Sulodexida para el tratamiento de la úlcera venosa de la pierna
References
Referencias de los estudios incluidos en esta revisión
Jump to:
Referencias de los estudios excluidos de esta revisión
Jump to:
Referencias adicionales
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT | |
| Participants | 235 people with leg ulcers due to CVI with a diameter greater than 2 cm. CVI was assessed by ultrasound (echo‐color‐Doppler) demonstration of valvular incompetence, or reflux in the superficial veins or post‐thrombotic changes and or reflux in the deep veins, or both Ulcer size ≦ 10 cm2 (sulodexide/control): 104/92 ulcers; > 10 cm2: 35/35 ulcers. | |
| Interventions | Intervention: sulodexide + local treatment Local treatment: wound care and compression therapy. Quote: "Wound care included the following steps as appropriate: mechanical cleansing of ulcer; detersion and removal of debris; local application of proteolytic enzymes (collagenases or proteases), or autolysis with hydrogel; antisepsis; dressing of the wound. High compression bandages were applied according to the local condition and the patient's compliance. The allowed materials for compression therapy were stretch elastic bandages, adhesive bandages, self adherent bandages, zinc oxide bandages, 4‐layer bandages." | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind; placebo controlled. Quote: "Patients received local treatment and were blindly allocated to sulodexide or matching placebo." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "At end of study ulcer tracings were sent in a random order to a blind, independent, off‐site assessor who calculated the total ulcerated area (in cm2) with a standardised computer system." |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis. A total of 31 (25.8%) patients in sulodexide group and 24 (21.8%) in the control withdrawn during treatment, the reasons were as follow: lost to follow up (9 vs. 6), protocol violation (1 vs. 2), non compliance (0 vs. 1), informed consent withdrawn (3 vs. 1), adverse events (7 vs. 7), ulcer recovered (6 vs. 5) and other reasons (5 vs. 2). |
| Selective reporting (reporting bias) | Low risk | Protocol could not be reviewed, however all outcomes in the 'Methods' were reported in the 'Results' section |
| Other bias | Low risk | Sample size was calculated |
| Methods | RCT | |
| Participants | 20 patients with ulcers caused by lower limb venous insufficiency | |
| Interventions | Intervention: sulodexide + local treatment | |
| Outcomes |
| |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the abstract section |
| Allocation concealment (selection bias) | Unclear risk | Not described in the abstract section |
| Blinding of participants and personnel (performance bias) | High risk | Not blind design |
| Blinding of outcome assessment (detection bias) | High risk | Not blind design |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described in the abstract section |
| Selective reporting (reporting bias) | Unclear risk | Cannot be judged from the abstract |
| Other bias | Unclear risk | Cannot be judged from the abstract |
| Methods | RCT | |
| Participants | 94 patients suffering from venous leg ulcers, secondary to post‐thrombotic syndrome or due to primary phlebopathology. Diagnosis was confirmed by venous echo Doppler Ulcer duration ≦ 6 months (sulodexide/control): 35/27 participants; 7 months to 12 months: 12/10 participants; > 12 months: 5/5 participants | |
| Interventions | Intervention: sulodexide + local treatment | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By a randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blind design |
| Blinding of outcome assessment (detection bias) | High risk | Not blind design |
| Incomplete outcome data (attrition bias) | Low risk | No missing participants or outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol could not be reviewed, however all outcomes in the 'Methods' were reported in the 'Results' section |
| Other bias | Low risk | No other source of bias identified |
| Methods | RCT Carried out from May 2005 until March 2006 | |
| Participants | 114 patients with venous leg ulcers, diagnosis was confirmed by colour Doppler, along with symptoms, Perthe test and Trendelinburg test Ulcer size ≦ 10 cm2 (sulodexide/control): 48/41 patients; > 10 cm2: 13/12 patients | |
| Interventions | Intervention: sulodexide + local treatment | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Not blind design |
| Blinding of outcome assessment (detection bias) | High risk | Not blind design |
| Incomplete outcome data (attrition bias) | Low risk | No missing participants or outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol could not be reviewed, however all outcomes in the 'Methods' were reported in the 'Results' section |
| Other bias | Low risk | No other source of bias identified |
