Tratamiento de la fatiga en la esclerosis lateral amiotrófica / enfermedad de la motoneurona
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised, placebo‐controlled trial | |
| Participants | 27 people with a diagnosis of clinically definite, probable, or laboratory‐supported ALS, FVC of 90% or greater, and an ALSFRS score of 30 or greater. Mean age in years (SD): resistance exercise 56 (7); control 51 (7) Gender (% female): resistance exercise 4 (30.8%); control 7 (50%) Mean time since diagnosis in months (SD): resistance exercise 20 (12); control 15 (13) | |
| Interventions | Intervention (N = 13) Moderate‐load and moderate‐intensity resistance exercise programme and stretching for upper and lower extremities. Intervention participants received a personalised 'moderate intensity resistance exercise programme' by an unblinded research physical therapist. The programmes were developed according to individual tolerance and limitations. Control (N = 14) Stretching exercises for upper and lower extremities only. | |
| Outcomes | Primary | |
| Funding | Funded by the ALS Association (US) | |
| Conflicts of interest | The authors report no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. Described as "randomized" but details of randomisation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Participants assigned by "selecting an opaque envelope that contained group assignment" |
| Blinding of participants and personnel (performance bias) | High risk | Participants not blinded. In addition, the trial authors reported that "[t]he physical therapists who initially prescribed the exercise programs were unblinded to group assignment and were responsible for collecting the logs, making the telephone contacts, interviewing subjects about exercise side effects and compliance, and revising the exercise program." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessor blinded. Participants asked not to reveal allocation to assessor. |
| Incomplete outcome data (attrition bias) | Low risk | 4 usual care participants (40%) and five resistance participants (62.5%) did not complete the study (P = 0.70). Two participants from each arm who dropped out were taking riluzole. No participants who discontinued did so because they believed that the exercise programme itself was worsening their condition. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in ClinicialTrials.gov registration |
| Other bias | Low risk | No other biases apparent |
| Methods | RCT with delayed‐start design | |
| Participants | 26 people with ALS/MND with normal respiratory function. Mean age in years (SD): intervention 57 (9); control 56 (8) Gender (% female): intervention 6 (46.1%); control 2 (15.4%) Mean time since diagnosis in months (SD): intervention 11 (5); control 12 (6) | |
| Interventions | Respiratory exercise using a inspiratory muscular training (IMT) device. All participants instructed to use the IMT twice daily; each session 10 minutes Intervention (N = 13) In the intervention groups the resistance was set to between 30% to 40% of maximum inspiratory pressure (MIP). Participants started the exercise programme at entry and were followed for 8 months. Control (N = 13) Sham exercise programme. In the sham intervention group the resistance in the IMT was set to the lowest possible level. Participants followed a placebo exercise programme at entry, then the active exercise programme for 4 months. | |
| Outcomes | Primary Functional status, measured by the ALSFRS Secondary Respiratory tests Neurophysiological measurements Fatigue, measured on the FSS Depression, measured on the Hamilton Rating Scale for Depression Sleepiness, measured on the ESS Functional Independence, measured on Functional Independence Measure Quality of life, measured on EuroQoL‐5D | |
| Funding | Fundacão para a Ciência e a Tecnologia | |
| Conflicts of interest | The authors report no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants independently randomised in blocks of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | High risk | Trainer aware of participants group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | ALSFRS administered by a blind assessor |
| Incomplete outcome data (attrition bias) | Unclear risk | For one analysis, incomplete data imputed as 25% of the lower percentile value observed in the remaining participants of the same group |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | Not discussed |
| Methods | RCT with 3:1 (intervention:placebo) randomisation | |
| Participants | 32 participants with probable or definite ALS by modified El Escorial criteria, FVC ≥ 50% and able to communicate verbally or with an assistive device. Mean age in years (SD): modafinil 59 (13); placebo 56 (5) Gender (% female): modafinil 11 (44%); placebo 3 (43%) Mean time since diagnosis in months (SD): modafinil 16 (18); placebo 29 (33) | |
| Interventions | Intervention (N = 25) Modafinil in doses beginning with 100 mg/day and increasing in the event of no response to 300 mg/day for four weeks Control (N = 7) Placebo | |
| Outcomes | Primary | |
| Funding | Not reported | |
| Conflicts of interest | The authors report no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "3:1 block randomisation provided by the Research Pharmacy at New York State Psychiatric Institute" |
| Allocation concealment (selection bias) | Low risk | "The sequence was concealed until the intervention was assigned" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was maintained and assessed for both doctor and participants, but "blinding was questionable since participants knew there was a 3:1 chance of getting modafinil versus placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) | Low risk | 16% dropout, 9% due to adverse events. However, there were complete data for the outcomes of interest in this review. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or prespecified outcomes |
