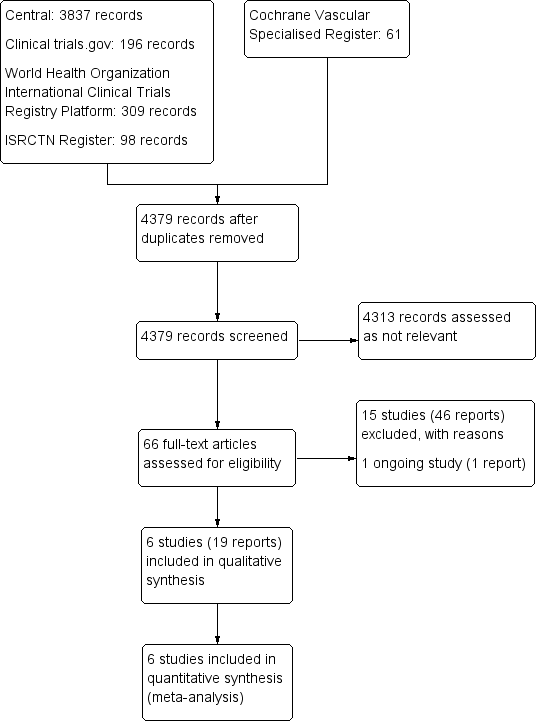
Prevención secundaria de la tromboembolia venosa recurrente después del tratamiento de anticoagulación oral inicial en pacientes con tromboembolia venosa sin causa aparente
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design : double‐blind, randomised, placebo‐controlled study Length of follow‐up : 4 years Dates of study : May 2003 to August 2011 Location : 56 sites in 5 countries (Australia, New Zealand, Canada, India, Argentina) Setting : hospital | |
| Participants | Number : 822: aspirin 411, placebo 411 Age, mean (SD) years : aspirin 55 (16.0), placebo 54 (15.8) Sex : aspirin 226 M/185 F, placebo 221 M/190 F Ethnic group : not stated Inclusion criteria : Male and female patients were eligible for inclusion if they were at least 18 years of age and had a first unprovoked episode of objectively diagnosed symptomatic DVT involving the popliteal vein or more proximal leg veins or an acute PE. VTE was considered to be unprovoked if it occurred in the absence of the following transient risk factors during the preceding 2 months: confinement to bed for longer than 1 week, major surgery, trauma requiring a cast, pregnancy or the puerperium, and use of the oral contraceptive pill or hormone replacement therapy. All patients were required to have completed initial anticoagulation therapy with heparin followed by warfarin (or an effective alternative anticoagulant) for between 6 weeks and 24 months. Exclusion criteria : Patients were not eligible for inclusion if the first unprovoked episode of VTE had occurred more than 2 years before enrolment; if they had an indication or contraindication for the use of aspirin, other antiplatelet therapy, or a non‐steroidal anti‐inflammatory drug; if they had an indication for continuing oral anticoagulation therapy; or if they had other medical problems that would interfere with participation in the trial or would limit life expectancy. Previous DVT or PE : no Type of VTE at initial diagnosis : apixaban 236 DVT/112 PE/59 DVT + PE, placebo 232 DVT/119 PE/56 DVT+ PE Creatinine clearance : not stated | |
| Interventions | Intervention 1 : aspirin 100 mg daily Intervention 2 : placebo 100 mg daily Duration of treatment : 2 to 4 years | |
| Outcomes | Primary : recurrence of VTE (composite of symptomatic, objectively confirmed DVT, non‐fatal PE, or fatal PE) and bleeding (major or clinically relevant non‐major bleeding). Major bleeding was defined as overt bleeding that was associated with a decrease in haemoglobin of at least 2 g per decilitre, or that necessitated transfusion of 2 or more units of blood, involved a critical site (e.g. retroperitoneal or intracranial bleeding), was disabling, required surgical intervention, or contributed to death. Bleeding episodes that did not meet the definition of major bleeding were considered to be clinically relevant only if they led to discontinuation of the study drug for longer than 14 days. Secondary : major vascular events (a composite of VTE, myocardial infarction, stroke, or cardiovascular death) and a measure of the net clinical benefit (a reduction in rate of the composite of VTE myocardial infarction, stroke, major bleeding, or death from any cause) Time frame for measuring outcomes : median 37.2 months | |
| Notes | 5 (1%) apixaban and 4 (1%) placebo patients had < 3 months' anticoagulation before randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed through a central web‐based randomisation system". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed through a central web‐based randomisation system". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind, matching placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All primary and secondary events were adjudicated by an independent event adjudication committee whose members were unaware of the group assignments". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
| Methods | Study design : multi‐centre, randomised, double‐blind, active‐controlled, event‐driven study Length of follow‐up : 12 months Dates of study : March 2014 to March 2016 Location : 31 countries Setting : 230 hospitals | |
