Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized Controlled Trial | |
| Participants | 100 subjects, 54 male, 46 female, median age of 59 (males were 1.5 years higher than females). Median back pain duration was 14 years, median duration of sciatica was 2 years Setting: Neurology department in a hospital in Norway | |
| Interventions | 1) Surgery – partial or total laminectomy, medial facetecomy, discectomy, and/or removal of osteophytes from the vertebral margins or facet joints. No fusions. (n=13) 2) Conservative therapy ‐ "lumbar orthosis use for 1 month worn during the day for all activities plus instruction and back school.” (n=18) | |
| Outcomes | 1) VAS 2) Verbal Rating Scale 3) Subjective Change (Better, Worse, or Unchanged) 4) Work Status 5) Subjective rating from evaluating physician and study team (Excellent, Fair, Unchanged, Worse)
Follow‐up: 6 months, 1, 4 and 10 years | |
| Notes | VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Low risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Low risk | |
| Was the timing of the outcome assessment similar in all groups? | Unclear risk | Information currently not available |
| Methods | Randomized Controlled Trial | |
| Participants | 73 subjects in total, 37 with spinal stenosis, 36 with acute herniated nucleus pulposus, 37 males, 36 female, average age of 48.5 years in the experimental group and 49.5 years in the placebo group. Experimental group average 36.6 months in symptom duration, placebo group averaged 29.4 months Setting: Orthopedic surgery department in the United States | |
| Interventions | 1) Steroid group ‐ 2mL of sterile water containing 80mg of methylprednisolone acetate combined with 5mL of 1% procaine was injected into the epidural space in the region between the 3rd and 4th lumbar vertebrae with the patient in the lateral decubitus position lying on the side of the painful limb (n=42, 20 with stenosis) 2) Placebo group ‐ 2mL of saline combined with 5mL of 1% procaine was injected into the epidural space in the region between the 3rd and 4th lumbar vertebrae with the patient in the lateral decubitus position lying on the side of the painful limb. (n=31, 17 with stenosis)
All patients were advised to take mild analgesics (aspirin or acetaminophen) during the post‐injection period. Second injection given if less than 50% improvement after 24 hours ‐ considered treatment failure | |
| Outcomes | 1) Subjective percentage of improvement with 75% required to be considered a treatment improvement, if less than 50% after 24 hours was considered a treatment failure 2) Re‐injection Rates 3) Surgery Rates
Follow‐up: 24 hours, every 3 months up to 30 months, averaging 20.2 months in the steroid group and 21.5 months in the control group
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | High risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 39 subjects with an average of 6 years of pain, average age of 56.6 years of age, 20 males and 19 females
Setting: Orthopedic hospital in Finland | |
| Interventions | 1)100IU Calcitonin injection every other day for 4 weeks. (n=20) 2) Placebo treatment (Miacalcic Sandoz 100IU) every other day for 4 weeks. (n=19) | |
| Outcomes | 1) VAS 2) Treadmill Test 3) Coping with ADLs 4) Digitest Ergojump Contact Test 5) Blood Tests
Follow up: I, 3, 4, 6, and 12 months | |
| Notes | ADLs = Activities of Daily Living, VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | Unclear risk | Information currently not available |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Information currently not available |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 53 subjects, 38 males and 15 female. Group 1 averaged 70 years of age and 79 days of symptoms on average, group 2 averaged 69 years of age and an average of 82 days of symptoms, group 3 averaged 72 years of age and 94 days of symptoms on average Setting: Anesthesia department in Japan | |
| Interventions | 1) Epidural injection with 8 mL of saline, repeated twice in the first week. (n=16) 2) Epidural injection with 8 mL of 1% mepivacaine, repeated twice in the first week. (n=18) 3) Epidural injection with a mixture of 8 mL of 1% mepivacaine and 40 mg of methylprednisone, repeated twice in the first week. (n=19) | |
| Outcomes | 1) Walking distance which was graded according to distance (Excellent, Good, or Poor)
Follow‐up: 1 week, 1 month, 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Unclear risk | Information currently not available |
| Was the care provider blinded to the intervention? | Unclear risk | Information currently not available |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | High risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 45 subjects, 13 males, 32 females, average ages in groups of 57.4, 49.13, and 53.06. 7 subjects with pain duration of 3‐6 months, 7 with pain duration of 6‐12 months, and 31 with pain duration of greater than 12 months Setting: Rehabilitation center in Turkey | |
| Interventions | 1) Stretching and strengthening exercises for lumbar, abdominal, leg muscles as well as low‐intensity cycling exercises were given as therapeutic exercises. Ultrasound was applied with 1mHz, 1.5W/cm2 intensity, in continuous mode on the back muscle for 10 minutes (n=17) 2) Same as group 1 with ultrasound on off‐mode (n=17) 3) No exercise ‐ no treatment (n=16) | |
| Outcomes | 1) VAS (out of 10) 2) Treadmill test at 3 km/h for maximum of 15 minutes or 750m 3) ODI 4) Analgesic Consumption 5) Physiatrist Assessment
Follow‐up: End of 3‐week treatment period only | |
| Notes | VAS = Visual Analogue Scale, ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | Low risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 29 subjects, 21 male, 8 female, average ages of 62.6, 61.1, and 53.1 years in the three groups, average pain duration of 5.7 years, 5.0 years, and 5.7 years in the three groups Setting: Medical school department of physical medicine and rehabilitation in Turkey | |
| Interventions | 1) Conservative inpatient physical therapy program 5 days a week for 2 weeks. PT included applications of ultrasound 1.5 W/cm2 for 10min, hot pack for 20min, and TENS for 20min to the lumbar region (n=13) 2) Lumbar epidural steroid injections, 10 mL of solution containing 60mg of triamcinolon acetonide (1.5 mL), 15 mg of 0.5% bupivacain hydrochloride (3 mL), and 5.5 mL of physiologic saline (0.9%NaCl) was injected in 3.5 minutes (n=10) 3) Control group (n=10)
All patients included were trained to pursue a home‐based therapeutic exercise program performed twice daily for a period of 6 months, and oral diclofenac sodium 75mg was administered to all patients twice daily for 2 weeks | |
| Outcomes | 1) VAS 2) Treadmill Walk Test 3) Nottingham Health Profile 4) RMDI 5) Functional testing including finger to floor distance, sit‐to‐stand, and a weight carrying test
Follow‐up: 2 weeks, 1, 3, and 6 months | |
| Notes | ODI = Oswestry Disability Index, RMDI = Roland Moris Disability Index, VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 94 subjects, 22% of surgical subjects were male, 45% of non‐operative subjects were male. Nonoperative group had average age of 62.9 years, surgical group had average age of 63.9 years. Surgical group averaged 14 years since onset of symptoms, non‐surgical group average 16 years since onset of symptoms. Minimum of 6 months of symptoms for study inclusion Setting: Research Center in Finland | |
| Interventions | 1) Segmental decompressive surgery with facetectomy (n=50) 2) Non‐operative treatment – NSAIDS when indicated and seen one to three times by a physiotherapist, in addition to the standard visit at each follow‐up. The physiotherapist gave all patients educational brochure. The patients were encouraged to use their back in a normal way. Pain‐relieving body postures were taught as well as basic ergonomics related to lifting and carrying. Individually structured programs included trunk muscle endurance and stretching‐type exercises. Additional individual physiotherapy consisting of passive treatment methods (such as ultrasound and transcutaneous nerve stimulation) (n=44) The patients in the surgical group also received the brochure and the instructions described above | |
| Outcomes | 1) 11‐point numerical pain rating scale for back and leg pain 2) Walking ability (distance without a break) also via treadmill test 3) General health status on a 5‐point scale (Very Good, Quite Good, Average, Quite Poor, or Very Poor) 4) ODI 5) Ability to complete certain ADL without difficulty, some difficulty, marked difficulties or not at all 6) Radiographic examination Follow‐up: 6 months, 1, 2 and 6 years | |
| Notes | ADLs = Activities of Daily Living, ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 79 subjects, 24 males and 24 females, with an average age of 69.6 years in the Limaprost group and 72.2 in the etodolac group Setting: Orthopedic surgery in a medical faculty in Japan | |
| Interventions | 1) Oral prostaglandin E1 derivative (15 g Limaprost) three times daily for eight weeks (n=39) 2) 400 mg of etodolac (NSAID) twice daily for eight weeks (n=40) | |
| Outcomes | 1) SF‐36 2) Verbal Pain Rating Scales 3) Walking Distance 4) LBP Severity 5) Leg Pain Severity 6) Leg Numbness Severity 7) Treatment Satisfaction
Follow‐up: 8 weeks | |
| Notes | LBP = Low Back Pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Unclear risk | Information currently not available |
| Was the care provider blinded to the intervention? | Unclear risk | Information currently not available |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Unclear risk | Information currently not available |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 55 subjects with an average age of 68.5 years and an average of 36.2 weeks of the condition in the intervention group and 29.8 weeks in the placebo group, 33 males and 22 females Setting: Spinal center in the United States | |
| Interventions | 1) 400 IU intranasal calcitonin daily for 6 weeks followed by open label 6‐week extension (n=36) 2) Placebo nasal spray daily for 6 weeks, followed by open label 6‐week extension, during which all patients received 400IU calcitonin (n=19) | |
| Outcomes | 1) VAS 2) Walking Capacity (time and distance to stopping) 3) ODI 4) Stenosis Specific Questionnaire 5) Satisfaction with pain levels, functional status, and treatment received 6) SF‐36 7) Symptom Diary Follow‐up: 12 weeks | |
| Notes | VAS = Visual Analogue, ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | Low risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 41 subjects with 10 in a double blind RCT crossover, 37 males and 4 females with ages between 41 and 67 years Setting: Infirmary in England | |
| Interventions | 1) 100 IU salmon calcitonin injection four times per week, sometimes with Maxalon for nausea (n=5) 2) Matching placebo (n=5) | |
| Outcomes | 1) Walking chart and ability to walk more than 1 mile 2) ODI Follow‐up: 10 weeks | |
| Notes | Only responders randomized, ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Unclear risk | Information currently not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information currently not available |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Unclear risk | Information currently not available |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Low risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | High risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 42 subjects, 35 male, 7 female, average of 53.6 years in 20 subjects and 56.7 years in 22 subjects, median duration of back pain reported was 11 years for 19 subjects, and 14 years for 22 subjects. Median duration of claudication was 1.25 years for 20 subjects and 4.5 years for 22 subjects Setting: Infirmary in England | |
| Interventions | 1) 100 IU of salmon calcitonin injected subcutaneously four times per week for eight weeks (n=20) 2) 1 mL of saline injected four times per week for eight weeks (n=22) | |
| Outcomes | 1) VAS 2) Claudication threshold and tolerance for walking at constant speed with verbal description of walking pain on a 5‐point pain rating scale 3) 3 level mobility assessment 4) Analgesic requirements 5) 3 level sleep disturbance 6) Treatment success defined as 100% improvement in walking distance and able to walk 800m Follow‐up: 4 and 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | Unclear risk | Information currently not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information currently not available |
| Was the drop‐out rate described and acceptable? | Low risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Information currently not available |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 68 subjects, 35 males, 33 females, average age of 58 years, 12 week median pain duration Setting: Hospital in Singapore | |
| Interventions | 1) Unweighted treadmill training ‐ Weeks 1 and 2, participants walked with a relatively pain‐free gait which translated to 30–40% of body weight. In Weeks 3 to 6, participants were encouraged to walk at a moderate intensity. The duration of each treadmill session was limited by participant tolerance or to a maximum of 30 minutes. Two times per week for 6 weeks = 12 sessions (n=33) 2) Cycling on upright bicycle ‐ During Weeks 1 and 2, participants cycled at their comfortable pace at 50 to 60 rpm. Participants were instructed to assume a flexed posture. In Weeks 3 to 6, participants were encouraged to exercise at a moderate intensity and the duration of each cycling session was limited by participant tolerance or to a maximum of 30 minutes. Two times per week for 6 weeks for 12 sessions (n=35) | |
| Outcomes | 1) VAS for pain over past week 2) Patient perceived benefit on a 6‐point scale 3) ODI 4) RMDI 5) Walking Ability
Follow‐up: 3 and 6 weeks | |
| Notes | ODI = Oswestry Disability Index, RMDI = Roland Moris Disability Index, VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | Low risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Low risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 45 subjects 31 males and 14 females, average ages of 57.65 years in the calcitonin group and 54.45 years in the paracematol group Setting: Physical and Rehabilitation Medicine Department in Turkey | |
| Interventions | 1) 200 IU intranasal calcitonin daily for 8 weeks (n=23) 2) Up to 1500mg of paracematol daily for 8 weeks (n=22)
Both groups took part in a physical therapy and exercise program five times per week for 15 sessions | |
| Outcomes | 1) VAS 2) Walking Capacity 3) RMDI 4) Ranges of Motion
Follow up: 8 weeks | |
| Notes | RMDI = Roland Moris Disability Index, VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | Low risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | Unclear risk | Information currently not available |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 40 subjects, 30 males, 10 females, average of 67 years in the intervention group and 70.2 years in the placebo group, average of 38.7 months with symptoms in the calcitonin group and 30.9 months in the placebo group Setting: University hospital in England | |
| Interventions | 1) Placebo nasal spray NaCl for four weeks (n=20) 2) 200 IU nasal salmon calcitonin for 4 weeks (n=20) | |
| Outcomes | 1) VAS 2) Shuttle Walking Test 3) 4 point subjective outcome of overall assessment (Excellent, Good, Fair, Poor) 4) ODI 5) Modified Somatic Perception Questionnaire 6) Modified Zung Depression Score
Follow‐up: Baseline, 4, 10, 16 weeks | |
| Notes | ODI = Oswestry Disability Index, VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | High risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Low risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | 152 subjects, 68 males and 84 females with an average age of 66.8 years. 44 of the subjects had symptoms for less than one month, 98 had symptoms for more than one month Setting: Hospital in Thailand | |
| Interventions | 1) Conservative treatment consisting of education, activity modification, exercise and physical therapy. NSAIDs, muscle relaxants, and analgesics as necessary. Vitamin B1, B6, and B12 three times per day (n=82) 2) Conservative treatment plus methylcobalamin (Methlcobalin ESAI), 1.5mg per day in 3 divided doses after meals for 6 months (n=70) | |
| Outcomes | 1) Presence of pain on spinal motion 2) Claudication distance 3) Medication intake (NSAIDs, muscle relaxants, and steroids)
Follow‐up: every month for two years | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | Unclear risk | Information currently not available |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Controlled Trial | |
| Participants | Subjects with image‐confirmed degenerative spondylolisthesis: 304 subjects in clinical trial, 303 in the observational cohort, 31% male in the surgical group, 33% male in the surgical group. Average age of 64.7 years in the surgical group and 68.2 years in the non‐surgical group. Subjects had symptoms for at least 12 weeks Setting: Multicentred orthopedic departments in the United States | |
| Interventions | 1) Assigned to surgery (standard laminectomy with or without fusion) (n=159) 2) Assigned to non‐surgical treatment – usual non‐operative care (n=145) 3) Chose surgery (n=173) 4) Chose non‐surgical treatment (n=130) | |
| Outcomes | 1) SF‐36 Bodily Pain 2) SF‐36 Bodily Function 3) Low Back Pain Bothersomeness Scale 4) Leg Pain Bothersomeness Scale 5) ODI 6) Subjective self‐reported improvement, satisfaction with current symptoms and care 7) Stenosis Bothersomeness Index Follow‐up: 6 w, 3 and 6 mo, 1, 2 and 4 yrs | |
| Notes | ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Information currently not available |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Low risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Clinical Trial | |
| Participants | 289 in the RCT, 365 in the observational cohort. 62% male in the surgical groups, 59% male in the non‐surgical groups. Average age of 63.8 in the surgical group, 66.1 in the non‐surgical group. 60% in the surgical group and 55% in the non‐surgical group had symptoms for over 6 months Setting: Multicentered ‐ orthopedic departments in the United States | |
| Interventions | 1) Assigned to surgery – standard laminectomy with or without fusion (n=138) 2) Assigned to non‐surgical treatment ‐ usual non‐operative care ‐ recommended to include at least active physical therapy, education or counseling with home exercise instruction, and the administration of NSAIDs, if tolerated (n=151) 3) Chose surgery (n=219) 4) Chose non‐surgical treatment (n=146) | |
| Outcomes | 1) SF‐36 Bodily Pain 2) SF‐36 Bodily Function 3) Low Back Pain Bothersomeness Scale 4) Leg Pain Bothersomeness Scale 5) ODI 6) Subjective self‐reported improvement, satisfaction with current symptoms and care 7) Stenosis Bothersomeness Index
Follow‐up: 6 weeks, 3 and 6 months, 1, 2 and 4 years | |
| Notes | ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | Low risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Information currently not available |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Low risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Clinical Trial | |
| Participants | 58 subjects, 31 males, 27 female, 29 (group 1) with an average age of 70 years, 29 (group 2) with an average age of 68.9, median low back pain duration of 108 months in Group 1 (n=29) and 60 months in Group 2 (n=29), lower extremity median pain duration of 48 months in Group 1 (n=29) and 24 months in Group 2 (n=29) Setting: University in the United States | |
| Interventions | 1) Flexion Exercise and Walking Group – 45‐60 minutes twice per week for 6 weeks. ‐ Lumbar flexion exercises along with performance of a progressive level self pace treadmill walking program, and subtherapeutic ultrasound. The duration of each treadmill session was based on patient’s tolerance on that specific day and could extend up to 45 minutes (n=29) 2) Manual Therapy, Exercise and Walking Group ‐ 45‐60 minutes twice per week for 6 weeks ‐ Manual physical therapy (thrust and non‐thrust) to the thoracic and lumbar spine, pelvis, and lower extremities and specific exercises at discretion based on the underlying impairments. Patients received specific exercises to address impairments in mobility, strength, and/or coordination. Exercises were performed in the clinic and as part of a home exercise program. Patients also underwent a body‐weight supported treadmill ambulation program using a cable and trunk harness system to unload a specific amount of weight from the patient while the patient walks as comfortably as possible on a treadmill (n=29) | |
| Outcomes | 1) Global Rating of Change (15 point scale) 2) Numerical Pain Rating Scale for lower limb 3) Walking Tolerance test 4) ODI 5) Medication consumption 6) Satisfaction subscale of the Spinal Stenosis Scale 7) Additional use of healthcare resources
Follow‐up: 6 weeks, 1 year, long‐term mail survey (averaging 29 months) | |
| Notes | NPRS = Numerical Pain Rating Scale, ODI = Oswestry Disability Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Clinical Trial | |
| Participants | 55 subjects, 22 males, 33 females, average age of 50.8 years Setting: Hospital department of physical medicine and rehabilitation in Turkey | |
| Interventions | 1) 900 mg of gabapentin per day increased weekly by 300 mg to a maximum of 2400 mg (n=28) 2) Placebo (n=27)
Both groups received physical therapy exercises, a lumbosacral corset with steel bracing and NSAID treatments | |
| Outcomes | 1) VAS – low back and leg pain during movement 2) Walking Distance 3) Presence or absence of motor and/or sensory deficits
Follow‐up: 15 days, 1, 2, 3, 4 months | |
| Notes | VAS = Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Was the drop‐out rate described and acceptable? | Unclear risk | Information currently not available |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Information currently not available |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
| Methods | Randomized Clinical Trial | |
| Participants | 30 subjects, 37 male and 26 female. Steroid group averaged 46.5 years of age and 36.6 months of symptoms, control group averaged 49 years of age and 29.4 months of symptoms Setting: Medical facility in Egypt | |
| Interventions | 1) Steroid injection ‐ 5mL of hydrocortisone acetate suspension, 2x2mL carbocaine, 4% volume completed with sterile saline to 30mL (n=18) 2) Control ‐ 2x2mL of carbocaine, 4% injected into epidural space. Volume completed with sterile saline to 30mL (n=12) | |
| Outcomes | 1) Subjective percentage of improvement where 75% or more was deemed successful and surgery after injection was considered a failure
Follow‐up: 24 hours, then every three months up to 36 months averaging 20.2 months in the steroid group and 21.5 months control group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | Unclear risk | Information currently not available |
| Was the patient blinded to the intervention? | High risk | |
| Was the care provider blinded to the intervention? | Unclear risk | Information currently not available |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Low risk | |
| Was the compliance acceptable in all groups? | Unclear risk | Information currently not available |
| Was the timing of the outcome assessment similar in all groups? | Low risk | |
| Methods | Randomized Clinical Trial | |
| Participants | 191 subjects, 57% male and 43% female in the X STOP group. 52% male and 48% female in the non‐operative group. Average age of 70 years in the X STOP group and 69.1 years in the non‐operative group. Average of 3.5 years symptom duration in the X STOP group and 4.7 years in the non‐operative group. Setting: Spine center in the United States | |
| Interventions | 1) X STOP Interspinous Process Decompression System (n=100) 2) Non‐operative treatment – subjects received an epidural steroid injection on enrolment and were eligible for additional injections as needed, as well as NSAIDS, analgesic agents, and physical therapy. Physical therapy consisted of education on back care and modalities such as ice packs, heat packs, massage, stabilization exercises, and pool therapy. Braces such as abdominal binders and corsets were permitted, but body jackets and chair back braces were not (n=91) | |
| Outcomes | 1) SF‐36 2) ZCQ 3) Worker’s Compensation Claims 4) ODI 5) Radiographic Changes
Follow‐up: Surgery: 7 (2 years) Control: 19 (2 years) | |
| Notes | ODI, ZCQ = Zurich Claudication Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information currently not available |
| Allocation concealment (selection bias) | High risk | |
| Was the patient blinded to the intervention? | Low risk | |
| Was the care provider blinded to the intervention? | Low risk | |
| Blinding of outcome assessment (detection bias) | High risk | |
| Was the drop‐out rate described and acceptable? | High risk | |
| Were all randomized participants analyzed in the group to which they were allocated? | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Were co‐interventions avoided or similar? | Unclear risk | Information currently not available |
| Was the compliance acceptable in all groups? | High risk | |
| Was the timing of the outcome assessment similar in all groups? | High risk | |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not published in English | |
| Not published in English | |
| No imaging confirmation requirement for inclusion | |
| Not neurogenic claudication | |
| No imaging confirmation requirement for inclusion | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Patients with leg pain were excluded | |
| Not an RCT | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Neurogenic claudication not explicitiy stated as inclusion criterion | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Not neurogenic claudication but sciatica or radiculopathy as inclusion criterion | |
| Mixed population not analysed separately | |
| Not an RCT | |
| No data on number of patients with lumbar spinal stenosis, patients did not explicitly have neurogenic claudication, subacute population | |
| Not an RCT | |
| Neurogenic claudication was not an inclusion criterion | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Mixed population, neurogenic claudication was not an inclusion criterion | |
| Mixed population, neurogenic claudication was not an inclusion criterion | |
| Duplicate of accepted study (see Tafazal 2007) | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Not neurogenic claudication but radiculopathy as inclusion criterion | |
| Neurogenic claudication was not an explicit requirement, patients had nerve root pain, patients were subacute | |
| Not published in English |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 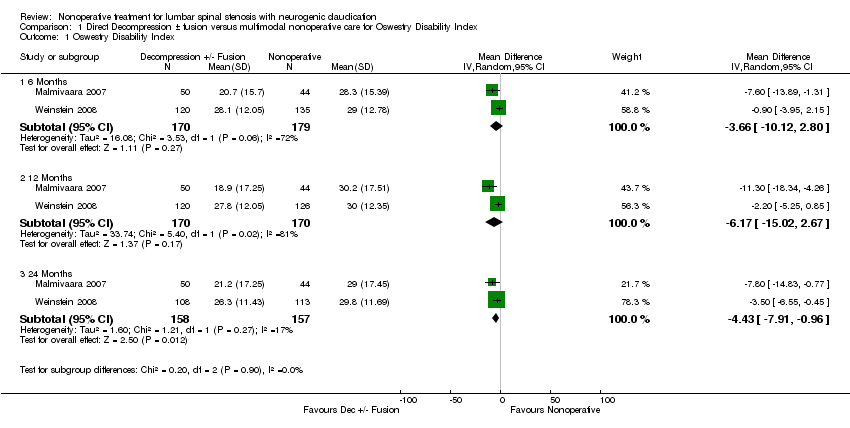 Comparison 1 Direct Decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, Outcome 1 Oswestry Disability Index. | ||||
| 1.1 6 Months | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐3.66 [‐10.12, 2.80] |
| 1.2 12 Months | 2 | 340 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐15.02, 2.67] |
| 1.3 24 Months | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐7.91, ‐0.96] |

Selection process of included articles.
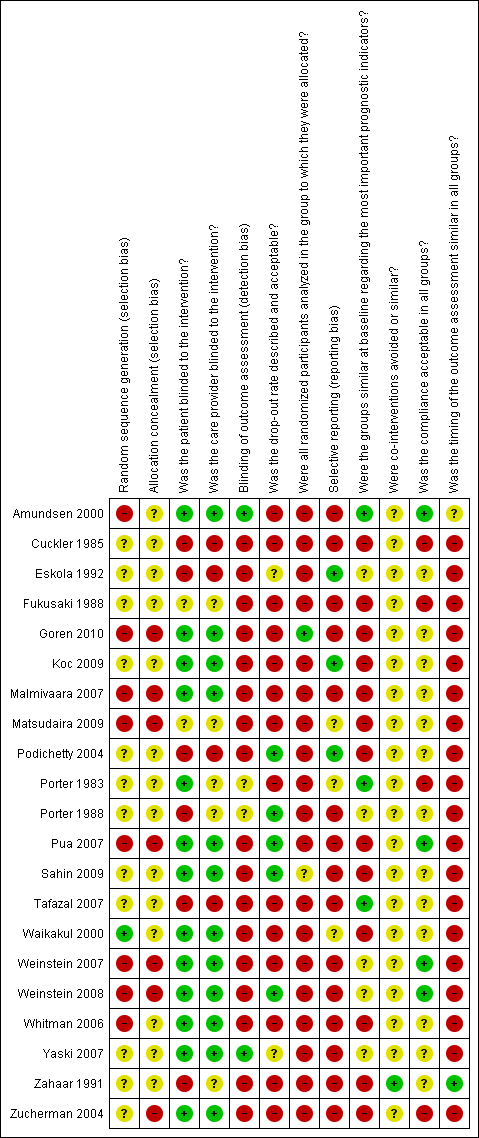
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
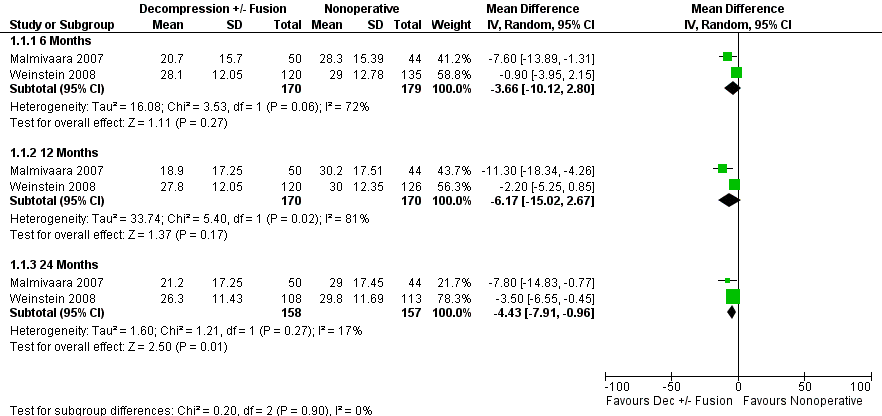
Forest plot of comparison: 1 Direct decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, outcome: 1.1 Oswestry Disability Index.

Comparison 1 Direct Decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, Outcome 1 Oswestry Disability Index.
| Comparisons and trials | Risk of bias | GRADE assessment and outcomes/measures (walking ability, pain, function, and quality of life) at each follow‐up point | ||||||||
| Consistency | Directness | Precision | Selective reporting | Immediate | Short‐term | Intermediate | Long‐term | Quality of the evidence | ||
| Calcitonin | ||||||||||
| Calcitonin injection versus placebo injection | ||||||||||
| High High | No No | Yes Yes | No No | Yes Yes |
| = TWT = VAS | = TWT = VAS | = TWT = VAS | +000 +000 | |
| High | No | Yes | No | Yes |
| ? distance walked | ? distance walked |
| +000 | |
| High High | No No | Yes Yes | No No | Yes Yes |
| = distance walked = VAS |
|
| +000 +000 | |
| Calcitonin nasal spray versus placebo nasal spray | ||||||||||
| High High High High | No No No No | Yes Yes Yes | No No No No | Yes |
| = distance walked = time walked = SF‐36 = VAS |
|
| +000 +000 +000 +000 | |
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = Shuttle walk = VAS leg = VAS back = ODI = global |
|
| +000 +000 +000 +000 +000 | |
| Calcitonin nasal spray plus physical therapy versus paracetamol plus physical therapy | ||||||||||
| High High High | No No No | Yes Yes Yes | No No No |
|
| = distance walked = VAS = RMDI |
|
| +000 +000 +000 | |
| Oral medication | ||||||||||
| Oral prostaglandin versus etodolac (NSAID) | ||||||||||
|
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No | Yes |
| > distance walked ? SF‐36 = LBP > Leg pain > global |
|
| ++00 +000 ++00 ++00 ++00 |
| Methylocobalamin (vitamin B12) plus conservative care versus conservative care | ||||||||||
| High | No | Yes | No | Yes | > distance walked | > distance walked | +000 | |||
| Gabapentin versus placebo | ||||||||||
|
| High High High | No No No | Yes Yes Yes | No No No |
|
|
| > distance walked = VAS (1‐2 months) > VAS (3 months) | > distance walked > VAS | +000 +000 +000 |
| Physical therapy | ||||||||||
| Exercise plus ultrasound versus exercise plus sham ultrasound | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back = VAS leg = ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus sham ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Inpatient physical therapy versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = NHP |
| +000 +000 +000 +000 | |
| Unweighted treadmill walking plus exercise versus cycling plus exercise | ||||||||||
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = distance walked = ODI = RMDI = VAS = global |
|
| ++00 ++00 ++00 ++00 ++00 | |
| Manual therapy, exercise and unweighted treadmill versus flexion exercise, walking and sham ultrasound | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT > global = ODI = NPRS |
| = global | +000 +000 +000 +000 | |
| Epidural injection | ||||||||||
| Translaminar epidural steroid injections versus placebo injections | ||||||||||
| High | No | Yes | No | = global | =global | +000 | ||||
| Translaminar epidural steroids plus epidural block versus placebo injections | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural steroids plus epidural block versus epidural block injections | ||||||||||
| High | No | Yes | No |
| = distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural block versus placebo | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Intralaminar epidural steroid plus epidural block versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Intralaminar epidural steroid plus epidural block versus inpatient physical therapy | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Caudal epidural steroids versus placebo injections | ||||||||||
| High | No | Yes | No |
| = global |
|
| = global | +000 | |
| Multimodal nonoperative treatment | ||||||||||
| Indirect decompression using interspinous spacer (X‐Stop) versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| Zucherman 2004, Anderson 2006 | High | No | Yes | No | SR (short & intermediate) |
| > ZCQ | > ZCQ | > ZCQ | +000 |
| Indirect decompression using interspinous spacer (X_Stop) versus multimodal nonoperative care | ||||||||||
| Zucherman 2004, 2005, Hsu 2006 | High | No No | Yes Yes | No No | SR SR |
| > ZCQ > SF‐36 | > ZCQ > SF‐36 | > ZCQ > SF‐36 | +000 +000 |
| Direct decompression ± fusion versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | +000 +000 +000 +000 +000 | |
| Direct decompression ± fusion versus multimodal nonoperative care | ||||||||||
| High High | No No | Yes Yes | No No |
|
| ?* pain severity | ?* global | ?* pain severity ? global | +000 +000 | |
| Low Low Low Low Low Low | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | ++00 ++00 ++00 ++00 ++00 +000 | ||
| High High High High High High | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS = ODI | = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS ODI (see Figure 4) | > SF‐36 BP** = SF‐36 PF = LBPBS = LPBI = SBS ODI (Figure 4) | +000 +000 +000 +000 +000 +000 | |
| Follow‐up points: Immediate = up to 1 week, short‐term = >1 week ‐ 3 months, intermediate = 3 months ‐ 1 year, long‐term = > 1 year > statistically significant favouring intervention (first comparison), < statistically significant favouring control (second comparison), = no statistically significant difference between intervention and control groups TWT = Treadmill Walking Test, VAS = Visual Analog Scale for Pain Intensity, RMDI = Roland‐Morris Back Disability Index, NHP = Nottingham Health Profile, Global = Patient Perceived Improvement, SR = Selective Reporting, ODI = Oswestry Back Disability Index, ? = insufficient data, LBP = Low back Pain Severity Scale, Leg pain = Leg Pain Severity Scale, ? SF‐36 = No data on overall score, improvement in some subscales, NPRS = Numeric Pain Rating Scale, SF‐36 BP = SF‐36 Bodily Pain Subscale, SF‐36‐ PF = SF‐36 Physical Function Subscale, LBPBS ‐ Low Back Pain Bothersome Scale, LPBI = Leg Pain Bothersome Index, SBS ‐ Stenosis Bothersome Scale, SW = Subjective Walking, VAS leg = Visual Analog Scale for Leg Pain, VAS LB = Visual Analog Scale for Low Back Pain, VAS leg walking = Visual Analog Scale for Leg pain while walking, ?* = no between group statistical comparisons, ** = SF‐36 BP significantly better at 2 years but not 4 years. GRADE evidence: +000 = Very low quality evidence, ++00 = Low quality evidence, +++0 = Moderate quality evidence, ++++ = High quality evidence | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 Months | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐3.66 [‐10.12, 2.80] |
| 1.2 12 Months | 2 | 340 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐15.02, 2.67] |
| 1.3 24 Months | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐7.91, ‐0.96] |