| Other bias | Low risk | No other biases apparent |
| Methods | Pilot RCT | |
| Participants | 10 people with probable or definite ALS/MND randomised. Mean age in years (SD): intervention 59(9); control 60(9) Gender (% female): intervention 1 (20%); control 3 (60%) Mean time since diagnosis in months (SD): intervention 11 (3); control 12 (4). | |
| Interventions | Intervention (N = 5) Daily repetitive transcranial magnetic stimulation (5Hz) on upper‐ and lower‐limb cortical areas Sham stimulation using a specific sham coil that provided no cortical stimulation but did produce similar auditory and scalp sensations 2 weeks' treatment | |
| Outcomes | Functional status, measured on the ALSFRS‐R Outcomes measured 2 weeks after the end of treatment | |
| Funding | Not reported | |
| Conflicts of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[Participants] were allocated to the treatment: 5 active and 5 sham stimulation" |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Use of sham treatment suggests that participants were blinded; but no explicit mention of blinding made |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Four week assessments available for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol or prespecified outcomes |
| Other bias | Low risk | No other biases apparent |
ALS: amyotrophic lateral sclerosis; ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Scale; FVC: forced vital capacity; SD: standard deviation; SF‐36: Short Form 36 Health Survey
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No validated, patient‐reported fatigue outcome (study used VAS for fatigue). Double‐blind cross‐over trial to assess tolerability of 3‐4 diaminopyridine (3,4‐DAP) | |
| Non‐randomised study. Open‐label trial of modafinil. 2‐week trial of 200 mg or 400 mg doses. Significant reductions in fatigue (FSS) and sleepiness (ESS) evident after 2 weeks | |
| No validated, patient‐reported fatigue outcome (study used maximum voluntary isometric contraction). RCT of topiramate to slow disease progression | |
| No validated, patient‐reported fatigue outcome (study used VAS for fatigue). Double‐blind trial to assess efficacy of alpha‐tocopherol (vitamin E) to treat ALS/MND | |
| Fatigue not assessed as primary or secondary end point | |
| Fatigue not assessed as primary or secondary end point | |
| Fatigue not assessed as primary or secondary end point | |
| Fatigue not assessed as primary or secondary end point | |
| Fatigue assessed using individual items from a validated scale, not the validated scale itself | |
| Fatigue not assessed using validated, patient‐reported outcome measure | |
| Fatigue not assessed using validated, patient‐reported outcome measure | |
| Fatigue not assessed using validated, patient‐reported outcome measure | |
| Fatigue not assessed using validated, patient‐reported outcome measure |
ALS/MND: amyotrophic lateral sclerosis/motor neuron disease; ESS: Epworth Sleepiness Scale; FSS: fatigue severity scale; VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Modafinil versus placebo, Outcome 1 Efficacy outcomes (all at 4 weeks). | ||||
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sleepiness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 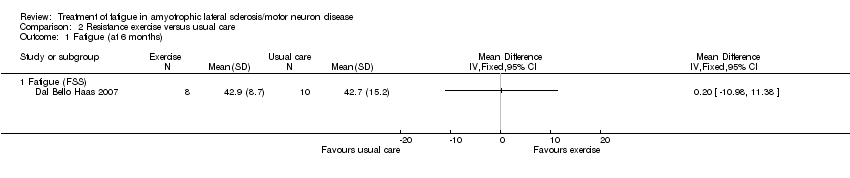 Comparison 2 Resistance exercise versus usual care, Outcome 1 Fatigue (at 6 months). | ||||
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Resistance exercise versus usual care, Outcome 2 Functional status (at 6 months). | ||||
| 2.1 Functional status (ALSFRS total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status (ALFRS lower extremity) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Resistance exercise versus usual care, Outcome 3 Quality of life (at 6 months). | ||||
| 3.1 Mental health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Physical function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Physical role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 General health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vitality (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Social function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Emotional role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Efficacy outcomes (all at 4 months) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.1
Comparison 3 Respiratory exercise versus sham intervention, Outcome 1 Efficacy outcomes (all at 4 months). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Sleepiness | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Depression | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Quality of life | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.5 Functional status | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.6 Functional status (ALSFRS‐bulbar) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.7 Functional status (ALSFRS‐respiratory) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||
| 1 Efficacy outcomes (all at 2 weeks) Show forest plot | Other data | No numeric data | ||||||||||||||||||||
| Analysis 4.1
Comparison 4 Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention, Outcome 1 Efficacy outcomes (all at 2 weeks). | ||||||||||||||||||||||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||||||||||||||||||||
| 1.2 Functional status | Other data | No numeric data | ||||||||||||||||||||

