Tratamiento antiadherencias después de una histeroscopia quirúrgica para la subfertilidad femenina
Appendices
Appendix 1. CGF Specialised Register search strategy
Procite platform
Keywords CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysteroscope " or "office hysteroscopy" or "operative hysteroscopy" or Title CONTAINS "hysteroscopy" or "hysteroscopy pain" or "hysteroscopy pain ‐surgical" or "hysteroscopy, techniques" or "hysteroscope " or "office hysteroscopy" or "operative hysteroscopy"
AND
Keywords CONTAINS "adhesiolysis" or "adhesion" or "adhesions" or "adhesions outcome" or "adhesion prevention" or "adhesion formation" or "pelvic adhesions" or "Sepracoat" or "icodextrin" or "hydrogel" or "hydrotubation" or "Seprafilm" or "intergel" or "Barrier Membrane" or "hyaluronan" or "hyaluronic acid" or "hyaluronidase" or "Promethazine" or "dextran" or "SprayGel" or "adhesion barrier" or "adhesion barriers" or "post‐operative adhesions" or "gynecologic surgical procedure" or "pelvic adhesions" or "amnion graft" or "antibiotics" or "*Estrogens" or "Estrogen" or "oestrogen" or "intrauterine device" or "Intrauterine Devices, Medicated" or "Intrauterine Releasing Devices" or Title CONTAINS "adhesiolysis" or "adhesion" or "adhesions" or "adhesions outcome" or "adhesion prevention" or "adhesion formation" or "pelvic adhesions" or "Sepracoat" or "icodextrin" or "hydrogel" or "hydrotubation" or "Seprafilm" or "intergel" or "Barrier Membrane" or "hyaluronan" (
11 records
Database: Search strategy for JB1900 in the Cochrane Gynaecology and Fertility specialised register was rerun and date limited from 01.01.15 (last search) to 07.06.17
Most recent update: 7 June 2017
Appendix 2. CENTRAL search strategy
#1MeSH descriptor: [Hysteroscopy] explode all trees (403)
#2hysteroscopic surgery (309)
#3operative hysteroscopy (212)
#4synechiolysis (6)
#5#1 or #2 or #3 or #4 (639)
#6barrier agent (816)
#7hyaluronic acid gel (218)
#8intrauterine balloon (116)
#9amnion graft (52)
#10estrogen treatment (6,178)
#11MeSH descriptor: [Intrauterine Devices] explode all trees (616)
#12MeSH descriptor: [Anti‐Bacterial Agents] explode all trees (10,749)
#13#6 or #7 or #7 or #8 or #9 or #10 or #11 or #12 (18,605)
#14intrauterine adhesions (119)
#15adhesion score (566)
#16reproductive outcome (4,098)
#17#14 or #15 or #16 (4,665)
#18#5 and #13 and #17 Publication Year from 2015 to 2017 (14)
Cochrane reviews (7)
Other reviews (0)
Trials (7)
14 records
Database: Cochrane Database of Systematic Reviews: Issue 6 of 12, June 2017
Most recent update: 6 June 2017
Appendix 3. MEDLINE search strategy (PubMed)
(((((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))) AND ((((reproductive outcome) OR adhesion score) OR intrauterine adhesions) OR "Gynatresia"[Majr])) AND (((((((((("Anti‐Bacterial Agents"[Majr]) OR "Intrauterine Devices"[Mesh]) OR estrogen treatment) OR amnion graft) OR intrauterine balloon) OR gel) OR hyaluronan) OR hyaluronic acid gel) OR barrier agents) OR adhesion prevention)) AND (((((synechiolysis) OR operative hysteroscopy) OR "Gynecologic Surgical Procedures"[Majr]) OR hysteroscopic surgery) OR "Hysteroscopy"[Majr])
21 records
Database: MEDLINE using PubMed
Most recent update: 6 June 2017
Appendix 4. Embase search strategy (Embase.com)
#1'hysteroscopy'/exp OR 'hysteroscopy'(10,800)
#2hysteroscopic AND 'surgery' (3,504)
#3gynaecological AND 'surgery' (15,150)
#4operative AND 'hysteroscopy'(1,775)
#5synechiolysis (82)
#6#1 OR #2 OR #3 OR #4 OR #5 (25,733)
#7'adhesion'/exp AND 'prevention' (2,460)
#8barrier AND agents (11,435)
#9hyaluronic AND 'acid'/exp AND 'gel'/exp (28)
#10'hyaluronan'/exp (33,533)
#11'gel'/exp (55,900)
#12'intrauterine'/exp AND 'balloon'/exp (602)
#13'amnion'/exp AND graft (735)
#14'estrogen'/exp AND treatment (79,899)
#15'intrauterine'/exp AND 'device'/exp (22,666)
#16'antibiotics'/exp (1,175,044)
#17#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 (1,365,328)
#18'intrauterine'/exp AND 'adhesions'/exp (465)
#19'adhesion'/exp AND score (937)
#20reproductive AND outcome (44,546)
#21#18 OR #19 OR #20 (45,864)
#22#6 AND #17 AND #21 (342)
#23'clinical trial'/exp (1,201,041)
#24'randomized controlled trial'/exp (447,991)
#25'randomization'/exp (73,693)
#26'single blind procedure'/exp (27,124)
#27'double blind procedure'/exp (137,917)
#28'crossover procedure'/exp (51,040)
#29'placebo'/exp (305,105)
#30randomi?ed AND controlled AND trial* AND [embase]/lim (554,250)
#31rct AND [embase]/lim (23,901)
#32'random allocation'/exp AND [embase]/lim (46,023)
#33'randomly allocated' AND [embase]/lim (23,118)
#34'allocated randomly' AND [embase]/lim (1,917)
#35allocated NEAR/2 random AND [embase]/lim (766)
#36'single blind$' AND [embase]/lim (29,481)
#37'double blind$' AND [embase]/lim (193,062)
#38(treble OR triple) NEAR/2 blind$ AND [embase]/lim (591)
#39placebo$ AND [embase]/lim (364,742)
#40'prospective study'/exp (372,790)
#41#23 OR #24 OR 25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 (1,883,550)
#42'case study'/exp (46,539)
#43'case report'/exp AND [embase]/lim (1,627,297)
#44'abstract report'/exp (89,710)
#45'letter'/exp (926,515)
#46#42 OR #43 OR #44 OR #45 (2,538,528)
#47#41 NOT #46 (1,819,026)
#48'animal'/exp (23,131,376)
#49'human'/exp (18,279,043)
#50#48 NOT #49 (4,852,333)
#51#47 NOT #50 (1,756,205)
#52#22 AND #51 (85)
#53#22 AND #51 AND [1‐3‐2015]/sd NOT [1‐6‐2017]/sd (32)
32 records
Database: Embase using Embase.com
Most recent update: 6 June 2017
Appendix 5. Web of Science search strategy
# 1TS = (hysteroscopy) (509)
# 2TS = (hysteroscopic surgery) (156)
# 3TS = (operative hysteroscopy) (122)
# 4TS = (synechiolysis) (8)
# 5#1 OR #2 OR #3 OR #4 (585)
# 6TS = (barrier agent)(3,157)
# 7TS =(hyaluronic acid gel)(482)
# 8TS = (intrauterine balloon)(70)
# 9TS = (amnion graft)(35)
# 10TS = (estrogen treatment) (5,460)
# 11TS = (intrauterine device) (701)
# 12TS = (antibiotics) (39,645)
# 13#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (49,338)
# 14TS =(intrauterine adhesions) (125)
# 15TS =(adhesion score) (690)
# 16TS = (reproductive outcome)(4,515)
# 17#14 OR #15 OR #16 (5,293)
# 18#5 AND #13 AND #17 (28)
# 19 TS =(randomized controlled trial) (82,310)
# 20 #18 AND #19 (11)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=2015‐2017
11 records
Database: Web of Science (WoS)
Most recent update: 6 June 2017
Appendix 6. CINAHL search strategy (EBSCOhost)
S1 TX hysteroscopy (466)
S2 TX hysteroscopic surgery (25)
S3 TX operative hysteroscopy (28)
S4 TX synechiolysis (2)
S5 S1 OR S2 OR S3 OR S4 (473)
S6 ""barrier agent"" (24,118)
S7 TX hyaluronic acid gel (26)
S8 TX intrauterine balloon (29)
S9 TX amnion graft (4)
S10 TX estrogen treatment (522)
S11 TX intrauterine device (1,577)
S12 TX antibiotics (35,078)
S13 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 (37,486)
S14 TX intrauterine adhesions (15)
S15 TX adhesion score (29)
S16 TX reproductive outcome (590)
S17 S14 OR S15 OR S16 (632)
S18 S5 AND S13 AND S17 (5)
S19 (MH "Clinical Trials") (87,486)
S20 PT clinical trial* (52,906)
S21 (MH "Randomized Controlled Trials") (29,785)
S22 PT randomized controlled trial* (30,863)
S23 (MH "Random Assignment") (34,135)
S24 TX Randomi*ation (5,181)
S25 TX single blind* (8,515)
S26 TX double blind* (707,324)
S27 TX triple blind* (137)
S28 ""TX treble blind*"" (38,806)
S29 TX Placebo* (31,331)
S30 TX prospective stud* (204,534)
S31 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 (975,773)
S32 S18 AND S31 (1)
1 record
Database: CINAHL using EBSCOHOST
Most recent update: 6 June 2017
Appendix 7. Items of the pilot‐tested data extraction form
1. Source
-
Study ID.
