||
| 4 systolic 24‐hour ABPM Show forest plot | 5 | 797 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐3.74, 4.29] |
| Analysis 1.4 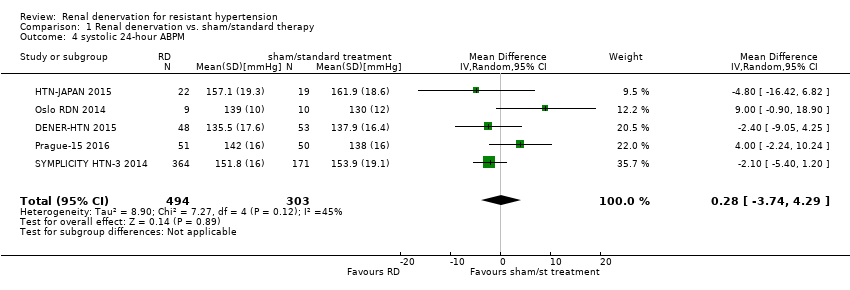 Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 4 systolic 24‐hour ABPM. | ||||
| 5 diastolic 24‐hour ABPM Show forest plot | 4 | 756 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐4.50, 6.36] |
| Analysis 1.5  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 5 diastolic 24‐hour ABPM. | ||||
| 6 systolic office BP Show forest plot | 6 | 886 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐15.26, 7.11] |
| Analysis 1.6  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 6 systolic office BP. | ||||
| 7 diastolic office BP Show forest plot | 5 | 845 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐7.30, 4.69] |
| Analysis 1.7  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 7 diastolic office BP. | ||||
| 8 serum creatinine Show forest plot | 3 | 736 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| Analysis 1.8  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 8 serum creatinine. | ||||
| 9 eGFR/creatinine clearance Show forest plot | 4 | 837 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐8.12, 3.95] |
| Analysis 1.9 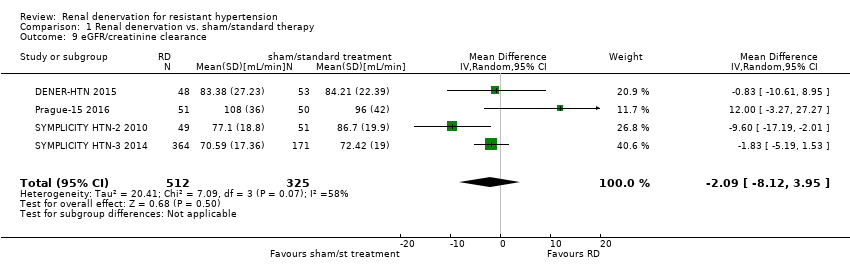 Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 9 eGFR/creatinine clearance. | ||||
| 10 bradycardia Show forest plot | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [1.19, 36.84] |
| Analysis 1.10  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 10 bradycardia. | ||||
| 11 femoral artery pseudoaneurysm Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.96 [0.44, 35.22] |
| Analysis 1.11  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 11 femoral artery pseudoaneurysm. | ||||
| 12 flank pain Show forest plot | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.48, 38.28] |
| Analysis 1.12  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 12 flank pain. | ||||
| 13 hypotensive episodes Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.07, 6.64] |
| Analysis 1.13  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 13 hypotensive episodes. | ||||
| 14 hypertensive crisis Show forest plot | 3 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| Analysis 1.14 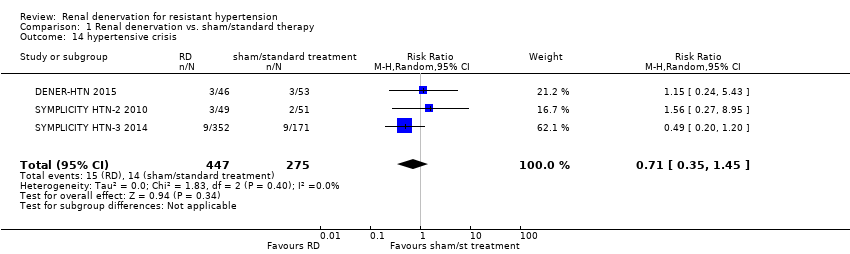 Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 14 hypertensive crisis. | ||||
| 15 hyperkalemia Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.01, 21.33] |
| Analysis 1.15  Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 15 hyperkalemia. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 1 Myocardial infarction.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 2 ischaemic stroke.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 3 unstable angina.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 4 systolic 24‐hour ABPM.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 5 diastolic 24‐hour ABPM.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 6 systolic office BP.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 7 diastolic office BP.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 8 serum creatinine.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 9 eGFR/creatinine clearance.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 10 bradycardia.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 11 femoral artery pseudoaneurysm.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 12 flank pain.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 13 hypotensive episodes.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 14 hypertensive crisis.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 15 hyperkalemia.
| Renal denervation versus sham denervation or standard treatment | |||||
| Patient or population: people with resistant hypertension | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect estimate | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Sham denervation/ Standard treatment | Renal denervation | ||||
| myocardial infarction | 14 per 1000 | 18 per 1000 (6 to 54) | RR 1.31 (0.45 to 3.84) | 742 (4 studies) | ⊕⊕⊝⊝ |
| ischaemic stroke | 12 per 1000 | 14 per 1000 (4 to 45) | RR 1.15 (0.36 to 3.72) | 823 (4 studies) | ⊕⊕⊝⊝ |
| unstable angina | 20 per 1000 | 12 per 1000 (2 to 101) | RR 0.63 (0.08 to 5.06) | 201 (2 studies) | ⊕⊕⊝⊝ |
| systolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.28 (‐3.74 to 4.29) | 797 | ⊕⊕⊕⊝ |
| diastolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.93 (‐4.50 to 6.36) | 756 | ⊕⊕⊕⊝ |
| systolic office BP (mmHg) | ‐ | ‐ | MD ‐4.08 (‐15.26 to 7.11) | 886 | ⊕⊕⊕⊝ |
| diastolic office BP (mmHg) | ‐ | ‐ | MD ‐1.30 (‐7.30 to 4.69) | 845 | ⊕⊕⊕⊝ |
| eGFR or creatinine clearance (mL/min/1.73m²) | ‐ | ‐ | MD ‐2.09 (‐8.12 to 3.95) | 837 | ⊕⊕⊕⊝ |
| *The assumed risk is the observed risk in the reference (control) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence Legend 1. Wide confidence intervals. 2. Only reported by less than half of the studies. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 4 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.45, 3.84] |
| 2 ischaemic stroke Show forest plot | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.72] |
| 3 unstable angina Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.06] |
| 4 systolic 24‐hour ABPM Show forest plot | 5 | 797 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐3.74, 4.29] |
| 5 diastolic 24‐hour ABPM Show forest plot | 4 | 756 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐4.50, 6.36] |
| 6 systolic office BP Show forest plot | 6 | 886 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐15.26, 7.11] |
| 7 diastolic office BP Show forest plot | 5 | 845 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐7.30, 4.69] |
| 8 serum creatinine Show forest plot | 3 | 736 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 9 eGFR/creatinine clearance Show forest plot | 4 | 837 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐8.12, 3.95] |
| 10 bradycardia Show forest plot | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [1.19, 36.84] |
| 11 femoral artery pseudoaneurysm Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.96 [0.44, 35.22] |
| 12 flank pain Show forest plot | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.48, 38.28] |
| 13 hypotensive episodes Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.07, 6.64] |
| 14 hypertensive crisis Show forest plot | 3 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| 15 hyperkalemia Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.01, 21.33] |