| Participants | Number : total participants in study 3365: rivaroxaban 2234, aspirin 1131; participants with unprovoked VTE: rivaroxaban: 921, aspirin: 468 Age, mean (SD) years : rivaroxaban 58.8 (14.7), aspirin 58.8 (14.7) Sex : rivaroxaban 1222 M/1012 F, aspirin 643 M/488 F Ethnic group : not stated Inclusion criteria : patients with objectively confirmed symptomatic DVT and/or PE who have completed 6 to 12 months of anticoagulant therapy with warfarin or another VKA, or with rivaroxaban, apixaban, or dabigatran, who have not interrupted therapy for more than 7 days before randomisation and who did not experience a symptomatic recurrence during the anticoagulation period Exclusion criteria : patients with an indication for therapeutic dose anticoagulants, hypersensitivity to investigational or comparator treatment, any other contraindication listed in the local labelling for investigational or comparator treatment, an indication for antiplatelet therapy or a conventional non‐steroidal anti‐inflammatory drug, hepatic disease associated with coagulopathy leading to a clinically relevant bleeding risk, active bleeding or high risk of bleeding contraindicating anticoagulant therapy, life expectancy < 6 months, concomitant use of human immunodeficiency virus protease inhibitors ‐ ketoconazole, itraconazole, voriconazole, and posaconazole, childbearing potential without proper contraceptive measures, pregnancy or breast feeding, and participation in a study with an investigational drug or medical device within 30 days before randomisation. Additional ineligibility criteria included a calculated creatinine clearance < 30 mL per minute or hepatic disease associated with a coagulopathy Creatinine clearance : 30 to < 50 mL/min: 89 rivaroxaban, 63 aspirin; 50 to < 80 mL/min: 581 rivaroxaban, 277 aspirin; ≥ 80 mL/min: 1561 rivaroxaban, 790 aspirin | |
| Interventions | Intervention 1 : rivaroxaban 10 mg once daily Intervention 2 : rivaroxaban 20 mg once daily Intervention 3 : aspirin 100 mg once daily Duration of treatment : 12 months | |
| Outcomes | Primary : recurrent symptomatic VTE (including fatal and non‐fatal PE and DVT) and clinically overt major bleeding (associated with a fall in haemoglobin ≥ 2 g/dL, leading to transfusion of ≥ 2 units of packed red blood cells or whole blood, or occurring in a critical site (e.g. intracranial, intraocular, pericardial, intra‐articular, intramuscular with compartment syndrome or retroperitoneal, or leading to death) Secondary : myocardial infarction, ischaemic stroke, non‐central nervous system embolism, all‐cause mortality, and clinically relevant non‐major bleeding (non‐major overt bleeding associated with study drug interruption longer than 14 days) Time frame for measuring outcomes : 12 months | |
| Notes | Please note that for the analysis in this review, we used data for participants with a diagnosis of an unprovoked index VTE only (number of participants rivaroxaban: 921, aspirin: 468). Data on secondary outcomes and VTE mortality for participants with a diagnosis of an unprovoked index VTE were not available in the main publication of the study, nor in the supplementary material. We contacted study authors to ask for these data, but they could not provide this information before completion of this review. Once the data are acquired, we will add them to future updates of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization with a block size of six was performed with the use of an interactive voice‐response system". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization with a block size of six was performed with the use of an interactive voice‐response system". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind. Rivaroxaban (20 mg and 10 mg) and matching placebo were provided as identical‐appearing, immediate‐release film‐coated tablets, whereas aspirin and matching placebo were provided as enteric‐coated tablets". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An independent committee whose members were unaware of the study‐group assignments adjudicated the qualifying initial diagnosis (deep‐vein thrombosis or pulmonary embolism) and all suspected outcomes that occurred during the study". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
