نقش مهار کنندههای خوراکی مستقیم ترومبین یا مهار کنندههای خوراکی فاکتور Xa برای درمان آمبولی ریوی
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010957.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 04 December 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Vascular Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
LR: drafted the protocol, selected studies for inclusion, extracted data, assessed the quality of studies, performed data analysis and wrote the review.
PK: commented on the protocol, selected studies for inclusion, extracted data, assessed the quality of the studies and commented on the review.
JM: selected studies for inclusion, extracted data, assessed the quality of the studies and commented on the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
LR: none known.
PK: I have received consultancy fees for attendance at advisory boards of Boehringer‐Ingelheim, Bayer and Daiitchi‐Sankyo and payment from Bayer for lectures at the 2013 anticoagulation master class. My institution was paid travel/accommodation/meeting expenses by Boehringer‐Ingelheim for my attendance at the 2013 ISTH meeting and staff and NHS costs by Boehringer‐Ingelheim and Daiitchi‐Sankyo for involvement in phase III trials of novel anticoagulants in venous thrombosis. Since Summer 2014 I have declined all invitations to advisory boards, or lectures on behalf of the pharmaceutical industry.
JM: I received travel, course fees, accommodation and meals from Medtronic as part of the Medtronic University program. This is an educational program, and includes registration and attendance at the European Vascular Course 2012. No financial remuneration was received by myself, other than costs of travel, accommodation, course fees and meals.
I received sponsorship to attend the Vascular Society annual meeting 2012 and 2014 in the form of registration fees and accommodation/travel costs.
I received sponsorship to attend a stenting master class, the Verve clinical meeting in 2013, and a technology forum in Phoenix, Arizona from Gore Medical. This was in the form of travel, accommodation and meals. No other financial remuneration was received.
I received sponsorship to attend the LINC 2015 meeting in Leipzig, Germany from Abbott Medical in the form of registration, accommodation, travel and meals.
I am a co‐founder of UKETS, a trainee initiative, which receives funding through sponsorship from endovascular technology and simulation companies. The majority of this is non‐financial (i.e. the companies supply trainers on the courses or allow use of their simulators), although some direct financial input is received from Vascutek and Mentice and is used to run events. No profit is derived from this initiative.
Medtronic, Gore Medical, Abbott Medical, Vascutek and Mentice do not manufacture any pharmaceuticals, including anticoagulants.
Acknowledgements
We would like to thank Dr Karen Welch for searching the Cochrane Vascular Specialised Register and the Cochrane Central Register of Controlled Trials. We would also like to thank Dr Marlene Stewart, Managing Editor of Cochrane Vascular, for her assistance and advice in completing this review
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Apr 14 | Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of pulmonary embolism | Review | Meixuan Li, Jing Li, Xiaoqin Wang, Xu Hui, Qi Wang, Shitong Xie, Peijing Yan, Jinhui Tian, Jianfeng Li, Ping Xie, Kehu Yang, Liang Yao | |
| 2015 Dec 04 | Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of pulmonary embolism | Review | Lindsay Robertson, Patrick Kesteven, James E McCaslin | |
| 2014 Feb 03 | Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of pulmonary embolism | Protocol | Lindsay Robertson, Patrick Kesteven | |
Differences between protocol and review
In a change from the protocol (Robertson 2014b), we excluded studies where treatment was for less than three months because a meta‐analysis of venous thromboembolism treatment strategies has demonstrated an increased rate of recurrence after less than three months anticoagulation but no significant difference with various longer periods of treatment (Boutitie 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Oral;
- Anticoagulants [*therapeutic use];
- Antithrombins [*therapeutic use];
- Dabigatran [therapeutic use];
- Factor Xa Inhibitors [*therapeutic use];
- Pulmonary Embolism [*drug therapy];
- Pyrazoles [therapeutic use];
- Pyridines [therapeutic use];
- Pyridones [therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Rivaroxaban [therapeutic use];
- Secondary Prevention;
- Thiazoles [therapeutic use];
- Venous Thromboembolism [prevention & control];
- Venous Thrombosis [prevention & control];
Medical Subject Headings Check Words
Humans;
PICOs

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
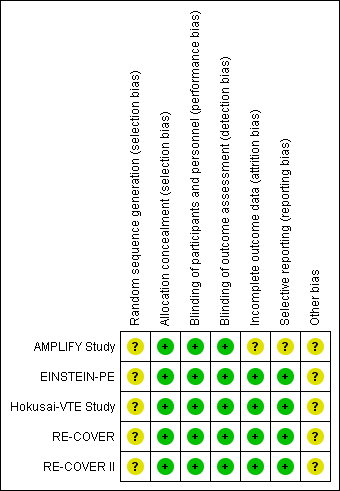
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.
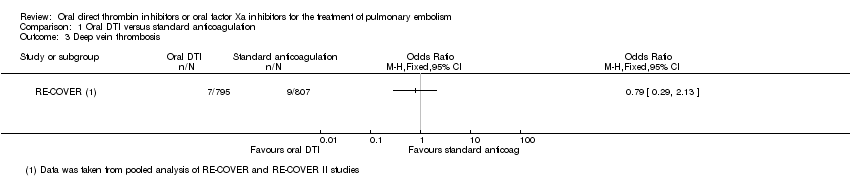
Comparison 1 Oral DTI versus standard anticoagulation, Outcome 3 Deep vein thrombosis.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 4 Major bleeding.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 3 Deep vein thrombosis.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 4 All‐cause mortality.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 5 Major bleeding.
| Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with Oral DTI | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.02 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 20 per 1000 | 20 per 1000 | |||||
| Recurrent venous thromboembolism | Study population | OR 0.93 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 31 per 1000 | 29 per 1000 | |||||
| Deep vein thrombosis5 | Study population | OR 0.79 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 11 per 1000 | 9 per 1000 | |||||
| All‐cause mortality | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not report on all‐cause mortality | |
| Major bleeding6 | Study population | OR 0.50 | 1527 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 10 per 1000 | 5 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
| Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with oral factor Xa | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.08 | 4509 | ⊕⊕⊕⊝ | ‐ | |
| 22 per 1000 | 24 per 1000 | |||||
| Recurrent venous thromboembolism5 | Study population | OR 0.85 | 6295 | ⊕⊕⊕⊕ | ‐ | |
| 24 per 1000 | 20 per 1000 | |||||
| Deep vein thrombosis6 | Study population | OR 0.72 | 4509 | ⊕⊕⊕⊕ | ‐ | |
| 11 per 1000 | 8 per 1000 | |||||
| All‐cause mortality | Study population | OR 1.16 | 4817 | ⊕⊕⊕⊝ | ‐ | |
| 16 per 1000 | 19 per 1000 | |||||
| Major bleeding8 | Study population | OR 0.97 | 4507 | ⊕⊕⊕⊕ | ‐ | |
| 14 per 1000 | 13 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent pulmonary embolism Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrent venous thromboembolism Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent pulmonary embolism Show forest plot | 2 | 4509 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.46, 2.56] |
| 2 Recurrent venous thromboembolism Show forest plot | 3 | 6295 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3 Deep vein thrombosis Show forest plot | 2 | 4509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 4 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Major bleeding Show forest plot | 2 | 4507 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.59, 1.61] |


