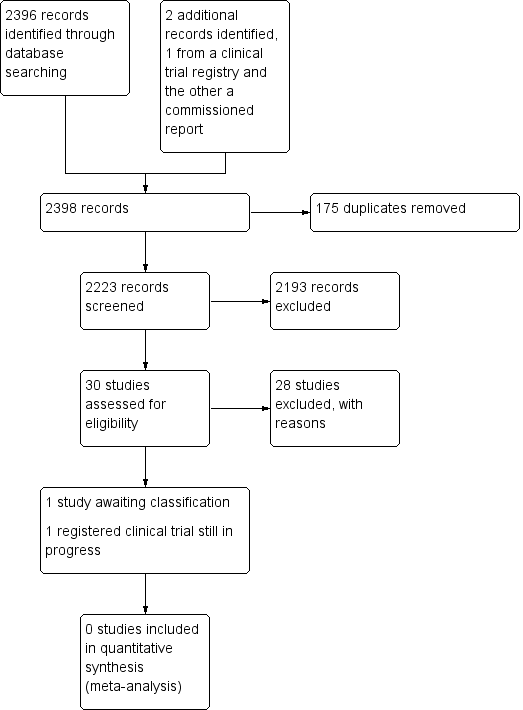
Equipos de especialistas en acceso vascular para la inserción de dispositivos y la prevención de fracasos
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Catheterization, Central Venous] explode all trees
#2 MeSH descriptor: [Catheterization, Peripheral] explode all trees
#3 MeSH descriptor: [Catheters, Indwelling] explode all trees
#4 MeSH descriptor: [Catheterization, Swan‐Ganz] explode all trees
#5 MeSH descriptor: [Catheterization] explode all trees
#6 (#1 or #2 or #3 or #4 or #5) and (team* or clinician* or specialist*):ti,ab
#7 ((clinician* or specialist*) near/4 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) near/4 team*)
#8 #6 or #7
Appendix 2. MEDLINE (Ovid SP) search strategy
1 ((Catheterization, Central Venous/ or Catheterization, Peripheral/ or Catheters, Indwelling/ or Catheterization/ or Catheterization, Swan‐Ganz/) and (team* or clinician* or specialist*).ti,ab.) or ((clinician* or specialist*) adj5 (inserter* or mainte* or vascular access*)).mp. or ((vascular access or intravenous therap* or IV) adj5 team*).mp.
2 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
3 1 and 2
Appendix 3. Embase (Ovid SP) search strategy
1. ((central venous catheterization/ or indwelling catheter/ or catheterization/ or pulmonary artery catheterization/) and (team* or clinician* or specialist*).ti,ab.) or ((clinician* or specialist*) adj4 (inserter* or mainte* or vascular access*)).mp. or ((vascular access or intravenous therap* or IV) adj5 team*).mp.
2. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh.
3. 1 and 2
Appendix 4. CINAHL (EBSCO) search strategy
S1 ( (MM "Catheterization") OR (MM "Catheterization, Central Venous") OR (MM "Catheterization, Peripheral") OR (MM "Catheterization, Peripheral Central Venous") ) AND ( team* or clinician* or specialist* ) or (AB ((clinician* or specialist*) N5 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) N5 team*) ) OR ( TI ((clinician* or specialist*) N5 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) N5 team*) )
S2 random* or multicenter* or prospective* or ((clinical or controlled) and trial*) or ((blind* or mask*) and (single or double or triple or treble))
S3 S1 and S2
Appendix 5. ISI Web of Science search strategy
#1 TI=(catheter* SAME (team* or clinician* or specialist*)) or TI=((clinician* or specialist*) SAME (inserter* or mainte* or vascular access*)) or TS=((vascular access or intravenous therap* or IV) SAME team*)
#2 TS=(random* or multicenter* or prospective* or ((clinical or controlled) SAME trial*) or ((blind* or mask*) SAME (single or double or triple or treble)))
#3 #1 and #2
Appendix 6. Data extraction tool
Data extraction sheet
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published (e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Report title (title of paper/ abstract/ report that data are extracted from) | |
| Report ID (ID for this paper/ abstract/ report) | |
| Reference details | |
| Report author contact details | |
| Publication type (e.g. full report/abstract/letter) | |
| Study funding sources (including role of funders) | |
| Possible conflicts of interest (for study authors) | |
| Notes: | |
2. Study eligibility
| Study characteristics | Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the protocol) | Yes | No | Unclear | Location in text (pg & ¶/fig/table) | |
| Type of study | Randomized controlled trial | |||||
| Participants | Adult/paediatric recipients of VAD Hospital/community setting | |||||
| Types of intervention | VAD insertion by a VAST Designation of VAST (infusion therapist, IV team, VA team, other specialist inserting VADs) | |||||
| Types of comparison | VAD insertion by medical doctor VAD insertion by nurse VAD insertion by physician assistant VAD insertion by technician | |||||
| Types of outcome measures | Primary outcomes First‐time insertion success Cost Complication or adverse events and time of
Secondary outcomes Device failure and time of as a result of
Patient satisfaction Staff satisfaction Length of hospital stay | |||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; IV: intravenous; pg: page; VA: vascular access; VAD: vascular access device; VAST: vascular access specialist team.
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
| Description Include comparative information for each group (i.e. intervention and controls) if available | Location in text (pg & ¶/fig/table) | ||
| Population description (from which study participants are drawn) | |||
| Setting (including location and social context) | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Notes: | |||
¶: paragraph; fig: figure; pg: page.
4. Methods
| Descriptions as stated in report/paper | Location in text (pg & ¶/fig/table) | ||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
| Unit of allocation (by individuals, cluster/ groups or body parts) | |||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval obtained for study | Yes No Unclear | ||
| Notes: | |||
¶: paragraph; fig: figure; pg: page.
5. Risk of bias assessment
| Domain | Risk of bias | Support for judgement | Location in text (pg & ¶/fig/table) | ||
| Low risk | High risk | Unclear | |||
| Random sequence generation (selection bias) | |||||
| Allocation concealment (selection bias) | |||||
| Blinding of participants and personnel (performance bias) | Outcome group: First time insertion success | ||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
| Blinding of outcome assessment (detection bias) | Outcome group: First‐time insertion success | ||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
| Incomplete outcome data (attrition bias) | First‐time insertion success | ||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
| Selective outcome reporting? (reporting bias) | |||||
| Other bias | |||||
| Notes: | |||||
¶: paragraph; fig: figure; pg: page; VAD: vascular access device.
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |
| Total no. randomized (or total population at start of study for NRCTs) | ||
| Clusters (if applicable, number, type, number of people per cluster) | ||
| Baseline imbalances | ||
| Withdrawals and exclusions (if not provided below by outcome) | ||
| Age | ||
| Sex | ||
| Race/ethnicity | ||
| Severity of illness Community/ inpatient Critical care area | ||
| Co‐morbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant socio‐demographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
¶: paragraph; fig: figure; NRCT: non‐randomized controlled trial; pg: page.
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention group 1
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |
| Intervention (e.g. VAD inserted by member of VAST) | ||
| VAD(i.e. type of VAD) | ||
| Description(e.g. clinical practice such as advanced technology ultrasound used) | ||
| Number randomized to group(specify whether number of people, VADs or clusters) | ||
| Training and education of VAST clinician | ||
| Timing(e.g. 5 day service, on call or 24/7) | ||
| Delivery(e.g. role and responsibility of VAST, autonomous/independent clinicians) | ||
| Providers and model (e.g. profession, nurse led/ medical model) | ||
| Co‐interventions | ||
| Economic variables | ||
| Resource requirements to replicate intervention (e.g. staff numbers, equipment) | ||
| Notes: | ||
¶: paragraph; fig: figure; pg: page; VAD: vascular access device; VAST: vascular access specialist team.
8. Outcomes
We will utilize this table for each primary and secondary outcome.
Outcome 1
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | ||
| Outcome name Primary 1 (P1) Secondary 1 (S1) | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
| Unit of measurement (if relevant) | |||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data | |||
| Assumed risk estimate (e.g. baseline or population risk noted in Background) | |||
| Power (sample size calculations) | |||
| Notes: | |||
¶: paragraph; fig: figure; ITT: intention to treat; pg: page.
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
9.1 Dichotomous outcome
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| Number missing participants and reasons | ||||||
| Number participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||
| Re‐analysis possible? | Yes No Unclear | |||||
| Re‐analysed results | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; pg: page.
9.2 Continuous outcome (repeat as necessary)
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results (We will specify is our results are catheters or days) | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Number missing participants and reasons | ||||||||||
| Number participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
| Unit of analysis (individuals, cluster/ groups or body parts) | ||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||||||
| Re‐analysis possible? | Yes No Unclear | |||||||||
| Re‐analysed results | ||||||||||
| Notes: | ||||||||||
¶: paragraph; fig: figure; pg: page; SD: standard deviation.
Other information
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| Number participants | Intervention | Control | ||||
| Number missing participants and reasons | ||||||
| Number participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||
| Re‐analysis possible? | Yes No Unclear | |||||
| Re‐analysed results | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; pg: page; SD: standard deviation; SE: standard error.
10. Applicability
| Have important populations been excluded from the study?(consider high‐risk populations, and possible differences in the intervention effect) | Yes No Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear | |
| Does the study directly address the review question? (any issues of partial or indirect applicability) | Yes No Unclear | |
| Notes: | ||
¶: paragraph; fig: figure; pg: page.
11. Other information
| Description as stated in report/paper | Location in text (pg & ¶/fig/table) | |
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
¶: paragraph; fig: figure; pg: page.
Appendix 7. 'Summary of findings' and quality of evidence
Insertion of a vascular access device by a VAST
Patients or population: people receiving vascular access device.
Setting: inpatient setting and outpatient/community setting.
Intervention: insertion or care (or both) of a vascular access device by a member of a VAST.
Comparison: clinicians inserting these devices who exist outside of a defined VAST.
Definitions: CVC: central venous catheter; IO: intraosseous; PICC: peripherally inserted central catheter; VAD: vascular access device; VAST: vascular access specialist team.
| Primary outcomes | Illustrative comparison risk | Relative effect | Number of participants | Quality of evidence | Comments |
| First‐time insertion success | |||||
| Economic cost‐benefit analysis | |||||
| Adverse events/complications (occlusion, phlebitis, infiltration/extravasation, thrombosis, catheter‐related bloodstream infection, pneumothorax/haemothorax, arterial puncture) | |||||
| Device failure with dwell time | |||||
| Secondary outcomes | Illustrative comparison risk | Relative effect | Number of participants | Quality of evidence | Comments |
| Patient satisfaction | |||||
| Staff satisfaction | |||||
| Length of hospital stay |
Sensitivity analysis
| Effectiveness: Paediatric Adult | |||||
| Specific VAD inserted by VAST (arterial, peripheral, PICC, CVC, tunnelled, IO, other ‐ will specify) | |||||
| VAST make‐up (model and members such as nursing/medical/technicians/physician assistants) |
CVC: central venous catheter; IO: intraosseous; PICC: peripherally inserted central catheter; VAD: vascular access device; VAST: vascular access specialist team.