| Methods | Study design : multi‐centre, randomised, double‐blind, parallel‐group trial Length of follow‐up : 42 months Dates of study : July 2007 to March 2012 Location : France Setting : 14 hospitals | |
| Participants | Number : 371: 184 warfarin, 187 placebo Age, mean (SD) years : warfarin 58.7 (17.9), placebo 57.3 (17.4) Sex : warfarin 78 M/106 F, placebo 103 M/84 F Ethnic group : not stated Inclusion criteria : patients 18 years of age or older who experienced a first episode of symptomatic unprovoked PE and had been treated initially for 6 uninterrupted months with a VKA. Unprovoked PE was defined as objectively confirmed symptomatic PE occurring in the absence of any major reversible risk factor for VTE within 3 months before diagnosis, including surgery with locoregional or general anaesthesia lasting longer than 30 minutes, trauma with or without plaster cast of the lower limbs, and bed rest for longer than 72 hours and in the absence of active cancer or cancer resolved within the 2 years before diagnosis. Objective tests confirming the index PE were ventilation‐perfusion lung scanning and spiral computerised tomography angiography. Exclusion criteria : Previously confirmed PE or proximal DVT, recurrent VTE, or bleeding during the initial 6‐month anticoagulation, known major thrombophilia, indication for VKA therapy for reasons other than VTE, increased bleeding risk, platelet count below 100 × 103/μL, major surgery planned within 18 months from randomisation, and life expectancy less than 18 months Previous DVT or PE : warfarin 17 DVT, placebo 14 DVT Type of VTE at initial diagnosis : PE Creatinine clearance : ≥ 30 to < 50 mL/min warfarin 16, placebo 7; ≥ 50 mL/min warfarin 164, placebo 173 | |
| Interventions | Intervention 1 : warfarin Intervention 2 : placebo Duration of treatment : 18 months | |
| Outcomes | Primary : symptomatic recurrent VTE (non‐fatal PE, fatal PE, and proximal DVT) and major bleeding at 18 months after randomisation (defined as major if it was clinically overt and associated with a fall in haemoglobin level ≥ 2.0 g per decilitre, or a need for transfusion of ≥ 2 units of red cells; if it was retroperitoneal or intracranial; or if it warranted permanent discontinuation of the study drug) Secondary : symptomatic recurrent VTE (non‐fatal PE, fatal PE, and proximal DVT) and major bleeding at 42 months after randomisation, and death unrelated to PE or major bleeding at 18 and 42 months Time frame for measuring outcomes : 18 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised using a central computerised Internet‐based system. Using a computer algorithm, an independent statistician generated the assignment list in randomly permuted blocks. Before the first patient was enrolled, this list was forwarded to a central anticoagulation clinic not involved in patient care". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised using a central computerised Internet‐based system". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind.....identical‐looking placebo....study INRs were determined at each patient's usual local laboratory and sent directly to the anticoagulation clinic. Patients and investigators remained unaware of the local results to maintain double‐blind conditions. For patients assigned to receive warfarin, the clinic returned the true INR results to investigators for dose adjustments. For those assigned to placebo, the clinic substituted computer‐generated sham INR results". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All outcomes were adjudicated blindly by an independent central critical events committee. All images for ventilation‐perfusion lung scanning, spiral computerised tomographic angiography, and ultrasonography were reviewed centrally by independent dedicated panels". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
| Methods | Study design : multi‐centre, randomised, double‐blind, placebo‐controlled trial Length of follow‐up : 2 years Dates of study : May 2004 to August 2010 Location : 25 European Centers in Austria, Denmark, France, Great Britain, Italy, and the Netherlands Setting : hospital | |
| Participants | Number : 403: 205 aspirin, 198 placebo Age, mean (SD) years : aspirin 61.9 (15.3), placebo 62.1 (15.1) Sex : aspirin 135 M/70 F, placebo 122 M/75 F Ethnic group : aspirin 203 (99%) white, placebo 195 (98.9%) white Inclusion criteria : patients aged 18 years or older who had been treated for 6 to 18 months with VKAs for a first‐ever, objectively confirmed, symptomatic, unprovoked proximal DVT, PE, or both. VTE was considered to be unprovoked when it occurred in the absence of any known risk factor. Exclusion criteria : known cancer; known major thrombophilia (antiphospholipid antibodies or lupus anticoagulant or homozygous factor V Leiden or prothrombin G21210A or double heterozygosity for factor V Leiden and prothrombin G21210A or deficiency of antithrombin, protein C or S); an indication for long‐term anticoagulant therapy other than VTE (as atrial fibrillation or prosthetic heart valve); previous symptomatic complications of atherosclerosis requiring treatment with aspirin or other antiplatelet agents; active bleeding or high risk for bleeding or a bleeding episode that occurred during the 6 to 18 months of anticoagulation; known allergy or intolerance to aspirin; life expectancy shorter than 6 months; anticipated non‐adherence to study medications; pregnancy or breast‐feeding; participation in another experimental pharmacotherapeutic program within 30 days before randomisation. Women with venous thromboembolism associated with the use of estro‐progestin therapy were excluded from the study. Previous DVT or PE : no Type of VTE at initial diagnosis : aspirin 122 DVT/83 PE, placebo 130 DVT/67 PE Creatinine clearance : not stated | |
| Interventions | Intervention 1 : aspirin 100 mg once daily Intervention 2 : placebo Duration of treatment : 2 years | |
| Outcomes | Primary : symptomatic recurrent VTE (DVT, fatal or non‐fatal PE) and major bleeding (defined as major if it was clinically overt and was associated with a fall in the haemoglobin level of ≥ 2.0 g per decilitre or a need for transfusion of ≥ 2 units of red cells; if it was retroperitoneal or intracranial; or if it warranted permanent discontinuation of the study drug) Secondary : arterial events (non‐fatal MI, unstable angina, stroke, transient Ischaemic attack, acute ischaemia of the lower limbs), all‐cause mortality, and clinically relevant, non‐major bleeding (any overt bleeding that required a medical intervention and did not meet any of the criteria for major bleeding) Time frame for measuring outcomes : 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote :"The randomisation sequence will be computer generated". |
| Allocation concealment (selection bias) | High risk | Quote: "Patients will be randomised according to the consecutive box number assigned to the study centre". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All suspected study outcomes were assessed by a central, independent adjudication committee whose members were unaware of the group assignments and who reviewed the imaging results". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
| Methods | Study design : multi‐centre, randomised, open trial Length of follow‐up : 2 years Dates of study : January 1995 to June 1998 Location : Italy Setting : hospital | |
| Participants | Number : 267: 134 extended anticoagulation, 133 anticoagulation withdrawn Age, mean (SD) years : 67.0 (12.4) Sex : 78 M/103 F Ethnic group : not stated Inclusion criteria : patients 15 to 85 years of age with a first episode of symptomatic, idiopathic, proximal deep vein thrombosis who had completed 3 uninterrupted months of oral anticoagulant therapy without having a recurrence of VTE or bleeding. Idiopathic DVT was defined as thrombosis occurring in the absence of cancer, known thrombophilia, or any transient risk factor for VTE (recent trauma, recent surgery or childbirth, prolonged immobilisation > days, oral contraception or pregnancy) Exclusion criteria : patients who required prolonged anticoagulant therapy for reasons other than VTE, major psychiatric disorders, and life expectancy less than 2 years Previous DVT or PE : no Type of VTE at initial diagnosis : DVT as demonstrated on compression ultrasonography or venography Creatinine clearance : not stated | |
| Interventions | Intervention 1 : warfarin Intervention 2 : no treatment Duration of treatment : 9 months | |