-
Report ID.
-
Review author ID.
-
Citation and contact details.
2. Eligibility
-
Confirm eligibility for review.
-
Reason for exclusion.
3. Trial characteristics
Study design
-
Random sequence generation.
-
Participant recruitment.
-
Participant inclusion and exclusion criteria.
-
Allocation concealment.
-
Blinding of participants, personnel and outcome assessors.
-
Completeness of outcome data.
-
Selective outcome reporting.
-
Other potential sources of bias.
Follow‐up
-
Duration of follow‐up.
-
Type of follow‐up.
Size of study
-
Number of women recruited.
-
Number of women randomly assigned.
-
Number of women excluded.
-
Number of women withdrawn and lost to follow‐up.
-
Number of women analysed.
Study setting
-
Single‐ or multicentre.
-
Location.
-
Timing and duration.
Diagnostic criteria
-
Screening by transvaginal sonography (TVS).
-
Screening by hysterosalpingography (HSG).
-
Screening by TVS and HSG.
-
Screening by other ultrasound diagnostic procedures, e.g. saline infusion sonography or gel instillation sonography.
-
Screening by hysteroscopy.
-
Diagnosis confirmed by hysteroscopy and biopsy.
4. Characteristics of study participants
Baseline characteristics
-
Age.
-
Primary or secondary subfertility.
-
Duration of subfertility.
-
Diagnostic workup: baseline follicle‐stimulating hormone, semen analysis, diagnosis of tubal pathology, confirmatory test of ovulation.
-
Other contributory causes to subfertility than uterine factor.
-
Previous treatments, e.g. in vitro fertilisation (IVF), intrauterine insemination (IUI) or other treatments.
Treatment characteristics
-
IUI natural cycle.
-
IUI controlled ovarian stimulation with anti‐oestrogens or gonadotropins.
-
IVF protocol and number of embryos transferred.
-
Intracytoplasmic sperm injection protocol and number of embryos transferred.
-
Detailed description of hysteroscopic procedure.
-
Detailed description of anti‐adhesion therapy.
5. Interventions
Total number of intervention groups
Absence of other interventions in treatment and control groups
For each intervention and comparison group of interest:
-
specific intervention;
-
intervention details;
-
timing of the intervention.
6. Outcomes
Outcomes and time points reported
Definition and unit of measurement for each of the following outcomes.
Primary outcome
-
Live birth.
-
Presence of intrauterine adhesions at second‐look hysteroscopy.
Secondary outcomes
-
Pregnancy.
-
Miscarriage.
-
Mean adhesion scores at second‐look hysteroscopy.
-
Severity of adhesions at second‐look hysteroscopy.
For each outcome of interest:
-
sample size;
-
missing participants;
-
summary data for each intervention group in 2 × 2 table;
-
estimate of effect with 95% confidence interval;
-
subgroup analyses.
7. Miscellaneous
-
Funding source.
-
Key conclusions of study authors.
-
Miscellaneous comments from study authors.
-
References to other relevant studies.
-
Correspondence required.
-
Miscellaneous comments by review authors.

Study flow diagram: summary of searches since 2015. PICO: population, intervention, comparator, outcome; RCT: randomised controlled trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.1 Live birth.
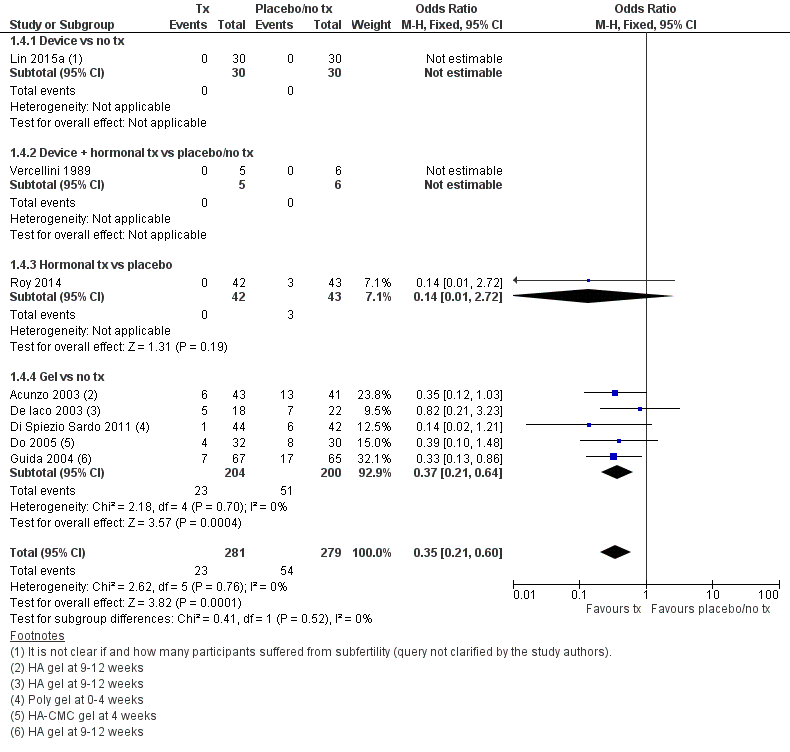
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.4 Presence of intrauterine adhesions at second‐look hysteroscopy.

Cates' plot of numbers needed to treat for an additional beneficial outcome (NNTB) for Analysis 1.4 assuming medium risk of 545 women per 1000 with intrauterine adhesions at second‐look hysteroscopy in the control group (no treatment or placebo). Randomly compared to control, the use of device with or without hormonal treatment or hormonal treatment or barrier gels (intervention) decreased the number of women with intrauterine adhesions at second‐look hysteroscopy to 234 women per 1000 (95% confidence interval 153 to 365 women per 1000). Figure drawn using www.nntonline.net.
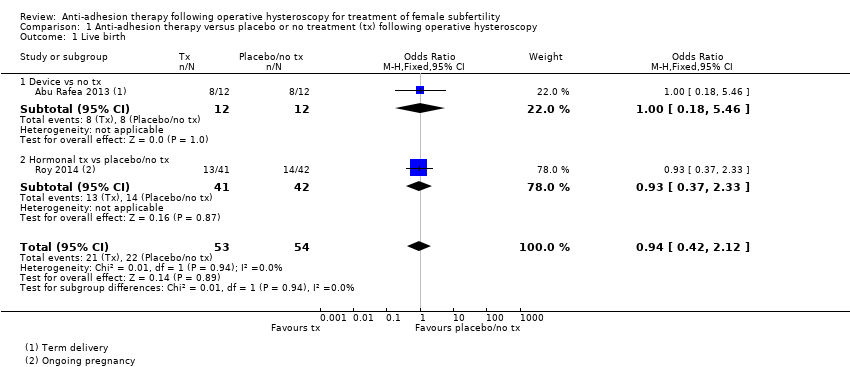
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 1 Live birth.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 2 Clinical pregnancy.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 3 Miscarriage.
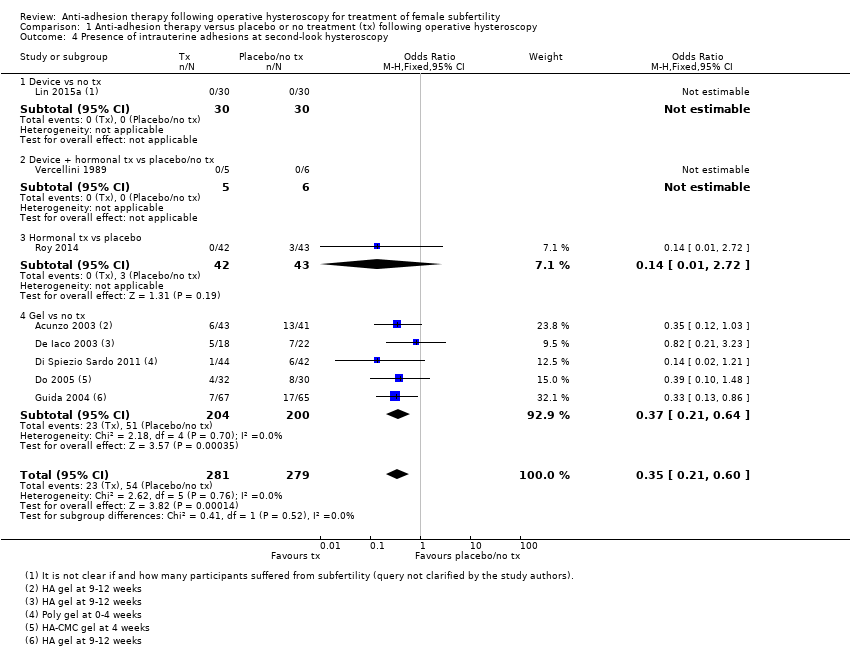
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions.
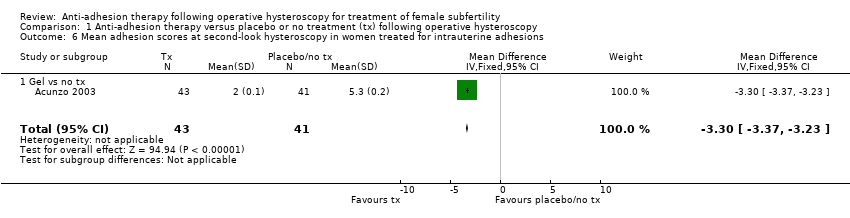
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions.
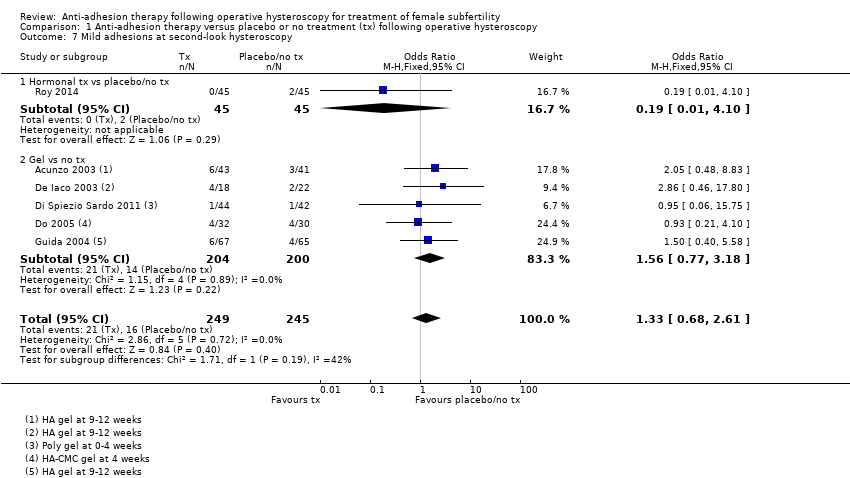
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 7 Mild adhesions at second‐look hysteroscopy.
