Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Phase II dose‐ranging randomised controlled study, participant and assessor blinded. 5 parallel groups. Participants enrolled into 3 consecutive cohorts based on efficacy and safety results of previous cohort.
Liposomal bupivacaine or control administered in a staged fashion starting after dissection but before prostheses insertion with the final injections administered before wound closure | |
| Participants | People undergoing primary unilateral total knee replacement under general anaesthesia (n = 138) Age 18‐75 years ASA 1‐3 Location: 10 centres (USA and Czech Republic) Dates: October 2007‐November 2008 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration using a standardised technique Control:
Intervention:
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00485693 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated via computer randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation separate to trial sites |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded. Personnel preparing and administering the study drug and control (who were not involved in post‐operative assessments) were not blinded but the injection technique was specified to decrease the risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Staff performing post operative assessment were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate, 6 of 138 participants did not complete the study |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of outcomes of interest as stated in study methods |
| Other bias | High risk | High risk of bias ‐ sample size < 50 participants/arm Unclear risk of bias ‐ funding provided by Pacira Pharmaceuticals as well as 2 authors were employees or consultants for Pacira |
| Methods | Phase III RCT, participant and assessor blinded. 2 parallel groups. Liposomal bupivacaine or control administered intra‐operatively (timing not specified) | |
| Participants | People undergoing primary first metatarsal bunionectomy under midazolam and/or propofol sedation followed by a Mayo block (n = 193) Age 18 years and older Location: 1 centre (USA) Dates: April 2000‐August 2009 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration. The infiltration technique was not specified Control:
Intervention:
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00890682 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated via computer randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation separate to trial sites |
| Blinding of participants and personnel (performance bias) | Unclear risk | It was not stated whether the surgeon was blinded and no standard injection technique was specified presenting a risk of performance bias. As such we considered this study to have an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Staff performing post operative assessment were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Data exist for all randomised participants |
| Selective reporting (reporting bias) | Low risk | All stated outcomes of interest reported |
| Other bias | Unclear risk | Unclear risk of bias ‐ sample size 50‐199 participants/arm Funding provided by Pacira Pharmaceuticals as well as author was employee of Pacira |
| Methods | Phase III RCT, participant and assessor blinded. 2 parallel groups. Liposomal bupivacaine or control administered at the end of surgery | |
| Participants | People undergoing excisional haemorrhoidectomy (Miller‐Morgan technique) under general anaesthesia (n = 189) Age 18 years and older ASA 1‐3 Location: 13 centres (Republic of Georgia, Poland and Serbia) Dates: May 2009‐August 2009 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration using a standardised technique Control:
Intervention:
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00890721 Trial also reported by Schmidt 2012 (Secondary reference of Gorfine 2011) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded as were personnel involved in administering the study drug and control. Furthermore, the injection technique was specified to decrease the risk of performance bias due to the risk of unbinding due to differences in appearance and viscosity of the trial drug with the control. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and the study team performing post‐operative assessments were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate, 3 of 189 participants did not complete the study |
| Selective reporting (reporting bias) | Low risk | All stated outcomes of interest reported |
| Other bias | Unclear risk | Unclear risk of bias ‐ sample size 50‐199 participants/arm Funded by Pacira Pharmaceuticals |
| Methods | Phase II dose‐ranging RCT, participant and assessor blinded. 4 parallel groups Participants enrolled into 2 consecutive cohorts based on efficacy and safety results of previous cohort:
Liposomal bupivacaine or control administered at the end of surgery | |
| Participants | People undergoing 2 or 3 column excisional haemorrhoidectomy (incision length > 3 cm) under general anaesthesia (n = 100) Age 18 years and older ASA 1‐3 Location: 9 centres (USA and Republic of Georgia) Dates: July 2007‐January 2008 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration using a standardised technique Control:
Intervention:
| |
| Outcomes | Outcomes
| |
| Notes | Clinical Trials reference: NCT 00529126 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Low risk | To reduce the risk of performance bias, drugs were dispensed by sheathed syringe by study members not involved with postoperative assessment. Furthermore the injection technique specified to further reduce the risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | All staff members involved in study related evaluation remained blinded throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate, 97 of 100 of participants completed the study |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of: all time point NRS, discharge readiness and EQ5D |
| Other bias | High risk | High risk of bias ‐ sample size < 50 participants/arm Unclear risk of bias ‐ support in preparation of the manuscript was provided by Peloton Advantage, supported by Pacira Pharmaceuticals |
| Methods | Phase II dose‐ranging parallel group RCT, participant and assessor blinded Participants enrolled into 4 consecutive cohorts based on efficacy and safety results of previous cohort
Liposomal bupivacaine or control administered at the end of surgery before wound closure | |
| Participants | People undergoing unilateral inguinal hernia repair (tension‐free technique) under general anaesthesia (n = 76) Age 18 years and older ASA 1‐2 Location: not specified Dates: December 2004‐December 2006 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration. The infiltration technique was not specified Control:
Intervention:
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 01203644 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | It is stated that the participant was blinded to treatment, however it is not specified whether the surgeon administering the treatment was blinded presenting an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is stated that the participant and the outcome assessor were blinded to treatment, however it is not clear whether other staff involved in the participants care were blinded presenting an unclear risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes specified reported |
| Other bias | High risk | High risk of bias ‐ sample size < 50 participants/arm Unclear risk of bias ‐ funding received from Pacira Pharaceuticals |
| Methods | Phase III RCT, participant and assessor blinded. 2 parallel groups. Liposomal bupivacaine or control administered at the end of surgery | |
| Participants | People undergoing 2 or 3 column excisional haemorrhoidectomy under general anaesthesia (n = 204) Age 18 years and older ASA 1‐4 Location: 20 centres (SA) Dates: August 2008‐February 2009 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration using a standardised technique Control:
Intervention:
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00744848 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | It was not stated whether the surgeon was blinded or whether a standard injection technique was specified. As such we considered this study to have an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is stated that the participant and the outcome assessor were blinded to treatment, however it is not clear whether other staff involved in the participants care were blinded presenting an unclear risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes specified on clinical trials registry reported |
| Other bias | Unclear risk | Unclear risk of bias ‐ sample size 50‐199 participants/arm Funded by Pacira Pharmaceuticals |
| Methods | Phase III RCT, participant and assessor blinded. 2 parallel groups. Liposomal bupivacaine or control administered intra‐operatively (timing not specified) | |
| Participants | People undergoing primary unilateral total knee replacement under general anaesthesia (n = 245) Age 18 years and older ASA 1‐4 Location: 19 centres (USA) Dates: August 2008‐January 2009 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration. The infiltration technique was not specified Control
Intervention
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00745290 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | It is stated that the participant was blinded to treatment, however it is not specified whether the surgeon administering the treatment was blinded presenting an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is stated that the participant and the outcome assessor were blinded to treatment, however it is not clear whether other staff involved in the participants' care were blinded presenting an unclear risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes specified on clinical trials registry reported |
| Other bias | Unclear risk | Unclear risk of bias ‐ sample size 50‐199 participants/arm Funded by Pacira Pharmaceuticals |
| Methods | Phase III RCT, participant and assessor blinded. 2 parallel groups. Liposomal bupivacaine or control administered at the end of surgery | |
| Participants | Women undergoing primary bilateral cosmetic submuscular breast augmentation under general anaesthesia (n = 134) Age 18 years and older ASA 1‐4 Location: 11 centres (USA) Dates: November 2008‐February 2009 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration. The infiltration technique was not specified Control
Intervention
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00813111 Long‐term follow‐up reported by Minkowitz 2012 (Secondary reference of Smoot 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated via computer randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation separate to trial sites |
| Blinding of participants and personnel (performance bias) | High risk | Staff and participants were blinded to treatment. However there was a high risk of performance bias with respect to the injection technique as no standard injection technique was specified with injections administered "by the surgeon’s preferred technique" presenting a high risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Staff performing outcome assessments blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | High risk | Trial terminated by study sponsor due to "administrative reasons" |
| Selective reporting (reporting bias) | Low risk | All outcomes specified reported |
| Other bias | Unclear risk | Unclear risk of bias ‐ sample size 50‐199 participants/arm Funding received from Pacira Pharmaceuticals |
| Methods | Phase II dose‐ranging RCT, participant and assessor blinded. 4 parallel groups Timing of liposomal bupivacaine or control administration not specified | |
| Participants | People undergoing primary open inguinal hernia repair under general anaesthesia (n = 98) Age 18 years and over ASA 1‐3 Location: 7 centres (USA) Dates: June 2007‐August 2008 | |
| Interventions | A single dose of the control or intervention drug was administered at the time of operation via wound infiltration. The infiltration technique was not specified. Control
Intervention
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Clinical Trials reference: NCT 00485433 This conference abstract also reported the outcomes of the study reported by Langford 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised; randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | It is stated that the participant was blinded to treatment, however it is not specified whether the surgeon administering the treatment was blinded presenting an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is stated that the participant and the outcome assessor were blinded to treatment, however it is not clear whether other staff involved in the participants' care were blinded presenting an unclear risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes specified on clinical trials registry reported |
| Other bias | High risk | High risk of bias ‐ sample size < 50 participants/arm Unclear risk of bias ‐ 2 authors were linked to Pacira Pharmaceutical |
AE – Adverse Events
ASA – American Society of Anaesthesiologists Score
AUC – Area Under Curve
BPI – Brief Pain Inventory
NRS – Numeric Rating Scale
RCT ‐ randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Open label sequential cohort study | |
| Open label sequential cohort study | |
| Review paper evaluating wound healing following liposomal bupivacaine at the surgical site | |
| Review paper evaluating the cardiac safety of liposomal bupivacaine after surgical site infiltration | |
| Review paper evaluating the efficacy of liposomal bupivacaine when infiltrated at the surgical site | |
| Open label sequential cohort study | |
| Open label sequential cohort study | |
| RCT evaluating the efficacy of liposomal bupivacaine at the surgical site during total knee replacement. Study excluded as not double blind with the outcome assessors not blinded to randomisation | |
| Review paper evaluating the efficacy of liposomal bupivacaine when infiltrated at the surgical site | |
| Open label sequential cohort study | |
| Review paper evaluating the pharmacokinetics of liposomal bupivacaine at the surgical site | |
| RCT evaluating the efficacy of liposomal bupivacaine at the laparoscopic port site during laparoscopic urologic surgery. Study excluded as liposomal bupivacaine assessed was not administered at the surgical site (kidney/renal tract/prostate) | |
| Open label sequential cohort study | |
| Open label sequential cohort study | |
| Women undergoing bilateral breast augmentation. Each participant was used as their own control and as such we excluded this study the review | |
| Women undergoing bilateral breast augmentation. Each participant was used as their own control and as such we excluded this study from the review | |
| RCT evaluating the efficacy of liposomal bupivacaine at the surgical site for the management of pain following total knee arthroplasty. This trial compared 266 mg liposomal bupivacaine mixed with 75 mg bupivacaine hydrochloride against an active control arm of 150 mg bupivacaine hydrochloride. At the time of writing the trial protocol it was not advised to mix liposomal bupivacaine with other drugs, in particular bupivacaine, due to the risk of premature de‐encapsulation of liposomal bupivacaine. As such studies evaluating liposomal bupivacaine with another drug were excluded from this review. In December 2015 an amendment to the FDA‐licensed indication was made which approved admixing liposomal bupivacaine with bupivacaine, including co‐administration in the same syringe. This amendment was made as it has been proposed that admixing with bupivacaine hydrochloride enhances early postoperative analgesia. As such in future updates of this review trials evaluating liposomal bupivacaine with bupivacaine hydrochloride will be included | |
| RCT evaluating the efficacy of liposomal bupivacaine at the surgical site compared with femoral nerve block for total knee replacement. Study excluded as participants in the femoral nerve block group who had persistent quadriceps inhibition after day 0 were also treated with a knee immobiliser which would be expected to impact on outcomes recorded (pain scores, opioid usage, range of movement). Additionally the trial was not double blind with the participants not blinded to randomisation. | |
| Review paper evaluating the safety of liposomal bupivacaine at the surgical site | |
| Open label sequential cohort study | |
| Open label sequential cohort study |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Ultrasound guided local infiltration analgesia for hip arthroscopy |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing hip arthroscopy |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Opioid consumption Pain scores |
| Starting date | July 2013 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Evaluation Exparel delivered in knee replacement |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing knee replacement |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Subjective pain Analgesic use Subject satisfaction |
| Starting date | Dec‐13 |
| Contact information | |
| Notes | Yet to recruit |
| Trial name or title | A prospective trial to reduce postoperative pain in implant based breast reconstruction |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing breast reconstruction |
| Interventions | Liposomal bupivacaine vs placebo vs bupivacaine hydrochloride vs botulinum toxin vs bupivacaine hydrochloride plus botulinum toxin |
| Outcomes | Pain score questionnaire |
| Starting date | April 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Early postoperative pain control following wrist operations |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing carpometacarpal arthroplasty or proximal row carpectomy operation |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Changes in pain |
| Starting date | May 2013 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | The effect of Exparel on post operative pain and narcotic use after colon surgery |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing elective colon resection (laparoscopic, robotic or open) |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | PCA (patient controlled analgesia) usage Oral pain medications Total IV (intravenous) narcotic used Total oral narcotic used Length of stay Return of bowel function Readmission Toradol Use Nausea medication Foley catheter removal Postoperative pain Postoperative satisfaction Home oral narcotic use |
| Starting date | February 2013 |
| Contact information | |
| Notes | Trial completed. Results not yet available ‐ contacted 29 January 2016 |
| Trial name or title | Comparison of two periarticular injection medications for adjunctive pain management following total knee arthroplasty (TKA) |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Measure pain intensity score (pre and postoperatively) by visual analogue scale (VAS) |
| Starting date | January 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Efficacy of rectal infiltration of Exparel for analgesic benefit following hemorrhoidectomy |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing haemorrhoidectomy |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Postoperative pain control Postoperative opioid consumption Postoperative nausea and vomiting Frequency of and pain during postoperative bowel movements Incidence of urinary retention |
| Starting date | April 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | PAIN ‐ Postoperative Analgesia INvestigation |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing sternotomy, thoracotomy, laparotomy or mini‐thoracotomy |
| Interventions | Liposomal bupivacaine vs bupivacine hydrochloride |
| Outcomes | Change in postoperative pain Overall opioid use Mean length of hospital stay Change from baseline in quality of life |
| Starting date | November 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Liposomal bupivacaine (Exparel) for postoperative pain control for open and laparoscopic abdominal hernia repair |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing open or laparoscopic abdominal hernia repair |
| Interventions | Liposomal bupivacaine vs standard care |
| Outcomes | Patient satisfaction with pain management after surgery Total length of time in post‐anaesthesia care unit (PACU) Change in postsurgical opioid consumption |
| Starting date | April 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Liposomal bupivacaine for pain control following anterior cruciate ligament reconstruction |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing anterior cruciate ligament reconstruction |
| Interventions | Liposomal bupivacaine vs no treatment |
| Outcomes | Postoperative pain Pain medication use Patient satisfaction with analgesia |
| Starting date | August 2014 |
| Contact information | |
| Notes | Trial completed. Results not yet available ‐ contacted 29 January 2016 |
| Trial name or title | Liposomal bupivacaine versus standard analgesia in TJA |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total joint arthroplasty (shoulder, hip, knee) |
| Interventions | Liposomal bupivacaine vs standard care |
| Outcomes | Length of stay in hospital (days) Time to postoperative rescue opioids (minutes) Readmission or ED visit due to pain control within 30 days |
| Starting date | July 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | A study of postsurgical pain control for lower extremity fractures |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing surgical fixation of a lower extremity fracture |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Change in pain visual analogue scale (VAS) Pain management satisfaction |
| Starting date | January 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Liposomal bupivacaine versus standard of care in total knee surgery |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee replacement |
| Interventions | Liposomal bupivacaine vs standard of care |
| Outcomes | Number physical therapy sessions required Visual analog scale (VAS) pain scores during admission (0‐10 scale) Length of stay Opioid consumption Incidence of opioid‐related adverse events |
| Starting date | August 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | THA lumbar plexus versus periarticular |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total hip arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride vs lumbar plexus block |
| Outcomes | Pain control comparison Pain management assessment 0‐3 months |
| Starting date | September 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Liposomal bupivacaine for post operative pain after knee replacement surgery |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing knee replacement |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Number physical therapy sessions required Visual analog scale (VAS) pain scores during admission (0‐10 scale) Length of stay (LOS, in days) Opioid consumption in oral morphine equivalents (OMEs, in milligrams) Incidence of opioid‐related adverse events (ORAEs) during admission Total cost of care (dollars) Hospital readmission |
| Starting date | August 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Efficacy of extended‐release liposomal bupivacaine for postoperative urogynecologic surgery |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing urogynecologic surgery |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Cumulative postoperative pain control Evaluate vaginal pain on postoperative day 7 |
| Starting date | October 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Trial liposomal bupivacaine following retropubic suburethral sling for stress urinary incontinence |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing retropubic suburethral sling for stress urinary incontinence |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Pain on postoperative day 1 Pain upon discharge from post‐anaesthesia care unit (PACU) Pain upon discharge from same day surgery Pain at 4 h after discharge home Total narcotic consumption Satisfaction with pain control at 1 week postoperative visit |
| Starting date | November 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Bupivacaine liposome suspension versus a concentrated multi drug periarticular injection |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride |
| Outcomes | Pain scores Morphine sulphate equivalent dose |
| Starting date | August 2013 |
| Contact information | |
| Notes | Trial completed. Results not yet available ‐ contacted 29 January 2016 |
| Trial name or title | Randomized trial of wound infiltration with extended‐release bupivacaine before laparoscopic or robotic hysterectomy |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing laparoscopic or robotic hysterectomy |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | Numerical Rating Scale (NRS) postoperative pain score on postoperative day 1 (POD1) NRS Pain score at 2 h NRS Pain score at 4 h NRS Pain score at 8 h NRS Pain score at 16 h NRS Pain score post‐op day 2 NRS Pain score post‐op day 3 NRS Pain score post‐op day 14 Quality of life as measured by the Brief Pain Inventory (BPI) Total opioid use prior to hospital discharge Total opioid use end of post‐op day 3 Total NSAID use end of post‐op day 3 Total opioid use at post‐op day 14 Total NSAID use at post‐op day 14 Adverse events |
| Starting date | July 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Multimodal pain management following primary TKA |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride vs continuous femoral nerve block |
| Outcomes | Time to discharge readiness |
| Starting date | September 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Exparel injection for postoperative orbital pain |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing enucleation or evisceration of the eye |
| Interventions | Liposomal bupivacaine vs bupivacine hydrochloride |
| Outcomes | Postoperative orbital pain Postoperative nausea and vomiting Quantity of oral narcotics used for postoperative pain control Patient satisfaction Postoperative complications |
| Starting date | February 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Liposomal bupivacaine in total knee arthroplasty |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs bupivacine hydrochloride |
| Outcomes | Mean visual analogue scale (VAS) pain scores Day 0, 1, 2 and 3 Complications |
| Starting date | June 2015 |
| Contact information | |
| Notes | Not yet recruiting |
| Trial name or title | EXPAREL® for pain after tonsillectomy |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing tonsillectomy |
| Interventions | Liposomal bupivacaine vs no intervention |
| Outcomes | Pain score (pain scores on a 0/10 scale) Pain medication usage (milligrams used) Oral intake (patient‐recorded oral intake) Patient complication (allergic reaction, swallowing dysfunction, hospital admission related to the study drug) Post‐tonsillectomy bleeding rate |
| Starting date | May 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Improvement of pain following robotic sacrocolpopexy and rectocele repair for pelvic organ prolapse |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing robotic sacrocolpopexy and rectocele repair for pelvic organ prolapse |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Global visual analogue score (VAS) for pain |
| Starting date | March 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Exparel for postoperative pain management in shoulder surgery |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing surgery for fractures of the shoulder and upper arm |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride (peripheral nerve block) |
| Outcomes | Quality of analgesia Time to discharge home Time to return to work Postoperative American Shoulder and Elbow surgeons (ASES) Subjective shoulder value (SSV) Constant score Incidence of nerve injury Postoperative opioid consumption |
| Starting date | June 2015 |
| Contact information | |
| Notes | Yet to recruit |
| Trial name or title | Femoral Nerve Block Compared to Exparel in Total Knee Replacement |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee replacement |
| Interventions | Liposomal bupivacaine vs femoral nerve block |
| Outcomes | Pain Score (VAS) Functional Outcome (Knee Society Score) |
| Starting date | January 2014 |
| Contact information | |
| Notes | Recruiting |
| Trial name or title | Liposomal bupivacaine with bupivacaine in ankle fracture ORIF |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing ankle fracture open reduction internal fixation |
| Interventions | Liposomal bupivacaine plus bupivacaine hydrochloride vs no treatment |
| Outcomes | Pain levels on a visual analog scale (VAS) |
| Starting date | December 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Pericapsular Exparel for pain relief in bunionectomy and related procedures |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing outpatient first metatarsophalangeal (MTP) joint procedure (bunionectomy, 1st MTP fusion, or cheilectomy) |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride |
| Outcomes | Opioid use as measured by questionnaire Pain relief measured by Defense and Veterans Pain Scale |
| Starting date | July 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | A randomized, double‐blind controlled trial of bupivacaine extended‐release liposome injection for postsurgical analgesia in patients undergoing open‐reduction internal fixation of the distal radius |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing open‐reduction internal fixation of the distal radius |
| Interventions | Liposomal bupivacaine vs placebo |
| Outcomes | Pain medication usage |
| Starting date | August 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Evaluation of EXPAREL for prolonged postsurgical analgesia in subjects undergoing third molar extraction |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing third molar extraction |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Area under the curve (AUC) of the numeric rating scale (NRS) at rest (NRS‐R) pain intensity scores through 48 h Treatment‐emergent adverse events Maximum plasma concentration Time to maximum plasma concentration Area under the plasma concentration‐versus‐time curve Apparent terminal elimination half‐life |
| Starting date | August 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Comparison of local anesthetic infusion pump versus DepoFoam bupivacaine for pain management |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing abdominoplasty |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride (continuous infiltration pump) |
| Outcomes | Recurrence of pain |
| Starting date | October 2014 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | A clinical trial of two periarticular multimodal drug injections in total hip arthroplasty |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total hip arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride vs bupivacaine hydrochloride |
| Outcomes | Pain score Narcotic consumption Length of stay |
| Starting date | January 2016 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Peri‐articular injection utilizing a pain cocktail with and without Exparel |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride |
| Outcomes | The visual pain scale from 1‐10 will be used to determine changes in pain control at 3, 12, 24, and 48 hour time intervals postoperatively |
| Starting date | October 2015 |
| Contact information | |
| Notes | Yet to recruit |
| Trial name or title | Impact of liposomal bupivacaine administered following placement of a transobturator suburethral sling |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing transobturator suburethral sling |
| Interventions | Liposomal bupivacaine vs placebo (NaCl 0.9%) |
| Outcomes | Visual analogue scale (VAS) Numeric rating scale (NRS) Likert scale to rate their level of satisfaction with their postoperative pain control |
| Starting date | February 2015 |
| Contact information | |
| Notes | Currently recruiting |
| Trial name or title | Exparel infiltration in anterior cruciate ligament reconstruction |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing anterior cruciate ligament reconstruction |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride (femoral nerve block) |
| Outcomes | Pain levels Morphine equivalents |
| Starting date | May 2014 |
| Contact information | |
| Notes | Yet to recruit |
| Trial name or title | Comparison of ropivacaine and liposomal bupivacaine for total knee arthroplasty |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing total knee arthroplasty |
| Interventions | Liposomal bupivacaine vs ropivacaine hydrochloride |
| Outcomes | Pain control measure on pain scale of 1‐10 Decreased maximal pain on pain scale of 1‐10 |
| Starting date | December 2015 |
| Contact information | |
| Notes | Yet to recruit |
| Trial name or title | Liposomal bupivacaine in implant based breast reconstruction |
| Methods | Parallel‐arm RCT |
| Participants | Participants undergoing breast reconstruction |
| Interventions | Liposomal bupivacaine vs bupivacaine hydrochloride |
| Outcomes | The effect of liposomal bupivacaine on average postoperative pain levels on postoperative day 1, 2, 3, 4, 5, 6 and 7 The effect of liposomal bupivacaine on postoperative opioid consumption The effect of liposomal bupivacaine on length of hospital stay The effect of liposomal bupivacaine on patient satisfaction with postoperative pain control The effect of liposomal bupivacaine on overall patient satisfaction The effect of liposomal bupivacaine on opioid‐related adverse events |
| Starting date | July 2015 |
| Contact information | |
| Notes | Currently recruiting |
ASA – American Society of Anaesthesiologists Score
ASES ‐ American Shoulder and Elbow surgeons
AUC – Area Under Curve
BPI ‐ Brief Pain Inventory
BPI – Brief Pain Inventory
ED – Emergency Department
IV ‐ Intravenous
LOS – Length of Stay
MTP ‐ Metatarsophalangeal
NRS – Numeric Rating Scale
NSAID – Non Steroidal Anti Inflammatory Drug
OME ‐ Oral Morphine Equivalents
ORAE – Opioid Related Adverse Event
ORIF – Open Reduction Internal Fixation
PACU ‐ Post Anaesthesia Care Unit
PCA ‐ Patient Controlled Analgesia
POD1 – Post Operative Day 1
RCT ‐ randomised controlled trial
SSV ‐ Subjective Shoulder Value
THA – Total Hip Arthroplasty
TJA – Total Joint Arthroplast
TKA – Total Knee Arthroplasty
TSA – Total Shoulder Arthroplasty
VAS – Visual Analogue Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative pain score 0 to 72 hours Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Liposomal bupivacaine vs control, Outcome 1 Cumulative pain score 0 to 72 hours. | ||||
| 1.1 vs placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 vs bupivacaine hydrocholoride | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participants not requiring postoperative opioids Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Liposomal bupivacaine vs control, Outcome 2 Participants not requiring postoperative opioids. | ||||
| 2.1 vs placebo | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 vs bupivacaine hydrochloride | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
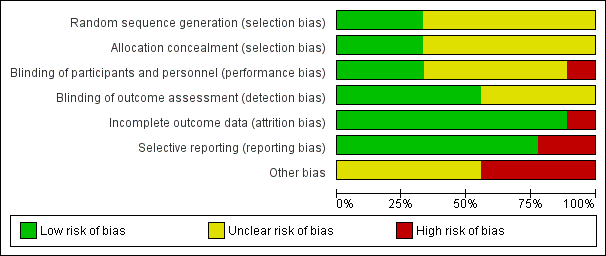
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
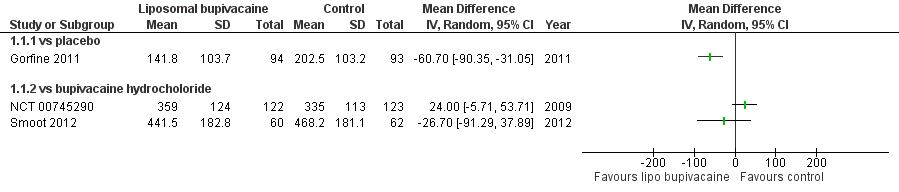
Forest plot of comparison: 1 Liposomal bupivacaine vs control, outcome: 1.1 Cumulative pain score 0 to 72 hours

Table of results for included simultaneous parallel‐arm trials

Forest plot of comparison: 1 Liposomal bupivacaine vs control, outcome: 1.2 Participants not requiring postoperative opioids

Illustrative example of an adaptive‐design trial. The decision to escalate, or de‐escalate a dose is conditional on the failure of the previous dose on the efficacy, or safety, or cost‐effectiveness of the intervention, introducing bias in any pooled analysis. The randomisation ratio is altered with each escalation/de‐escalation while the control group population is typically reported cumulatively for all dose levels

Table of results for adaptive‐design trials

Comparison 1 Liposomal bupivacaine vs control, Outcome 1 Cumulative pain score 0 to 72 hours.

Comparison 1 Liposomal bupivacaine vs control, Outcome 2 Participants not requiring postoperative opioids.
| Liposomal bupivacaine infiltration at the surgical site compared with placebo for the management of postoperative pain | |||
| Patient or population: aged 18 years and older undergoing elective surgery at any surgical site Settings: inpatient Intervention: surgical site infiltration of liposomal bupivacaine Comparison: surgical site infiltration of placebo | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Cumulative pain score from the end of operation (0 hours) to 72 hours (NRS 0 to 10) | A reduction in cumulative pain score associated with the use of liposomal bupivacaine was reported in one study. The mean cumulative pain score from the end of operation to 72 hours (NRS 0 to 10) in the placebo control group was 202.5 points with the mean cumulative pain score from the end of operation to 72 hours in the liposomal bupivacaine intervention group being 60.7 points lower (90.4 lower to 31.1 lower). | 189 participants (1 study) | ⊕⊝⊝⊝ |
| Serious adverse events | No reported drug‐related serious adverse events, no study withdrawals due to drug‐related adverse events | 382 participants (2 studies) | ⊕⊕⊝⊝ |
| Mean pain score at 12, 24, 48, 72 and 96 hours following surgery (NRS 0 to 10) | No data reported | No studies | |
| Time to first postoperative opioid dose over initial 72 hours | A longer time to first postoperative opioid dose associated with the use of liposomal bupivacaine was reported in two studies. In the placebo control group the time to first postoperative opioid was 4.3 and 1.2 hours compared to 7.2 and 14.3 hours in the liposomal bupivacaine groups respectively. The distribution of data was not reported. | 382 participants (2 studies) | ⊕⊕⊝⊝ |
| Total postoperative opioid consumption over first 72 hours | A reduction in total postoperative opioid consumption over first 72 hours associated with the use of liposomal bupivacaine was reported in one study. In the placebo control group the mean cumulative parenteral morphine equivalent dose over the first 72 hours was 29.1 mg and was 6.8 mg lower (12.8 mg lower to 0.9 mg lower) in the liposomal bupivacaine intervention group. | 189 participants (1 study) | ⊕⊝⊝⊝ |
| Percentage of participants not requiring postoperative opioids over initial 72 hours | One study reported a higher proportion of participants not requiring postoperative opioids over initial 72 hours associated with the use of liposomal bupivacaine (RR 0.82; 95% CI 0.72 to 0.94), and one study found no difference (RR 0.99; 95% CI 0.95 to 1.03). | 382 participants (2 studies) | ⊕⊝⊝⊝ |
| Incidence of adverse events within 30 days of surgery | The incidence of cardiac events and wound complications within 30 days of surgery were not reported in any study Adverse events within 30 days of surgery were reported in all studies with nausea, constipation and vomiting being the most common. | 382 participants (2 studies) | ⊕⊕⊝⊝ |
| CI: confidence interval; NRS: numeric rating scale; RR: risk ratio | |||
| GRADE Working Group grades of evidence | |||
| aWe downgraded the quality of this evidence due to the sparseness of data (‐1), indirectness (‐1) and risk of bias (‐1) due to the unclear risk of bias due to the sample size (50‐199). | |||
| Liposomal bupivacaine infiltration at the surgical site compared with bupivacaine hydrochloride for the management of postoperative pain | |||
| Patient or population: aged 18 years and older undergoing elective surgery at any surgical site Settings: inpatient Intervention: surgical site infiltration of liposomal bupivacaine Comparison: surgical site infiltration of bupivacaine hydrochloride | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Cumulative pain score from the end of operation (0 hours) to 72 hours (NRS 0 to 10) | No difference in cumulative pain score was reported in two studies. In one study the mean cumulative pain score from the end of operation to 72 hours (NRS 0 to 10) in the active control group was 335.0 points and 24.0 points higher (5.7 lower to 53.7 higher) in the liposomal bupivacaine intervention group. In the other study the mean cumulative pain score from the end of operation to 72 hours (NRS 0 to 10) in the active control group was 468.2 points and 26.7 points lower (91.3 lower to 37.9 higher) in the liposomal bupivacaine intervention group. Data were not pooled as differences in outcomes were expected due to differences in surgical interventions between studies. | 379 participants (2 studies) | ⊕⊝⊝⊝ |
| Serious adverse events | No reported drug‐related serious adverse events, no study withdrawals due to drug‐related adverse events | 583 participants (3 studies) | ⊕⊕⊕⊝ |
| Mean pain score at 12, 24, 48, 72 and 96 hours following surgery (NRS 0 to 10) | A reduction in mean pain score at 12 hours, but not 24, 48 or 72 hours, associated with the use of liposomal bupivacaine was reported in one study. Mean pain score at these time points were not reported in other studies. In the study that reported mean pain score (NRS 0 to 10) at 12 hours in the active control group it was 6.9 points and 1.3 points lower (2.4 lower to 0.2 lower) in the liposomal bupivacaine intervention group at this time point. | 134 participants (1 study) | ⊕⊝⊝⊝ |
| Time to first postoperative opioid dose over initial 72 hours | No data reported | No studies | |
| Total postoperative opioid consumption over first 72 hours | No difference in cumulative parenteral morphine equivalent dose over first 72 hours was reported in one study though no estimate of variance was provided and as such estimates of effect could not be calculated. | 134 participants (1 study) | ⊕⊝⊝⊝ |
| Percentage of participants not requiring postoperative opioids over initial 72 hours | No difference in the percentage of participants not requiring postoperative opioids over initial 72 hours was reported in one study (RR 0.95; 95% CI 0.86 to 1.05). | 134 participants (1 study) | ⊕⊝⊝⊝ |
| Incidence of adverse events within 30 days of surgery | The incidence of cardiac events and wound complications within 30 days of surgery were not reported in any study Adverse events within 30 days of surgery were reported in all studies with nausea, constipation and vomiting being the most common. | 583 participants (3 studies) | ⊕⊕⊕⊝ |
| CI: confidence interval; NRS: numeric rating scale; RR: risk ratio | |||
| GRADE Working Group grades of evidence | |||
| aWe downgraded the quality of this evidence one level due to the sparseness of data, a further level because Smoot 2012 was subject to a high risk of bias due to the risk of performance bias and attrition bias due to early termination of the study (as well as the unclear risk of bias due to the sample size (50‐199)), and a further level due to inconsistency. We did not pool of results as we predicted that participant characteristics, as well as nature of postoperative pain, would be different following breast augmentation and knee replacement. As such we expected there to be heterogeneity of the results due to population characteristics, not due to intervention characteristics. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative pain score 0 to 72 hours Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 vs placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 vs bupivacaine hydrocholoride | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participants not requiring postoperative opioids Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 vs placebo | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 vs bupivacaine hydrochloride | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |