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.

Comparison 1 Modafinil versus placebo, Outcome 1 Efficacy outcomes (all at 4 weeks).

Comparison 2 Resistance exercise versus usual care, Outcome 1 Fatigue (at 6 months).

Comparison 2 Resistance exercise versus usual care, Outcome 2 Functional status (at 6 months).

Comparison 2 Resistance exercise versus usual care, Outcome 3 Quality of life (at 6 months).
| Study | Respiratory exercise N | Sham intervention N | MD, 95% CI |
| Fatigue (FSS) | |||
| Pinto 2012 | 12 | 12 | ‐9.654, 95% CI ‐22.037 to 2.729 |
| Sleepiness | |||
| Pinto 2012 | 12 | 12 | 0.308, 95% CI ‐3.48 to 4.096 |
| Depression | |||
| Pinto 2012 | 12 | 12 | 1.769, 95% CI 0.018 to 3.52 |
| Quality of life | |||
| Pinto 2012 | 12 | 12 | 0.769, 95% CI ‐17.093 to 18.631 |
| Functional status | |||
| Pinto 2012 | 12 | 12 | 0.846, 95% CI ‐2.157 to 3.849 |
| Functional status (ALSFRS‐bulbar) | |||
| Pinto 2012 | 12 | 12 | ‐0.385, 95% CI ‐1.378, to 0.609 |
| Functional status (ALSFRS‐respiratory) | |||
| Pinto 2012 | 12 | 12 | 0.077, 95% CI ‐0.254 to 0.407 |
Comparison 3 Respiratory exercise versus sham intervention, Outcome 1 Efficacy outcomes (all at 4 months).
| Study | Number of participants | Analysis of variance (time x treatment arm |
| Fatigue (FSS) | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 4.0; P = 0.04 |
| Functional status | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 2.7; P > 0.05 |
Comparison 4 Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention, Outcome 1 Efficacy outcomes (all at 2 weeks).
| Modafinil compared to placebo in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Fatigue assessed with: Fatigue Severity Scale (FSS) | The mean FSS score was 43 | MD 11 lower | ‐ | 32 | ⊕⊝⊝⊝ | |
| Adverse events | Three adverse events led to discontinuation of modafinil (2 headache, 1 chest tightness). Anxiety (in 2 people), nausea (in 2), dizziness (in 1), and sialorrhoea (in 1; probably ALS‐related) also occurred with modafinil. Placebo group adverse events were not reported ‐ it is not clear whether there were none. | ‐ | 32 | ⊕⊝⊝⊝ | The trial reported the number of adverse events in the treatment group, but not numbers of events in the placebo group or number of people experiencing adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the CI of the effect estimate included appreciable benefit and little or no effect. | ||||||
| Exercise compared to usual care in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise | |||||
| Fatigue Fatigue Severity Scale (FSS) | The mean FSS score was 42.7 | MD 0.2 higher | ‐ | 18 | ⊕⊝⊝⊝ | ‐ |
| Adverse events | No adverse events were reported | ‐ | 18 | ⊕⊝⊝⊝ | None of the people who discontinued did so because they thought the exercise programme was making their condition worse. No participants reported excessive soreness, cramping, or fatigue. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence 3 times to low quality: twice for imprecision as the trial was very small and CI included appreciable benefit and appreciable harm. The third downgrading was for study limitations as the nature of the intervention prevented participant blinding. | ||||||
| Inspiratory muscle training compared to sham intervention in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk or value with sham intervention | Risk or value with inspiratory muscle training | |||||
| Fatigue Fatigue Severity Scale (FSS) follow up: 4 months | An illustrative mean FSS score in the absence of inspiratory muscle training is 42.71 | MD 9.654 lower | ‐ | 24 | ⊕⊝⊝⊝ | |
| Adverse events | The trialists stated that the exercise protocol had no adverse effects. | ‐ | 24 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The mean FSS score in the control group after 4 months was not available from Pinto 2012. The value given here, for illustrative purposes, is the control group mean at 6 months from Dal Bello Haas 2007. 2We downgraded the quality of evidence 3 times to very low: twice for imprecision and once for study limitations. The trial was very small and CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. | ||||||
| Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND | |||
| Patient or population: people with ALS/MND | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Fatigue assessed with: Fatigue Severity Scale (FSS) follow‐up: 2 weeks | No FSS scores were given. The investigators assessed fatigue with the FSS using 2‐way analysis of variance (within‐subjects factor time, between‐subjects treatment arm). The trial reported a significant effect for fatigue at the end of the follow‐up period. The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). | 10 | ⊕⊝⊝⊝ |
| Adverse events | No adverse events were reported. | 10 | ⊕⊝⊝⊝ |
| RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The risk of bias was unclear as the trial report provided too little detail for assessment. The trial involved 10 people. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sleepiness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Functional status (ALSFRS total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status (ALFRS lower extremity) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mental health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Physical function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Physical role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 General health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vitality (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Social function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Emotional role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 months) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Sleepiness | Other data | No numeric data | ||
| 1.3 Depression | Other data | No numeric data | ||
| 1.4 Quality of life | Other data | No numeric data | ||
| 1.5 Functional status | Other data | No numeric data | ||
| 1.6 Functional status (ALSFRS‐bulbar) | Other data | No numeric data | ||
| 1.7 Functional status (ALSFRS‐respiratory) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 2 weeks) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Functional status | Other data | No numeric data | ||