RCT: Randomized Controlled Trial
CVI: Chronic Venous Insufficiency
LSU: Lipasemic Units
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Review | |
| Not controlled trial, no use of sulodexide as a study intervention | |
| RCT, parallel design, no use of sulodexide as a study intervention | |
| RCT, cross over design, no use of sulodexide as a study intervention | |
| Review | |
| Quasi‐RCT. Quote: "Group I included patients with odd numbers, group II included patients with even numbers. The numbers were determined by the surgeon." | |
| RCT, parallel design, no use of sulodexide as a study intervention | |
| RCT, parallel design, not previously defined patients, with mixed arterial and venous ulcers of lower limbs |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed (overall) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.12] |
| Analysis 1.1  Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 1 Proportion of ulcers completely healed (overall). | ||||
| 2 Proportion of ulcers completely healed (sensitivity analysis) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| Analysis 1.2 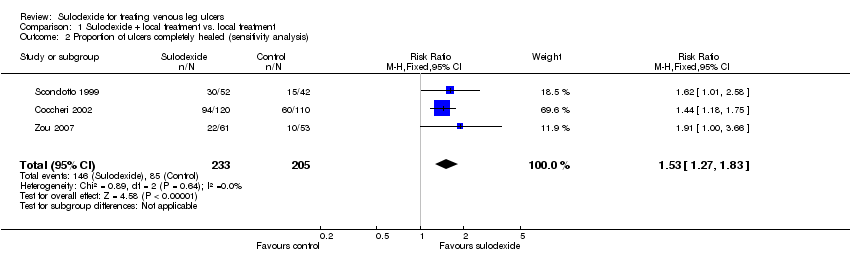 Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 2 Proportion of ulcers completely healed (sensitivity analysis). | ||||
| 3 Proportion of ulcers completely healed at 30 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 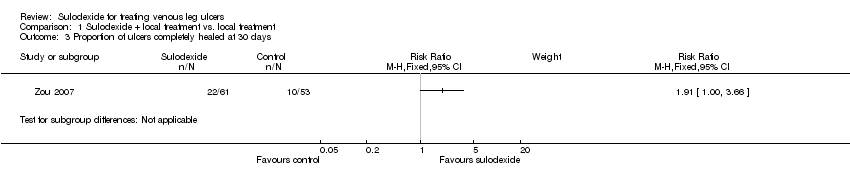 Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 3 Proportion of ulcers completely healed at 30 days. | ||||
| 4 Proportion of ulcers completely healed at 60 days Show forest plot | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.20, 2.28] |
| Analysis 1.4  Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 4 Proportion of ulcers completely healed at 60 days. | ||||
| 5 Proportion of ulcers completely healed at 90 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 5 Proportion of ulcers completely healed at 90 days. | ||||
| 6 Adverse effects Show forest plot | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.48, 4.34] |
| Analysis 1.6  Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 6 Adverse effects. | ||||

PRISMA flow diagram of literature screening
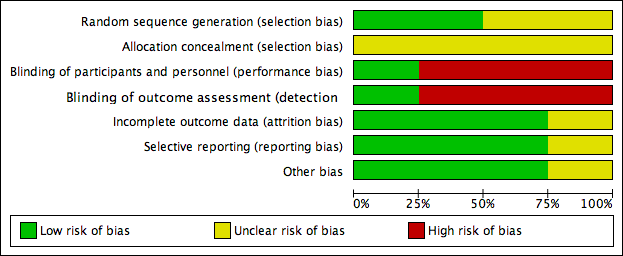
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 1 Proportion of ulcers completely healed (overall).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 2 Proportion of ulcers completely healed (sensitivity analysis).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 3 Proportion of ulcers completely healed at 30 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 4 Proportion of ulcers completely healed at 60 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 5 Proportion of ulcers completely healed at 90 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 6 Adverse effects.
| Sulodexide + local treatment compared to local treatment for treating venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| local treatment | Sulodexide + local treatment | |||||
| Proportion of ulcers completely healed (overall) | 298 per 1000 | 494 per 1000 | RR 1.66 | 438 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 30 days | 189 per 1000 | 360 per 1000 | RR 1.91 | 114 | ⊕⊝⊝⊝ | |
| Proportion of ulcers completely healed at 60 days | 250 per 1000 | 412 per 1000 | RR 1.65 | 324 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 90 days | 327 per 1000 | 524 per 1000 | RR 1.6 | 230 | ⊕⊕⊝⊝ | |
| Time to complete ulcer healing | Available data were limited and not analysed | |||||
| Change in absolute ulcer size | Available data were limited and not analysed | |||||
| Ulcer recurrence | Not reported | |||||
| Adverse effects | 31 per 1000 | 44 per 1000 | RR 1.44 | 344 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | Not reported | |||||
| Direct costs | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors). 2 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (single study with very wide confidence intervals). 3 Downgraded one level for risk of bias (lack of allocation concealment) and one level for imprecision (single study with very wide confidence intervals). 4 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed (overall) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.12] |
| 2 Proportion of ulcers completely healed (sensitivity analysis) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3 Proportion of ulcers completely healed at 30 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Proportion of ulcers completely healed at 60 days Show forest plot | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.20, 2.28] |
| 5 Proportion of ulcers completely healed at 90 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Adverse effects Show forest plot | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.48, 4.34] |