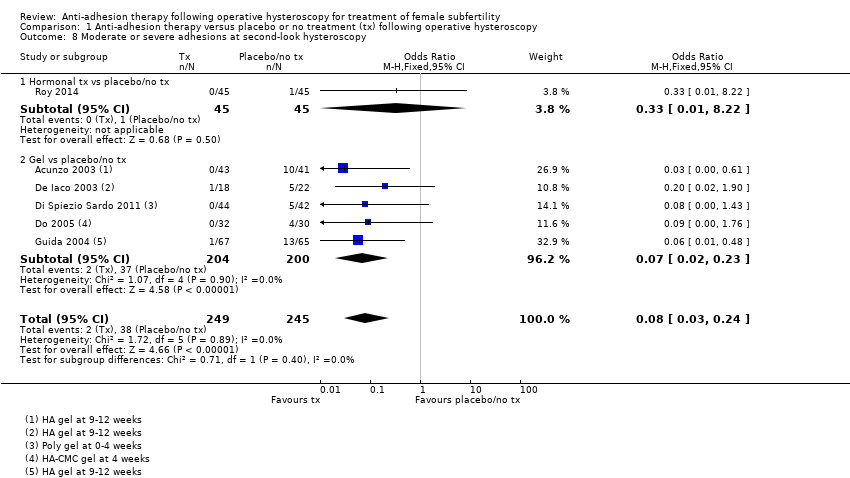
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 8 Moderate or severe adhesions at second‐look hysteroscopy.
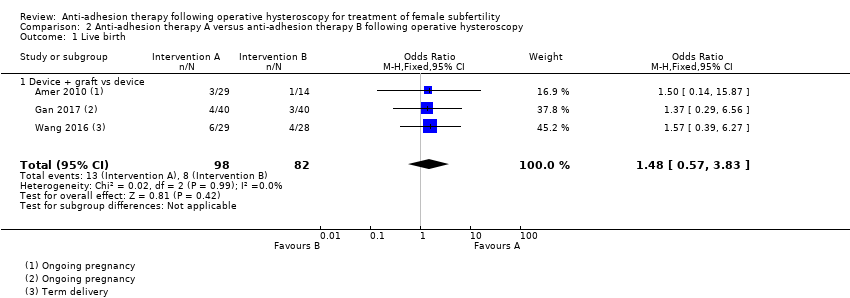
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 1 Live birth.
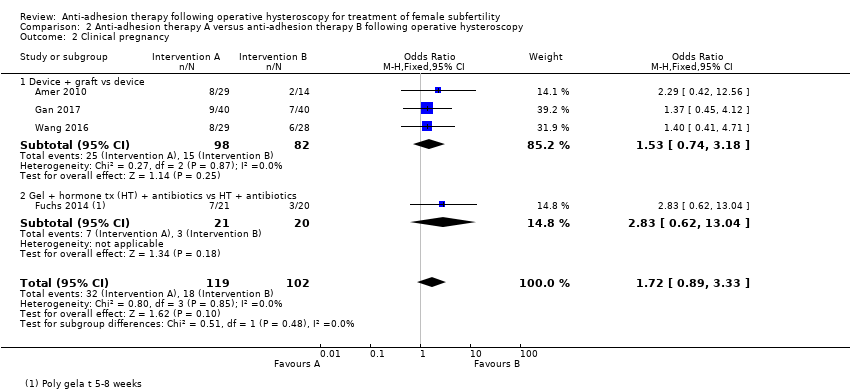
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 2 Clinical pregnancy.
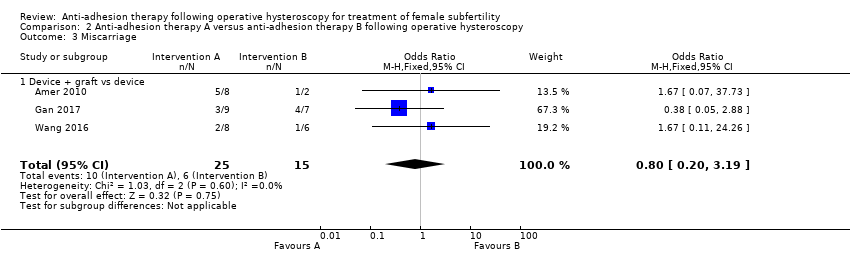
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 3 Miscarriage.
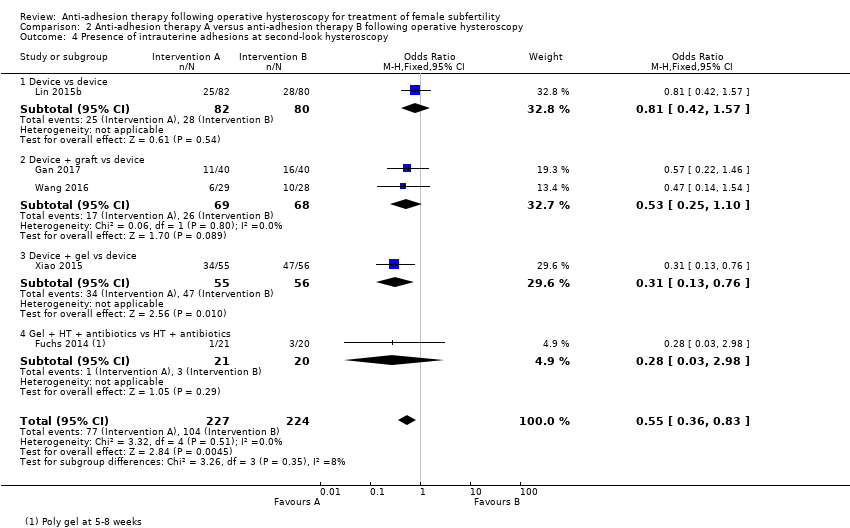
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.
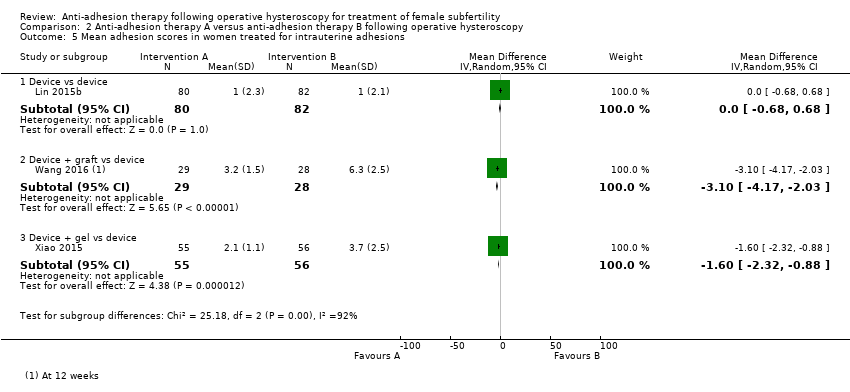
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 5 Mean adhesion scores in women treated for intrauterine adhesions.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 6 Mild adhesions at second‐look hysteroscopy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 7 Moderate or severe adhesions at second‐look hysteroscopy.
| Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology associated with subfertility or adverse pregnancy outcome Settings: single centre, Hysteroscopy Unit or Department of Obstetrics and Gynaecology of a university or non‐university tertiary care hospital Intervention: any anti‐adhesion therapy Comparison: no treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Anti‐adhesion therapy | |||||
| Live birth a | No treatment or placebo | Device or hormonal treatment | OR 0.94 (0.42 to 2.12) | 107 | ⊕⊝⊝⊝ | ‐ |
| Mean‐risk populationb | ||||||
| 407 per 1000 | 399 per 1000 | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (second‐look hysteroscopy at 4‐12 weeks after operative hysteroscopy) | No treatment or placebo | Device ± hormonal treatment or hormonal treatment or barrier gel | OR 0.35 g (0.21 to 0.60) | 560 (8 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk populationf | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population f | ||||||
| 545 per 1000 | 234 per 1000 | |||||
| High‐risk population f | ||||||
| 875 per 1000 | 376 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The two included studies reported term delivery (Abu Rafea 2013) or ongoing pregnancy (Roy 2014), which we used as a surrogate outcome for live birth. b The assumed risk for the mean‐risk population was the pooled risk of all live births in control groups of the two included studies. c Downgraded one level for serious risk of bias: one study was at high risk of bias in several domains, including allocation concealment. d Downgraded one level for serious imprecision; only 43 events in total. e Downgraded one level for serious indirectness, because only 30% (35/118) of all randomised women in this analysis were subfertile. f The assumed risk for low‐, medium‐ and high‐risk population based on presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and intrauterine adhesions (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). G Two studies reported no events (Lin 2015a; Vercellini 1989). h Downgraded one level for serious risk of bias: all eight studies had several limitations but none was at high risk for selection bias related to random sequence generation or allocation concealment. i Downgraded one level for serious indirectness, because in four of eight studies less than 50% of participants were subfertile and in four of eight studies it was unclear whether subfertile women were included. | ||||||
| Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology Settings: multicentric, Hysteroscopy Unit of Department of Obstetrics and Gynaecology of a university, university‐affiliated or non‐university tertiary care hospital Intervention: anti‐adhesion therapy A Comparison: anti‐adhesion therapy B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐adhesion therapy B | Anti‐adhesion therapy A | |||||
| Live birth a | Device | Device + graft | OR 1.48 (0.57 to 3.83) | 180 (3 RCTs) | ⊕⊕⊝⊝ Low c,d | ‐ |
| 98 per 1000 b | 138 per 1000 (60 to 315) | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (6‐12 weeks) | Device or hormonal treatment with antibiotics | Device ± graft/gel or gel + hormonal treatment + and antibiotics | OR 0.55 (0.36 to 0.83) | 451 (5 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk population e | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population e | ||||||
| 545 per 1000 | 403 per 1000 | |||||
| High‐risk population e | ||||||
| 875 per 1000 | 647 per 1000 | |||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The three included studies reported term delivery (Wang 2016) or ongoing pregnancy (Amer 2010; Gan 2017; Wang 2016), which we used as a surrogate outcome for live birth. b The assumed risk for the average‐risk population is the pooled risk of all the live births in the control groups of the three included studies. c Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. d Downgraded one level for serious imprecision‐ only 21 events in total. e The assumed risk for low/medium/high‐risk population is based on the presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and IUAs (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). f Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. g Downgraded one level for serious indirectness because, in two of five studies, less than 50% of participants were subfertile; in one of five studies, it was unclear if subfertile women were included and in two of five studies, the proportion of infertile women was not reported. | ||||||
| Outcome | Balloon group (intervention: n = 82) | IUD group (control: n = 80) | P value |
| AFS score before surgery (median, 95% CI) | 8 (5 to 12) | 8 (5 to 12) | 1.00 |
| Median reduction in AFS score | 7 (2 to 12) | 7 (0 to 12) | 1.00 |
| IUD: intrauterine device; n: number of participants. | |||
| Statistic | Fresh amnion graft (group 2: n = 14) | Dried amnion graft (group 3: n = 15) | No amnion graft (group 1: n = 14) | P value |
| Median | 1.5 | 2 | 2 | ‐ |
| IQR | 1 to 2 | 1 to 2 | 1 to 2 | 0.27 |
| IQR: interquartile range; n: number of participants. | ||||
| Statistic | Amnion graft (intervention: n = 40) | No graft (control: n = 40) | P value |
| Median | 2 | 4 | ‐ |
| IQR | 2 to 5 | 2 to 6 | 0.03 |
| IQR: interquartile range; n: number of participants. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 1.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 1.2 Hormonal tx vs placebo/no tx | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 2 Clinical pregnancy Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.01] |
| 2.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2.2 Hormonal tx vs placebo | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
| 3 Miscarriage Show forest plot | 2 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.18, 2.57] |
| 3.1 Device vs no tx | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 3.2 Hormonal tx vs placebo | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.01] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 8 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.60] |
| 4.1 Device vs no tx | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Device + hormonal tx vs placebo/no tx | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Hormonal tx vs placebo | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 4.4 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 5.1 Gel vs no tx | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 6.1 Gel vs no tx | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 7 Mild adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 7.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.10] |
| 7.2 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| 8 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.24] |
| 8.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 8.2 Gel vs placebo/no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 1.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 2 Clinical pregnancy Show forest plot | 4 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.89, 3.33] |
| 2.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 2.2 Gel + hormone tx (HT) + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 13.04] |
| 3 Miscarriage Show forest plot | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 3.1 Device + graft vs device | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| 4.1 Device vs device | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.57] |
| 4.2 Device + graft vs device | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.10] |
| 4.3 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 4.4 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
| 5 Mean adhesion scores in women treated for intrauterine adhesions Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Device vs device | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.68, 0.68] |
| 5.2 Device + graft vs device | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.17, ‐2.03] |
| 5.3 Device + gel vs device | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐2.32, ‐0.88] |
| 6 Mild adhesions at second‐look hysteroscopy Show forest plot | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 6.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 7 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 2 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.61] |
| 7.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.63] |
| 7.2 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |

