Dosis única de dipirona (metamizol) para el dolor postoperatorio agudo en adultos
Information
- DOI:
- https://doi.org/10.1002/14651858.CD011421.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 20 April 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
All review authors contributed to writing the revised protocol.
LH and SD searched for studies, selected studies for inclusion, and carried out data extraction.
RAM acted as arbitrator.
All authors were involved in analysis and writing the full review.
SD will be responsible for updating the review, if appropriate.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
No sources of support supplied
Declarations of interest
LH none known
SD none known
RAM has received institutional grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has attended boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Acknowledgements
This review received infrastructure support from the Oxford Pain Relief Trust.
Funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Apr 20 | Single dose dipyrone (metamizole) for acute postoperative pain in adults | Review | Leslie Hearn, Sheena Derry, R Andrew Moore | |
| 2014 Dec 05 | Single dose dipyrone (metamizole) for acute postoperative pain | Protocol | Leslie Hearn, Sheena Derry, R Andrew Moore | |
Differences between protocol and review
We have assessed the quality of the evidence using the GRADE approach and included a 'Summary of findings' table in the full review, which were not included in the Protocol.
Notes
For the latest update (2016) we did not identify any potentially relevant studies that were published after 1999. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Female; Humans; Male;
PICOs
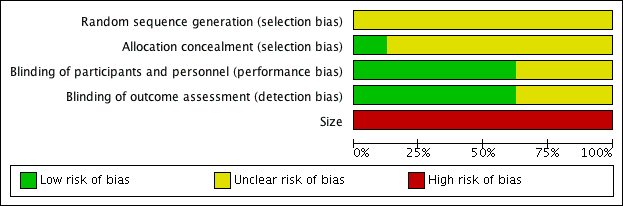
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Oral dipyrone 500 mg versus placebo, outcome: 1.1 Participants with ≥ 50% pain relief over 4 to 6 hours.

Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4 to 6 hours.
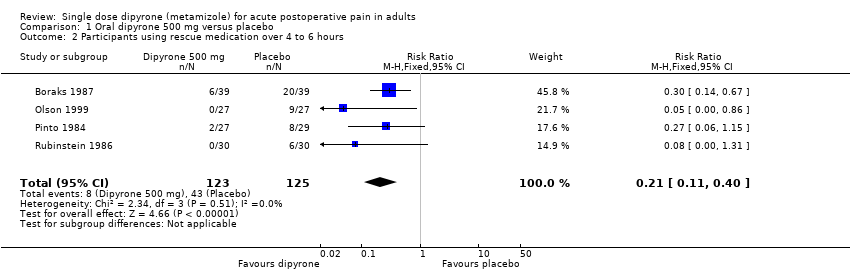
Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 2 Participants using rescue medication over 4 to 6 hours.
| Oral dipyrone 500 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with acute postoperative pain Settings: clinic Intervention: oral dipyrone 500 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Relative effect and NNT or NNTp | Number of studies, participants, events | Quality of the evidence | Comments | |
| intervention | comparator | |||||
| At least 50% of maximum pain relief over 4 to 6 h | 730 in 1000 | 320 in 1000 | RR 2.4 (95% CI 1.8 to 3.1) NNT 2.4 (1.9 to 3.1) | 5 studies, 288 participants, 151 events | Moderate | Small studies, few events |
| Participants remedicating within 4 to 6 h | 70 in 1000 | 340 in 1000 | RR 0.21 (0.11 to 0.40) NNTp 3.6 (2.7 to 5.4) | 4 studies, 248 participants, 51 events | Low | Small studies, very few events |
| Participants with at least one adverse event | Insufficient data for analysis | ‐ | ‐ | ‐ | ‐ | |
| Participants with a serious adverse event | None reported | None reported | ‐ | 5 studies, 288 participants, no events | Very low | Small studies, no events |
| CI: confidence interval; h: hour; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4 to 6 hours Show forest plot | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.84, 3.11] |
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |


