Tratamiento complementario con esteroides para la tuberculosis pulmonar
References
Referencias de los estudios incluidos en esta revisión
Jump to:
Referencias de los estudios excluidos de esta revisión
Jump to:
Referencias adicionales
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT | |
| Participants | Number of participants: 134 enrolled Inclusion criteria: Males and females aged 14‐70 years with acute, progressive PTB of moderately or far advanced extent, recent origin and bacteriologically proven | |
| Interventions | (1) Chemotherapy: Streptomycin sulphate (1 g/day) IM, sodium aminosalicylic acid (16 g daily, 12 g for females), isoniazid (100 mg 3 times/day). In patients over 50 years, combostep, a mixture of streptomycin and dihydro‐streptomycin was substituted for streptomycin. Para‐aminosalicylic acid only used in patients with fluid retention causing difficulties (2) Steroid: Chemotherapy and corticotrophin gel 60 units for four days (30 units every twelve hours), 50 units for four days (25 units every twelve hours), 40 units for three weeks (20 units every twelve hours). Then, a maintenance dose of 30 units once daily for six weeks gradually reduced over three weeks. Total duration 13 weeks | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: New York, USA Study dates: February 1957 to January 1958 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers utilised |
| Allocation concealment (selection bias) | Low risk | Allocation concealed (although method undescribed) |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | RCT | |
| Participants | Number of participants: 100 enrolled and randomized Inclusion criteria: West African (Ashanti) males aged 16 to 40, radiographic evidence of acute extensive pulmonary disease of recent origin and previously untreated, excreting tubercle bacilli in sputum and fully sensitive to anti‐TB drugs employed Exclusion criteria: Concomitant disease known to be adversely affected by corticosteroids (hypertension, cardiac failure, diabetes) | |
| Interventions | (1) Chemotherapy: Streptomycin sulphate (1 g/day), sodium para‐aminosalicylic acid (4 g, three times daily), isoniazid (100 mg three times daily), potassium citrate 20 g (three times daily) (2) Steroid: Chemotherapy + prednisolone, 5 mg four times per day. Started after one week of chemotherapy, continued for eight weeks. In 10th to 11th week dose gradually dropped and none given after 12th week (last week in hospital) | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Kumasi, Ghana Study dates: not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | RCT | |
| Participants | Number of participants: 4379 with 178 randomized | |
| Interventions | (1) Chemotherapy: Isoniazid, rifampicin, pyrazinamide and streptomycin and/or ethambutol for first three months. Isoniazid, rifampicin and ethambutol for following 6 months (2) Steroid: Chemotherapy + 20 mg Prednisilone twice per day IV/IM for 10 days, then orally for 30 days reduced by 10 mg every 10 days. | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Izmir, Turkey Study dates: January 1992 to December 1997 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Partial blinding |
| Methods | *RCT with two treatment arms* Generation of allocation sequence: random sampling numbers Inclusion of enrolled/randomized participants: 346 (85%) randomized | |
| Participants | Number of participants: 408 enrolled Inclusion criteria: Men and women aged 15‐60 years with acute PTB, newly diagnosed and of recent origin, more than one lung zone involved, treated for <= 3 weeks and with tubercle bacilli in sputum | |
| Interventions | (1) Chemotherapy group: Streptomycin 1 g/day, sodium para‐aminosalicylic acid 16 g/day in three or four divided doses, M and isoniazid 300mg daily in two equal doses for first six months. In month two to six, chemotherapy continued according to choice of each hospital or clinic, most (about 90%) received a combination of para‐aminosalicylic acid or isoniazid, about 10% also continued with streptomycin (2) Corticotrophin Arm: Chemotherapy + corticotrophin for three months. ACTH (corticotrophin ZN) 60 iu for four days, 40 iu for four days, 30 iu for 10 weeks (all daily in two equal doses). 20 iu for seven days, 10 iu for seven days (daily as single dose) and KCl with food (3) Prednisone Arm: Described in BTA ‐ Prednisone data | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: England and Wales Study dates: 27th September 1957 to 27th July 1961 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers utilised |
| Allocation concealment (selection bias) | Low risk | Randomization schedule held confidentially at co‐ordinating centre |
| Blinding (performance bias and detection bias) | Low risk | Physician and patient blinded |
| Methods | *RCT with two treatment arms* Generation of allocation sequence: random sampling numbers Inclusion of enrolled/randomized participants: 346 (85%) randomized | |
| Participants | Number of participants: 408 enrolled Inclusion criteria: Men and women aged 15‐60 years with acute PTB, newly diagnosed and of recent origin, more than one lung zone involved, treated for <= 3 weeks and with tubercle bacilli in sputum | |
| Interventions | (1) Chemotherapy group: Streptomycin 1 g/day, sodium para‐aminosalicylic acid 16 g/day in three or four divided doses, M and isoniazid 300 mg daily in two equal doses for first six months. In month two to six, chemotherapy continued according to choice of each hospital or clinic, most (about 90%) received a combination of para‐aminosalicylic acid or isoniazid, about 10% also continued with streptomycin (2) Corticotrophin Arm: Described in BTA ‐ Corticotrophin data (3) Prednisone Arm: Chemotherapy + prednisone for three months. 50 mg for four days, 37.5 mg for four days, 30 mg for 10 weeks (given every four hours excluding the night dose), ACTH 20 iu daily as single dose for seven days plus daily reduction of prednisolone to 20, 15, 10, 5 and 0 mg. ACTH 10 iu daily as a single dose for seven days and KCl with food | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: England and Wales Study dates: September 1957 to July 1961 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers utilised |
| Allocation concealment (selection bias) | Low risk | Randomization schedule held confidentially at co‐ordinating centre |
| Blinding (performance bias and detection bias) | Low risk | Physician and patient blinded |
| Methods | *RCT with two treatment arms* Generation of allocation sequence: random sampling numbers Inclusion of enrolled/randomized participants: 346 (85%) randomized | |
| Participants | Number of participants: 408 enrolled Inclusion criteria: Men and women aged 15‐60 years with acute PTB, newly diagnosed and of recent origin, more than one lung zone involved, treated for <= 3 weeks and with tubercle bacilli in sputum | |
| Interventions | (1) Chemotherapy group: Streptomycin 1 g/day, sodium para‐aminosalicylic acid 16 g/day in three or four divided doses, M and isoniazid 300 mg daily in two equal doses for first six months. In month two to six, chemotherapy continued according to choice of each hospital or clinic, most (about 90%) received a combination of para‐aminosalicylic acid or isoniazid, about 10% also continued with streptomycin (2) Corticotrophin Arm: Chemotherapy + corticotrophin for three months. ACTH (corticotrophin ZN) 60 iu for four days, 40 iu for four days, 30 iu for 10 weeks (all daily in two equal doses). 20 iu for seven days, 10 iu for seven days (daily as single dose) and KCl with food (3) Prednisone Arm: Chemotherapy + prednisone for three months. 50 mg for four days, 37.5 mg for four days, 30 mg for 10 weeks (given every four hours excluding the night dose), ACTH 20 iu daily as single dose for seven days plus daily reduction of prednisolone to 20, 15, 10, 5 and 0 mg. ACTH 10 iu daily as a single dose for seven days and KCl with food | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: England and Wales Study dates: September 1957 to July 1961 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers utilised |
| Allocation concealment (selection bias) | Low risk | Randomization schedule held confidentially at co‐ordinating centre |
| Blinding (performance bias and detection bias) | Low risk | Physician and patient blinded |
| Methods | RCT | |
| Participants | Number of participants: 39 randomized | |
| Interventions | (1) Chemotherapy: 1 g streptomycin, 200 mg isoniazid, 500,000 u penicillin by IV every 12 hours (penicillin only to prevent and treat infections associated with cortisone, and also in case on diagnostic error eg pneumonia) (2) Steroid: chemotherapy + IV cortisone for 15 days, 1st to 3rd day 300 mg/day, 4‐6 days 200 mg, 7‐9 days 100 mg, 10‐15 days 50 mg (total 2100 mg) | |
| Outcomes | Included in review:
| |
| Notes | Study location: Lisbon, Italy Study dates: 1955 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | No blinding however measures of mortality are unlikely to be affected by this |
| Methods | RCT | |
| Participants | Number of Participants: 213 randomized Exclusion criteria: Received collapse therapy at any time, chemotherapy previously given (protocol allowed patients to be admitted to trial who had been started on chemotherapy <one month prior to acceptance (n = 4)), bacilli known to be resistant to streptomycin, para‐aminosalicylic acid or isoniazid, active extra‐pulmonary disease, pregnant or within three months of parturition, other condition known to be adversely affected by steroids eg peptic ulcer, hypertension, cardiac failure, etc. | |
| Interventions | (1) Chemotherapy: If aged >40 years 5 g sodium para‐aminosalicylic acid and 100 mg isoniazid 2 times/day, 1 g streptomycin sulphate 3 times/week for 6 months | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Scotland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers utilised |
| Allocation concealment (selection bias) | Low risk | Allocation by co‐ordinating centre from pre‐arranged lists |
| Blinding (performance bias and detection bias) | Low risk | Patients, physicians and radiographer blinded |
| Methods | Quasi‐RCT | |
| Participants | Number of participants: 118 enrolled but 11 excluded and 7 lost to follow up Inclusion criteria: previously untreated cavitary PTB, admitted to pulmonary disease services of the Madison and Minneapolis Veterans Administration hospitals | |
| Interventions | (1) Chemotherapy: Isoniazid 300 mg, para‐aminosalicylic acid 12 mg, streptomycin sulphate 1 gm/day for one month, three times weekly after. Total duration not stated. (2) Steroid: Chemotherapy + prednisolone. 4 mg daily in four equal doses for 10 weeks, then dose reduced by 4 mg every four days for 12 days. | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Madison & Minneapolis, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No described |
| Allocation concealment (selection bias) | Low risk | Allocated via 'random assignment' |
| Blinding (performance bias and detection bias) | Low risk | Patients, physicians and radiographers blinded |
| Methods | Quasi‐RCT | |
| Participants | Number of participants: 16 | |
| Interventions | (1) Chemotherapy: Streptomycin 20 mg/lb body weight/day intra‐muscularly in a single daily dose & oral isoniazid total daily dose of 5 mg/lb body weight/day in divided doses three times daily for 12 weeks. (2) Steroid: Chemotherapy + triamcinolone 0.25 mg/lb body weight/day for four weeks, then 0.125 mg for four weeks then dose gradually reduced and stopped after a further two weeks | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Liverpool, UK Study dates: May 1958 ‐ Nov 1959 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quasi‐RCT, alternately allocated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Physician, patient and radiographer blinded |
| Methods | Quasi‐RCT | |
| Participants | Number of participants: 118 Inclusion criteria: not described Exclusion criteria: not described | |
| Interventions | (1) Chemotherapy: "standard anti‐TB drugs" ‐ no further details | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quasi‐randomized, alternately allocated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | RCT | |
| Participants | Number of participants: 109 | |
| Interventions | 1) Chemotherapy: 300 mg isoniazid, 12 g para‐aminosalicylic acid and 1 g streptomycin sulphate daily for one month then three times per week 2) Steroid: Chemotherapy + prednisolone 40 mg/day reduced by 2.5 mg every five days, distributed as equally as possible in four doses. Reduction continued until daily total of 20 mg reached. Continued on 20 mg until two roentographs taken two weeks apart showed little further change. Then reduction of 2.5 mg again made every five days until total discontinuation. Total dose averaged 1040 mg in first month, overall dosage averaged 2372 mg over an average of three months and 16 days | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: New York, USA Study dates: not known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Allocation using concealed envelopes |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Methods | RCT | |
| Participants | Participants: 202 | |
| Interventions | 1) Chemotherapy: Standard HIV associated TB regimen (weight adjusted doses of isoniazid, rifampin, pyrazinamide and ethambutol) 2) Steroid: Chemotherapy and Prednisilone 2.75 mg/kg daily for four weeks then tapered over the next four weeks (8 weeks total) | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Uganda | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized utilising computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Allocated blindly using sequentially drawn lots |
| Blinding (performance bias and detection bias) | Low risk | Physician and patients blinded |
| Methods | RCT | |
| Participants | Number of participants: 27 enrolled Inclusion criteria: endobronchial lesions suggestive of endobronchial TB (such as cheese like material, stenosis, granular, ulceration or inflammatory changes) observed by bronchoscopy with either caseating necrosis on tissue biopsy, positive stains or cultures of acid‐fast bacilli on the sputum, bronchial washing or brushing | |
| Interventions | (1) Chemotherapy: Isoniazid 10 mg/kg/day, pyridoxine at 50‐100mg/day, para‐aminosalicylic acid 12 g/day* (2) Steroid: Prednisolone 48 mg/day for 2 weeks, then dosage was decreased by 50% every 3‐4 days to reach discontinuation* *First 6 patients in each arm received 100 IU of ACTH this was given as 40 IU/day per day for the last 3 days of therapy. | |
| Outcomes | Included in review:
Not included in review
| |
| Notes | Study location: Baltimore, USA Study dates: 1959 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Methods | RCT | |
| Participants | Number of participants: 118 enrolled | |
| Interventions | (1) Chemotherapy: Isoniazid 20 mg/kg/day and 200 mg/kg/day para‐aminosalicylic acid, usually for one year (2) Steroid: prednisone 5 mg/kg for two days, 3 mg/kg for two days, 2 mg/kg for two days, then 1 mg/kg to end fourth week (28 days), 0.5 mg/kg fifth week, 0.25 mg/kg sixth week, total duration of use: 37 days | |
| Outcomes | Included in review:
| |
| Notes | Study location: New York, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Codes sealed from view |
| Blinding (performance bias and detection bias) | Low risk | Physicians and patients blinded |
| Methods | RCT | |
| Participants | Number of participants: 34 | |
| Interventions | 1) Chemotherapy: Isoniazid, rifampin, pyrazinamide, streptomycin or ethambutol or both 2) Steroid: Chemotherapy + Prednisolone 0.5 mg doses, approximately 1.0 mg/kg per day for four or eight weeks and then tapered followed up with bronchoscopy | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not sufficiently described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Methods | RCT *3 control arms* | |
| Participants | Participants: 530 | |
| Interventions | 1) Chemotherapy: either Rifampicin 7 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five months Rifampicin 5 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for three months No rifampicin ‐ 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five monthsIn phase 2 patients were only enrolled onto this chemotherapeutic regimen. 2) Steroid: Chemotherapy (a, b or c) and Prednisilone 20 mg 3 times daily for one week, then 10 mg once and 5 mg twice a day for five weeks, then 5 mg twice a day for one week and then 5 mg daily for a final week (8 weeks in total) | |
| Outcomes | Published and included in review:
Not included in review:
| |
| Notes | Study location: South India, Madras | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | RCT *3 control arms* | |
| Participants | Participants: 530 | |
| Interventions | 1) Chemotherapy: either Rifampicin 7 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five months Rifampicin 5 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for three months No rifampicin ‐ 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five monthsIn phase 2 patients were only enrolled onto this chemotherapeutic regimen. 2) Steroid: Chemotherapy (a, b or c) and Prednisilone 20 mg 3 times daily for one week, then 10 mg once and 5 mg twice a day for five weeks, then 5 mg twice a day for one week and then 5 mg daily for a final week (8 weeks in total) | |
| Outcomes | Published and included in review:
Not included in review:
| |
| Notes | Study location: South India, Madras | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | RCT | |
| Participants | Participants: 530 | |
| Interventions | 1) Chemotherapy: either Rifampicin 7 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five months Rifampicin 5 months ‐ 12 mg/kg rifampicin, 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for three months No rifampicin ‐ 400 mg of isoniazid (incorporating 6 mg pyridoxine), 40 mg/kg pyrazinamide, 0.75 g streptomycin sulphate daily for two months. Then 0.75 g streptomycin, 15 mg/kg isoniazid (incorporating 6 mg pyridoxine), and 70 mg/kg pyrazinamide twice weekly for five monthsIn phase 2 patients were only enrolled onto this chemotherapeutic regimen. 2) Steroid: Chemotherapy (a, b or c) and Prednisilone 20 mg 3 times daily for one week, then 10 mg once and 5 mg twice a day for five weeks, then 5 mg twice a day for one week and then 5 mg daily for a final week (8 weeks in total) | |
| Outcomes | Published and included in review:
Not included in review:
| |
| Notes | Study location: South India, Madras | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Incomplete blinding |
| Methods | Open RCT Allocation concealment: Not described | |
| Participants | Number of participants: 29 | |
| Interventions | 1) Chemotherapy: Isoniazid 10 mg/kg to a maximum dose of 300 mg/kg, rifampicin 15 mg/kg to a maximum dose of 600 mg/kg for a year with ethambutol 20 mg/kg for two months. When cultures were positive or sensitivity tests showed choice of treatment unsuitable, treatment regimen was adjusted 2) Steroid: Chemotherapy + daily dose of prednisolone 2 mg/kg for 15 days tapered and stopped between 1.5 and three months | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described sufficiently "children were divided at random" |
| Allocation concealment (selection bias) | High risk | Open trial |
| Blinding (performance bias and detection bias) | High risk | Open trial |
| Methods | *RCT with 2 treatment arms* Generation of allocation sequence: Random assignment of centrally labelled medication sets | |
| Participants | Number of participants: 566 | |
| Interventions | 1) Chemotherapy: Daily 1 gm streptomycin, 35‐45 g/kg body weight pyrazinamide, isoniazid 4‐6 mg/kg body weight and 10‐12 g para‐aminosalicylic acid for 12 weeks. 2) Steroid for 5 weeks: Chemotherapy + prednisolone 20 mg for three days, 15 mg for the next four days, 10 mg each day for 3 weeks 5 mg for four days and 2.5 mg for next three days 3) Steroid for 9 weeks: Chemotherapy + prednisolone 20 mg for three days, 15 mg for the next four days, 10 mg each day for 7 weeks 5 mg for four days and 2.5 mg for next three days | |
| Outcomes | Included in review:
Not included in review
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally controlled randomization |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by central centre, opaque bottles sent to treatment centres |
| Blinding (performance bias and detection bias) | Low risk | Patients, physicians and outcome assessors blinded |
| Methods | *RCT with 2 treatment arms* Generation of allocation sequence: Random assignment of centrally labelled medication sets | |
| Participants | Number of participants: 566 | |
| Interventions | 1) Chemotherapy: Daily 1 gm streptomycin, 35‐45 g/kg body weight pyrazinamide, isoniazid 4‐6 mg/kg body weight and 10‐12 g para‐aminosalicylic acid for 12 weeks. 2) Steroid for 5 weeks: Chemotherapy + prednisolone 20 mg for three days, 15 mg for the next four days, 10 mg each day for 3 weeks 5 mg for four days and 2.5 mg for next three days 3) Steroid for 9 weeks: seeUSPHS 1965 ‐ 9 week data | |
| Outcomes | Included in review:
Not included in review
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally controlled randomization |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by central centre, opaque bottles sent to treatment centres |
| Blinding (performance bias and detection bias) | Low risk | Patients, physicians and outcome assessors blinded |
| Methods | *RCT with 2 treatment arms* Generation of allocation sequence: Random assignment of centrally labelled medication sets | |
| Participants | Number of participants: 566 | |
| Interventions | 1) Chemotherapy: Daily 1 g streptomycin, 35‐45 g/kg body weight pyrazinamide, isoniazid 4‐6 mg/kg body weight and 10‐12 g para‐aminosalicylic acid for 12 weeks. 2) Steroid for 5 weeks: see USPHS 1965 ‐ 5 week data 3) Steroid for 9 weeks: Chemotherapy + prednisolone 20 mg for three days, 15 mg for the next four days, 10 mg each day for 7 weeks 5 mg for four days and 2.5 mg for next three days | |
| Outcomes | Included in review:
Not included in review
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally controlled randomization |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by central centre, opaque bottles sent to treatment centres |
| Blinding (performance bias and detection bias) | Low risk | Patients, physicians and outcome assessors blinded |
| Methods | RCT | |
| Participants | Number of participants: 100 Exclusion criteria: Non‐tuberculous diagnostic problems, those previously treated with anti‐TB chemotherapy, those with contraindications; pregnancy, renal disease, hypertension, peptic ulcerative disease and diabetes | |
| Interventions | 1) Chemotherapy: 300 mg isoniazid daily and 9 to 12 gm of aminosalicylic acid daily 2) Steroid: Chemotherapy plus 5 mg of prednisolone every six hours for 10 days, every eight hours for 10 days, every 12 hours for 40 days, every day for four days, then 2.5 mg every day for four days. | |
| Outcomes | Included in review:
Not included in review:
| |
| Notes | Study location: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Patient, physician and outcome assessors blinded |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Retrospective case control, peritoneal TB not PTB. | |
| All received steroids, no control group. | |
| Control group not concurrent. | |
| A case series. | |
| Did not examine steroid efficacy as an adjunctive TB therapy. | |
| Did not examine steroid efficacy as an adjunctive TB therapy. | |
| A review. | |
| A case report. | |
| All receive steroids, no control group. | |
| A set of case reviews. | |
| Meningeal TB not PTB. | |
| A review. | |
| All receive steroids, no control group. | |
| A review. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| TB pleurisy not PTB. | |
| Meningeal TB not PTB. | |
| Non‐steroidal intervention. | |
| Not TB patients. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| Pleural TB not PTB. | |
| Meningeal TB not PTB. | |
| A review. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| TB pleurisy not PTB. | |
| Central nervous system TB not PTB. | |
| Pericardial TB not PTB. | |
| A case series. | |
| Didn't examine steroid efficacy as an adjunctive therapy. | |
| A review. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| Contacted authors regarding eligibility criteria, no reply. | |
| A case series. | |
| Looking at efficacy of tuberculin and steroid (not chemotherapy and steroids). | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, looked at immunotherapy. | |
| A review. | |
| Part of a review series. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| Commentary on Schoeman 1997. | |
| Contacted authors regarding eligibility criteria, no reply. | |
| Meningeal TB not PTB. | |
| Non‐TB haemoptysis, not PTB. | |
| Meningeal TB not PTB. | |
| Didn't examine steroid efficacy as an adjunctive PTB therapy. | |
| TB pleurisy not PTB. | |
| Meningeal TB not PTB. | |
| A review. | |
| Meningeal TB not PTB. | |
| Letter referring to Galarza 1995. | |
| Control group not concurrent. | |
| Letter to editor, not novel data. | |
| Pleural TB not PTB. | |
| Didn't examine steroid efficacy as an adjunctive PTB therapy, looked at prednisolone with immunotherapy. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| TB IRIS not PTB. | |
| TB IRIS not PTB. | |
| Severe cases favourably allocated to steroid treatment group. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, role of aspirin. | |
| Systematic review of pericarditis. | |
| Meningeal TB not PTB. | |
| No control group. Didn't examine efficacy of steroid and chemotherapy compared to lone chemotherapy, compared efficacy of two types of steroid. | |
| A review. | |
| No control group, did not compare steroid use to 'placebo or no treatment'. | |
| No control group, does not compare steroid use to 'placebo or no treatment'. | |
| Participants did not have pleurisy ‐ cases of pulmonary TB. | |
| An editorial. | |
| A review. | |
| A review of 233 pericardial TB cases, not PTB cases. | |
| Didn't examine steroid efficacy as an adjunctive PTB therapy. | |
| Didn't examine steroid efficacy as an adjunctive PTB therapy. | |
| Didn't examine steroid efficacy as an adjunctive PTB therapy. | |
| Tuberculous Pericarditis, not PTB | |
| Pharmacokinetic study of Isoniazid | |
| Meningeal TB not PTB. | |
| Meningeal TB not PTB (not novel data same information as Schoeman 1997). | |
| Not looking at steroid efficacy as an adjunctive TB therapy, adjunctive thalidomide therapy. | |
| TB pericarditis, not PTB. | |
| Contacted authors regarding eligibility criteria, no reply. | |
| Meningeal TB, not PTB. | |
| Pleural effusion, not PTB. | |
| A review. | |
| A letter. | |
| Contacted authors regarding eligibility criteria, no reply. | |
| Pericardial TB, not PTB. | |
| Pericardial TB, not PTB. | |
| Pericardial TB, not PTB. | |
| Milliary TB, no distinction between organ type. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, looked at metabolic rate in patients who have TB and pneumonia. | |
| Tuberculous pleurisy, not PTB. | |
| Tuberculous pleurisy, not PTB. | |
| Updated by Horne 1960. | |
| Meningeal TB, not PTB | |
| Looked at the pathway through which steroids improve meningeal TB outcomes, not PTB. | |
| TB meningitis, not PTB | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| Preliminary results from USPHS 1965 ‐ 5 week data. | |
| Controls not concurrent. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy. | |
| Controls not concurrent. | |
| Controls not concurrent. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, looked at risk factors for not taking corticosteroid for TB pericarditis. | |
| Pleural TB, not PTB. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, looked at TB diagnostic techniques. | |
| Didn't examine steroid efficacy as an adjunctive TB therapy, steroids as therapy for tuberculous pyrexia. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 18 | 3815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.15] |
| Analysis 1.1 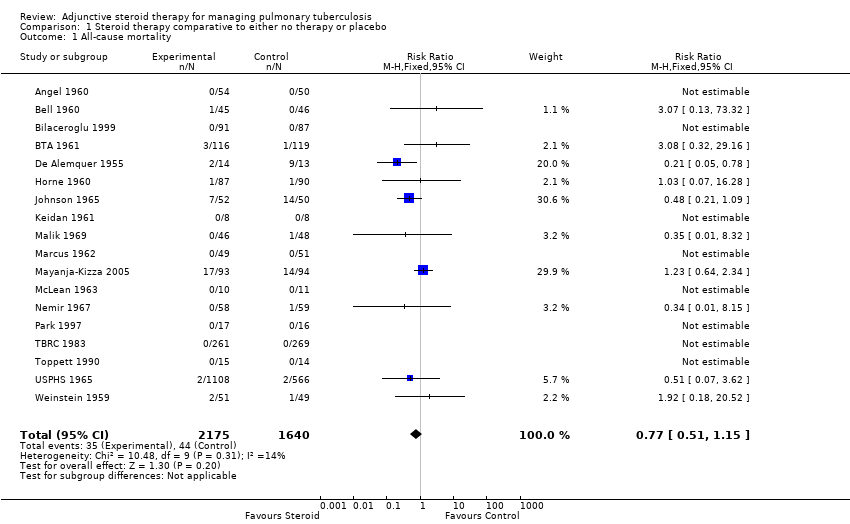 Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 1 All‐cause mortality. | ||||
| 2 Sputum conversion by 2 months Show forest plot | 13 | 2750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.09] |
| Analysis 1.2 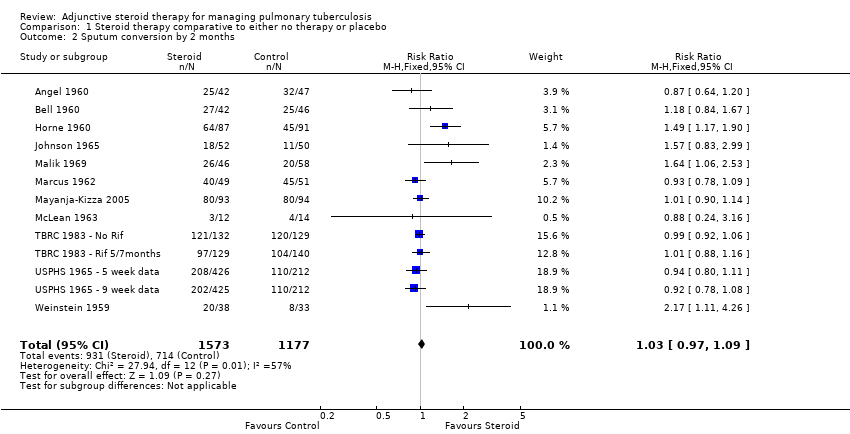 Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 2 Sputum conversion by 2 months. | ||||
| 3 Sputum conversion at 6 months Show forest plot | 10 | 2150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| Analysis 1.3  Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 3 Sputum conversion at 6 months. | ||||
| 4 Treatment Failure Show forest plot | 10 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.98, 1.05] |
| Analysis 1.4 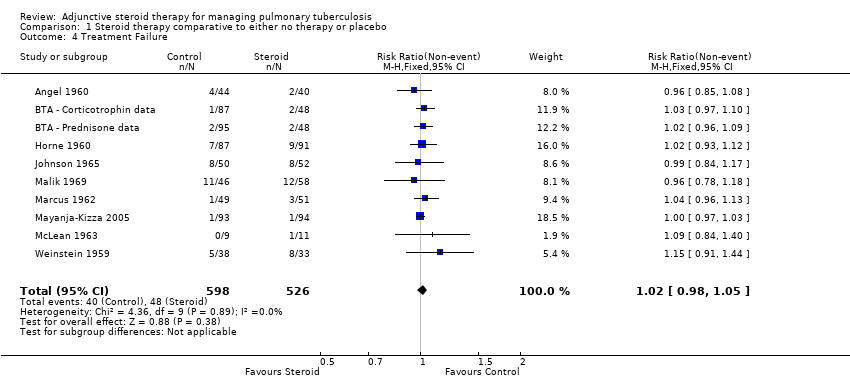 Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 4 Treatment Failure. | ||||
| 5 Relapse Show forest plot | 5 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.07] |
| Analysis 1.5  Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 5 Relapse. | ||||
| 6 Clinical Improvement at 1 month Show forest plot | 5 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.09, 1.24] |
| Analysis 1.6  Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 6 Clinical Improvement at 1 month. | ||||
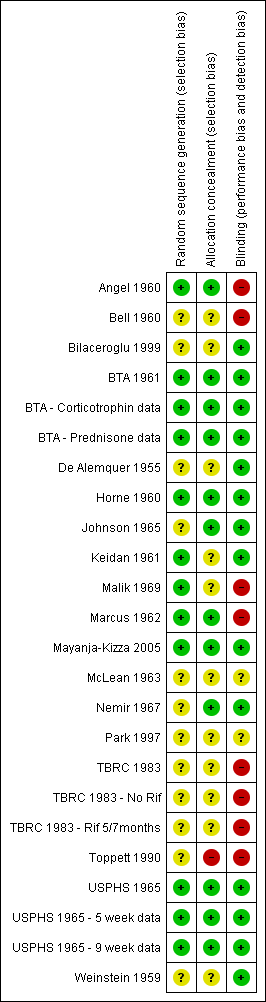
Risk of bias summary: review authors' judgements regarding each risk of bias item for each included study.

Funnel plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.2 Sputum conversion by 2 months.

Funnel plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.6 Clinical Improvement at 1 month.

Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.1 All‐cause mortality.

Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.2 Sputum conversion by 2 months.

Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.3 Sputum conversion at 6 months.

Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.4 Treatment Failure.
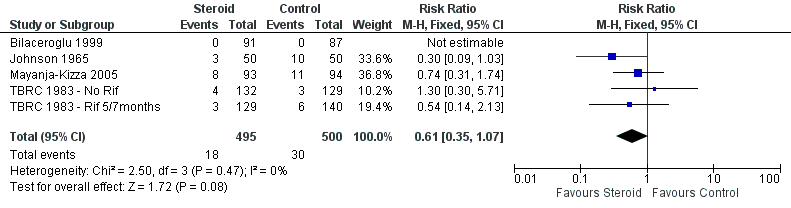
Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.5 Relapse.

Forest plot of comparison: 1 Steroid therapy comparative to either no therapy or placebo, outcome: 1.6 Clinical Improvement at 1 month.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 1 All‐cause mortality.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 2 Sputum conversion by 2 months.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 3 Sputum conversion at 6 months.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 4 Treatment Failure.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 5 Relapse.

Comparison 1 Steroid therapy comparative to either no therapy or placebo, Outcome 6 Clinical Improvement at 1 month.
| Steroid therapy comparative to either no therapy or placebo for managing pulmonary tuberculosis | ||||||
| Patient or population: patients with managing pulmonary tuberculosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroid therapy comparative to either no therapy or placebo | |||||
| All‐cause mortality | 27 per 1000 | 21 per 1000 | RR 0.77 | 3815 | ⊕⊕⊝⊝ | |
| Sputum Conversion by 2 months | 656 per 1000 | 722 per 1000 | RR 1.1 | 1475 | ⊕⊕⊝⊝ | |
| Sputum conversion at 6 months | Study population | RR 1.01 | 875 | ⊕⊕⊕⊝ | ||
| 911 per 1000 | 920 per 1000 | |||||
| Clinical Improvement at 1 month | Study population | RR 1.16 | 497 | ⊕⊝⊝⊝ | ||
| 794 per 1000 | 921 per 1000 | |||||
| *The assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not downgraded for risk of bias: Most of these studies were conducted in the 1960s and provided minimal detail on trial methods. However, the most recent trial from 2005 was well conducted and showed no evidence of clinical benefit with steroids. | ||||||
| Study ID | Outcome Definition | Culture or Smear (if culture not reported) Negative | ||||
| Time point (month) | Steroid | Control | Reported Statistical Significance | |||
| Culture negative; three samples of sputum or gastric washings were sent for culture at start of treatment then at monthly intervals. | Baseline | 0/54 | 0/50 | No significant difference | ||
| 1 | 7/34 | 12/38 | ||||
| 2 | 25/42 | 32/47 | ||||
| 3 | 29/46 | 39/42 | P < 0.001 significantly greater among controls | |||
| 4 | 39/45 | 41/45 | No significant difference | |||
| 5 | 40/44 | 38/40 | ||||
| 6 | 45/47 | 43/46 | ||||
| Culture negative; 24 hour specimens of sputum were collected until treatment started, then collected monthly. Concentrates prepared by tri‐sodium phosphate method and incubated for 12 weeks. | Baseline | 0/45 | 0/46 | No significant difference | ||
| 1 | 16/44 | 11/45 | ||||
| 2 | 27/42 | 25/46 | ||||
| 3 | 31/41 | 35/43 | ||||
| Culture conversion rate (Sputum taken twice weekly) | Reported as 'comparable' | P = 0.0794 | ||||
| Culture negative; direct smear and culture exam taken monthly and analysed using local lab facilities | Baseline | 9/111 | 5/118 | Not reported | ||
| 1 | 36/100 | 31/109 | ||||
| 3 | 70/89 | 77/104 | ||||
| 6 | 86/87 | 91/95 | ||||
| 9 | 67/67 | 74/74 | ||||
| 12 | 40/40 | 39/40 | ||||
| Culture negative; direct smear and culture exam taken monthly and analysed using local lab facilities | Baseline | 4/115 | 5/118 | |||
| 1 | 36/109 | 31/109 | ||||
| 3 | 80/104 | 77/104 | ||||
| 6 | 93/95 | 91/95 | ||||
| 9 | 80/80 | 74/74 | ||||
| 12 | 45/45 | 39/40 | ||||
| Culture negative; direct sputum smear, gastric lavage or laryngeal swabs | 1 | 40/87 | 28/91 | No significant difference | ||
| 2 | 64/87 | 45/91 | P < 0.01 | |||
| 3 | 67/87 | 62/91 | No significant difference | |||
| 4 | 75/87 | 75/91 | ||||
| 5 | 80/87 | 82/91 | ||||
| 6 | 86/87 | 91/91 | ||||
| 9 | 75/77 | 80/80 | ||||
| 12 | 77/77 | 80/80 | ||||
| Specimens of sputum were collected every two weeks for four months and then every month thereafter Reversal of infectiousness; the first of three consecutive monthly specimens negative for tubercle bacilli on microscopy and culture | 2 | 18/52 | 11/50 | No significant difference | ||
| 4 | 30/52 | 31/50 | ||||
| 6 | 38/52 | 37/50 | ||||
| 8 | 46/52 | 42/50 | ||||
| 10 | 49/52 | 44/50 | ||||
| 12 | 48/52 | 46/50 | ||||
| Sputum negative; disappearance of sputum in bacilli was monitored for monthly | 1 | 11/46 | 14/58 | No significant difference | ||
| 2 | 26/46 | 20/58 | P < 0.05 | |||
| 3 | 28/46 | 28/58 | P < 0.05 | |||
| 6 | 35/46 | 46/58 | No significant difference | |||
| Culture negative; specimens of sputum were collected every two weeks until 2 months then at 3 months and 6 months | 1 | 35/49 | 31/51 | Not reported | ||
| 2 | 40/49 | 45/51 | ||||
| 3 | 45/49 | 45/51 | ||||
| 6 | 48/49 | 48/51 | ||||
| Culture negative; sputum cultures obtained at month 1 and 2 and examined for AFB | 1 | 58/93 | 35/94 | P = 0.001 | ||
| 2 | 80/93 | 80/94 | No significant difference | |||
| Smear or sputum negative; Smear or culture of sputum or gastric aspirate | 1 | 0/13 | 0/14 | No significant difference | ||
| 2 | 3/12 | 4/14 | ||||
| 3 | 5/11 | 8/14 | ||||
| 4 | 8/12 | 11/14 | ||||
| 5 | 8/11 | 13/13 | ||||
| 6 | 9/9 | 10/11 | ||||
| Culture from bronchial aspiration and gastric washings on 3 successive morning on admission; culture positive after therapy | During therapy | 4/58 | 2/59 | Not reported | ||
| After therapy | 2/58 | 2/59 | Not reported | |||
| Culture negative; Sputum smears examined by fluorescence microscopy and cultured using a modification of Petroffs method. TB chemotherapeutic with rifampicin for either 5 or 7 months. | 1 | 59/132 | 70/129 | No significant difference | ||
| 2 | 121/132 | 120/129 | ||||
| 3 | 1284/132 | 123/129 | ||||
| Culture negative; Sputum smears examined by fluorescence microscopy and cultured using a modification of Petroffs method.TB chemotherapeutic regimen without rifampicin. | 1 | 46/129 | 36/140 | No significant difference | ||
| 2 | 97/129 | 104/140 | ||||
| 3 | 116/129 | 123/140 | ||||
| Culture negative | 1 | 121/426 | 101/424 | Not reported | ||
| 2 | 208/426 | 220/424 | Not reported | |||
| 3 | 316/426 | 308/424 | Not reported | |||
| 4 | 365/426 | 342/424 | Not reported | |||
| 5 | 387/426 | 374/424 | Not reported | |||
| 6 | 389/426 | 372/424 | Not reported | |||
| 8 | 410/426 | 392/424 | Not reported | |||
| Culture negative | 1 | 107/425 | 101/424 | Not reported | ||
| 2 | 202/425 | 220/424 | Not reported | |||
| 3 | 263/425 | 308/424 | Not reported | |||
| 4 | 330/425 | 342/424 | Not reported | |||
| 5 | 368/425 | 374/424 | Not reported | |||
| 6 | 395/425 | 372/424 | Not reported | |||
| 8 | 389/425 | 392/424 | Not reported | |||
| Sputum negative; Cumulative sputum conversion | Baseline | 16/38 | 6/33 | Not reported | ||
| 2 | 20/38 | 8/33 | ||||
| 8 | 33/38 | 25/33 | ||||
| Study ID | Outcome Definition | Steroid | Control | Reported Statistical Significance | |
| Culture positive at 5 months | 4/44 | 2/40 | Not reported | ||
| Culture positive at 6 months | 1/87 | 4/95 | |||
| Culture positive at 6 months | 2/95 | 4/95 | |||
| Culture positive at 5 months | 7/87 | 9/91 | |||
| Culture or smear positive at 6 months | 8/50 | 8/52 | |||
| Study specific indicator, failure to convert by 12 months | 2/50 | 4/52 | |||
| Culture or smear positive at 6 months | 11/46 | 12/58 | |||
| Culture or smear positive at 6 months | 1/49 | 3/51 | |||
| Study specific indicator, not described further | 1/93 | 1/94 | No significant difference | ||
| Culture positive at 6 months | 0/9 | 1/11 | Not reported | ||
| Sputum positive at end of study (6 to 16 months) | 2/38 | 6/33 | |||
| Study ID | Method | Steroid | Control | Reported Statistical Significance | |
| Smear or culture positive TB within 3 years follow up | 0/91 | 0/87 | Not reported | ||
| Number readmitted for TB during up to five years of follow up | 3/50 | 10/50 | P < 0.05 | ||
| Those needing re‐treatment up to two years after first regimen of anti‐tuberculous therapy. | 8/93 | 11/94 | No difference | ||
| Two or more of six cultures positive in any three consecutive monthly exams | 4/132 | 3/129 | No difference | ||
| 3/129 | 6/140 | ||||
| Study ID | Outcome Definition | Time Point (months) | Steroid Group | Control Group | Reported Statstical Significance | |
| Immediate and pronounced non‐specific improvement in general condition reported by nurses and patients | N/A, described as being immeasurable numerically | |||||
| Number of participants judged by treating physician to be in 'fair' or 'good' condition as opposed to 'severely ill' or 'poor' condition. | 1 | 44/44 | 39/46 | Improvement from baseline greater in steroid group P < 0.01 | ||
| 2 | 42/42 | 46/46 | No difference | |||
| Number of patients judged by treating physician to have had 'considerable', 'moderate' or 'slight improvement' as opposed to 'no change' or 'deterioration' | 1 | 96/99 | 100/114 | Not reported | ||
| 3 | 91/92 | 102/107 | ||||
| 6 | 85/86 | 94/95 | ||||
| Number of patients judged by treating physician to have had considerable, moderate or slight improvement | 1 | 113/116 | 100/114 | |||
| 3 | 107/108 | 102/107 | ||||
| 6 | 98/98 | 94/95 | ||||
| Participants initially with; any degree of cough or sputum, or moderate to severe symptoms, who saw moderate to marked improvement in condition. | 10 weeks | 39/48 | 28/46 | P < 0.05 | ||
| Participants showing definite‐striking improvement | 1 | 8/8 | 0/8 | Not reported | ||
| Improvement in 'well‐being and strength' | 7 weeks | 49/49 | 48/51 | Not reported | ||
| 'Rapid symptom response'; method of assessment unclear | Not reported | 13/13 | 1/14 | P < 0.0001 | ||
| 'Improved'; Method of assessment unclear. | 1 | 23/31 | 14/31 | No difference | ||
| 2 | 10/16 | 9/18 | ||||
| 3 | 5/6 | 3/4 | ||||
| 6 | 0/1 | 0/2 | ||||
| 7 | 0/1 | 0/1 | ||||
| time point unclear | 39/58 | 27/59 | P < 0.05 | |||
| Author | Outcome Definition | Steroid Group | Control Group | Reported Statistical Significance | |
| Mean time till patients afebrile, temperature not specified | 5 days | 19.4 days | Not reported | ||
| Mean time till patients afebrile (<37.5°C) | 25 days | 47 days | Not reported | ||
| Mean temperature change 72 hours after treatment initiation | ‐1.2 ºC | + 0.2ºC | P = 0.003 | ||
| Patients with an average temperature of 99°F or more (1 month) | 23/100 | 52/113 | Not reported | ||
| 17/113 | 52/113 | ||||
| Patients with an average temperature of 99°F or more (3 months) | 12/92 | 24/104 | |||
| 36/106 | 24/104 | ||||
| Patients with an average temperature of 99°F or more (6 months) | 7/92 | 10/104 | |||
| 7/106 | 10/104 | ||||
| Median fever duration | 1 day | 6 days | Not reported | ||
| Mean fever duration | 1 day | 13 days | |||
| Mean number of days for temperature to permanently drop below 100°F | 1 day | 26 day | Not reported | ||
| Mean number of febrile days | 2.9 days | 10.2 days | Not reported | ||
| Study ID | Outcome | Time Point | Steroid Group | Control Group | Reported Statistical Significance | |
| Mean gain in weight from baseline (lbs)* | 1 months | 13 | 4 | Not reported | ||
| 2 months | 19 | 8 | ||||
| 3 months | 24 | 11 | P < 0.001 | |||
| 4 months | 25 | 13 | Not reported | |||
| 5 months | 26 | 15 | ||||
| 6 months | 27 | 17 | P < 0.001 | |||
| Mean gain in weight from baseline (lbs) | 1 month | 2.75 | 2.13 | No statistically significant differences in mean weight of trial arms at any time point. | ||
| 2 months | 7.56 | 4.93 | ||||
| 3 months | 8.05 | 8.09 | ||||
| Mean gain in weight (kg) | from day 18 to 70 | 7.2 | 4.2 | P = 0.0022 | ||
| Mean gain in weight from baseline (lbs) | 1 month | 12 | 6 | Not reported | ||
| 3 months | 21 | 11 | ||||
| 6 months | 24 | 17 | P = 0.1 | |||
| 12 months | 23 | 21 | ||||
| Mean gain in weight from baseline (lbs) | 1 month | 9 | 6 | P = 0.1 | ||
| 3 months | 26 | 11 | ||||
| 6 months | 24 | 17 | No statistically significant difference | |||
| 12 months | 21 | 21 | ||||
| Mean gain in weight from baseline(lbs) | 1 month | 8 | 6 | Not reported | ||
| 2 months | 17 | 11 | ||||
| 3 months | 21 | 15 | ||||
| 4 months | 21 | 15 | ||||
| 5 months | 21 | 16 | ||||
| 6 months | 21 | 16 | ||||
| Patients initially under 130 lbs who gained 15 lbs or more (n) | 2 months | 8/23 | 0/17 | P<0.025 | ||
| Number of patients initially 15 lbs or more under ideal weight who gained 15 lbs or more by | 2 months | 9/24 | 2/21 | P<0.05 | ||
| Number of patients initially more than 20 lbs under ideal weight who gained 15 lbs or more by | 2 months | 9/24 | 2/19 | |||
| Mean gain in weight from baseline (lbs) | 1 month | 8.6 | 4.4 | Not significant | ||
| 2 months | 12.3 | 5.1 | P<0.05 | |||
| 3 months | 13.3 | 7.2 | ||||
| 6 months | 16.51 | 11.74 | Not significant | |||
| Mean gain in weight from baseline (lbs) | 6 months | 25 | 16 | P<0.01 | ||
| *Read from graph, | ||||||
| Author | Outcome definition | Steroid Group | Control Group | Statistical Significance |
| Average length of stay for those discharged | 53.4 +/‐ 3.1 days | 71.3 +/‐ 5.6 days | P=0.0284 | |
| Average length of stay for those discharged | 10 months | 11 months | Not reported | |
| Average length of stay for those discharged | 188.7 days | 207.4 days | Not reported |
| Study ID | Outcome definition | Time point (months) | Steroid | Control | Statistical Significance Reported |
| Max expiratory flow rate (body temperature and pressure saturated; litres/minute) | Baseline | 189.8 (SD 101.7) | 205.1 (SD 114.4) | Significantly higher in the corticotrophin group, no value given | |
| 3 | 241.1 (SD 137.7) | 227.3 (SD 115.4) | |||
| 6 | 238.7 (SD 125.2) | 228.4 (SD 100.2) | |||
| Mean vital capacity (maximal inhalation; standard temperature and pressure dry) | Baseline | 2,649 (SD 751) | 2,523 (SD 838) | Increased to the same extent in both groups, no value given | |
| 3 | 2,940 (SD 757) | 2,728 (SD 790) | |||
| 6 | 2,995 (SD 817) | 2,874 (SD 786) | |||
| Max breathing capacity (body temperature and pressure saturated; litres/minute) | Baseline | 88.6 (SD 36.3) | 82.0 (SD 26.5) | Increased to the same extent in both groups, no value given | |
| 3 | 102.6 (SD 25.6) | 97.8 (SD 32.3) | |||
| 6 | 100.4 (SD 28.7) | 99.1 (SD 31.2) | |||
| Abnormal maximal voluntary ventilation | 6 | 12/46 | 20/58 | P < 0.05 | |
| 12 | 19/46 | 20/58 | No significant difference | ||
| Abnormal vital capacity | 6 | 14/46 | 12/58 | ||
| 12 | 19/46 | 24/58 | |||
| Abnormal maximal expiratory flow | 6 | 17/46 | 21/58 | ||
| 12 | 19/46 | 24/58 | |||
| Diffusion capacity normal | 1 | 34/49 | 35/51 | No significant difference | |
| 2 | 34/49 | 35/51 | |||
| 3 | 34/49 | 36/51 | |||
| 6 | 34/49 | 37/51 | |||
| Maximal Mid expiratory flow rate (1/sec) | 1 | 2 | 2 | No significant difference | |
| 2 | 2.1 | 2.1 | |||
| 3 | 2.1 | 2.3 | |||
| 6 | 2.2 | 2.3 | |||
| Vital capacity normal | 1 | 35/49 | 36/51 | No significant difference | |
| 2 | 36/49 | 37/51 | |||
| 3 | 36/49 | 37/51 | |||
| 6 | 39/49 | 40/51 | |||
| Lung volumes (6 variables measured) | Not reported | Data not extracted | No significant difference in any of the measures | ||
| Ventilation Effects (6 variables measured) | |||||
| Perfusion Effects (6 variables measured) | |||||
| Diffusion Effects (3 variables measured) | |||||
| Mean improvement in forced vital capacity (%) | 2 | 9.2 | 10.4 | No significant difference | |
| Mean improvement in forced expiratory capacity (%) | 13.1 | 9.4 | No significant difference | ||
| Author | Outcome definition | Steroid | Control | Statistical significance |
| Sepsis, venous thrombosis, mental changes, and partial deafness | Numbers not given, stated equal in each treatment arm. | Not reported | ||
| Hypersensitivity reactions | 4/54 | 8/54 | ||
| Hypertension | 1/45 | 0/46 | ||
| Toxicity | 0/45 | 0/46 | ||
| Drug resistance | 18/91 | Not reported | ||
| Developed co‐morbidities | 28/275 | 13/133 | ||
| Chemotherapy regimen changed due to PAS intolerance | 3/275 | 0/133 | ||
| Chemotherapy regimen changed due to streptomycin toxicity | 6/275 | 6/133 | ||
| Chemotherapy regimen changed due to hypersensitivity reactions | 7/275 | 9/133 | ||
| Vestibular disturbance | 2/87 | 5/91 | ||
| Hypersensitivity | 5/87 | 4/91 | ||
| Hypertension, diabetes, peptic ulcer, psychosis and infections | Incidence equal in each treatment arm (6%, 4%, 0%, 0%, and 4% respectively) | |||
| Changes to chemo regimen due to hypersensitivity, intolerance, drug resistance and ineffectiveness | 29/52 | 24/50 | P < 0.05 | |
| Related respiratory illness (5 years follow up) | 11/52 | 15/50 | Not reported | |
| Acne | 23/52 | 9/50 | ||
| Mooning of the face | 34/52 | 8.5/50 | ||
| Bronchitis, pneumonia or respiratory insufficiency | 4/52 | 11/50 | Not reported | |
| Hypersensitivity reactions | 19/52 | 7/50 | P < 0.025 | |
| Hypersensitivity reactions | 1/49 | 4/51 | Not reported | |
| ≥ 1 adverse event | 87/93 | 82/94 | P = 0.38 | |
| ≥ 1 severe or life threatening event within 3 years | 22/93 | 18/94 | P = 0.19 | |
| Candidiasis | 32/93 | 36/94 | Not reported | |
| Hyperglycemia | 9/93 | 3/94 | P = 0.036 | |
| Abdominal pain | 17/93 | 13/94 | P = 0.47 | |
| Hepatitis | 12/93 | 6/94 | P = 0.09 | |
| Fluid retention | 28/93 | 4/94 | P < 0.001 | |
| Pruritis | 33/93 | 33/94 | P = 0.95 | |
| Herpes simplex | 10/93 | 4/94 | Not reported | |
| Herpes Zoster | 16/93 | 17/94 | P = 0.99 | |
| Kaposi sarcoma | 0/93 | 2/94 | P = 0.49 | |
| Pneumonia | 16/93 | 16/94 | P = 0.93 | |
| Urinary tract infection | 14/93 | 7/94 | P = 0.19 | |
| Hypertension | 12/93 | 4/94 | P = 0.039 | |
| Number of complications | 1/14 | 5/13 | Not reported | |
| Number of side effects | 3/14 | 13/13 | ||
| Rebound phenomena ‐ fever | 8/13 | 0/14 | ||
| Infectious disease | 1/19 | 5/29 | ||
| Viral disease | 2/19 | 9/29 | ||
| bacterial disease | 2/19 | 3/29 | ||
| Incidence of adverse events | Figures not reported, stated no difference between groups | |||
| Athralgia | Figures not reported, stated no difference between groups | |||
| Swelling of feet or face | 62/344 | 3/339 | P = 0.00001 | |
| GI disturbances | 21/344 | 1/339 | P = 0.00001 | |
| Induced hyperglycaemia | 2/344 | 0/339 | Not reported | |
| Severe adverse event requiring discontinuation | 2/851 | 1/424 | Not reported | |
| Hepatitis | 4/851 | 2/424 | Not reported | |
| Sensitivity to Streptomycin‐Pyrazinamide | 68/851 | 22/424 | More patients showed intolerance in steroid groups | |
| Senstivity to Isoniazid‐PAS | 38/851 | 26/424 | Not reported | |
| Blood pressure | No differences in mean SBP or DBP at any time point | |||
| Fasting plasma glucose | No differences at any time points | |||
| Acne | 14/426 | 11/424 | Not reported | |
| Moonface | 10/426 | 3/424 | Not reported | |
| Euphoria | 6/426 | 4/424 | Not reported | |
| Oedema | 3/426 | 9/424 | Not reported | |
| Acne | 17/425 | 11/424 | Not reported | |
| Moonface | 11/425 | 3/424 | Not reported | |
| Euphoria | 11/425 | 4/424 | Not reported | |
| Oedema | 8/425 | 9/424 | Not reported | |
| Author | Outcome definition | Steroid | |
| Acne | 11/54 | ||
| Mooning of face | 22/54 | ||
| Fluid retention | 7/54 | ||
| Transient diabetes mellitus | 6/54 | ||
| DBP over 100 mm | 2/54 | ||
| Paroxysmal nocturnal dyspnoea | 1/54 | ||
| Withdrawal phenomena | None | ||
| Mooning of face | None | ||
| Steroid side effects | None | ||
| Steroid therapy changed or stopped | 15/111 | ||
| Mooning of the face | 7/72 | ||
| Rebound Phenomena | 6/111 | ||
| Hypertension | 7/111 | ||
| Pyschiatric disturbance | 6/111 | ||
| Steroid therapy changed or stopped | 7/116 | ||
| Mooning of the face | 30/116 | ||
| Rebound Phenomena | 34/116 | ||
| Hypertension | 6/116 | ||
| Glycosuria | 10/116 | ||
| Pyschiatric problems | 2/116 | ||
| Marked obesity | 3/87 | ||
| Hypertension | 11/87 | ||
| Mooning of the face | 18/87 | ||
| Hirsuitism | 1/87 | ||
| Transient glycosuria | 6/87 | ||
| Transient rash on prednisolone withdrawal | 17/87 | ||
| Withdrawn from prednisolone therapy | 2/87 | ||
| Acute thrombophlebitis | 1 | ||
| Herpes Zoster | 1 | ||
| Diabetes | 1 | ||
| Marked weight gain (23lb) | 1 | ||
| Moderate Acne | 1 | ||
| Temporary elevation of blood pressure | 3 | ||
| Abdominal distension | 7 | ||
| Steroid therapy suspended | 2 | ||
| Adverse events | None | ||
| Increased infection rate | None | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 18 | 3815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.15] |
| 2 Sputum conversion by 2 months Show forest plot | 13 | 2750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.09] |
| 3 Sputum conversion at 6 months Show forest plot | 10 | 2150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.04] |
| 4 Treatment Failure Show forest plot | 10 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.98, 1.05] |
| 5 Relapse Show forest plot | 5 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.07] |
| 6 Clinical Improvement at 1 month Show forest plot | 5 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.09, 1.24] |

