Orale direkte Thrombinhemmer oder orale Faktor‐Xa‐Hemmer für die Behandlung von Lungenembolie
Abstract
Background
Pulmonary embolism is a potentially life‐threatening condition in which a clot can travel from the deep veins, most commonly in the leg, up to the lungs. Previously, a pulmonary embolism was treated with the anticoagulants heparin and vitamin K antagonists. Recently, however, two forms of direct oral anticoagulants (DOACs) have been developed: oral direct thrombin inhibitors (DTI) and oral factor Xa inhibitors. The new drugs have characteristics that may be favourable over conventional treatment, including oral administration, a predictable effect, lack of frequent monitoring or re‐dosing and few known drug interactions. To date, no Cochrane review has measured the effectiveness and safety of these drugs in the long‐term treatment (minimum duration of three months) of pulmonary embolism.
Objectives
To assess the effectiveness of oral DTIs and oral factor Xa inhibitors for the long‐term treatment of pulmonary embolism.
Search methods
The Cochrane Vascular Trials Search Co‐ordinator searched the Specialised Register (last searched January 2015) and the Cochrane Register of Studies (last searched January 2015). Clinical trials databases were also searched for details of ongoing or unpublished studies. We searched the reference lists of relevant articles retrieved by electronic searches for additional citations.
Selection criteria
We included randomised controlled trials in which patients with a pulmonary embolism confirmed by standard imaging techniques were allocated to receive an oral DTI or an oral factor Xa inhibitor for the long‐term (minimum duration three months) treatment of pulmonary embolism.
Data collection and analysis
Two review authors (LR, JM) independently extracted the data and assessed the risk of bias in the trials. Any disagreements were resolved by discussion with the third author (PK). We used meta‐analyses when we considered heterogeneity low. The two primary outcomes were recurrent venous thromboembolism and pulmonary embolism. Other outcomes included all‐cause mortality and major bleeding. We calculated all outcomes using an odds ratio (OR) with a 95% confidence interval (CI).
Main results
We included five randomised controlled trials with a total of 7897 participants. Two studies tested oral DTIs (dabigatran) and three studies tested oral factor Xa inhibitors (one rivaroxaban, one edoxaban and one apixaban).
Analysis showed no difference in the effectiveness of oral DTIs and standard anticoagulation in preventing recurrent pulmonary embolism (OR 1.02, 95% CI 0.50 to 2.04; two studies; 1602 participants; high quality evidence), recurrent venous thromboembolism (OR 0.93, 95% CI 0.52 to 1.66; two studies; 1602 participants; high quality evidence), deep vein thrombosis (DVT) (OR 0.79, 95% CI 0.29 to 2.13; two studies; 1602 participants; high quality evidence) and major bleeding (OR 0.50, 95% CI 0.15 to 1.68; two studies; 1527 participants; high quality evidence).
For oral factor Xa inhibitors, when we combined the three included studies together in meta‐analyses, there was significant heterogeneity for recurrent pulmonary embolism (OR 1.08, 95% CI 0.46 to 2.56; two studies; 4509 participants; I2 = 58%; moderate quality evidence). The oral factor Xa inhibitors were no more or less effective in the prevention of recurrent venous thromboembolism (OR 0.85, 95% CI 0.63 to 1.15; three studies; 6295 participants; high quality evidence), DVT (OR 0.72, 95% CI 0.39 to 1.32; two studies; 4509 participants; high quality evidence), all‐cause mortality (OR 1.16, 95% CI 0.79 to 1.70; one study; 4817 participants; moderate quality evidence) or major bleeding (OR 0.97, 95% CI 0.59 to 1.62; two studies; 4507 participants; high quality evidence). None of the studies measured quality of life.
Authors' conclusions
There is no evidence of a difference between oral DTIs and standard anticoagulation in the prevention of recurrent pulmonary embolism. Using the GRADE criteria, the quality of evidence was high. The evidence of the effectiveness of oral factor Xa inhibitors for the prevention of recurrent pulmonary embolism was too heterogenous to combine in a pooled analysis. For the outcomes recurrent venous thromboembolism, DVT, all‐cause mortality and major bleeding there is no evidence of a difference between DOACs and standard anticoagulation. According to GRADE criteria, the quality of evidence was moderate to high.
PICOs
Laienverständliche Zusammenfassung
Neuartige orale Gerinnungshemmer für die Behandlung von Lungenembolie
Hintergrund
Die venöse Thromboembolie ist eine Erkrankung, bei der sich ein Blutgerinnsel in den tiefen Venen (am häufigsten in den Beinen) bildet. Dieses Blutgerinnsel kann sich dann lösen und die Lungenarterien verstopfen (Lungenembolie). Die Lungenembolie ist lebensbedrohlich und kommt in 3 bis 4 von 10.000 Personen vor. Die Wahrscheinlichkeit eine Lungenembolie zu erleiden erhöht sich durch folgende Risikofaktoren: frühere Blutgerinnsel, längere Zeiträume ohne körperliche Bewegung (z.B. bei Reisen im Flugzeug oder Bettruhe), Krebs, Östrogenexposition (Schwangerschaft, orale Verhütungsmittel oder Hormonersatztherapie), Trauma und Blutkrankheiten wie Thrombophilie (abnorme Blutgerinnung). Die Diagnose einer Lungenembolie wird durch die Bestimmung der Risikofaktoren sowie eine Computertomographie (Suche nach einem Blutgerinnsel) gestellt. Bestätigt sich eine Lungenembolie wird der Patient mit einem Gerinnungshemmer behandelt. Dieses Medikament verhindert die Bildung weiterer Blutgerinnsel. Bis vor Kurzem waren Heparin, Fondaparinux und Vitamin‐K‐Antagonisten die Mittel der Wahl. Diese Medikamente können jedoch Nebenwirkungen verursachen und unterliegen Beschränkungen. Vor kurzem wurden zwei weitere Klassen neuartiger oraler Gerinnungshemmer entwickelt, bei welchen es sich um sogenannte direkte Thrombinhemmer (direkte Thrombin‐Inhibitoren, DTI) und Faktor‐Xa‐Hemmer handelt. Es gibt bestimmte Gründe, warum orale DTI und Faktor‐Xa‐Hemmer die vermeintlich bessere Wahl sind. Sie können oral eingenommen werden, ihre Wirkung ist vorhersehbar, eine häufige Überwachung oder Anpassungen der Dosierung sind nicht erforderlich und sie haben wenige bekannte Wechselwirkungen mit anderen Arzneimitteln. In diesem Review wird die Wirksamkeit und Sicherheit dieser neuen Arzneimittel mit der herkömmlichen Behandlung verglichen.
Hauptergebnisse
Die Recherche relevanter Studien bis Januar 2015 ergab 5 Studien mit insgesamt 7897 Teilnehmern. In den Studien wurden orale DTI bzw. Faktor‐Xa‐Hemmer mit der herkömmlichen Behandlung verglichen. Wir prüften, ob eine 3‐monatige Behandlung weitere Blutgerinnsel und Lungenembolie vorbeugten. Die wichtigsten Sicherheitsendpunkte umfassten Tod und Nebenwirkungen wie z.B. Blutungen. Dieser Review zeigt, dass es keine Evdenz für einen Unterschied zwischen direkten Gerinnungshemmern und der herkömmlichen Behandlung gibt, was das Vorbeugen weiterer Blutgerinnsel in der Lunge oder in den Beinen angeht. Zur oralen Faktor‐Xa‐Hemmer und der Prävention von rezidivierenden Blutgerinnsel in der Lunge waren die Studien zu verschieden, um eine sinnvolle Schlussfolgerung zu ziehen. Zudem gab es keine Evidenz für Unterschiede bezüglich der wiederkehrenden venösen Thrombosen, tiefen Beinvenenthrombosen und Anzahl der Todesfälle oder Blutung. Keine Studie hat die gesundheitsbezogene Lebensqualität untersucht.
Qualität der Evidenz
Für die Endpunkte rezidivierende Lungenembolie und Gesamtmortalität beim Vergleich zwischen orale Faktor‐Xa‐Hemmer und Standard‐Gerinnungshemmung haben wir die Qualität der Evidenz von hoch auf moderat herabgestuft. Der Grund dafür sind die Unterschiede in den Ergebnissen zwischen den Studien und die kleine Anzahl Studien, die in diesen Review eingeschlossen wurden. Die Qualität der Evidenz für alle anderen Endpunkte war hoch.
Authors' conclusions
Summary of findings
| Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with Oral DTI | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.02 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 20 per 1000 | 20 per 1000 | |||||
| Recurrent venous thromboembolism | Study population | OR 0.93 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 31 per 1000 | 29 per 1000 | |||||
| Deep vein thrombosis5 | Study population | OR 0.79 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 11 per 1000 | 9 per 1000 | |||||
| All‐cause mortality | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not report on all‐cause mortality | |
| Major bleeding6 | Study population | OR 0.50 | 1527 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 10 per 1000 | 5 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
| Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with oral factor Xa | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.08 | 4509 | ⊕⊕⊕⊝ | ‐ | |
| 22 per 1000 | 24 per 1000 | |||||
| Recurrent venous thromboembolism5 | Study population | OR 0.85 | 6295 | ⊕⊕⊕⊕ | ‐ | |
| 24 per 1000 | 20 per 1000 | |||||
| Deep vein thrombosis6 | Study population | OR 0.72 | 4509 | ⊕⊕⊕⊕ | ‐ | |
| 11 per 1000 | 8 per 1000 | |||||
| All‐cause mortality | Study population | OR 1.16 | 4817 | ⊕⊕⊕⊝ | ‐ | |
| 16 per 1000 | 19 per 1000 | |||||
| Major bleeding8 | Study population | OR 0.97 | 4507 | ⊕⊕⊕⊕ | ‐ | |
| 14 per 1000 | 13 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
Background
Description of the condition
Pulmonary embolism is a potentially life‐threatening condition in which a blood clot blocks the supply to the lungs. Pulmonary embolism is often a consequence of a thrombus in the deep veins of the legs (deep vein thrombosis (DVT)) that dislodges and travels in the blood to the pulmonary arteries. The prevalence of pulmonary embolism has been estimated as 3 to 4 per 10,000 people, although the true prevalence is hard to measure due to underestimation by diagnostic imaging and overestimation by postmortem data. DVT is present in approximately 70% to 80% of people with a pulmonary embolism, yet only 15% of pulmonary embolism cases have symptoms of DVT (Huerta 2007). One complication of pulmonary embolism is chronic thromboembolic pulmonary hypertension (CTPH). CTPH occurs when the clot obstructs the pulmonary arteries causing excessive pressure in the pulmonary artery and stress to the right ventricle. CTPH is less common but it can result in heart failure (NICE 2012a).
Risk factors for pulmonary embolism are similar to those for DVT and are classified as provoked or unprovoked (Kearon 2012). Provoked pulmonary embolism occurs following surgery or pregnancy, or by a non‐surgical transient risk factor such as a history of venous thromboembolism, venous insufficiency, chronic heart failure, thrombophilia, obesity, immobility (such as prolonged travel, acute medical illness or hospitalisation), cancer, oestrogens (pregnancy, use of oral contraceptives or hormone replacement therapy) and trauma (SIGN 2010).
Diagnosis of pulmonary embolism is made by general assessment of the patient's medical history, physical examination and clinical pre‐test probability. However, it can be particularly challenging as the symptoms (dyspnoea, pleuritic chest pain, retrosternal chest pain, cough and haemoptysis) are not specific (NICE 2012a). In severe cases, the right ventricle fails leading to dizziness, syncope, tachypnoea, tachycardia, hypoxia, elevated jugular venous pressure, systemic hypotension and cardiogenic shock (NICE 2012a). The UK National Institute for Health and Care Excellence recommend that people presenting with a suspected pulmonary embolism should be assessed using a two‐level pulmonary embolism Wells score (NICE 2012a; Wells 2000). Points are awarded for clinical features present including clinical signs of DVT, heart rate greater than 100 beats per minute, recent immobilisation or surgery, previous DVT, haemoptysis and malignancy (Wells 2000). For patients with a low pre‐test probability, the use of a D‐dimer assay combined with a clinical prediction rule has a high negative predictive value and avoids the need for unnecessary imaging (Qaseem 2007). However, for patients who have intermediate or high pre‐test probability of pulmonary embolism, imaging is essential. Patients with a score of greater than 4 are judged to be likely to have had a pulmonary embolism and should undergo immediate diagnostic imaging. If this cannot be performed immediately, patients should be given immediate interim parenteral anticoagulant therapy until the imaging test is done. Patients with a negative diagnosis in whom a DVT is likely should be given a proximal leg vein ultrasound scan (NICE 2012a).
There are two types of imaging technique used to diagnose pulmonary embolism: computed tomography pulmonary angiogram (CTPA) and ventilation perfusion (V/Q) scan.
1. Computed tomography pulmonary angiogram
CTPA involves injecting a contrast agent intravenously and performing a computed tomography (CT) scan of the chest to visualise the pulmonary arteries and detect any thrombi in the pulmonary arteries down to the subsegmental branches. The procedure has over 90% specificity and sensitivity in diagnosing pulmonary embolism in the main, lobar and segmental pulmonary arteries (Riedel 2004). However, the radiation dose administered to the patient is much larger than that of a V/Q scan, and thus patients who have a CTPA may be at an increased life‐time risk of cancer (Anderson 2009). CTPA is contraindicated in patients who have an allergy to contrast media, renal impairment or in whom the risk of radiation is too high. In these patients, a V/Q scan is performed instead (NICE 2013).
2. Ventilation perfusion scan
A V/Q scan comprises of two parts: the ventilation part where the patient breathes in a radioisotope (in the form of a gas or an aerosol) and the perfusion part where the patient is given an intravenous injection of the isotope. A gamma camera is used to detect where the isotopes are in the lungs and the images show which areas of the lungs are ventilated but not perfused (NICE 2012a). Another version of this test, the V/Q single photon emission computed tomography (V/Q SPECT) has been developed. The camera is rotated around the patient thus generating three‐dimensional images and leading to a more accurate diagnosis (Laurence 2012).
Description of the intervention
Until recently, standard treatment of a pulmonary embolism was with an indirect thrombin inhibitor, namely unfractionated heparin (UFH), low molecular weight heparin (LMWH) or vitamin K antagonists (VKAs). These drugs block the action of thrombin either by "activating naturally occurring thrombin inhibitors or by inhibiting specific factors in the coagulation system that subsequently impact on thrombin generation or activity" (Weitz 2003). Present guidelines recommend initial therapy for pulmonary embolism with a parenteral anticoagulant (UFH or LMWH or fondaparinux) and initial VKA initiation (Kearon 2012). Recommendations include the use of LMWH or fondaparinux over UFH for initial therapy of pulmonary embolism. Although heparin and VKAs are effective anticoagulants, there are limitations associated with each. LMWH must be administered parenterally and may be associated with an increased risk of bleeding and haemodynamic instability (Kearon 2012). Meanwhile VKAs have a narrow therapeutic window, require frequent monitoring and dosage adjustments, and can have multiple interactions with other drugs (Ageno 2012).
Two further classes of oral anticoagulants have been developed: direct thrombin inhibitors (DTI) and factor Xa inhibitors. DTIs and factor Xa inhibitors have characteristics that may be favourable over heparin and VKAs, including ease of oral administration, a predictable effect, lack of frequent monitoring or re‐dosing and fewer known drug interactions (compared with VKA) (Fox 2012).
How the intervention might work
Oral direct thrombin inhibitors
DTIs work by binding directly to the enzyme thrombin without the need for a co‐factor such as antithrombin. Unlike heparins and VKAs, DTIs can inhibit both soluble thrombin and fibrin‐bound thrombin (Kam 2005). Other advantages include a more predictable anticoagulant effect because of their lack of binding to other proteins, lack of an antiplatelet effect and no suspected concern of heparin‐induced thrombocytopenia (HIT) (Lee 2011). There are several types of DTIs.
1. Dabigatran
Dabigatran etexilate is a reversible oral DTI that is metabolised to its active ingredient, dabigatran, in the gastrointestinal tract (Ageno 2012). It does not require anticoagulation monitoring, is excreted by the kidneys and has a half‐life of 12 to 17 hours. As well as a treatment for venous thrombosis, this drug has been involved in many large randomised studies of atrial fibrillation (Connolly 2009), acute coronary syndromes (Oldgren 2011), and prevention of thrombosis following orthopaedic surgery (Eriksson 2007), and in patients with mechanical heart valves (Van de Werf 2012). In common with the other novel oral anticoagulants, dabigatran is associated with a lower incidence of intracranial haemorrhage (compared with VKA). However, again compared with VKA, dabigatran showed a higher incidence of indigestion and heartburn and a higher incidence of gastrointestinal bleeding. Dabigatran, in the atrial fibrillation studies, showed a tendency (although ultimately not statistically significant) to increased incidence of myocardial infarction (Baetz 2008).
2. Ximelagatran
Ximelagatran is a prodrug that is metabolised to melagatran as it is better absorbed from the gastrointestinal tract (Kam 2005). It has a plasma half‐life of three hours, has a predictable response after oral administration and does not require coagulation monitoring. Ximelagatran was found to be effective in the treatment of venous thromboembolism but caused unacceptable liver toxicity (Boudes 2006), and was, therefore, never licensed.
Oral factor Xa inhibitors
Factor Xa inhibitors bind directly to the active site of factor Xa, thus blocking the activity of the clotting factor. Unlike indirect factor Xa inhibitors such as fondaparinux, direct factor Xa inhibitors "inactivate free FXa and FXa incorporated with the prothrombinase complex equally well" and do not require interaction with the inhibitor antithrombin (Eriksson 2009). They have been shown to be non‐inferior to VKA but without the need for regular blood test monitoring. They appear to have fewer drug interactions (compared with VKA) and no food or alcohol interactions
1. Rivaroxaban
Rivaroxaban is a reversible direct factor Xa inhibitor. For the initial treatment of acute pulmonary embolism, the recommended dosage of rivaroxaban is 15 mg twice daily for the first 21 days followed by 20 mg once daily for continued treatment and prevention of recurrence (NICE 2012b). The plasma half‐life, if renal function is normal, is estimated to be 8 to 10 hours (Spyropoulos 2012).
2. Apixaban
Apixaban is an oral, small molecule, reversible inhibitor of factor Xa with a plasma half‐life of 8 to 15 hours, taken twice daily (Eriksson 2009).
3. Betrixaban
Betrixaban is an orally administered direct factor Xa inhibitor. It also has a half‐life of 15 hours, offers the convenience of once daily dosing and may exhibit fewer drug interactions than warfarin (Palladino 2013).
4. Edoxaban
Edoxaban is an oral direct inhibitor of activated factor X that is rapidly absorbed with a half‐life of 9 to 11 hours. Edoxaban has a dual mechanism of elimination with one‐third eliminated via the kidneys and the remainder excreted in the faeces. It also offers the convenience of once‐daily dosing (Eikelboom 2010), and is used in conjunction with LMWH for five days.
Why it is important to do this review
The effectiveness of oral DTIs and oral factor Xa inhibitors for the treatment of venous thromboembolism has been studied in several randomised controlled trials (EINSTEIN‐DVT Study (EINSTEIN Investigators), ODIXa‐DVT Study (Agnelli 2007), Botticelli Study (Botticelli Investigators), AMPLIFY Study (Agnelli 2013), RE‐COVER II Study (Schulman 2011), THRIVE Studies (Eriksson 2003)). One non‐Cochrane systematic review has examined the effectiveness of DTIs and factor Xa inhibitors versus VKAs in the treatment of acute venous thromboembolism (Fox 2012). The primary outcome was venous thromboembolism and results were not presented for DVT and pulmonary embolism separately. To date, no systematic review has been conducted examining the effectiveness of oral inhibitors in the treatment of pulmonary embolism alone.
A separate Cochrane systematic review assessing the effectiveness of oral DTIs and oral factor Xa inhibitors for the treatment of DVT was published recently (Robertson 2015).
Objectives
To assess the effectiveness of oral DTIs and oral factor Xa inhibitors for the long‐term treatment of pulmonary embolism.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in which patients with a confirmed pulmonary embolism were allocated to receive an oral DTI or an oral factor Xa inhibitor for the treatment of pulmonary embolism. We included published studies and studies in progress if preliminary results were available. We placed no restrictions on publication status and non‐English studies were eligible for inclusion in the review. We exclude DTIs and factor Xa inhibitors that were not given by the oral route.
Types of participants
Patients with a pulmonary embolism, confirmed by standard imaging techniques (CTPA, V/Q scan).
Types of interventions
-
Oral DTIs (e.g. dabigatran, ximelagatran) (although ximelagatran was withdrawn from the market in 2006 due to safety issues, we have included it in the review to make the results as comprehensive as possible).
-
Oral factor Xa inhibitors (e.g. rivaroxaban, apixaban, betrixaban, edoxaban).
-
Other anticoagulants (e.g. LMWH, UFH, VKAs).
Comparisons included:
-
One oral DTI versus another oral DTI.
-
One oral factor Xa inhibitor versus another oral factor Xa inhibitor.
-
Oral DTI versus oral factor Xa inhibitor.
-
Oral DTI or oral factor Xa inhibitor versus another anticoagulant.
Treatment had to be for a minimum duration of three months as this is standard anticoagulation practice for a pulmonary embolism.
Types of outcome measures
Primary outcomes
-
Recurrent pulmonary embolism, confirmed by standard imaging techniques (CTPA, V/Q scan).
-
Recurrent venous thromboembolism (clinically overt DVT, confirmed by standard imaging techniques including proximal leg vein ultrasound scan or D‐dimer test, or both; or clinically overt pulmonary embolism, confirmed by CTPA or V/Q scan, or both).
-
Clinically overt DVT confirmed by standard imaging techniques (proximal leg vein ultrasound scan, venography) or D‐dimer test, or both.
Secondary outcomes
-
All‐cause mortality.
-
Adverse effects of treatment including major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005).
-
-
Fatal bleeding.
-
Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome.
-
Bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells.
-
Any combination of points 1 to 3.
-
-
Health‐related quality of life, as reported in included studies.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched January 2015) and the Cochrane Register of Studies (CRS) (http://www.metaxis.com/CRSWeb/Index.asp) (last searched January 2015). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com)
The TSC also searched the following trial databases for details of ongoing and unpublished studies using the terms apixaban or betrixaban or dabigatran or edoxaban or rivaroxaban or ximelagatran.
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
-
ClinicalTrials.gov (clinicaltrials.gov/).
-
ISRCTN Register (http://www.isrctn.com/).
Searching other resources
We searched the reference lists of relevant articles retrieved by the electronic searches for additional citations.
Data collection and analysis
Selection of studies
One review author (LR) used the selection criteria to identify trials for inclusion and the second review author (JM) independently confirmed this selection. We resolved any disagreements by discussion.
Data extraction and management
Two review authors (LR, JM) independently extracted the data from the included studies. We recorded information about the trial design, diagnosis of pulmonary embolism, baseline characteristics of participants and type of prophylaxis. We recorded recurrent pulmonary embolism (fatal and non‐fatal) and DVT data as the primary outcome measures. We collected data on all‐cause mortality and adverse effects of treatment including clinically relevant bleeding and health‐related quality of life in accordance with the secondary outcome measures. We contacted authors of included studies if further information or clarification was required. We resolved any disagreements in data extraction and management by discussion and sought the opinion of the third author (PK) and an expert, if required.
Assessment of risk of bias in included studies
Two review authors (LR, JM) independently used the Cochrane 'Risk of bias' tool for assessing risk of bias for each of the included studies (Higgins 2011). The tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting and any other relevant biases. We judged each of these domains as either high, low or unclear risk of bias according to Higgins 2011, and provided support for each judgement. We presented the conclusions in a 'Risk of bias' table. We resolved any disagreements by discussion with the third author (PK).
Measures of treatment effect
We based the analysis on intention‐to‐treat data from the individual clinical trials. As the primary and secondary outcomes were all binary measures, we computed odds ratios (ORs) using a fixed‐effect model and calculated the 95% confidence intervals (CI) for the effect sizes.
Unit of analysis issues
The unit of analysis in this review was the individual patient.
Dealing with missing data
We sought information about drop‐outs, withdrawals and other missing data and, if not reported, we contacted study authors for this information.
Assessment of heterogeneity
We assessed heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, the Chi2 test for homogeneity with a 10% level of significance and using the I2 statistic to measure the degree of inconsistency between the studies. An I2 result of greater than 50% represented moderate to substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to assess publication bias by funnel plots if a sufficient number of studies (10 or more) were available in the meta analyses. There are many reasons for funnel plot asymmetry, and we planned consult the Cochrane Handbook for Systematic Reviews of Interventions to aid the interpretation of the results (Sterne 2011).
Data synthesis
The review authors independently extracted the data. One review author (LR) input the data into Review Manager 5 (RevMan 2014), and the second review author (JM) cross‐checked data entry. We resolved any discrepancies by consulting the source publication.
We used a fixed‐effect model to meta‐analyse the data. If the I2 statistic indicated heterogeneity greater than 50%, we performed a random‐effects model analysis instead of a fixed‐effect model analysis.
Subgroup analysis and investigation of heterogeneity
-
History of venous thromboembolism.
-
Age.
-
Active cancer (treatment within last six months or palliative).
-
Pregnancy.
-
Major surgery requiring general or regional anaesthesia in the previous 12 weeks.
-
Recent period of immobility (bedridden three or more days in the previous 12 weeks).
-
Thrombophilia (genetic or acquired).
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies that we judged to be at high risk of bias. We also planned to perform sensitivity analyses with and without ximelagatran a priori given that this drug is no longer available. However, we found no studies that tested ximelagatran in patients with a pulmonary embolism.
'Summary of findings' table
We presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data for all outcomes of this review (Types of outcome measures) in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. We used the GRADEprofiler (GRADEpro) software to assist in the preparation of the 'Summary of findings' table (www.guidelinedevelopment.org).
Results
Description of studies
Results of the search
See Figure 1.

Study flow diagram.
Included studies
Five randomised controlled trials met the inclusion criteria for this review (AMPLIFY Study; EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). See Characteristics of included studies.
The AMPLIFY Study was a double‐blind study in which 5395 patients with a DVT or pulmonary embolism were randomised to receive oral apixaban 10 mg twice daily for the first seven days, followed by 5 mg twice daily for six months or enoxaparin 1 mg/kg body weight every 12 hours for at least five days and warfarin concomitantly for six months. Patients were followed up for six months. Outcomes included a composite of recurrent symptomatic venous thromboembolism (fatal or non‐fatal pulmonary embolism and DVT), mortality related to venous thromboembolism, major bleeding and clinically relevant non‐major bleeding.
The EINSTEIN‐PE study was an open‐label study in which 4832 patients were randomised to receive oral rivaroxaban 15 mg twice daily for the first three weeks, followed by 20 mg once daily (n = 2419) or enoxaparin 1.0 mg per kg of body weight twice daily and either warfarin or acenocoumarol, started within 48 hours of randomisation (n = 2413). Participants were followed up at three, six and 12 months and outcomes included recurrent pulmonary embolism, recurrent DVT, major bleeding and all‐cause mortality.
The Hokusai‐VTE Study was a double‐blind study in which 4921 participants were randomised to receive 60 mg oral edoxaban once daily (n = 2468) or dose‐adjusted warfarin therapy and edoxaban‐like placebo (n = 2453). Outcomes were measured monthly for one year. Results were presented for all patients with a venous thromboembolism but specific outcome data for the subset of participants with a pulmonary embolism were obtained through communication with the author.
RE‐COVER was a phase III non‐inferiority, double‐blind, double‐dummy trial in which patients with a venous thromboembolism (n = 2539) were given 150 mg dabigatran twice daily or warfarin. In addition, initial treatment with an approved parenteral anticoagulant (unfractionated heparin administered intravenously or low molecular weight heparin administered subcutaneously) was started before patients were randomised. Treatment was for a period of six months and included sham monitoring of international normalised ratio (INR) and sham titration of warfarin in the control group. To gain regulatory approval, the study was repeated with an identical design (RE‐COVER II).
Excluded studies
See Characteristics of excluded studies.
We excluded 13 studies (Ageno 2014; AMPLIFY Extended Study; Botticelli DVT Study; Einstein‐DVT Dose Study; Einstein DVT Study; EINSTEIN Extension Study; ODIXa‐DVT Study; Piazza 2014; REMEDY; RE‐SONATE; THRIVE; THRIVE I; THRIVE III). We excluded five studies as patients had a DVT only (Botticelli DVT Study; Einstein‐DVT Dose Study; Einstein DVT Study; ODIXa‐DVT Study; Piazza 2014). We excluded one study as although all patients had a venous thromboembolism, specific data on the subgroup with a pulmonary embolism was not published (THRIVE I). We made attempts to contact the authors for these data but were unsuccessful. We excluded three studies as they were extended studies testing the effectiveness of DOACs as prophylaxis rather than the treatment of pulmonary embolism (AMPLIFY Extended Study; EINSTEIN Extension Study; REMEDY). We excluded the THRIVE study as treatment was for less than three months, while we excluded the THRIVE III study as the control arm was a placebo. We excluded one study as it was not a randomised controlled trial (Ageno 2014). Finally, we excluded the REMEDY study from this review as participants were already included in the RE‐COVER and RE‐COVER II studies.
Risk of bias in included studies

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
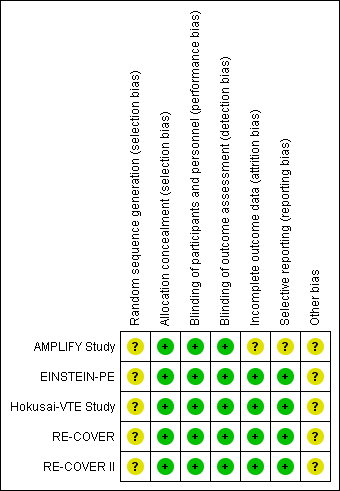
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All five included studies stated that they used a computerised system to conceal treatment allocation. However, the authors did not state the method by which the random sequence was generated and therefore we deemed the risk of selection bias relating to random sequence generation to be unclear. All five included studies reported that treatment allocation was concealed with the use of a computerised system and we therefore judged them at low risk of selection bias for allocation concealment.
Blinding
The EINSTEIN‐PE study was open‐label as the treatment arms comprised of rivaroxaban administered orally and subcutaneous enoxaparin. Therefore, blinding of participants and personnel was not possible. However, we judged that the lack of blinding in the control group was unlikely to have affected the outcome and therefore judged it to have a low risk of performance bias. The AMPLIFY Study, RE‐COVER, RE‐COVER II and Hokusai‐VTE Study were double‐blind and therefore we judged them to be at low risk of performance bias.
All studies used independent committees, whose members were unaware of the study group assignments, to adjudicate all suspected outcomes and the results of baseline imaging tests. Therefore we judged all included studies to be at low risk of detection bias.
Incomplete outcome data
Four studies accounted for all missing data and we judged them to be at low risk of attrition bias (EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). The AMPLIFY Study inappropriately excluded a number of randomised patients from the intention‐to‐treat (ITT) analysis. Furthermore, a large number of patients within each treatment group were classified as discontinuing the study for "other reasons" with no given explanations and therefore we deemed the risk of attrition bias to be unclear
Selective reporting
Protocols were available for four studies (EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). Furthermore, the study outcomes were clearly pre‐specified and data on the outcomes were presented. Therefore we judged these studies to be at low risk of reporting bias. The AMPLIFY Study pre‐defined minor bleeding as a secondary outcome but data were not reported in the paper and therefore we deemed the risk of reporting bias in this study to be unclear.
Other potential sources of bias
All five studies were funded by the pharmaceutical companies that manufacture dabigatran, rivaroxaban and edoxaban. This potentially could have influenced the time frame of reported safety outcomes and therefore we deemed the risk of other bias to be unclear. In addition, the AMPLIFY Study analysed non‐inferiority using an ITT analysis. When compared with the per‐protocol analysis, ITT favoured the finding of non‐inferior results. This may have skewed the result in favour of an increased efficacy of apixaban.
Effects of interventions
See: Summary of findings for the main comparison Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism; Summary of findings 2 Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism
We identified two studies that compared an oral direct thrombin inhibitor (DTI) versus standard anticoagulation with warfarin (RE‐COVER; RE‐COVER II), and two studies that compared an oral factor Xa inhibitor versus standard anticoagulation with warfarin (EINSTEIN‐PE; Hokusai‐VTE Study). We did not find any studies comparing one DTI with another DTI, one factor Xa inhibitor with another factor Xa inhibitor, or an oral DTI with a factor Xa inhibitor.
1. Oral direct thrombin inhibitor versus standard anticoagulation
In the meta‐analysis of oral DTIs versus standard anticoagulation, we used data from a paper, Schulman 2011, which combined the RE‐COVER and RE‐COVER II studies. This is reflected in the data analysis tables and 'Summary of findings' table by showing only one study for this comparison (summary of findings Table for the main comparison).
Recurrent pulmonary embolism
Two studies on a combined total of 1602 patients measured recurrent pulmonary embolism (RE‐COVER; RE‐COVER II). The rate of recurrent pulmonary embolism was similar between patients treated with dabigatran (16 events/795 participants) and those treated with standard anticoagulation (16 events/807 participants) leading to an odds ratio (OR) of 1.02 (95% confidence interval (CI) 0.50 to 2.04) (Analysis 1.1).
Recurrent venous thromboembolism
Two studies on a combined total of 1602 patients measured recurrent venous thromboembolism (RE‐COVER; RE‐COVER II). The rate of recurrent pulmonary embolism was similar between patients treated with dabigatran (23 events/795 participants) and those treated with standard anticoagulation (25 events/807 participants) leading to an OR of 0.93 (95% CI 0.52 to 1.66) (Analysis 1.2).
Deep vein thrombosis (DVT)
Two studies on a combined total of 1602 patients measured DVT (RE‐COVER; RE‐COVER II). The rate of DVT was similar between patients treated with dabigatran (seven events/795 participants) and those treated with standard anticoagulation (nine events/807 participants) leading to an OR of 0.79 (95% CI 0.29 to 2.13) (Analysis 1.3).
All‐cause mortality
Neither study presented results on all‐cause mortality for the specific group of participants with pulmonary embolism.
Adverse effects of treatment
Both studies, RE‐COVER and RE‐COVER II, measured major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). The rate of major bleeding was similar between patients treated with oral DTIs (four events/759 participants) and those treated with standard anticoagulation (eight events/768 participants) leading to an OR of 0.50 (95% CI 0.15 to 1.68) (Analysis 1.4).
Health‐related quality of life
Health‐related quality of life was not an outcome in any of the included studies.
2. Oral factor Xa inhibitor versus standard anticoagulation
See summary of findings Table 2.
Recurrent pulmonary embolism
We included two studies on a combined total of 4509 patients in a meta‐analysis (EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent pulmonary embolism was similar between patients treated with oral factor Xa inhibitors (45 events/2253 participants) and those treated with standard anticoagulation (50 events/2256 participants), leading to an OR of 1.08 (95% CI 0.46 to 2.56). The I2 statistic was 58%, indicating significant heterogeneity. Therefore we used a random‐effects model in place of the planned fixed‐effect model (Analysis 2.1). The AMPLIFY Study did not present recurrent pulmonary embolism data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis.
Recurrent venous thromboembolism
We included three studies on a combined total of 6295 patients in a meta‐analysis (AMPLIFY Study; EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent venous thromboembolism was similar between patients treated with oral factor Xa inhibitors (84 events/3153 participants) and those treated with standard anticoagulation (98 events/3142 participants), leading to an OR of 0.85 (95% CI 0.63 to 1.15) (Analysis 2.2).
DVT
We included two studies on a combined total of 4509 patients in a meta‐analysis (EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent DVT was similar between patients treated with oral factor Xa inhibitors (18 events/2553 participants) and those treated with standard anticoagulation (25 events/2256 participants), leading to an OR of 0.72 (95% CI 0.39 to 1.32) (Analysis 2.3). The AMPLIFY Study did not present DVT data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis.
All‐cause mortality
One study measured all‐cause mortality (EINSTEIN‐PE). The rate was similar between patients treated with the oral factor Xa inhibitor rivaroxaban (2.40%; 58 events/2412 participants) and those treated with standard anticoagulation (50 events/2405 participants), leading to an OR of 1.16 (95% CI 0.79 to 1.70) (Analysis 2.4). The AMPLIFY Study did not present all‐cause mortality data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis
Adverse effects of treatment
Both studies, EINSTEIN‐PE and Hokusai‐VTE Study, measured major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). The rate of major bleeding was similar between patients treated with oral factor Xa inhibitors (30 events/2253 participants) and those treated with standard anticoagulation (31 events/2254 participants) leading to an OR of 0.97 (95% CI 0.59 to 1.61) (Analysis 2.5). The AMPLIFY Study did not present adverse effects of treatment data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis
Health‐related quality of life
Health‐related quality of life was not an outcome in any of the included studies.
Discussion
Summary of main results
Recurrent pulmonary embolism
Meta‐analyses showed that the rate of recurrent pulmonary embolism was similar between the oral direct thrombin inhibitor (DTI) dabigatran and standard anticoagulation, indicating that neither was more or less effective. For factor Xa inhibitors, there was substantial heterogeneity when we combined data from the two studies in a meta‐analysis. Therefore no meaningful conclusions can be drawn from this pooled analysis.
Recurrent venous thromboembolism
Meta‐analyses showed that the rate of recurrent venous thromboembolism was similar between the oral DTI dabigatran and standard anticoagulation, indicating that neither was more or less effective. Similarly, for oral factor Xa inhibitors, the rate of recurrent venous thromboembolism was similar to standard anticoagulation, indicating that neither was more or less effective.
Deep vein thrombosis (DVT)
Meta‐analyses showed that both oral DTIs and factor Xa inhibitors were no more effective than standard anticoagulation in preventing DVT.
All‐cause mortality
One study measured all‐cause mortality in patients treated with the oral factor Xa inhibitor rivaroxaban and found that it was no more effective in preventing deaths than standard therapy.
Major bleeding
Results of our meta‐analysis indicate that direct oral anticoagulants (DOACs) offer no reduction in major bleeding compared to standard anticoagulation. The included studies all used the strict definition of major bleeding provided by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005).
Health‐related quality of life
Health‐related quality of life was not reported in the included studies.
Overall completeness and applicability of evidence
This review assessed whether long‐term treatment with new oral anticoagulants, such as DTIs and factor Xa inhibitors, reduced the rate of recurrent venous thromboembolism, all‐cause mortality and major bleeding in patients with a pulmonary embolism. Two studies tested DTIs and three studies tested factor Xa inhibitors within similar study populations. With the exception of all‐cause mortality and health‐related quality of life, all of the addressed outcomes were analysed and reported by the trialists. Statistical heterogeneity was high for recurrent pulmonary embolism in the studies testing factor Xa inhibitors. This was unexpected as each individual study had strict inclusion criteria that resulted in the overall patient population of this review having almost identical conditions. Furthermore, for each particular drug, the concentrations used across studies were similar.
Subgroup analyses could not be performed because of the lack of patient level data. These analyses might be important to guide clinical management in patients with different risk factors for pulmonary embolism.
Although many consider DVT and pulmonary embolism to be manifestations of the same disorder, we elected to study these two conditions separately as there is evidence of clinically significant differences between them. The majority of recurrent events occur at the same site as the original thrombosis (in other words, in a patient presenting with a pulmonary embolism, a recurrent event after treatment is much more likely to be another pulmonary embolism); both oral contraceptive use and Factor V Leiden mutation are more likely to be associated with DVT than pulmonary embolism; on the other hand, lung disease is much more likely to be associated with pulmonary embolism. A review on the effectiveness of oral DTIs and factor Xa inhibitors for the long‐term treatment of DVT was recently published (Robertson 2015).
We did not find any studies comparing:
-
one oral DTI versus another anticoagulant;
-
one oral DTI versus another oral DTI;
-
one oral factor Xa inhibitor versus another oral factor Xa inhibitor;
-
oral DTI versus oral factor Xa inhibitor.
A recent cost‐effectiveness analysis conducted by the National Institute for Health Care and Excellence (NICE) used data from the RE‐COVER, RE‐SONATE and REMEDY trials to measure the cost‐effectiveness of DOACs versus standard anticoagulation for the treatment of DVT and pulmonary embolism (NICE 2014). While dabigatran and rivaroxaban were not compared directly, the report found no difference in efficacy between the two drugs and that the costs were also very similar.
Quality of the evidence
With the exception of selection and funding bias where the risk was unclear, the risk of bias was low in all included studies, reflecting good methodological quality. One of the five included studies was open‐label because of the complexity of monitoring international normalised ratio (INR) in the standard anticoagulation arm. However, all outcomes were assessed by observers who were blinded to the treatment and all safety outcomes were adjudicated by a central independent committee in each study. We could not investigate publication bias because we could not assess asymmetry in a funnel plot with the limited number of studies included in the meta‐analysis. All included studies were funded by the pharmaceutical company that formulated the particular drug being tested in the study. This could have led to funding bias. Currently there is no Cochrane tool to estimate the risk of this so we classified this as a potential other risk of bias. Funding by the pharmaceutical company could also have influenced the timeframe of reported safety outcomes and this has to be considered. All five included studies reported using a computerised system to generate the randomisation sequence. However, no further information was provided and for this reason we deemed that the risk of selection bias for random sequence generation was unclear.
For the comparison of oral DTIs versus standard anticoagulation, we graded the quality of the evidence as high. For oral factor Xa inhibitors versus standard anticoagulation, we downgraded the evidence for the outcome recurrent pulmonary embolism to moderate due to substantial heterogeneity that could not be explained. We also downgraded the evidence for all‐cause mortality to moderate as only one study was included for this outcome. However, for the outcomes recurrent venous thromboembolism, DVT and major bleeding, the evidence remained high as the outcomes were direct and effect estimates were consistent and precise, as reflected in the narrow confidence intervals around the ORs. See summary of findings Table for the main comparison; summary of findings Table 2.
One of the limitations of this review is the small number of included studies included. However, there were large numbers of participants within each study.
Potential biases in the review process
The search was as comprehensive as possible and we are confident that we have included all relevant studies. However, the possibility remains that some relevant trials, particularly in the 'grey' literature (for example conference proceedings), have been missed. Two review authors independently performed study selection and data extraction in order to minimise bias in the review process. We strictly adhered to the inclusion and exclusion criteria set out in the protocol in order to limit subjectivity. We performed data collection according to the process suggested by Cochrane. We also followed Cochrane processes as described by Higgins 2011 for assessing the risk of bias. For two of the included studies, RE‐COVER and RE‐COVER II, we took data from a pooled analysis published in one paper (Schulman 2011). This was the best available evidence. We tried to obtain data directly from the trialists but to no avail.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first review to measure the efficacy and safety of oral anticoagulants in patients with a pulmonary embolism. The same oral anticoagulants have been assessed in eight other systematic reviews (Antoniazzi 2103; Castellucci 2013; Fox 2012; Gomez‐Outes 2014; Hirschl 2014; Kang 2014; Sardar 2014; van der Huille 2014), but in patients with a venous thromboembolism. Five reviews found that novel oral anticoagulants are associated with less bleeding than conventional treatment (Antoniazzi 2103; Fox 2012; Gomez‐Outes 2014; Hirschl 2014; van der Huille 2014).
The review by Fox 2012 performed meta‐analysis by brand rather than class of drug and found no difference in recurrent venous thromboembolism between the two treatment groups. Rivaroxaban was the only drug found to be significantly associated with fewer major bleeding episodes (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.39 to 0.84). All‐cause mortality did not differ between the two treatment groups.
The review by van der Huille 2014 showed no difference between the two treatment groups in terms of recurrent venous thromboembolism, fatal pulmonary embolism and all‐cause mortality. However, the novel oral anticoagulants were associated with a significant reduced risk of major bleeding (relative risk (RR) 0.60, 95% CI 0.41 to 0.88) and fatal bleeding (RR 0.36, 95% CI 0.15 to 0.87).
Hirschl 2014 found no differences between DOACs and standard treatment regarding recurrent venous thromboembolism and death. However, bleeding was reduced by rivaroxaban (RR 0.55, 95% CI 0.38 to 0.81), apixaban (RR 0.31, 95% CI 0.17 to 0.55) and edoxaban (RR 0.81, 95% CI 0.71 to 0.93).
The review by Gomez‐Outes 2014 found that the risk of recurrent venous thromboembolism was similar between the two treatment groups (RR 0.91, 95% CI 0.79 to 1.06) but the DOACs were associated with reduced major bleeding (absolute risk difference of ‐0.6%, 95% CI ‐1.0% to ‐0.3%).
The review by Kang 2014 found that DOACs did not differ in the risk of mortality or recurrent venous thromboembolism. However, dabigatran was associated with increased major bleeding compared to apixaban (RR 2.69, 95% CI 1.19 to 6.07) and edoxaban also had a higher bleeding rate compared to apixaban (RR 2.74, 95% CI 1.40 to 5.39).
The review by Antoniazzi 2103 included patients with venous thromboembolism and atrial fibrillation. Eight studies were included and results showed that the risk of major bleeding was lower in patients treated with dabigatran (RR 0.83, 95% CI 0.78 to 0.97).
The reviews by Castellucci 2013 and Sardar 2014 compared oral anticoagulants and antiplatelet drugs but the focus was on the secondary prevention of venous thromboembolism rather than treatment.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.
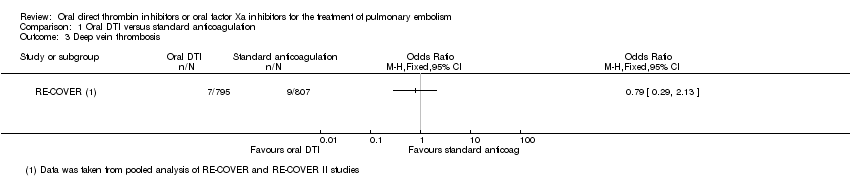
Comparison 1 Oral DTI versus standard anticoagulation, Outcome 3 Deep vein thrombosis.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 4 Major bleeding.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 3 Deep vein thrombosis.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 4 All‐cause mortality.

Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 5 Major bleeding.
| Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with Oral DTI | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.02 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 20 per 1000 | 20 per 1000 | |||||
| Recurrent venous thromboembolism | Study population | OR 0.93 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 31 per 1000 | 29 per 1000 | |||||
| Deep vein thrombosis5 | Study population | OR 0.79 | 1602 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 11 per 1000 | 9 per 1000 | |||||
| All‐cause mortality | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not report on all‐cause mortality | |
| Major bleeding6 | Study population | OR 0.50 | 1527 | ⊕⊕⊕⊕ | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 10 per 1000 | 5 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
| Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard anticoagulation | Risk with oral factor Xa | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.08 | 4509 | ⊕⊕⊕⊝ | ‐ | |
| 22 per 1000 | 24 per 1000 | |||||
| Recurrent venous thromboembolism5 | Study population | OR 0.85 | 6295 | ⊕⊕⊕⊕ | ‐ | |
| 24 per 1000 | 20 per 1000 | |||||
| Deep vein thrombosis6 | Study population | OR 0.72 | 4509 | ⊕⊕⊕⊕ | ‐ | |
| 11 per 1000 | 8 per 1000 | |||||
| All‐cause mortality | Study population | OR 1.16 | 4817 | ⊕⊕⊕⊝ | ‐ | |
| 16 per 1000 | 19 per 1000 | |||||
| Major bleeding8 | Study population | OR 0.97 | 4507 | ⊕⊕⊕⊕ | ‐ | |
| 14 per 1000 | 13 per 1000 | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent pulmonary embolism Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrent venous thromboembolism Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent pulmonary embolism Show forest plot | 2 | 4509 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.46, 2.56] |
| 2 Recurrent venous thromboembolism Show forest plot | 3 | 6295 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3 Deep vein thrombosis Show forest plot | 2 | 4509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 4 All‐cause mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Major bleeding Show forest plot | 2 | 4507 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.59, 1.61] |