| Outcomes | Primary : recurrence of symptomatic, objectively confirmed VTE Secondary : death and major bleeding. Deaths were classified as the result of PE, bleeding, or another identifiable cause, or as unexplained. Bleeding was defined as major if it was clinically overt and was associated with a decrease in haemoglobin level ≥ 20 g/L or the need to transfuse ≥ 2 units of red blood cells, if it was retroperitoneal or intracranial, warranted permanent discontinuation of therapy with the study drug, or required rehospitalisation. Time frame for measuring outcomes : 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed centrally". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open trial" Comment: Blinding could have been possible by using identical‐looking placebo and sham INRs. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "independent, blinded assessment of the outcome events....all suspected outcome events and all deaths were reviewed centrally by an independent, external adjudication committee whose members were unaware of the treatment group assignments". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
| Methods | Study design : multi‐centre, randomised, open trial Length of follow‐up : 2 years Dates of study : January 1997 to December 2000 Location : Italy Setting : hospital | |
| Participants | Number : 181: 90 extended anticoagulation, 91 anticoagulation withdrawn Age, mean (SD) years : 67.0 (12.4) Sex : 78 M/103 F Ethnic group : not stated Inclusion criteria : patients 15 to 85 years of age with a first episode of symptomatic, idiopathic, objectively confirmed pulmonary embolism who had completed 3 uninterrupted months of oral anticoagulant therapy without having a recurrence or bleeding. Idiopathic PE was defined as PE occurring in the absence of cancer, known thrombophilia, or any transient risk factor for VTE (recent trauma, recent surgery childbirth, prolonged immobilisation > days, oral contraception or pregnancy). Exclusion criteria : PE associated with known cancer or thrombophilia, prolonged anticoagulant therapy required for reasons other than VTE, major psychiatric disorders, and life expectancy less than 2 years Previous DVT or PE : no Type of VTE at initial diagnosis : PE Creatinine clearance : not stated | |
| Interventions | Intervention 1 : warfarin Intervention 2 : no treatment Duration of treatment : 9 months | |
| Outcomes | Primary : recurrence of symptomatic, objectively confirmed VTE Secondary : death and major bleeding. Deaths were classified as resulting from PE, bleeding, or another identifiable cause, or as unexplained. Bleeding was defined as major if it was clinically overt and was associated with a decrease in haemoglobin level of ≥ 20 g/L or the need to transfuse ≥ 2 units of red blood cells, if it was retroperitoneal or intracranial, warranted permanent discontinuation of therapy with the study drug, or required rehospitalisation. Time frame for measuring outcomes : 9 months | |
| Notes | 11.6% of participants had received thrombolytic treatment. In total, 326 participants were included in the study, but for the purpose of this review, we included only the 181 participants with idiopathic PE. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed centrally". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open trial" Comment: Blinding could have been possible by using identical‐looking placebo and sham INRs. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "independent, blinded assessment of the outcome events....all suspected outcome events and all deaths were reviewed centrally by an independent, external adjudication committee whose members were unaware of the treatment group assignments". |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Data on all prespecified primary and secondary outcomes were reported. |
| Other bias | Low risk | Study appears to be free from other sources of bias. |
DVT: deep vein thrombosis.
g: grams.
g/dL: grams per decilitre.
g/L: gram per litre.
INR: international normalised ratio.
mg: milligrams.
MI: myocardial infarction.
PE: pulmonary embolism.
SD: standard deviation.
μL: microlitres.
VKA: vitamin K antagonist.
VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| 13% of apixaban and 12% of placebo participants had a previous VTE. Trial authors were contacted for data on participants with unprovoked VTE only, but they did not reply. | |
| Study was on pregnant women with a history of VTE. | |
| Study assesses effectiveness of rivaroxaban for initial treatment of PE. | |
| Over 34% of study participants (412/1196) had an unprovoked VTE. Risk factors for VTE included recent surgery or trauma, immobilisation, oestrogen therapy, active cancer, or puerperium. Trial authors were contacted for data on participants with unprovoked VTE only, but they did not reply. | |
| Participants had high levels of Factor VIII; therefore the VTE was not unprovoked. | |
| Study was on acutely ill, hospitalised patients who were at elevated risk of VTE. | |
| Over a quarter of study participants had a known thrombophilia (26% Factor V Leiden mutation, 5% G20210A prothrombin gene mutation, and 5% antiphospholipid antibodies). Trial authors were contacted for data on participants with no known thrombophilia, but they did not reply. | |
| Study looked at recurrent VTE rates during initial 24 weeks of treatment. | |
| Over a quarter of study participants had a known thrombophilia (22% of warfarin‐treated and 26.6% of placebo‐treated participants had Factor V Leiden mutation; 4.7% of warfarin and 4.8% of placebo had G20210A prothrombin mutation). Trial authors were contacted for data on participants with no known thrombophilia, but they did not reply. | |
| Study looked at initial anticoagulant treatment for acute VTE rather than extended prophylaxis. | |
| Over 18% of study participants (525/2856) had a known thrombophilia. Trial authors were contacted for data on participants with no known thrombophilia, but they did not reply. | |
| Over 10% of study participants (155/1343) had a known thrombophilia. Trial authors were contacted for data on participants with no known thrombophilia, but they did not reply. | |
| Trial studied the effectiveness of sulodexide, which is a low molecular weight heparin. | |
| Trial studied idraparinux, which is an injectable anticoagulant. | |
| The primary outcome of this study was the size of thrombotic masses, which is not relevant to our review. |
PE: pulmonary embolism.
VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Prolonged Anticoagulation After a First Episode of Idiopathic Proximal Deep Vein Thrombosis (PADIS TVP) |
| Methods | Multi‐centre, double‐blind, randomised, parallel‐group trial |
| Participants | Inclusion criteria :
Exclusion criteria :
|
| Interventions | Intervention 1 : warfarin Intervention 2 : placebo Duration : 18 months |
| Outcomes | Primary : symptomatic recurrent venous thromboembolism and serious bleeding Secondary : mortality due to a cause other than recurrent venous thromboembolism or serious bleeding |
| Starting date | July 2007 |
| Contact information | Francis Couturaud, University Hospital, Brest |
| Notes |
DVT: deep vein thrombosis.
INR: international normalised ratio.
PE: pulmonary embolism.
VKA: vitamin K antagonist.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 VTE‐related mortality Show forest plot | 4 | 1862 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| Analysis 1.1  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 1 VTE‐related mortality. | ||||
| 1.1 Warfarin vs placebo | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| 2 Recurrent VTE Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.03] |
| Analysis 1.2  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 2 Recurrent VTE. | ||||
| 2.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.15, 1.80] |
| 2.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.92] |
| 3 Major bleeding Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.87, 3.85] |
| Analysis 1.3  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 3 Major bleeding. | ||||
| 3.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.89, 8.92] |
| 3.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.47, 3.47] |
| 4 All‐cause mortality Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| Analysis 1.4  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 4 All‐cause mortality. | ||||
| 4.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.53, 2.17] |
| 4.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 5 Clinically relevant non‐major bleeding Show forest plot | 4 | 1672 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| Analysis 1.5  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 5 Clinically relevant non‐major bleeding. | ||||
| 5.1 Warfarin vs placebo | 2 | 448 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| 6 Stroke Show forest plot | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| Analysis 1.6  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 6 Stroke. | ||||
| 6.1 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| 7 Serious adverse events (myocardial infarction) Show forest plot | 3 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.87] |
| Analysis 1.7  Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 7 Serious adverse events (myocardial infarction). | ||||
| 7.1 Warfarin vs placebo | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.16] |
| 7.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent VTE Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 1 Recurrent VTE. | ||||
| 2 Major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 2 Major bleeding. | ||||
| 3 Clinically relevant non‐major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 3 Clinically relevant non‐major bleeding. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 1 VTE‐related mortality.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 2 Recurrent VTE.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 3 Major bleeding.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 4 All‐cause mortality.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 5 Clinically relevant non‐major bleeding.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 6 Stroke.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 7 Serious adverse events (myocardial infarction).

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 1 Recurrent VTE.

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 2 Major bleeding.

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 3 Clinically relevant non‐major bleeding.
| Extended prophylaxis compared to placebo in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with extended prophylaxis | |||||
| VTE‐related mortalitya | Study population | OR 0.98 | 1862 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of VTE‐related mortality. | |
| 2 per 1000 | 2 per 1000 | |||||
| Recurrent VTEc | Study population | OR 0.63 | 2043 | ⊕⊕⊕⊝ | ||
| 170 per 1000 | 114 per 1000 | |||||
| Major bleedinge | Study population | OR 1.84 | 2043 | ⊕⊕⊝⊝ | ||
| 11 per 1000 | 20 per 1000 | |||||
| All‐cause mortalityg | Study population | OR 1.00 | 2043 | ⊕⊕⊕⊝ | ||
| 38 per 1000 | 38 per 1000 | |||||
| Clinically relevant non‐major bleedingh | Study population | OR 1.78 | 1672 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of clinically relevant non‐major bleeding. | |
| 6 per 1000 | 11 per 1000 | |||||
| Strokei | Study population | OR 1.15 | 1224 | ⊕⊕⊕⊝ | ||
| 10 per 1000 | 11 per 1000 | |||||
| Myocardial infarction | Study population | OR 1.00 | 1495 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
| VTE extended prophylaxis compared to another VTE extended prophylaxis in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with aspirin | Risk with rivaroxaban | |||||
| VTE‐related mortalitya | See comments. | See comments. | See comments. | See comments. | Data on VTE‐related mortality are yet available for participants with unprovoked VTE. | |
| Recurrent VTEb | Study population | OR 0.28 | 1389 | ⊕⊕⊕⊝ | ||
| 56 per 1000 | 16 per 1000 | |||||
| Major bleedingd | Study population | OR 3.06 | 1389 | ⊕⊕⊕⊝ | ||
| 2 per 1000 | 7 per 1000 | |||||
| All‐cause mortalitye | See comments. | See comments. | See comments. | See comments. | Data on all‐cause mortality are not yet available for participants with unprovoked VTE. | |
| Clinically relevant non‐major bleedingf | 19 per 1000 | 16 per 1000 (7 to 37) | OR 0.84 (0.37 to 1.94) | 1389 | ⊕⊕⊕⊝ | |
| Strokeg | See comments. | See comments. | See comments. | See comments. | Data on stroke are not yet available for participants with unprovoked VTE. | |
| Myocardial infarction | See comments. | See comments. | See comments. | See comments. | Data on myocardial infarction are not yet available for participants with unprovoked VTE. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 VTE‐related mortality Show forest plot | 4 | 1862 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| 1.1 Warfarin vs placebo | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| 2 Recurrent VTE Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.03] |
| 2.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.15, 1.80] |
| 2.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.92] |
| 3 Major bleeding Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.87, 3.85] |
| 3.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.89, 8.92] |
| 3.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.47, 3.47] |
| 4 All‐cause mortality Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| 4.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.53, 2.17] |
| 4.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 5 Clinically relevant non‐major bleeding Show forest plot | 4 | 1672 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| 5.1 Warfarin vs placebo | 2 | 448 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| 6 Stroke Show forest plot | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| 6.1 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| 7 Serious adverse events (myocardial infarction) Show forest plot | 3 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.87] |
| 7.1 Warfarin vs placebo | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.16] |
| 7.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent VTE Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Clinically relevant non‐major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |