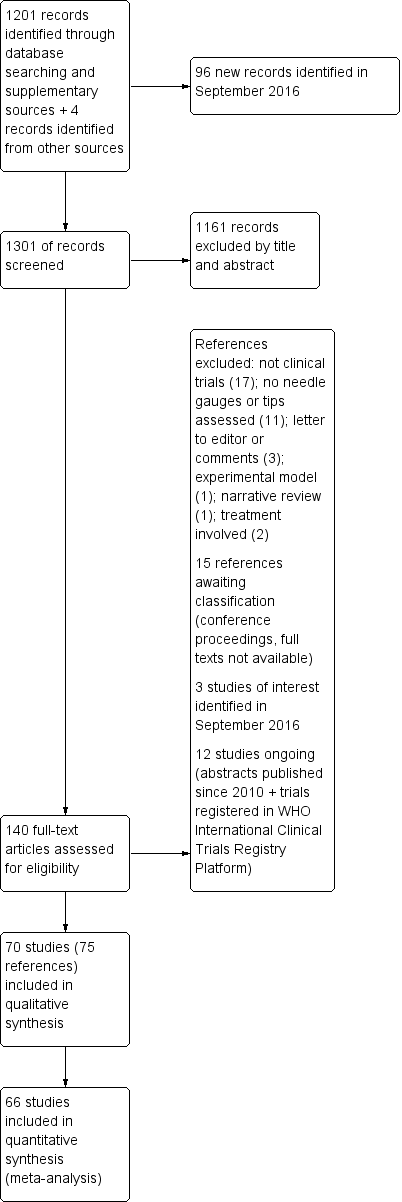
طراحی قطر (gauge) و سر سوزن برای پیشگیری از سردرد متعاقب بیحسی نخاعی (PDPH)
References
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants | 1. 208 patients enrolled (obstetric patients undergoing elective caesarean section) Patients randomized to:
2. No randomized patients were excluded 3. No patients lost to follow‐up 4. Main characteristics of patients: unclear. "There were no significant differences between the patients in the two groups in terms of age, weight, height, previous history of c‐section and past history of headache" | |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned to…" (page 150) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomly assigned to…" (page 150) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (patients scheduled for surgery in the lower part of the body)
2. 2 patients randomized to Quincke group were excluded due to failures in identification of subarachnoidal space 3. No patients lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… were randomised into two groups after written informed consent was obtained" (page 535) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients did not know which needle was used" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "After 72 hours all patient were contacted personally or by telephone, and questioned in a structured interview about problems or symptoms which could have been a result of the spinal anaesthetic. This interview was done by a nurse anaesthetist who was unaware of which needle that had been used, and whether any problems had occurred during the anaesthetic." (page 536) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of the funder during the study was unclear (Medisinsk forskning I Finnmark) |
| Methods |
| |
| Participants | 1. 400 women enrolled (consecutive patients receiving spinal anaesthesia for orthopaedic operations of the lower extremities) Patients randomized to:
2. No randomized patients lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to (…)" (page 166) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Neither the interviewer nor the patient were aware of the kind of needle that had been used". (page 167) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Neither the interviewer nor the patient were aware of the kind of needle that had been used" (page 167) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 354 women enrolled (ASA 1 and 2 undergoing spinal anaesthesia for elective caesarean section) Patients randomized to:
2. 4 patients (2 for each group) with failure to identify the subarachnoid space were excluded Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized, using a randomization table, into two groups (…)". (page 1132) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients were blinded to the needle utilized". (page 1132) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were assessed after operation by an investigator blinded to the needle and not involved in their perioperative care". (page 1132) |
| Incomplete outcome data (attrition bias) | Low risk | 1.31% patients enrolled were not analysed |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (patients from different surgical departments of Nawaz Sharif Social Security Hospital Lahore having different surgical procedures on the lower abdomen and lower limbs such as hernias, amputations, debridements, vesicolithotomy, total hip replacements, tibial nailing or plating, external fixators, caesarian sections and hysterectomies) Excluded patients: patients with systemic disease such as uncontrolled diabetes mellitus and hypertension, congestive cardiac failure, severe anaemia, pulmonary oedema, coagulopathies and vertebral column deformities Patients randomized to:
2. No patients were excluded 3. Main characteristics of patients:
| |
| Interventions |
Co‐intervention: needle directed cephalic slightly upwards towards umbilicus | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Two groups consisting 100 patients each were randomly chosen". (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Several outcomes unreported in results section: speed of CSF back flow, aspiration of CSF, ease of injection |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (less than 45 years of age, presenting for daycare surgery on the lower half of the body to be performed under spinal anaesthesia) Excluded patients: unclear
2. 9 patients failed to return their questionnaires and 2 patients were recorded as failures 3. Main characteristics of patients:
| |
| Interventions | Spinal anaesthesia with either a 27 G Quincke needle or 27 G Whitacre
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated to receive spinal anaesthesia" (page 780) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients were not aware of which needle had been used to perform the anaesthesia". (page 780) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The replies to the questionnaires were assessed by one of the authors who was not aware of which needle had been used to perform the anaesthesia". (page 780) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 133 children having LP as part of their standard treatment protocol for leukaemia, recruited during visits to the Day Surgery Unit of the Royal Children’s Hospital. Aged 4 to 15 years at the time of first procedure. Exclusion criteria: excluded if they had insufficient LPs remaining in their planned treatment, had significant coexisting medical problems causing headache or were routinely using 25 G needles at parental request, or if there were significant social or communication problems. 3. 40 were excluded for meeting exclusion criteria
4. 93 were allocated to a random sequence of 4 LPs, 2 with 22 G (A) and 2 with 25 G (B), and completed 341 LPs
2. No randomized patients were excluded 3. 18 patients lost to follow‐up: 2 children had their last LP after their 16th birthday (excluded). 16 did not complete all 4 procedures for reasons such as moving interstate or finishing their treatment protocol, giving a total of 341 procedures (167 with the 22 G and 174 with the 25 G needle) 4. Main characteristics of patients (not specifying groups):
| |
| Interventions |
| |
| Outcomes | Primary outcomes:
Secondary outcomes: assessed by a 1‐page questionnaire and telephone interview on days 1, 3 and 7 following the procedure
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment allocation was computer‐generated by an independent statistician." (page 204) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated a sequential study number which corresponded to a large envelope containing four smaller sealed envelopes, labelled a, b, c and d, containing details of the needle sizes to be used for four procedures" (page 204) |
| Blinding of participants (performance bias) | Low risk | Quote: "All LPs were performed under general anaesthesia by the same experienced doctor (CC) who was given the relevant sealed envelope immediately before the procedure. This doctor was not involved in data collection after the procedure. All other staff and study participants were blinded to the needle gauge." (page 204) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A study researcher blinded to the needle size recorded the time from first needle insertion to successful commencement of CSF collection and the time required for collection of 22 drops of CSF (approximately 1 mL) (…) Following each procedure, parents were given a one‐page questionnaire to take home which asked them to record details of any headache in the child on days 1, 3 and 7 following the procedure (…) A researcher also phoned families on days 1, 3 and 7 after each procedure to ensure the data were recorded, and confirm the nature of any headache." (page 204) |
| Incomplete outcome data (attrition bias) | Low risk | 8.3% (31 out of 372 procedures) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 158 patients enrolled during a 12‐month period (ASA I and II, aged from 20 to 40 years, undergoing lower limb orthopaedic surgery) Exclusion criteria: presence of hypovolaemia, coagulation disorders, infection at the puncture site, use of general anaesthesia, history of headaches, chronic back pain or pregnancy Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On arrival at the operating room, the patients were assigned to one of two groups using a randomization table: (…)." (page 548) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "After surgery, close follow‐up of patients was performed by an investigator blinded to the study protocol". (page 549) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Information about non‐specific headaches is unclear |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients ASA I and II, aged 18 to 45 years and scheduled for knee arthroscopy were randomly assigned to 2 groups Unclear if patients were enrolled but not randomized Exclusion criteria: history of migraine headaches, previous PDPH Patients randomized to:
2. 6 patients were excluded from analysis because exclusion criteria had been missed or they could not be contacted Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "…were randomly assigned to two groups." (page 1107) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The interview was conducted by an anaesthetist unaware of the anaesthetic technique used..." (page 1107) |
| Incomplete outcome data (attrition bias) | Low risk | 6 patients (3%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (healthy obstetric patients requiring caesarean section) Exclusion criteria: patients in whom labour epidural analgesia had been attempted or performed previously, or in whom a spinal anaesthetic had been attempted with other needles 4 patients in the Sprotte group (3 Sprotte with fentanyl and 1 Sprotte with plain local anaesthetic) and 2 in the Quincke group (1 randomized to receive fentanyl and 1 Sprotte with plain local bupivacaine) were not available for follow‐up
2. 6 (6%) patients randomized were excluded because exclusion criteria had been missed or because they could not be contacted
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "This randomized, blinded study (…)." (page 222) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all patients were evaluated daily during the first 4 postoperative days by the designated nurse, who was blinded to the type of needle and medication used (...) Investigators conducting telephone follow‐up were blinded to the type of needle and anesthetic solution used". (page 223) |
| Incomplete outcome data (attrition bias) | Low risk | 4 patients (8%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 80 patients undergoing different surgical procedures and receiving regional anaesthesia were randomized. Unclear if patients were enrolled but not randomized. Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Individuals were randomly assigned to receive…" (page 241) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 1555 patients enrolled (ASA I‐II patients undergoing lower abdominal surgery and hospitalization no more than 24 hours) Exclusion criteria: history of PDPH in previous surgeries Number of patients randomized per group: unclear 2. 33 patients randomized were excluded due to (unclear numbers by group):
3. 1522 patients were analysed in 2 groups:
4. No patients were lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Distribution of patients in each group were randomly using the last two digits of their medical history" (page 183) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Once the patient had started mobilisation was visited by a team member who did not know the type of needle used and asked specifically for headache occurrence." (page 183) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 313 patients aged 18 to 55 years were enrolled (scheduled for non‐obstetric outpatient surgery below the umbilicus to be performed during spinal anaesthesia) Exclusion criteria: unclear Patients randomized to:
2. 12 patients were excluded from analysis
3. 301 patients were analysed (lost to follow‐up: 3.83%)
3. Main characteristics of patients:
| |
| Interventions | 1. 27 G Pencan group: 0.40 mm O.D. B Braun, Germany 2. 27 G Quincke group: Spinocan 27 G, B. Braun, Germany. The bevel of the Quincke‐type spinal needle was kept parallel to the longitudinal direction of the dural sac. | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was performed using the sealed envelope technique.…" (page 643) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was performed using the sealed envelope technique.…" (page 643) |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients were blinded to the choice of spinal needle, and only the needle size of the spinal needle was documented in the anaesthetic record." (page 644) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients were followed up by a single anaesthesiologist (HF) also blinded to the choice of spinal needle." (page 644) |
| Incomplete outcome data (attrition bias) | Low risk | 3.83% patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 412 patients undergoing thoracic/cervical or lumbar myelographies were enrolled Exclusion criteria: unclear Patients randomized to:
Also patients inside each group were randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "In a prospective randomized trial the incidence of complaints after lumbar puncture…" (page 922) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 40 patients healthy ASA I patients under 40 years of age were enrolled. Indications for surgery varied, but all operations were subumbilical. Exclusion criteria: patients complaining of pre‐existing headache or backache Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "a restricted randomised double‐blind study to ensure equal numbers in each group was initiated…" (page 350). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The use of a 0.90 mm introducer needle ensured that the patients were unable to differentiate between the two spinal needles." (page 350) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Postoperatively, patients were visited by two of the authors (MCH and RMW), who had no knowledge of which needle size had been used for spinal anaesthesia." (page 350) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 308 patients aged 18 to 45, ASA I, undergoing surgery in lower limbs Exclusion criteria: column injuries, cognitive or coagulation comorbidities, infection in site of lumbar puncture Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to one of two groups…" (page 162) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 100 ASA Grade I and II of either sex in the age group between 25 to 45 years, who were to receive spinal anaesthesia to undergo subumbilical surgery Exclusion criteria: obstetric patients, patients with abnormalities of spine, soft tissue infection at the site of needle insertion, acute ear infection and respiratory tract infection, coagulation disorders and neurological symptoms Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
Co‐intervention: patients were premedicated with tablet diazepam 5 mg a night before and 5 mg on the morning of surgery. Morphine sulphate 0.15 mg/kg and promethazine 0.5 mg/kg was also administered intramuscularly to all patients 45 minutes before anaesthesia. | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly divided into two groups…" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All the patients were visited at the end of 24 hours and then on the third and fourth post‐operative day by an anaesthetist who was not present during the performance of spinal anaesthesia." (page 2) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 493 ASA Grade I to III patients undergoing orthopaedic surgery of the lower limbs were included 19 patients receive 2 surgeries, therefore the authors included 512 procedures in this study. However, 12 patients were excluded due to anatomical factors (1 procedure). 500 procedures randomized to:
Also patients were assigned to different regimes of mobilization (not analysed in the present review) 2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According with a random list patients were assigned to one of four groups" (page 861) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Examiners and patients had no knowledge of the needle type used (double‐blind)." (page 861) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Examiners and patients had no knowledge of the needle type used (double‐blind)." (page 861) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 128 patients referred to lumbar, thoracic, cervical or total column myelography were included 128 patients assigned to:
2. 15 patients were lost to follow‐up and excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were numbered sequentially: in even‐numbered patients a 22 gauge needle was used and for odd‐numbered patients, a 25 gauge needle " (page 487) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 11% of patients (15) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 688 women undergoing caesarean section in whom spinal anaesthesia was clinically indicated Exclusion criteria: anticoagulation therapy, aortic valve disease, NYHA class 3 or 4 cardiac symptomology, sepsis at the site of injection, severe pre‐eclampsia and systemic hypotension Patients randomized to:
2. 7 patients were not studied because 1 withdrew and 5 were entered twice A further 16 were excluded from the analysis of headache due to protocol deviations Patients analysed (3.34% lost to follow‐up):
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was achieved using sealed envelopes, which contained the needle to be used as well as the documentation" (page 1006) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was achieved using sealed envelopes, which contained the needle to be used as well as the documentation" (page 1006) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all patients were seen within 48 h of surgery by a member of the study team who had not been involved with the performance of the block" (page 1007) |
| Incomplete outcome data (attrition bias) | Low risk | 3.34% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 60 women ASA I or II scheduled for elective caesarean section were enrolled Exclusion criteria: abnormal lumbar spaces, coagulopathy, infection, pre‐eclampsia or obesity Patients randomized to:
2. No patients were excluded at follow‐up 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized, pulling out of a hat method (…)". (page 379) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients and the assessor of postoperative complications but not the attending anesthetists were blinded to the needle used" (page 380) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Postoperatively, the patients were visited daily for five days by an anaesthetist not involved in the perioperative care (..)" (page 1007) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 693 patients under 50 years undergoing spinal anaesthesia were included Exclusion criteria: patients with diseases that could affect CSF pressure were excluded from the study Patients divided into 2 groups:
2. No patients reported as lost to follow‐up 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "693 patients younger than 50 years, submitted to spinal anesthesia, were divided into two groups corresponding to each type of disposable needle used" (page 3) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients did not know which type of needle to use" (page 3) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were reported as lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 730 ambulatory surgery patients, 18 years or older, ASA I or II, and electing to receive spinal anaesthesia, were enrolled Exclusion criteria: patients with history of migraine headache or chronic back pain Number of patients randomized to each group: unclear 2. 72 patients (9.86%) were excluded at follow‐up Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomly assigned (…)". (page 734) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "In operating room, while patients were blinded to the needle size used (…)". (page 380) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "One of the nurse investigators (..), who had no knowledge of the patient´s needle assignment, made (…)" (page 1007) |
| Incomplete outcome data (attrition bias) | Low risk | 9.86% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Severity of headache and back pain are mentioned, but results are not reported. Adverse events, additional to PDPH, were not reported. |
| Other bias | Unclear risk | The role of the funder in this research is unclear |
| Methods |
| |
| Participants | 1. 53 patients who underwent elective orthopaedic knee or hip surgery under spinal anaesthesia were enrolled (age > 60 years, ASA classes I–II, recumbent in bed for the first 24 hours postoperatively, and administration of intravenous patient‐controlled analgesia for the first 48 hours postoperatively) Exclusion criteria: history of migraine headache, previous history of PDPH, cardiovascular or central nervous disease, and coagulation abnormality Patients were randomized to:
2. 3 patients (5.66%) were excluded due to severe hypotension, heart problems after operation or refusal to participate in follow‐up Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The 53 patients were randomly allocated to either the experimental group (…)" (page 1316) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Incomplete outcome data (attrition bias) | Low risk | 5.66% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 100 patients enrolled (Dutch patients, both sexes, > 18 years of age) Patients randomized to:
2. 1 randomized patient was excluded due to:
3. 0 patients lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At random allocation a few minutes before lumbar puncture, through telephone via the trial bureau |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomization unit. The allocation sequence was unknown to the investigators. |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome was assessed by a medical doctor not involved in the lumbar puncture and blinded to the intervention type |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient excluded from the study |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 60 ASA physical status 1 and 2 children aged one to 13 years, scheduled for day case operations of the lower abdomen, genital area or lower extremities, were enrolled Patients were randomized to:
2. No patients were excluded at follow‐up 3. Main characteristics of patients:
| |
| Interventions |
Co‐intervention: at the end of the operation the children were given ibuprofen 10 mg/kg‐1 as a suppository for pre‐emptive pain therapy | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: " The children were randomly allocated (…)" (page 116) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (ASA I‐II children, aged 2 to 128 months, and scheduled for day care surgery) Exclusion criteria: known contraindication to spinal puncture, such as an increased intracranial pressure, haemorrhagic diathesis or infection at the puncture site. Children with neurologic disorders or allergy to bupivacaine or other local anaesthetics were also excluded. Patients randomized to:
2. 5 patients randomized were excluded due to:
3. 1 patients lost to follow‐up:
4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated to receive spinal anaesthesia with either (…)" (page 1077) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The parents were blinded to the needle used." (page 1077) |
| Incomplete outcome data (attrition bias) | Low risk | 6 patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 57 patients enrolled (ASA I‐II children, aged 8 months to 15 years, with cancer or neurological symptoms having a diagnostic and/or therapeutic LP) Exclusion criteria: unclear Patients randomized to:
2. No exclusions 3. No losses to follow‐up: 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The 53 patients were randomly allocated to either the experimental group (…)Those children having repeated LPs remained in the same needle group throughout the study" (page 1316) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Incomplete outcome data (attrition bias) | Low risk | 5.66% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 215 patients enrolled (ASA I‐II children, aged 1 to 18 years, undergoing surgery below the umbilicus)
2. 1 patient randomized was excluded from the complication analysis 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation schedule was generated by a computer and concealed until the patient arrived in the operating theatre (…)" (page 211) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this item as low or high risk |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients, parents and post‐anaesthesia care unit (PACU) nurses were unaware of the type of needle used. (…)" (page 211) |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "using an open‐randomised, parallel‐groups, and prospective design (…)" (page 211) |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 60 consecutive outpatients (ASA) physical status I–III, ages ranging between 18 and 60 years, scheduled for unilateral lower limb surgery, with spinal block being used as the sole anaesthetic without any intraoperative sedation were enrolled Exclusion criteria: previous history of intolerance to the study drug or related compounds and existing contraindications for spinal anaesthesia, patients with a body mass index (BMI) of 30 kg/m2, those with a history of alcoholism, drug abuse, or psychological or other emotional problems, patients who were pregnant or lactating Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
In both groups a 20 G introducer was applied | |
| Outcomes | Primary outcome:
Secondary outcomes:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "using a sealed envelope technique, the patients were randomized to two groups". (page 225) |
| Allocation concealment (selection bias) | Low risk | Quote: "using a sealed envelope technique, the patients were randomized to two groups" (page 225) |
| Blinding of participants (performance bias) | Low risk | Quote: "patients, nurses, and the anesthetist performing the motor and sensory block assessments were blinded for the spinal needle type used". (page 225) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 63 patients enrolled (consecutive patients older than 18 years scheduled for a diagnostic or therapeutic lumbar puncture as a part of their routine clinical management)
2. 5 patients randomized were excluded due to:
3. 0 patients lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary A. Incidence of PDPH B. Adverse events: not reported C. Severity PDPH D. Any headache subsequent to a lumbar puncture: not reported | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to undergo LP with a standard (...).Patients were randomized only once. Therefore, those who required repeated LPs had them done with the same needle type." (page 1492) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The study was blinded to the patient. However, because the different needles have different structures, the physician knew which needle was used and could not be blinded to the needle." (page 1492) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Follow‐up was performed by a physician, blinded to the randomization, on days 2 (...)". (page 1492) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 300 patients (ASA I or II) aged 15 to 40 years (196 male, 104 female) undergoing elective orthopaedic procedures were enrolled Exclusion criteria: migraine or chronic severe headache, infection, local anaesthetic allergy or a preference for general anaesthesia Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
All punctures were done with a 20 G introducer (Braun, Germany) | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to have (…)" (page 58) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported. Presence of associated symptoms is mentioned in methods, but not reported. |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 298 patients enrolled (patients consenting to spinal anaesthesia for elective and emergency caesarean section)
3. Losses to follow‐up or exclusions: not reported 2. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The needle to be used was assigned in a random manner: (…)". (page 58) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 160 patients attending for myelography were included. Further details were not provided. Exclusion criteria: patients with marked obstruction of CSF flow Number of patients randomized by arm: unknown 2. 14 patients (8.75%) were excluded from analysis due to incomplete follow‐up or death Patients analysed:
3. Main characteristics of patients (in general):
| |
| Interventions |
After the study, patients rested in bed with head elevated for 24 hours and were encouraged to consume fluids | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomized to undergo (..)". (page 1102) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients were questioned by an independent observer at 24 hours (…)". (page 1102) |
| Incomplete outcome data (attrition bias) | Low risk | 8% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 389 patients undergoing orthopaedic surgery or general surgery were enrolled Exclusion criteria: refusal of technique, allergy to anaesthesia, neurological comorbidities or coagulation conditions Patients randomized to:
2. 12 patients (3%) were excluded from analysis, due to protocol deviations Patients analysed:
Telephone interview was complete in (lost to follow‐up: 10%):
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized into two groups (..)" (page 449) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "patients were interviewed by telephone to the second and seventh day after surgery, by an anesthesiologist unaware of who and who and how the puncture was made, and following a specific and as a template for all patients (…)" (page 450) |
| Incomplete outcome data (attrition bias) | Low risk | 10% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 100 consecutive patients undergoing diagnostic LP were enrolled Exclusion criteria: contraindications against any type of LP Patients randomized to:
2. 10 patients (10%) were excluded from analysis, due to protocol deviations Patients analysed:
3. Main characteristics of patients (only analysed patients):
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The study was carried out as a prospective randomized blind study on a general neurological ward (..)" (page 376) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The LP was carried out by a resident who was asked not to disclose the type of needle to the patient or to the masked examiner." (page 377) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All examinations were carried out by an examiner who was unaware of the puncture technique and observations were recorded on standardised check‐lists (…)" (page 377) |
| Incomplete outcome data (attrition bias) | Low risk | 10% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients enrolled (obstetric female patients aged 20 to 35 belonging to ASA I undergoing elective or emergency lower segment caesarean section) Patients randomized to:
2. Losses to follow‐up and exclusions were not reported 2. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to one of the two groups Q or W according to computer generated numbers". (page 420) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Post‐operatively, follow up was done up to 7 days after the surgery or till the time of discharge by an anaesthesiologist who had no knowledge of the spinal needle." (page 421) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Severity of post‐dural puncture headache was assessed according to Corbey severity grading and visual analogue scale (VAS). This information is not reported. |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 215 American Society of Anesthesiology Class I to II postpartum patients presenting for elective postpartum bilateral tubal ligations under spinal anaesthesia were enrolled Patients randomized to:
2. 11 patients (5.1%) were excluded from analysis, because of loss to follow‐up, cancellation of surgery or inability to identify the sub‐arachnoid space Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomized by means of a computer‐generated random number table into either" (page 360) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Postoperatively, an investigator who was blinded to the group assignment interviewed the patients daily while in the hospital" (page 360) |
| Incomplete outcome data (attrition bias) | Low risk | 5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of funder is unclear |
| Methods |
| |
| Participants | 1. 107 consecutive patients (inpatient and outpatient) referred to the Department of Radiology for lumbar myelography were enrolled Number of patients randomized per arm: unclear 2. 7 patients (6.5%) were excluded from analysis because they were operated within the first 7 days or they did not returned the questionnaire 146 patients were analysed:
3. Main characteristics of patients: 58 men, 42 women, age range: 20 to 82 years, mean: 50.5 years) | |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomized into two groups (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 6.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 778 patients undergoing myelography from 26 April 1993 to 7 September 1994, at a large, tertiary care, academic hospital were eligible for this study Of the 778 eligible patients, 340 consented to participate in the study 340 patients were randomized to:
2. 26 patients received another needle than the randomized needle; additionally, 49 patients were not followed up. Authors include all data (as randomized) in the final analysis. 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequential order of Whitacre and Quincke needle assignments was randomly assigned by computer in blocks of 10 to ensure an equal number of patients in each needle group." (page 772) |
| Allocation concealment (selection bias) | Low risk | Quote: "When a patient consented to enter the study, the fluoroscopy technologist received the needle assignment from the chief radiologic technologist, who kept the randomization list." (page 772) |
| Blinding of participants (performance bias) | Low risk | Quote: "There was no formal masking of the research nurses or patients; however, there was probable masking in effect. At follow‐up, most patients did not seem to know their needle group assignment." (page 772) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The principal investigator (S.B.P.), who was masked to the needle group assignment, coded the patient diagnosis by chart review." (page 772) |
| Incomplete outcome data (attrition bias) | Low risk | 14.4% of patients were lost to follow‐up. However, all randomized patients were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of the funder in this research is unclear |
| Methods |
| |
| Participants | 1. 160 ASA grade I or II patients undergoing orthopaedic surgery or manipulations to reduce lower limb fractures were included Exclusion criteria: patients with a history of migraine or frequent headaches and neurological problems Patients divided into 2 groups:
2. No patients reported as lost to follow‐up 3. Main characteristics of patients: not fully reported | |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly allocated, on the basis of date (but not the year) of their birth, to receive spinal anaesthesia with either (...)" (page 560) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Two of the authors, who were unaware of the needle gauge employed, examined each patient on the first, second and third postoperative days and inquired about the occurrence of headache" (page 561) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 234 ASA I‐II outpatients undergoing elective arthroscopy of the knee joint Exclusion criteria: contraindication to regional anaesthesia Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to receive (…)" (page 73) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "and were interviewed by one of the authors (blind with respect to needle size(…)" (page 74) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 108 patients enrolled (patients referred for myelograms) Exclusion criteria: inability to sit or stand, inability to reliably communicate, a situation that would tend to decrease the presence and reporting of spinal headache 108 patients randomized to:
2. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "108 were randomized to a 22‐gauge" (page 1290) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An observer contacted each subject by telephone 5‐14 days after the myelogram. The observer did not know which type of needle had been used on the subjects." (page 1290) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 90 patients enrolled (female patients of 20 to 38 years old, undergoing caesarian sections) Exclusion criteria: ASA above III Number of patients randomized per arm: unclear 2.Number of patients excluded (who required more than one prick): unclear. 3 patients were excluded from analysis for unknown reasons. Number of analysed patients:
3. No patients lost to follow‐up 4. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "ninety female patients of 20 to 38 years of age, undergoing caesarian sections were randomly distributed to either 25 or 27 gauge Quincke needle groups" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were interviewed first through third post‐operative days about the occurrence of headache and their satisfaction regarding spinal anesthesia." (page 1) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 admitted for elective total unilateral hip replacement were enrolled Number of patients randomized per arm: unclear 2. 17 patients (8.5%) were excluded from analysis, in the pre and postoperative period. It was impossible to perform the spinal procedure with the prescribed 25 G needle in 4 patients; 7 had an incomplete block, of whom 5 had a supplementary general anaesthetic and 2 another spinal injection; 4 had major cardiovascular complications, 1 had a classical migraine first noticed postoperatively and another needed a further spinal anaesthetic on the second postoperative day because the artificial hip dislocated. 183 patients were analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated in a double blind manner (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "Spinal anaesthesia was performed by the department anaesthetists, but did not include the authors." (page 571) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "The patients in study 1 were interviewed by one of the authors on the fourth day after surgery (...)" (page 571) |
| Incomplete outcome data (attrition bias) | Low risk | 8.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 200 patients aged between 20 and 40 years and admitted for either elective or acute orthopaedic, lower abdominal or urogenital surgery were enrolled Number of patients randomized per arm: unclear 2. 7 patients (3.5%) were excluded. It was impossible to perform the spinal procedure with a 25 G needle in 1 patient; 2 needed a supplementary general anaesthetic, 1 another spinal anaesthetic, while 3 had a history of migraine first noticed postoperatively 193 patients were analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated in a double blind manner (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "Spinal anaesthesia was performed by the department anaesthetists, but did not include the authors." (page 571) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "The patients in study 1 were interviewed by one of the authors on the fourth day after surgery (..)" (page 571) |
| Incomplete outcome data (attrition bias) | Low risk | 8.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 73 patients enrolled (women in active labour who requested labour analgesia and accepted a combined spinal‐epidural technique) Patients randomized to:
2. 6 (8.21%) patients lost to follow‐up because no cerebrospinal fluid was obtained with the Sprotte needle Patients analysed:
3. Main characteristics of patients were not provided. Quote: "The two groups were similar with regard to cervical dilation, parity, height, weight, and initial pain score." (page 575) | |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized to have the spinal component (..)" (page 574) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | 8.21% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 91 patients undergoing spinal anaesthesia for operations in the departments of orthopaedics, general surgery and urology from August 2006 to October 2007 were enrolled Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated into (…)" (page S157) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 676 outpatients (ASA physical status I‐II, aged 18 to 60 years) given spinal anaesthesia for elective day‐case surgery were enrolled Exclusion criteria: use of oral opioids or regular use of nonsteroidal anti‐inflammatory drugs, history of allergy to any study medication, patient refusal, contraindication for spinal anaesthesia, abuse of drugs or alcohol, headache preoperatively on the morning of surgery and body mass index not within normal limits (17 to 28) Number of patients randomized per arm: unclear 2. 54 patients (33 in the Quincke group and 21 in the Whitacre group) were excluded from the study for various reasons such as if they received midazolam for sedation (15/10), general anaesthesia was required because of insufficient spinal block (12/6), pethidine was required for postoperative shivering (2/2), or pain medication different from the study protocol had been given to the patient in the ward or at home (2/1) Of the remaining 622 patients, 529 patients returned the questionnaire (85.1%) and were available for the final analysis Total of exclusions: 147 (21.74%) 3. Patients analysed:
4. Main characteristics of patients:
| |
| Interventions |
The choice of whether to use an introducer needle (22 G (0.7 mm) 30 mm long, Yale1 needle, Becton Dickinson Ltd) was left to the individual anaesthesiologist performing the spinal block. | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was computer‐generated and double‐blind except for the anaesthetist performing (…)" (page 475) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients, surgeons, as well as the postoperative ward personnel did not know which spinal needle had been used." (page 475) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The anaesthesiologist who analyzed patient outcome was unaware of the spinal needle type used." (page 475) |
| Incomplete outcome data (attrition bias) | High risk | 21% patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 216 patients ASA I to III, undergoing in‐house and ambulatory anorectal surgery, performed in lithotomy position, were enrolled Exclusion criteria: contraindications against spinal anaesthesia, patients considered to be ASA status IV–I, operation techniques other than in lithotomy position and prior participation in the study. After inclusion of 216 patients, the study was terminated when interim analysis showed unexpected high rates of PDPH in both study groups. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Upon arrival in the operating theatre the patients were randomly allocated 1:1 using sealed envelopes in blocks of 20 to receive a spinal saddle block with either a 25‐G or a 29‐G Quincke type spinal needle" (page 776) |
| Allocation concealment (selection bias) | Low risk | Quote: "Upon arrival in the operating theatre the patients were randomly allocated 1:1 using sealed envelopes in blocks of 20 to receive a spinal saddle block with either a 25‐G or a 29‐G Quincke type spinal needle" (page 776) |
| Blinding of participants (performance bias) | Low risk | Quote: "Study participants were blinded to the type of needle used." (page 776) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A consultant anaesthesiologist who was blinded towards the needles used and who was not involved in the study assessed the incidence of PDPH" (page 776) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 363 patients (male/female, 18 to 80 years; American Society of Anesthesiologists (ASA) physical grade I–III) undergoing in‐house and ambulatory anorectal surgery, performed in lithotomy position, were enrolled and randomized Exclusion criteria: general contraindications against spinal anaesthesia, patients' history of recurrent headaches or a previous PDPH, patients considered to be ASA grade IV–VI, operation techniques other than in lithotomy position and prior participation in the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients (in general):
Quote: "The groups did not differ in their demographic data" (page 99) | |
| Interventions | 1. Group A: 27 G PP needle, 10 minutes pre‐operative time in upright sitting position. 27 G PP needle with introducer (Pencan ® 0.42 × 88 mm– G27 × 3½, B. Braun, Melsungen, Germany) 2. Group B: 27 G Q needle, 10 minutes pre‐operative time in upright sitting position. 27 G Q needle with introducer (Spinocan ® 0.42 × 88 mm–G27 × 3½, B‐Braun, Melsungen, Germany) 1. Group C: 27 G PP needle, 30 minutes pre‐operative time in upright sitting position. 27 G PP needle with introducer (Pencan ® 0.42 × 88 mm– G27 × 3½, B. Braun, Melsungen, Germany) 1. Group D: 27 G Q needle, 30 minutes pre‐operative time in upright sitting position. 27 G Q needle with introducer (Spinocan ® 0.42 × 88 mm–G27 × 3½, B‐Braun, Melsungen, Germany) A vertical bevel direction was used. | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "upon arrival in the operating theatre, the patients were randomised via sealed envelopes in order to assign each patient into one of four study groups" (page 98) |
| Allocation concealment (selection bias) | Low risk | Quote: "upon arrival in the operating theatre, the patients were randomised via sealed envelopes in order to assign each patient into one of four study groups" (page 98) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "A consultant anaesthesiologist who was blinded towards the needles used and who was not involved in the study assessed the incidence of PDPH" (page 99) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 388 ASA I‐III patients, aged 15 to 80 years, who were scheduled for subumbilical surgery, were enrolled Exclusion criteria: obstetric patients Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
Both needles were used with a 20 G introducer to facilitate puncture | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "the patients were randomly assigned" (page 462) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 375 ASA physical status I and II caesarean section and postpartum tubal ligation patients at 4 hospitals participated in the study Exclusion criteria: unclear Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions | 1. 22 G Sprotte needle 2. 24 G Sprotte needle All patients received an infusion of at least 1000 mL of lactated Ringer's solution over 30 minutes prior to the block | |
| Outcomes | Outcomes were not classified as primary or secondary 1. Complication: headache 2. PDPH 3. Severity of PDPH | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to receive" (page 43) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were visited at least once by the anesthesiologist during the postoperative period, and nurses on the obstetrics floor were instructed to notify the anesthesiologist of any complication, including headache. In addition, patients were contacted by telephone 1 week or more after discharge by an investigator who was blinded to the type of needle used." (page 43) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 800 young patients (16 to 40 years old) with ASA risk I/II scheduled for endoscopic urological procedures under spinal anaesthesia between January 2008 and December 2009 were enrolled in this study Exclusion criteria: history of headache, use of oral opioids or non‐steroidal anti‐inflammatory drugs, or contraindications to spinal anaesthesia Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided by computer‐generated random numbers into four groups of 200 patients each." (page 25) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Postoperatively, all patients were visited successively for three days by a staff member, who was unaware of the type of needle used, to inquire about headache." (page 25) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 480 American Society of Anesthesiologists physical status classification (ASA) I‐II women, aged 18 to 45 years, undergoing elective caesarean section, were enrolled Exclusion criteria: patient refusal, contraindication to spinal anaesthesia for infectious haemodynamic, haemostatic or neurological reasons, emergency caesarean section, severe pre‐eclampsia or failure of the spinal anaesthesia. Patients with more than one attempt were excluded from the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary.
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were selected randomly by balloting." (page 10) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patient, surgeon and the assessor in the ward did not know which spinal needle was used." (page 10) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Postoperatively, all patients were assessed daily for 4‐days by an investigator, blinded to the type and size of the needle used.." (page 11) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 96 women (ASA I and II) scheduled for elective post‐partum tubal ligation under spinal anaesthesia were enrolled Exclusion criteria: abnormal lumbar spaces due to deformities of the spine or obesity Exclusion criteria: patient refusal, contraindication to spinal anaesthesia for infectious haemodynamic, haemostatic or neurological reasons, emergency caesarean section, severe pre‐eclampsia or failure of the spinal anaesthesia. Patients with more than one attempt were excluded from the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly, using computer generated numbers." (page 707) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients were evaluated daily throughout their hospital course by an observer blinded to group assignment and then interviewed by telephone one week after discharge from hospital for the presence of headache, backache, or any other complication". (page 707) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 150 women of ASA grade I undergoing spinal anaesthesia for elective caesarean section were enrolled Patients randomized to:
2. 6 patients (4%) were excluded from further analysis because of a failure to identify the subarachnoid space with the trial needle 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each woman was allocated by random number selection to one of." (page 589) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "At 24 h also, the second "blind " anaesthetist visited the patient. His duty was to check that the questionnaire had been completed and to record the patient's temperature. If a headache had been reported, he completed a second questionnaire ascertaining the onset and distribution of the headache, the effect of posture and if there was any visual or auditory disturbance." (page 590) |
| Incomplete outcome data (attrition bias) | Low risk | 4% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 212 women of ASA grade I undergoing spinal anaesthesia for elective caesarean section were enrolled Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated to receive a subarachnoid block using either a 25G or a 27G Whitacre (...)" (page 859) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were interviewed daily on the 1st to 5th postoperative days, by an anaesthetist unaware of the needle size used (..)". (page 860) |
| Incomplete outcome data (attrition bias) | Low risk | 8 patients (3.7%) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 100 patients enrolled (either sex, age group 14 to 75, ASA I and II, admitted for elective or emergency lower segment caesarian section and other surgical procedures) Losses at follow‐up and reasons for exclusions not reported 2. Patients randomized to:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated into four groups" (page 711) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All the patients were blinded to the needle utilized. The anaesthetist conducting the procedure was not blinded as the two needles have different appearance making blinding impossible". (page 711) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 100 patients enrolled (either sex, age group 14 to 75, ASA I and II, admitted for elective or emergency lower segment caesarian section and other surgical procedures) Losses at follow‐up and reasons for exclusions not reported 2. Patients randomized to:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated into four groups" (page 711) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All the patients were blinded to the needle utilized. The anaesthetist conducting the procedure was not blinded as the two needles have different appearance making blinding impossible". (page 711) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 700 patients enrolled (ASA I/II/III patients were scheduled for lower abdominal or extremity surgery (orthopaedic, trauma, urology, visceral, gynaecology) and underwent the same protocol) Patients randomized to:
23 randomized patients (15 group B, 18 group S) were excluded due to:
2. 0 patients lost to follow‐up 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a randomization protocol that was created by a computerized program for each study site". (page 513) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "During postoperative Day 2 and 4, all patients were visited by an anesthesiologist who was blinded to the type of spinal needle" (page 514) |
| Incomplete outcome data (attrition bias) | Low risk | 23 patients (3.2%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 230 patients enrolled (who had a neurologic indication for an LP (e.g. MS, neuroborreliosis or other CNS infections), between 18 and 59 years, no recent headache (at least up to 1 week before LP, years; 2) no recent headache, i.e. at least up to 1 week), no evidence of increased intracranial pressure, no LP in the last 4 weeks, ability to be mobilized and no previous headache or other pain medication. Patients randomized to:
2. No exclusions or loses to follow‐up were reported 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to one or the other group according to Efron." (page 2311) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 60 ASA I and II primi and multipara parturient undergoing elective caesarean section aged 19 to 40 years. Exclusion criteria: parturient refusal, weight more than 75 kg, eclampsia/pre‐eclampsia, bleeding disorders Patients randomized to:
2. 6 (8.21%) patients lost to follow‐up because no cerebrospinal fluid was obtained with the Sprotte needle 3. Main characteristics:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "60 ASA I and II primi and multipara parturient undergoing elective caesarean section aged 19‐40 years were randomly divided (...)" (page 264) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 300 co‐operative ASA I and II who had spinal anaesthesia for minor orthopaedic or urologic operations, and who were not expected to need a blood transfusion Patients randomized to:
2. 256 patients (86.5%) returned the second questionnaire and were included in the study
1. Main characteristics:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomized into three groups of equal size" (page 284) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 13.5% of patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 300 patients undergoing surgery under spinal anaesthesia Patients randomized to:
2. Patients analysed: 2% failure rate, thus 6 patients were excluded from analysis. 94% were interviewed postoperatively. 3. Main characteristics:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Three hundred patients undergoing surgery under spinal anaesthesia were randomly allocated (...)". (page 723) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "All the spinal anaesthetics were performed by the authors"... "The patients were contacted by one of the authors one week after the surgery." (page 723) |
| Incomplete outcome data (attrition bias) | Low risk | 18 patients (6%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 116 patients enrolled (patients attending the investigation ward on a regional neurology unit for elective diagnostic lumbar puncture) Not randomized (n = 15) Consent refused (n = 8) Incomplete training for senior house officers (n = 7) 99 patients randomized to:
2. 2 patients (2%) did not receive the allocated intervention in each arm and they were excluded. 97 patients randomized to: standard needle (48, 46.56%); atraumatic needle (49, 47.53%) 6. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done by a computer generated code stored in opaque envelopes that were serially numbered and sealed" (page 987) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done by a computer generated code stored in opaque envelopes that were serially numbered and sealed" (page 987) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "One week after lumbar puncture, the patients were telephoned by a single observer who was blinded to needle allocation" (page 987) |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients were lost to follow‐up (2%) |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Unclear risk | The role of funder is unclear |
| Methods |
| |
| Participants | 1. 100 patients enrolled (healthy volunteers rated normal on physical and neurological examinations)
2. No randomized patients were excluded from the study
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Members of each successive pair of incoming volunteers were randomly" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The subjects were blinded with respect to size of needle used. They were all interviewed by the same neurologist (W.W.T.), who was also blinded as to needle size" (page 2) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "They were all interviewed by the same neurologist (W.W.T.), who was also blinded as to needle size" (page 2) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 96 patients less than 45 years of age undergoing elective or emergency surgery were enrolled Exclusion criteria: obstetric patients Number of patients randomized to each group: unclear 2. 3 patients were excluded from further analysis due to incomplete interviews Patients analysed:
3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized to receive spinal anesthesia with either the 24 gauge Sprotte needle or the 27 G Quincke needle." (page 608) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients were interviewed in person or by telephone (if discharged from the hospital) by an anesthetist not involved with the case or by a research nurse. Both were blinded to the spinal needle used" (page 608) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients were interviewed in person or by telephone (if discharged from the hospital) by an anesthetist not involved with the case or by a research nurse. Both were blinded to the spinal needle used" (page 608) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 284 patients enrolled (patients referred for myelography)
2. 6 patients were excluded following failed lumbar puncture with 26 G needles 3. Main characteristics of patients:
| |
| Interventions |
All lumbar punctures were performed with the patient in the left lateral decubitus position Patients were routinely ambulatory following the examination | |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly assigned to the 22 G or the 26 G needle group." (page 338) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were given a questionnaire to complete on discharge from hospital 24h after the myelogram. Late complications were obtained by telephone" (page 338) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
| Methods |
| |
| Participants | 1. 40 patients ASA I‐II, aged from 18 to 50 years, undergoing subumbilical surgery were enrolled Exclusion criteria: history of headache, refusal of method, hypertension Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
| |
| Interventions |
| |
| Outcomes | Outcomes were not classified as primary or secondary
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "(we) developed a clinical trial with 40 patients" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Follow‐up to detect patients who developed PDPH was realized by an anesthesiologist different from the one who performed the procedure" (page 67) |
| Incomplete outcome data (attrition bias) | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Acronyms and abbreviations used in this table
ASA: American Society of Anesthesiologists; ASN: Atraucan spinal needle; BMI: body mass index; CI: confidence interval; c‐section: caesarean section; CSF: cerebrospinal fluid; G: gauge; IQR: interquartile range; L2‐3 to L3‐4: lumbar vertebrae 2‐3 to 3‐4; LP: lumbar puncture; NRS: numerical rating scale; NYHA: New York Heart Association; PDPH: post‐dural puncture headache; RCT: randomized controlled trial; SD: standard deviation; SEM: standard error of the mean; VAS: visual analogue scale; vs: versus; WSN: Whitacre spinal needle
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| In this study, the authors did not evaluate the use of a specific type of needle or its gauge for the evaluation of PDPH. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study is not a randomized controlled trial. | |
| This study was excluded because an intervention to treat PDPH is included. | |
| This study was excluded because it was performed without any random allocation process. | |
| In this study, the units of randomization were anaesthesiologists in training (learning curve) and not a specific type of needle. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was a letter to the editor. | |
| This study was excluded because it was a comment. | |
| This study was excluded because the unit of randomization was the procedure and not the patient. | |
| In this study, the authors used a pulsatile cerebrospinal fluid model to test a spinal needle. | |
| This study was excluded because it was a letter to the editor. | |
| This study was excluded because an intervention to treat PDPH is included. | |
| This study was excluded because the intervention assessed in this review was not addressed. | |
| This study was excluded because it did not use an adequate method of randomization (odd and even hospital record numbers). | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it evaluated the duration of time sitting and spinal needle type on the maximal spread of local anaesthetics and not the presence of PDPH. | |
| This study was excluded because the unit of randomization was the procedure and not the patient. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study is not a randomized controlled trial. | |
| This study was excluded because it was a narrative review. | |
| This study was excluded because it evaluated different positions (oxford position, lateral and sitting positions) during spinal‐epidural anaesthesia and not the use of different types of needles. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study is not a randomized controlled trial. | |
| This study was excluded because it evaluated the effects of reinsertion of the stylet after a spinal anaesthesia procedure on PDPH and not the type or size of the needle. | |
| This study was excluded because it evaluated the effects of reinsertion of the stylet after a spinal anaesthesia procedure on PDPH and not the type or size of the needle. | |
| This study was excluded because it was a narrative review. | |
| This study was excluded because it evaluated different types of anaesthesia techniques (sequential combined spinal epidural block versus spinal block) and not different types of needles. | |
| This study was excluded because the authors randomized the days on which each different needle would be used and not the patients. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was performed without any random allocation process. | |
| This study was excluded because it was not focused on the prevention of PDPH. |
PDPH: post‐dural puncture headache
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single blinded, interventional, experimental study |
| Participants | A total of 100 females, aged 18 to 35 years, ASA physical status I and II, with singleton pregnancy undergoing elective or emergency caesarean section under spinal anaesthesia |
| Interventions | Participants were randomly allocated to receive spinal anaesthesia either by using 25 G Quincke or 25 G Whitacre needles. Patients were followed for 3 days postoperatively |
| Outcomes | The primary outcome was the assessment of headache and the relation to posture. Secondary outcomes were onset of headache, its duration, severity and response to the treatment |
| Notes | — |
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 400 patients who received spinal anaesthesia for operation of the lower extremities |
| Interventions | Patients were randomly assigned to 2 groups (25 G Whitacre and a 25 G Quincke needles) and were interviewed postoperatively on days 1, 3, 5 and 7 to assess PDPH |
| Outcomes | The primary outcome was PDPH. Secondary outcomes were: duration of PDPH, non‐postural headache and the duration of non‐postural headache. |
| Notes | — |
| Methods | Prospective, randomized and single‐blinded clinical trial |
| Participants | Patients older than 14 years were scheduled for a diagnostic or therapeutic lumbar puncture |
| Interventions | 2 kinds of spinal needle: atraumatic or S‐type or traumatic or Q‐type |
| Outcomes | Development of PDPH according to the International Headache Association criteria |
| Notes | — |
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 158 patients, ASA I and II, ranging in age from 20 to 40 years undergoing lower limb orthopaedic surgery |
| Interventions | Patients were randomly assigned to 2 groups (26 G Atraucan and 27 G Whitacre needles) for the realization of spinal anaesthesia |
| Outcomes | The primary outcome was: frequency and degree of PDPH. Secondary outcomes were: performance of the subarachnoid technique and intraoperative side effects. |
| Notes | — |
| Methods | Prospective, randomized, experimental study in healthy participants |
| Participants | 330 parturients scheduled for caesarean section |
| Interventions | 25, 26 or 27 G pencil point, Whitacre type (with introducer) needles |
| Outcomes | Puncture failure rates, post‐dural puncture headache |
| Notes | — |
| Methods | Prospective, single‐blind, randomized study |
| Participants | A total of 100 women undergoing elective and emergency caesarean delivery under spinal anaesthesia were recruited |
| Interventions | Patients were randomly allocated to receive spinal anaesthesia either by using 2 spinal needles (Becton Dickinson Whitacre sizes 25 G and 26 G needles) |
| Outcomes | Incidence of PDPH |
| Notes | — |
| Methods | Randomized, prospective study |
| Participants | A total of 113 patients referred for lumbar, thoracic, cervical or total column myelography |
| Interventions | Participants were numbered sequentially; in even‐numbered patients a 22 G needle was used and for odd‐numbered patients, a 25 G needle |
| Outcomes | The primary outcome was the incidence of headache following myelography. Secondary outcomes were: the influence of needle type, sex, myelogram type and operators. |
| Notes | — |
| Methods | Prospective, randomized trial |
| Participants | 149 patients undergoing lumbar transforaminal epidural steroid injection for radicular leg pain |
| Interventions | Whitacre and Quincke type needles |
| Outcomes | After final confirmation of intravascular injection with digital subtraction angiography, total procedure time and amount of radiation exposure during the procedure were measured |
| Notes | — |
| Methods | Only title is available |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | — |
| Methods | Prospective, randomized study |
| Participants | A total of 197 patients aged below 40 years were included in this study |
| Interventions | Participants were randomized to receive spinal analgesia using one of the following needles: Sprotte G24, Spinocan G27 or Atraucan G26 |
| Outcomes | The primary outcome of this study was the incidence of postoperative complications including post‐dural puncture headache (PDPH) |
| Notes | — |
| Methods | Prospective, randomized study |
| Participants | A total of 90 adult patients who underwent elective surgical operations under spinal anaesthesia were evaluated |
| Interventions | Patients were randomly allocated to 3 groups of 30 each to receive spinal anaesthesia using 20‐ G, 22 G or 24‐gague spinal needles |
| Outcomes | The primary outcome was: incidence of headache and frequency of hearing loss |
| Notes | — |
| Methods | Prospective, randomized study |
| Participants | A total of 106 patients, aged below 40 years, scheduled for surgery in the lower part of the body were chosen for this study |
| Interventions | Patients were allocated randomly to have spinal analgesia with either a Sprotte 24 G or an Atraucan 26 G spinal needle |
| Outcomes | The primary outcome was: incidence of PDPH. Secondary outcomes were: ease of needle insertion and number of puncture attempts |
| Notes | — |
| Methods | Prospective, randomized study |
| Participants | A total of 56 patients were recruited in this study |
| Interventions | Patients underwent spinal anaesthesia for extra‐corporeal shockwave lithotripsy using either a Sprotte 24 G (n = 28) or Vygon 29 G or Quincke type needle (n = 28) |
| Outcomes | Frequency of PDPH |
| Notes | — |
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 60 nulliparous women |
| Interventions | Participants were randomized to receive an epidural infusion of either 0.125% plain bupivacaine or 0.0625% bupivacaine with 2.5µg/ml fentanyl |
| Outcomes | The primary outcome was pain and motor block. Secondary outcomes were maternal side effects and cardio‐tocograph abnormalities |
| Notes | — |
| Methods | Prospective, controlled study |
| Participants | 30 ASA I or II patients |
| Interventions | Lumbar puncture was carried out with 26 G in group I and 18 G in group II |
| Outcomes | Complications during spinal anaesthesia included: vomiting, nausea, allergia and low blood pressure. Postspinal headache. |
| Notes | — |
| Methods | Prospective, randomized study |
| Participants | A total of 92 pregnant patients undergoing elective caesarean section |
| Interventions | Patients undergoing lumbar puncture were randomized to 2 groups (Group I: 22 G Quincke disposable needle and group II: 22 G Quincke reusable needle) |
| Outcomes | The primary outcome was the assessment of PDPH. There were no secondary outcomes. |
| Notes | — |
| Methods | Prospective follow‐up study |
| Participants | A total of 400 patients were included in this study |
| Interventions | Patients were randomly selected to have a spinal anaesthesia using either a 27 G Quincke‐type needle or a 27 G pencil point needle |
| Outcomes | The primary outcome was the severity of needle damage according to the type and number of attempts |
| Notes | — |
| Methods | Prospective study |
| Participants | 200 patients between 18 and 45 years of age belonging to ASA grade I and II of either sex |
| Interventions | Spinal anaesthesia with 25 G or 29 G Quincke type spinal needle |
| Outcomes | Incidence, type, severity, duration, day of onset and site of post‐dural puncture headache were recorded for the first 5 postoperative days |
| Notes | — |
ASA: American Society of Anesthesiologists; G: gauge; PDPH: post‐dural puncture headache
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'Incidence and severity of post dural puncture headache after spinal anaesthesia for caesarean section; a comparison between 25G Quincke cutting and 25G Pencan pencil point spinal needles' |
| Methods | Study design: double‐blind randomized controlled trial |
| Participants | Patients and methods: 200 adult female patients aged 20 to 40 years, ASA I and II, presenting for elective or emergency caesarean deliveries under spinal anaesthesia were randomly divided into 2 groups of 100 patients each |
| Interventions | In group P, spinal anaesthesia was performed by Pencan needle while in group Q spinal anaesthesia was performed by Quincke cutting needle using a standardized technique |
| Outcomes | Level of block (sympathetic, sensory, motor) was assessed intraoperatively. Patients were followed for 3 consecutive days postoperatively for headache, its onset, severity and associated symptoms |
| Starting date | August 2009 to August 2010 |
| Contact information | Ahmed J |
| Notes | — |
| Trial name or title | 'The association between needle types and headache' |
| Methods | Not known |
| Participants | 664 ASA I‐II group elective caesarean patients who had no contraindications for spinal anaesthesia were included to this study. The thickness of the needle and the shape of tip of the spinal needle was recorded after anaesthesia. The education period of the anaesthesia performer, number of attempts, the space used for anaesthesia (L3‐4, LL4‐5) and movement of patient during anaesthesia were recorded |
| Interventions | Patients were randomly divided into 2 groups: group I (Atraucan 26G n = 323) and group II (Quincke 26G n = 342) |
| Outcomes | Patients were questioned about headache for 72 hours. Chi2 and comparison of proportions were used for statistical evaluations |
| Starting date | Not known |
| Contact information | AkdemirMS |
| Notes | — |
| Trial name or title | 'Post‐dural puncture headache is markedly reduced when 25 Sprotte needles are used' |
| Methods | To evaluate the frequency of post‐dural puncture headache (PDPH) using 4 types of needles with a prospective, rater‐blind study |
| Participants | 365 lumbar punctures were performed using 4 different types of needles as follows: 39 with 20 G Quincke traumatic needle, 62 with 22G Sprotte needle, 133 with 25G Whitacre needle, 131 with 25G Sprotte needle |
| Interventions | 25 G Whitacre needle, 25 G Sprotte needle |
| Outcomes | The patient was blinded to the needle used; a neurologist, blinded to the type of the needle, interviewed the patient for PDPH. Safety and time consumption were evaluated. |
| Starting date | Not known |
| Contact information | Bertolotto A |
| Notes | — |
| Trial name or title | '25G Sprotte needle strongly reduces the risk of post‐lumbar puncture headache in clinical practice' |
| Methods | To evaluate the frequency of post‐lumbar puncture headache (PLPH) using 5 types of needles |
| Participants | 363 lumbar punctures were performed using 5 different types of needles as follows: 39 with 20 G Quincke traumatic needle, 11 with 22 G Quincke needle, 53 with 22 G Whitacre needle, 134 with 25 G Whitacre needle, 126 with 25 G Sprotte needle |
| Interventions | 25 G Whitacre needle, 25 G Sprotte needle |
| Outcomes | The patient was blinded to the needle used; a neurologist, blinded to the type of the needle, interviewed the patient for PLPH. Safety and time consumption were evaluated. |
| Starting date | Unclear |
| Contact information | Bertolotto A |
| Notes | — |
| Trial name or title | 'Comparison of 22/27g Microtip vs 25g Pencan spinal needle; insertion characteristic and complications' |
| Methods | Single‐blind, randomized study |
| Participants | A total of 101 parturients admitted for elective lower segment caesarian sections under spinal anaesthesia were admitted in the study |
| Interventions | Patients were randomly assigned to have Pencan (n = 50) or Microtip (n = 51) needle |
| Outcomes | The outcomes of this study were: ease of needle insertion, first attempt success rate and CSF flow rate as well as incidence of paraesthesia, post‐dural puncture headache (PDPH) and backache (PDPB), transient neurological symptoms (TNS) |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
| Trial name or title | 'Comparison of Sprotte and Quincke needles with respect to post dural puncture headache' |
| Methods | Randomization: randomized |
| Participants | Inclusion criteria: age 16 to 65, patients with major surgery of the lower limb or lower abdominal segment under spinal anaesthesia Age minimum: 16 Age maximum: 65 Gender: both male and female |
| Interventions | Intervention 1: Sprotte spinal needle. Intervention 2: Quincke spinal needle |
| Outcomes | Headache Hypotension Nausea & vomiting Nuchal rigidity Time point (all outcomes): every 4 hours until 24 hours after surgery. Method of measurement (all outcomes): checklist and physical exam. |
| Starting date | 21 April 2010 |
| Contact information | Afsane Norouzi |
| Notes | — |
| Trial name or title | 'CSE for caesarean section: Gertie Marx versus Pencan spinal needles' |
| Methods | Compared Gertie Marx spinal needle with PENCAN needle to determine which one is preferred by obstetric patients |
| Participants | Following IRB approval and informed consent, 124 ASA I‐II parturients, who requested neuraxial block for C/S, were included. The epidural space was located with ESPOCAN 18 gauge epidural 'Braun' needle (B. Braun Medical Inc.) at L3‐4 or L4‐5 interspace with loss of resistance to air technique using a midline approach in the lateral or sitting flexed position |
| Interventions | Patients were then randomized to 1 of 2 groups. Group I: 59 patients had a 25 G PENCAN spinal needle placed in the subarachnoid space. Group II: 65 had a 26 G Gertie Marx spinal needle (IMD Inc. USA) placed in the subarachnoid space. |
| Outcomes | An investigator recorded patients' height, weight, parity, position, the distance of the epidural space from the skin, technical problems, paraesthesia and pain upon insertion of the spinal needle, time to incision, difficulty with catheter insertion, post‐dural puncture headache, transient radicular irritability, duration of procedure and overall satisfaction with the technique use |
| Starting date | Not known |
| Contact information | Lorthe J |
| Notes | — |
| Trial name or title | 'Effect of small versus large epidural needles on postdural puncture headache study' |
| Methods | Allocation: randomized |
| Participants | Inclusion criteria: Age minimum: 18 years Age maximum: N/A Gender: female |
| Interventions | Device: => 18 G Tuohy‐type needle Device: 19 G Tuohy‐type epidural needle, 23 G catheter |
| Outcomes | Incidence of post‐dural puncture headache (time frame: within the first 14 days of epidural placement) Anaesthesiologist satisfaction with the 19 G Tuohy epidural needle and 23 G catheter compared with traditional Tuohy‐type epidural needles and traditional catheters (time frame: during labour and delivery) Degree of dysfunction and disability related to PDPH symptoms (time frame: within first 14 days post‐epidural placement and, if necessary, up to 1 year post‐epidural placement) |
| Starting date | June 2007 |
| Contact information | Pamela J Angle |
| Notes | — |
| Trial name or title | 'Comparison of two spinal needles regarding postdural puncture headache' |
| Methods | Time perspective: prospective |
| Participants | Inclusion Criteria: Age minimum: 18 Years |
| Interventions | Two kind of spinal anaesthesia needles will be used:
|
| Outcomes | Post‐dural puncture headache in patients receiving spinal anaesthesia for caesarean section (time frame: 1 week) Backache in patients receiving spinal anaesthesia for caesarean section (time frame: 1 week) |
| Starting date | June 2013 |
| Contact information | Ruslan Abdullayev |
| Notes | — |
| Trial name or title | 'Post‐dural puncture headache ‐ needles and biomarkers in CSF' |
| Methods | Allocation: randomized |
| Participants | Inclusion criteria: Age minimum: 18 years Age maximum: 60 years Gender: both |
| Interventions | Device: atraumatic needle Device: traumatic needle |
| Outcomes | Post‐dural puncture headache (PDPH) (time frame: at day 7 post LP) Levels of inflammatory mediators in CSF (time frame: during lumbar puncture) Levels of metabolites in CSF (time frame: during lumbar puncture) Levels of neuropeptides in CSF (time frame: during lumbar puncture) |
| Starting date | February 2012 |
| Contact information | Francis Odeh, MD, PhD |
| Notes | — |
| Trial name or title | 'Combined spinal epidural (CSE) for cesarean section: Gertie Marx versus Pencan spinal needles' |
| Methods | Prospective, randomized study |
| Participants | A total of 124 ASA I‐II parturients who requested neuraxial block for caesarean section were included in this study |
| Interventions | Patients were randomized into 2 groups (Group I: n = 59 has a 25 G PENCAN spinal needle placed in the subarachnoid space and Group II: n = 65 had a 26 G Gertie Marx spinal needle in the subarachnoid space |
| Outcomes | Need to rotate or reinsert the epidural needle, the efficacy of the block, side effects from the block, difficulty with catheter insertion and the sensory level overall satisfaction |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
| Trial name or title | 'Post dural puncture headache after spinal anaesthesia for caesarean section: a comparison of 25G Quincke, 27G Quincke and 27G Whitacre spinal needles' |
| Methods | Comparative, randomized, double‐blind, interventional study |
| Participants | A total of 480 ASA I‐II full‐term pregnant women, 18 to 45 years of age, scheduled for elective caesarean section, under spinal anaesthesia |
| Interventions | Participants were randomized into 3 groups: Group I (25 G Quincke spinal needle: n = 168), Group II (27 G Quincke spinal needle: n = 160) and Group III (27 G Whitacre spinal needle: n = 152) |
| Outcomes | The primary outcome was the frequency of PDPH. Secondary outcomes were the severity and onset of PDPH. |
| Starting date | From October 2005 to December 2006 |
| Contact information | Not known |
| Notes | — |
Acronyms and abbreviations used in this table
ASA: American Society of Anesthesiologists; ASN: Atraucan spinal needle; BMI: body mass index; CI: confidence interval; c‐section: caesarean section; CSF: cerebrospinal fluid; G: gauge; IQR: interquartile range; L2‐3 to L3‐4: lumbar vertebrae 2‐3 to 3‐4; LP: lumbar puncture; NRS: numerical rating scale; NYHA: New York Heart Association; PDPH: post‐dural puncture headache; RCT: randomized controlled trial; SD: standard deviation; SEM: standard error of the mean; VAS: visual analogue scale; WSN: Whitacre spinal needle
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH by indication Show forest plot | 36 | 9378 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.72, 2.67] |
| Analysis 1.1  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 1 PDPH by indication. | ||||
| 1.1 Anaesthesia only | 30 | 8401 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.60, 3.04] |
| 1.2 Myelography only | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.34, 3.00] |
| 1.3 Diagnostic lumbar puncture only | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.38, 3.58] |
| 2 PDPH by gauge Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 2 PDPH by gauge. | ||||
| 2.1 22 gauge | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.56, 2.97] |
| 2.2 25 gauge | 5 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.56, 3.95] |
| 2.3 27 gauge | 11 | 4076 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.81, 4.53] |
| 3 PDPH by gender Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 3 PDPH by gender. | ||||
| 3.1 Only women | 9 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.62, 4.17] |
| 4 PDPH/anaesthesia: type of surgery Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 4 PDPH/anaesthesia: type of surgery. | ||||
| 4.1 Caesarean section | 8 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.60, 6.10] |
| 4.2 Orthopaedic procedures | 3 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.58, 3.19] |
| 4.3 Other surgeries | 19 | 6083 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.50, 3.51] |
| 5 PDPH by position Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 5 PDPH by position. | ||||
| 5.1 Lateral position | 9 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.39, 9.24] |
| 5.2 Sitting position | 11 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.52, 2.94] |
| 6 PDPH by age Show forest plot | 36 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 6 PDPH by age. | ||||
| 6.1 No distinctions by age | 34 | 9063 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.73, 2.73] |
| 6.2 Only < 18 years | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.56, 5.12] |
| 7 AE: paraesthesia Show forest plot | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.96] |
| Analysis 1.7  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 7 AE: paraesthesia. | ||||
| 8 AE: backache Show forest plot | 12 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| Analysis 1.8  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 8 AE: backache. | ||||
| 9 Severe PDPH by indication Show forest plot | 24 | 6420 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.20, 2.94] |
| Analysis 1.9  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 9 Severe PDPH by indication. | ||||
| 9.1 Anesthesia | 19 | 5542 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.88, 3.53] |
| 9.2 Myelography | 4 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.68, 4.28] |
| 9.3 Diagnostic lumbar puncture | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.18, 7.63] |
| 10 Any headache by indication Show forest plot | 18 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.17, 1.57] |
| Analysis 1.10  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 10 Any headache by indication. | ||||
| 10.1 Anaesthesia | 16 | 3656 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 10.2 Myelography | 2 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.21] |
| 11 PDPH sensitivity analysis Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.26, 6.15] |
| Analysis 1.11  Comparison 1 Traumatic needle versus atraumatic needle, Outcome 11 PDPH sensitivity analysis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge. | ||||
| 1.1 23 G vs 25 G | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 1.2 25 G vs 27 G | 4 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.39] |
| 1.3 25 G vs 29 G | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.46, 9.78] |
| 1.4 26 G vs 27 G | 1 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.55, 16.43] |
| 1.5 21 G vs 25 G | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 2 PDPH by type of surgery Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 2 PDPH by type of surgery. | ||||
| 2.1 Caesarean section | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.64, 2.57] |
| 2.2 Orthopaedic surgeries | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.58] |
| 2.3 Other surgeries | 6 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.23, 7.03] |
| 3 PDPH by age Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 3 PDPH by age. | ||||
| 3.1 No distinctions about age | 8 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.11, 3.95] |
| 3.2 Only children | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 3.3 Only > 60 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 4 PDPH by position Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 4 PDPH by position. | ||||
| 4.1 Lateral position | 5 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.98, 3.16] |
| 4.2 Sitting position | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.56] |
| 5 AE: backache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 5 AE: backache. | ||||
| 6 Severe PDPH by gauge Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 6 Severe PDPH by gauge. | ||||
| 6.1 23 G vs 25 G | 1 | 53 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.2 25 G vs 27 G | 3 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 6.3 25 G vs 29 G | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.4 21 G vs 25 G | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7 Any headache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 7 Any headache. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge. | ||||
| 1.1 22 G vs 24 G | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.81] |
| 1.2 22 G vs 25 G | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.50] |
| 1.3 24 G vs 25 G | 2 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [1.00, 31.67] |
| 1.4 25 G vs 26 G | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.90] |
| 1.5 25 G vs 27 G | 2 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.59, 23.64] |
| 1.6 26 G vs 27 G | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.30, 10.73] |
| 1.7 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.58, 4.37] |
| 2 PDPH by type of surgery Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 2 PDPH by type of surgery. | ||||
| 2.1 Caesarean section | 6 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.64, 5.79] |
| 2.2 Orthopaedic procedures | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.30, 5.07] |
| 2.3 Other surgeries | 5 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.83] |
| 3 PDPH by gender Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 3 PDPH by gender. | ||||
| 3.1 Only women | 8 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.51, 2.20] |
| 4 PDPH by position Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 4 PDPH by position. | ||||
| 4.1 Sitting position | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.06] |
| 4.2 Lateral position | 5 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.65, 5.41] |
| 5 AE: paraesthesia Show forest plot | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.31, 15.30] |
| Analysis 3.5  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 5 AE: paraesthesia. | ||||
| 6 AE: backache Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 6 AE: backache. | ||||
| 7 Severe PDPH by gauge Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 7 Severe PDPH by gauge. | ||||
| 7.1 22 G vs 24 G | 1 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7.2 22 G vs 25 G | 1 | 234 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 24 G vs 25 G | 1 | 304 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.4 25 G vs 26 G | 2 | 311 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.5 25 G vs 27 G | 1 | 212 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.6 26 G vs 27 G | 1 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.7 27 G vs 29 G | 1 | 389 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Any headache by gauge Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 8 Any headache by gauge. | ||||
| 8.1 22 G vs 25 G | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.85, 5.51] |
| 8.2 24 G vs 25 G | 2 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.49, 2.77] |
| 8.3 25 G vs 26 G | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.99] |
| 8.4 25 G vs 27 G | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.65, 5.39] |
| 8.5 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |
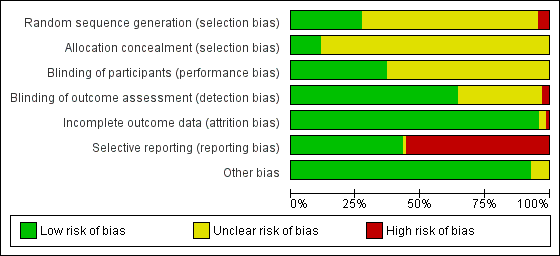
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
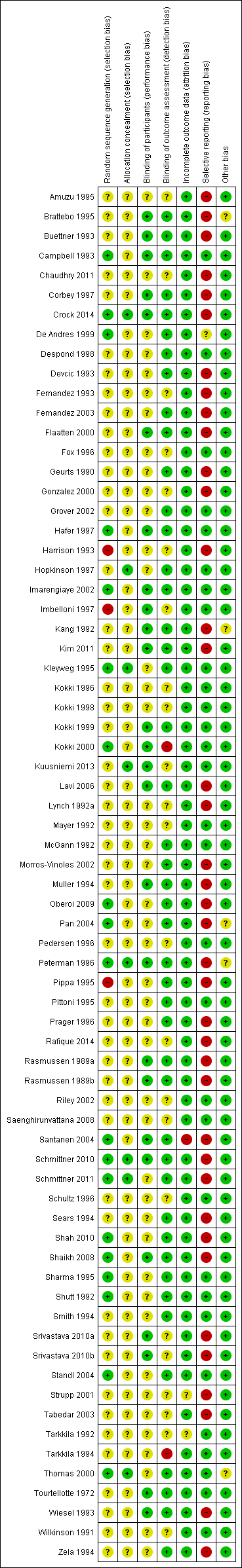
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
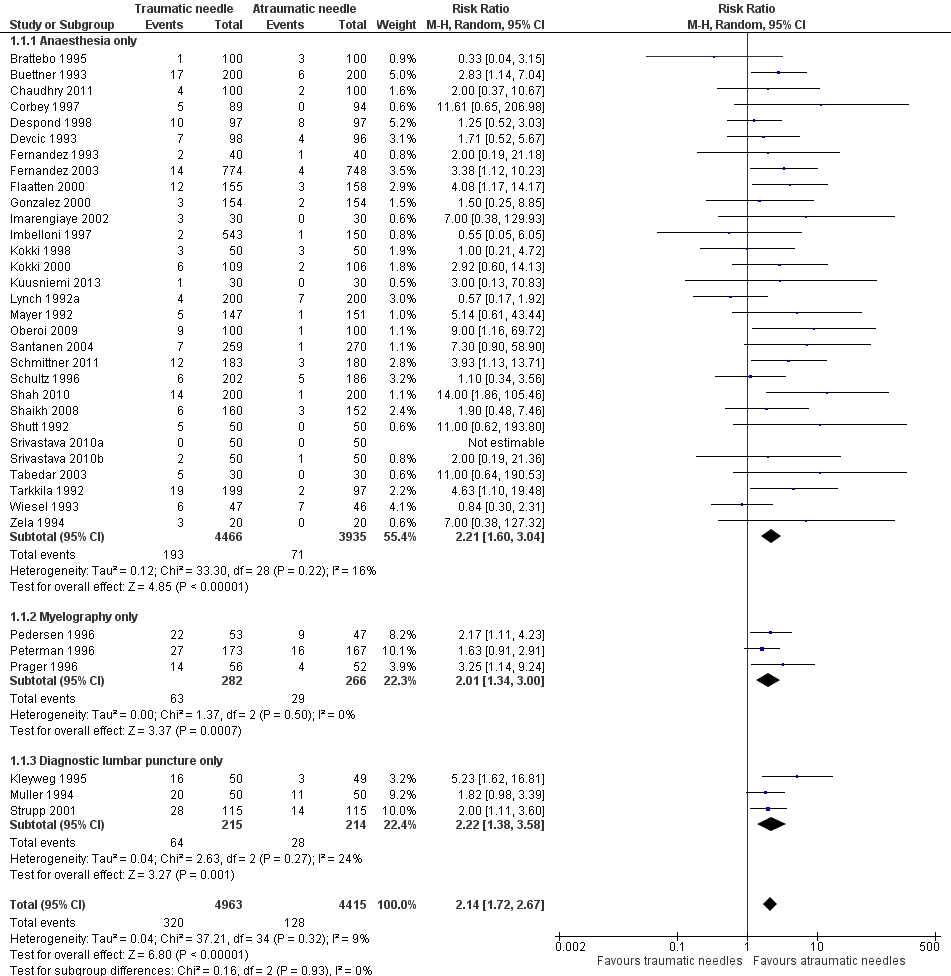
Forest plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
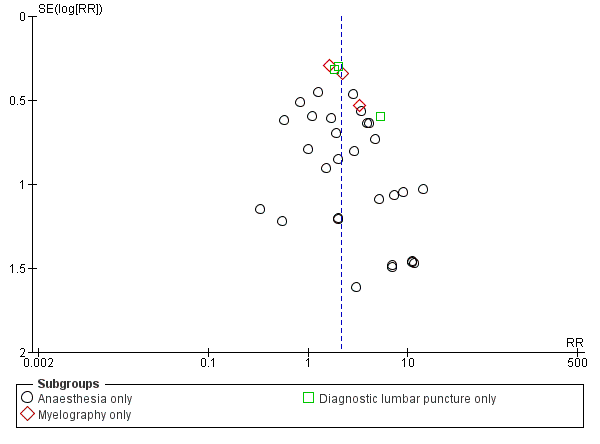
Funnel plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
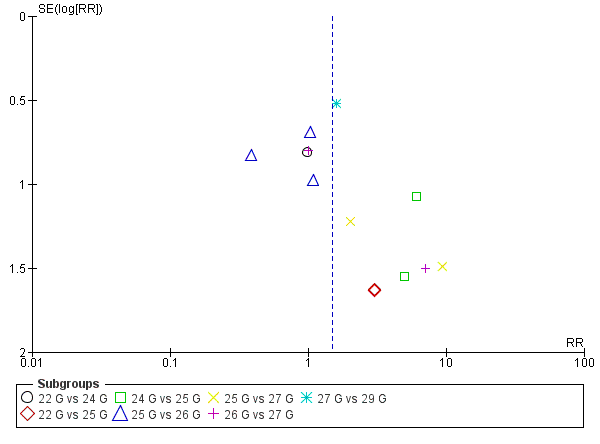
Funnel plot of comparison: 3 Atraumatic needles: different gauges, outcome: 3.1 PDPH major gauge versus minor gauge by number.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 1 PDPH by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 2 PDPH by gauge.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 3 PDPH by gender.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 4 PDPH/anaesthesia: type of surgery.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 5 PDPH by position.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 6 PDPH by age.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 7 AE: paraesthesia.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 8 AE: backache.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 9 Severe PDPH by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 10 Any headache by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 11 PDPH sensitivity analysis.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 3 PDPH by age.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 4 PDPH by position.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 5 AE: backache.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 6 Severe PDPH by gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 7 Any headache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 3 PDPH by gender.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 4 PDPH by position.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 5 AE: paraesthesia.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 6 AE: backache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 7 Severe PDPH by gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 8 Any headache by gauge.
| Traumatic needles compared to atraumatic needles for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needles | Traumatic needles | |||||
| Onset of PDPH | 30 per 1000 | 64 per 1000 | RR 2.14 | 9378 | ⊕⊕⊕⊝ | — |
| Adverse events: paraesthesia | 52 per 1000 | 50 per 1000 | RR 0.96 | 573 | ⊕⊕⊕⊝ | — |
| Adverse events: backache | 155 per 1000 | 147 per 1000 | RR 0.94 | 3027 | ⊕⊕⊕⊝ | — |
| Severe PDPH | 0 per 1000 | 10 per 1000 | RD 0 | 6420 | ⊕⊕⊝⊝ | — |
| Any headache | 221 per 1000 | 290 per 1000 | RR 1.35 | 4104 | ⊕⊕⊕⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Traumatic needle(major gauge) compared to traumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Traumatic needle ‐ smaller gauge | Traumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.86 to 6.47 | 2288 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Adverse events: paraesthesia ‐ not reported | See comment | See comment | Not estimable | — | See comment | We did not identify any studies reporting this outcome. |
| Adverse event: backache | — | — | RR ranged | 948 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Severe PDPH | — | — | RD ranged | 1128 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Any headache | — | — | RR ranged | 771 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Imprecision downgraded by one level due to few events reported in each arm. 3Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit. | ||||||
| Atraumatic needle (major gauge) compared to atraumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needle ‐ smaller gauge | Atraumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.38 to 9.3 | 3134 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: paraesthesia | — | — | RR ranged from 1.03 to 7.61 | 439 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: backache | — | — | RR ranged | 526 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Severe PDPH | — | — | RD ranged | 1983 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Any headache | — | — | RR ranged | 1791 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH by indication Show forest plot | 36 | 9378 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.72, 2.67] |
| 1.1 Anaesthesia only | 30 | 8401 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.60, 3.04] |
| 1.2 Myelography only | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.34, 3.00] |
| 1.3 Diagnostic lumbar puncture only | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.38, 3.58] |
| 2 PDPH by gauge Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 22 gauge | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.56, 2.97] |
| 2.2 25 gauge | 5 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.56, 3.95] |
| 2.3 27 gauge | 11 | 4076 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.81, 4.53] |
| 3 PDPH by gender Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 9 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.62, 4.17] |
| 4 PDPH/anaesthesia: type of surgery Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Caesarean section | 8 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.60, 6.10] |
| 4.2 Orthopaedic procedures | 3 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.58, 3.19] |
| 4.3 Other surgeries | 19 | 6083 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.50, 3.51] |
| 5 PDPH by position Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Lateral position | 9 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.39, 9.24] |
| 5.2 Sitting position | 11 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.52, 2.94] |
| 6 PDPH by age Show forest plot | 36 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 No distinctions by age | 34 | 9063 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.73, 2.73] |
| 6.2 Only < 18 years | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.56, 5.12] |
| 7 AE: paraesthesia Show forest plot | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.96] |
| 8 AE: backache Show forest plot | 12 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 9 Severe PDPH by indication Show forest plot | 24 | 6420 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.20, 2.94] |
| 9.1 Anesthesia | 19 | 5542 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.88, 3.53] |
| 9.2 Myelography | 4 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.68, 4.28] |
| 9.3 Diagnostic lumbar puncture | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.18, 7.63] |
| 10 Any headache by indication Show forest plot | 18 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.17, 1.57] |
| 10.1 Anaesthesia | 16 | 3656 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 10.2 Myelography | 2 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.21] |
| 11 PDPH sensitivity analysis Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.26, 6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 23 G vs 25 G | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 1.2 25 G vs 27 G | 4 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.39] |
| 1.3 25 G vs 29 G | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.46, 9.78] |
| 1.4 26 G vs 27 G | 1 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.55, 16.43] |
| 1.5 21 G vs 25 G | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 2 PDPH by type of surgery Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.64, 2.57] |
| 2.2 Orthopaedic surgeries | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.58] |
| 2.3 Other surgeries | 6 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.23, 7.03] |
| 3 PDPH by age Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 No distinctions about age | 8 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.11, 3.95] |
| 3.2 Only children | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 3.3 Only > 60 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 4 PDPH by position Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Lateral position | 5 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.98, 3.16] |
| 4.2 Sitting position | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.56] |
| 5 AE: backache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Severe PDPH by gauge Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 23 G vs 25 G | 1 | 53 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.2 25 G vs 27 G | 3 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 6.3 25 G vs 29 G | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.4 21 G vs 25 G | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7 Any headache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 22 G vs 24 G | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.81] |
| 1.2 22 G vs 25 G | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.50] |
| 1.3 24 G vs 25 G | 2 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [1.00, 31.67] |
| 1.4 25 G vs 26 G | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.90] |
| 1.5 25 G vs 27 G | 2 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.59, 23.64] |
| 1.6 26 G vs 27 G | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.30, 10.73] |
| 1.7 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.58, 4.37] |
| 2 PDPH by type of surgery Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 6 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.64, 5.79] |
| 2.2 Orthopaedic procedures | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.30, 5.07] |
| 2.3 Other surgeries | 5 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.83] |
| 3 PDPH by gender Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 8 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.51, 2.20] |
| 4 PDPH by position Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Sitting position | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.06] |
| 4.2 Lateral position | 5 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.65, 5.41] |
| 5 AE: paraesthesia Show forest plot | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.31, 15.30] |
| 6 AE: backache Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Severe PDPH by gauge Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 22 G vs 24 G | 1 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7.2 22 G vs 25 G | 1 | 234 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 24 G vs 25 G | 1 | 304 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.4 25 G vs 26 G | 2 | 311 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.5 25 G vs 27 G | 1 | 212 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.6 26 G vs 27 G | 1 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.7 27 G vs 29 G | 1 | 389 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Any headache by gauge Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 22 G vs 25 G | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.85, 5.51] |
| 8.2 24 G vs 25 G | 2 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.49, 2.77] |
| 8.3 25 G vs 26 G | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.99] |
| 8.4 25 G vs 27 G | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.65, 5.39] |
| 8.5 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |