Aflibercept para la degeneración macular neovascular senil
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees
#2 MeSH descriptor: [Retinal Degeneration] explode all trees
#3 MeSH descriptor: [Retinal Neovascularization] explode all trees
#4 MeSH descriptor: [Choroidal Neovascularization] explode all trees
#5 MeSH descriptor: [Macula Lutea] explode all trees
#6 ((macul* or retina* or choroid*) near/4 degener*)
#7 ((macul* or retina* or choroid*) near/4 neovasc*)
#8 maculopath*
#9 (macul* near/2 lutea*)
#10 (macul* near/3 dystroph*)
#11 (macul* near/2 syndrome)
#12 ((macul* or geographic) near/2 atroph*)
#13 ((macul* or retina*) near/2 edema*)
#14 (AMD or ARMD or CNV)
#15 {or #2‐#14}
#16 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees
#17 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees
#18 MeSH descriptor: [Endothelial Growth Factors] explode all trees
#19 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees
#20 (anti* near/2 VEGF*)
#21 (endothelial near/2 growth near/2 factor*)
#22 (aflibercept or VEGF Trap* or Trap Eye or "AVE 005" or AVE005 or Zaltrap or ZIV‐aflibercept or "AVE 0005" or AVE0005 or eylea or vasculotropin trap)
#23 {or #16‐#22}
#24 #15 and #23
Appendix 2. MEDLINE (OvidSP) search strategy
1. Randomized Controlled Trial.pt.
2. Controlled Clinical Trial.pt.
3. (randomized or randomized).ab,ti.
4. placebo.ab,ti.
5. drug therapy.fs.
6. randomly.ab,ti.
7. trial.ab,ti.
8. groups.ab,ti.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp animals/ not humans.sh.
11. 9 not 10
12. exp Macular Degeneration/
13. exp Retinal Degeneration/
14. exp Retinal Neovascularization/
15. exp Choroidal Neovascularization/
16. exp Macula Lutea/
17. ((macul* or retina* or choroid*) adj4 degener*).tw.
18. ((macul* or retina* or choroid*) adj4 neovasc*).tw.
19. Maculopath*.tw.
20. (macul* adj2 lutea*).tw.
21. (macul* adj3 dystroph*).tw.
22. (macul* adj2 syndrome).tw.
23. ((macul* or geographic) adj2 atroph*).tw.
24. ((macul* or retina*) adj2 edema*).tw.
25. (AMD or ARMD or CNV).tw.
26. or/13‐24
27. exp angiogenesis inhibitors/
28. exp angiogenesis inducing agents/
29. exp endothelial growth factors/
30. exp vascular endothelial growth factors/
31. (anti* adj2 VEGF*).tw.
32. (endothelial adj2 growth adj2 factor*).tw.
33. (aflibercept or VEGF Trap* or Trap Eye or "AVE 005" or AVE005 or Zaltrap or ZIV‐aflibercept or "AVE 0005" or AVE0005 or eylea or vasculotropin trap).tw.
34. (15C2VL427D or 845771‐78‐0 or 862111‐32‐8).rn.
35. or/27‐34
36. 11 and 26 and 35
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE.com search strategy
#1 'randomized controlled trial'/exp
#2 'randomization'/exp
#3 'double blind procedure'/exp
#4 'single blind procedure'/exp
#5 random*:ab,ti
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 'animal'/exp OR 'animal experiment'/exp
#8 'human'/exp
#9 #7 AND #8
#10 #7 NOT #9
#11 #6 NOT #10
#12 'clinical trial'/exp
#13 (clin* NEAR/3 trial*):ab,ti
#14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti
#15 'placebo'/exp
#16 placebo*:ab,ti
#17 random*:ab,ti
#18 'experimental design'/exp
#19 'crossover procedure'/exp
#20 'control group'/exp
#21 'latin square design'/exp
#22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
#23 #22 NOT #10
#24 #23 NOT #11
#25 'comparative study'/exp
#26 'evaluation'/exp
#27 'prospective study'/exp
#28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti
#29 #25 OR #26 OR #27 OR #28
#30 #29 NOT #10
#31 #30 NOT (#11 OR #23)
#32 #11 OR #24 OR #31
#33 'retina maculopathy'/exp
#34 'retina degeneration'/exp
#35 'retina macula degeneration'/exp
#36 'retina neovascularization'/exp
#37 'subretinal neovascularization'/exp
#38 'retina macula lutea'/exp
#39 ((macul* OR retina* OR choroid*) NEAR/4 degener*):ab,ti
#40 ((macul* OR retina* OR choroid*) NEAR/4 neovasc*):ab,ti
#41 maculopath*:ab,ti
#42 (macul* NEAR/2 lutea*):ab,ti
#43 (macul* NEAR/3 dystroph*):ab,ti
#44 (macul* NEAR/2 syndrome):ab,ti
#45 ((macul* OR geographic) NEAR/2 atroph*):ab,ti
#46 ((macul* OR retina*) NEAR/2 edema*):ab,ti
#47 amd:ab,ti OR armd:ab,ti OR cnv:ab,ti
#48 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47
#49 'angiogenesis'/exp
#50 'angiogenesis inhibitor'/exp
#51 'angiogenic factor'/exp
#52 'endothelial cell growth factor'/exp
#53 'vasculotropin'/exp
#54 (anti* NEAR/2 vegf*):ab,ti
#55 endothelial:ab,ti AND growth:ab,ti AND factor*:ab,ti
#56 'aflibercept'/exp
#57 aflibercept:ab,ti OR (vegf NEXT/1 trap*):ab,ti OR 'trap eye':ab,ti OR 'ave 005':ab,ti OR ave005:ab,ti OR zaltrap:ab,ti OR 'ziv aflibercept':ab,ti OR 'ave 0005':ab,ti OR ave0005:ab,ti OR eylea:ab,ti OR 'vasculotropin trap':ab,ti
#58 15c2vl427d:rn OR '845771 78 0':rn OR '862111 32 8':rn
#59 '301253 48 5':rn
#60 #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59
#61 #32 AND #48 AND #60
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh])
#2 ((macul*[tw] OR retina*[tw] OR choroid*[tw]) AND degener*[tw]) NOT Medline[sb]
#3 ((macul*[tw] OR retina*[tw] OR choroid*[tw]) AND neovasc*[tw]) NOT Medline[sb]
#4 Maculopath*[tw] NOT Medline[sb]
#5 (macul*[tw] AND lutea*[tw]) NOT Medline[sb]
#6 (macul*[tw] AND dystroph*[tw]) NOT Medline[sb]
#7 (macul*[tw] AND syndrome[tw]) NOT Medline[sb]
#8 ((macul*[tw] OR geographic[tw]) AND atroph*[tw]) NOT Medline[sb]
#9 ((macul*[tw] OR retina*[tw]) AND edema*[tw]) NOT Medline[sb]
#10 (AMD[tw] OR ARMD[tw] OR CNV[tw]) NOT Medline[sb]
#11 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 (anti VEGF*[tw] OR antiVEGF*[tw]) NOT Medline[sb]
#13 (endothelial[tw] AND growth[tw] AND factor*[tw]) NOT Medline[sb]
#14 (aflibercept[tw] OR VEGF Trap*[tw] OR Trap Eye[tw] OR "AVE 005"[tw] OR AVE005[tw] OR Zaltrap[tw] OR ZIV‐aflibercept[tw] OR "AVE 0005"[tw] OR AVE0005[tw] OR eylea[tw] OR vasculotropin trap[tw]) NOT Medline[sb]
#15 #12 OR #13 OR #14
#16 #1 AND #11 AND #15
Appendix 5. LILACS search strategy
((Macul$ OR Mácul$ OR Retina$ OR Retiniana OR Choroid$ OR Coroide) AND (Degenera$ OR Neovasculariza$) OR MH:C11.768.585$ OR MH:C11.768.585.439$ OR MH: C11.768.725$ OR MH:C23.550.589.500.725$ OR MH:C11.941.160.244$ OR MH:C23.550.589.500.145$ OR MH:A09.371.729.522$ OR maculopath$ OR AMD OR ARMD OR CNV) AND ("Recombinant Fusion Proteins" OR "Proteínas Recombinantes de Fusión" OR "Proteínas Recombinantes de Fusão" OR MH:D12.776.828.300$ OR "Angiogenesis Inhibitors" OR "Inhibidores de la Angiogénesis" OR "Inibidores da Angiogênese" OR "Angiogenic Antagonists" OR MH:D27.505.696.377.077.099$ OR MH:D27.505.696.377.450.100$ OR MH:D27.505.954.248.025$ OR "Angiogenesis Inducing Agents" OR "Inductores de la Angiogénesis" OR "Indutores da Angiogênese" OR MH:D27.505.696.377.077.077$ OR "Factores de Crecimiento Endotelial" OR "Fatores de Crescimento Endotelial" OR MH:D12.644.276.390$ OR MH:D12.776.467.390$ OR MH:D23.529.390$ OR MH:D12.644.276.100.800$ OR MH:D12.776.467.100.800$ OR MH:D23.529.100.800$ OR antiVEGF$ OR anti‐VEGF$ OR (endothelial AND growth AND factor$))
Appendix 6. metaRegister of Controlled Trials search strategy
aflibercept or VEGF Trap* or Trap Eye OR AVE 005 or AVE005 or Zaltrap or ZIV‐aflibercept or AVE 0005 or AVE0005 OR eylea or vascular endothelial growth factor trap or vasculotropin trap or anti‐VEGF
Appendix 7. ClinicalTrials.gov search strategy
(anti‐VEGF AND ("Macular Degeneration" OR AMD OR ARMD OR "Retinal Degeneration" OR "Retinal Neovascularization" OR "Choroidal Neovascularization" OR CNV)) OR (aflibercept OR "VEGF Trap" OR "Trap Eye" OR "AVE 005" OR AVE005 OR Zaltrap OR ZIV‐aflibercept OR "AVE 0005" OR AVE0005 OR eylea OR "vascular endothelial growth factor trap" OR "vasculotropin trap")
Appendix 8. ICTRP search strategy
aflibercept OR VEGF Trap* OR "Trap Eye" OR "AVE 005" OR AVE005 OR Zaltrap OR ZIV‐aflibercept OR "AVE 0005" OR AVE0005 OR eylea OR "vascular endothelial growth factor trap" OR "vasculotropin trap" OR Macular degeneration AND anti‐VEGF OR AMD AND anti‐VEGF OR ARMD AND anti‐VEGF OR "Retinal Degeneration" AND anti‐VEGF OR "Retinal Neovascularization" AND anti‐VEGF OR "Choroidal Neovascularization" AND anti‐VEGF OR CNV AND anti‐VEGF

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
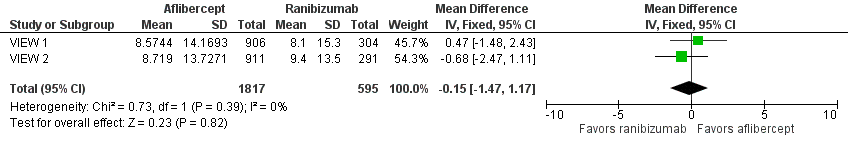
Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.1 Mean change in BCVA in ETDRS letters at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.2 Gain of ≥ 15 letters of BVCA at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.4 Absence of fluid on optical coherence tomography (OCT) at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.7 Mean change in vision‐related quality‐of‐life scores at 1 year.
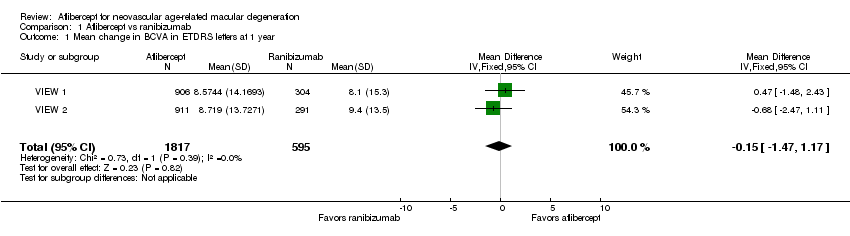
Comparison 1 Aflibercept vs ranibizumab, Outcome 1 Mean change in BCVA in ETDRS letters at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 2 Gain of ≥ 15 letters of BVCA at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 3 Loss of ≥ 15 letters of BVCA at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 4 Absence of fluid on optical coherence tomography (OCT) at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 5 Mean change in size of the choroidal neovascularization at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 6 Mean change in central retinal thickness at 1 year.
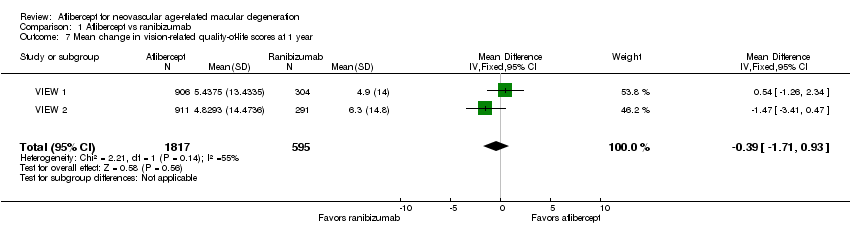
Comparison 1 Aflibercept vs ranibizumab, Outcome 7 Mean change in vision‐related quality‐of‐life scores at 1 year.
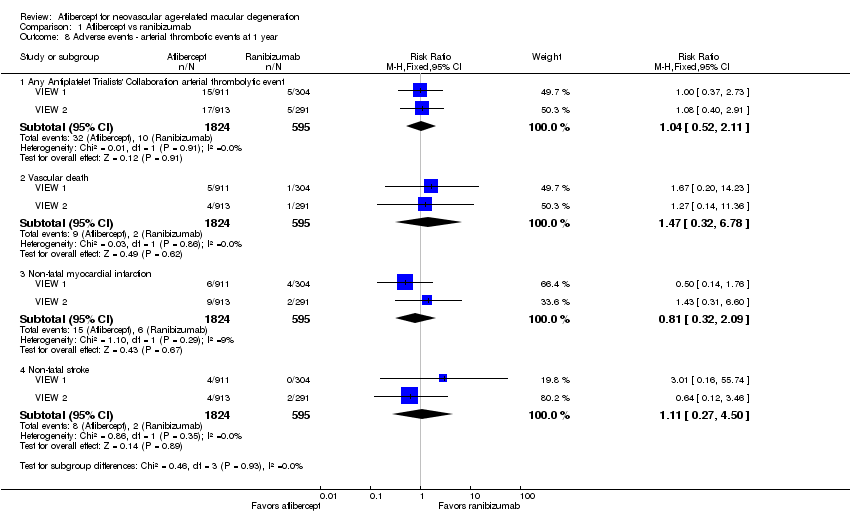
Comparison 1 Aflibercept vs ranibizumab, Outcome 8 Adverse events ‐ arterial thrombotic events at 1 year.
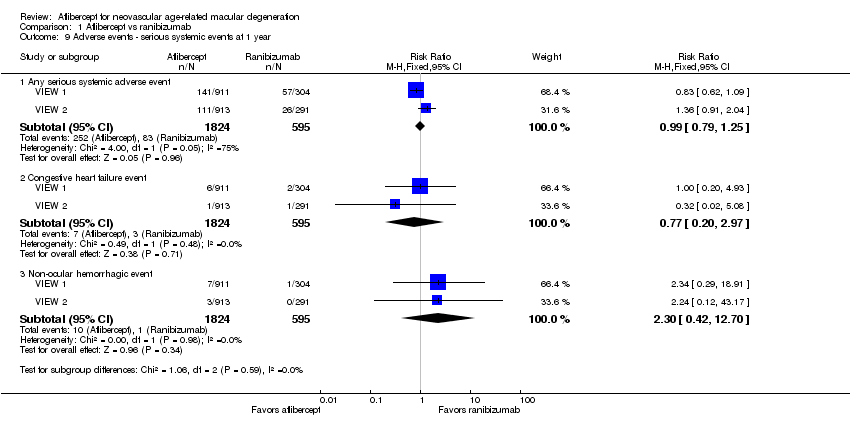
Comparison 1 Aflibercept vs ranibizumab, Outcome 9 Adverse events ‐ serious systemic events at 1 year.
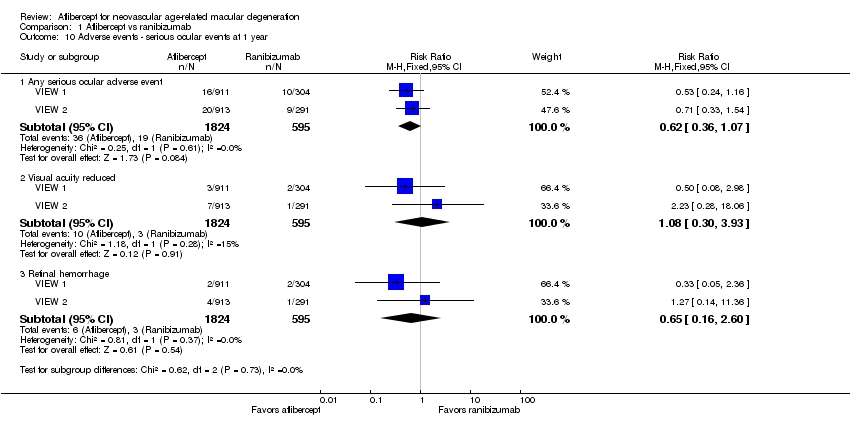
Comparison 1 Aflibercept vs ranibizumab, Outcome 10 Adverse events ‐ serious ocular events at 1 year.
| Aflibercept vs ranibizumab for neovascular age‐related macular degeneration | ||||||
| Patient or population: people with age‐related macular degeneration Settings: clinical centers Intervention: intravitreal injections of aflibercept Comparison: intravitreal injections of ranibizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ranibizumab | Aflibercept | |||||
| Mean change in BCVA in ETDRS letters at 1 year (number of letters) | Mean change in visual acuity across ranibizumab groups ranged from gains of 8.57 letters to 8.71 letters | Mean change in visual acuity in aflibercept groups was on average 0.15 fewer letters gained (95% CI 1.47 fewer letters to 1.17 more letters) | MD ‐0.15 | 2412 | ⊕⊕⊕⊕ | |
| Gain of ≥ 15 letters of BVCA at 1 year | 324 per 1000 | 314 per 1000 | RR 0.97 | 2412 (2) | ⊕⊕⊕⊕ | |
| Absence of fluid on optical coherence tomography (OCT) at 1 year | 595 per 1000 | 630 per 1000 | RR 1.06 | 2291 (2) | ⊕⊕⊕⊕ | |
| Quality‐of‐life measures at 1 year (National Eye Institute‐Visual Function Questionnaire [NEI‐VFQ]) | Mean improvement in composite NEI‐VQF score ranged across control groups from 4.9 to 6.3 points | Mean improvement in composite NEI‐VQF score in intervention groups was on average 0.39 points lower (95% CI 1.71 points lower to 0.93 points higher) | MD ‐0.39 | 2412 (2) | ⊕⊕⊕⊕ | |
| Adverse events ‐ serious systemic events at 1 year | 139 per 1000 | 138 per 1000 | RR 0.99 (0.79 to 1.25) | 2419 (2) | ⊕⊕⊕⊝ | |
| Adverse events ‐ serious ocular events at 1 year | 32 per 1000 | 20 per 1000 | RR 0.62 (0.36 to 1.07) | 2419 (2) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The unit of analysis is the individual (one study eye per person). | ||||||
| GRADE Working Group grades of evidence aAdverse events downgraded to moderate quality as the number of events is small (wide confidence intervals) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA in ETDRS letters at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.47, 1.17] |
| 2 Gain of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 3 Loss of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 4 Absence of fluid on optical coherence tomography (OCT) at 1 year Show forest plot | 2 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 5 Mean change in size of the choroidal neovascularization at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.78, 0.29] |
| 6 Mean change in central retinal thickness at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐4.94 [‐15.48, 5.61] |
| 7 Mean change in vision‐related quality‐of‐life scores at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.71, 0.93] |
| 8 Adverse events ‐ arterial thrombotic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Any Antiplatelet Trialists' Collaboration arterial thrombolytic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.52, 2.11] |
| 8.2 Vascular death | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.78] |
| 8.3 Non‐fatal myocardial infarction | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.09] |
| 8.4 Non‐fatal stroke | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.27, 4.50] |
| 9 Adverse events ‐ serious systemic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Any serious systemic adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 9.2 Congestive heart failure event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.20, 2.97] |
| 9.3 Non‐ocular hemorrhagic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.42, 12.70] |
| 10 Adverse events ‐ serious ocular events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Any serious ocular adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 10.2 Visual acuity reduced | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.30, 3.93] |
| 10.3 Retinal hemorrhage | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.16, 2.60] |


