Agonistas de los receptores activados por proliferadores peroxisómicos gamma para la prevención del accidente cerebrovascular recurrente y otros eventos vasculares en personas con accidente cerebrovascular o accidente isquémico transitorio
Resumen
Antecedentes
Los agonistas de los receptores activados por proliferadores peroxisómicos gamma (PPAR‐γ) son fármacos sensibilizadores a la insulina utilizados para el tratamiento de la resistencia a la insulina. Además de reducir la glucosa en la diabetes, estos fármacos también pueden proteger contra la hiperlipidemia y la arteriosclerosis, que son factores de riesgo del accidente cerebrovascular. Esta es una actualización de una revisión publicada por primera vez en enero de 2014 y actualizada posteriormente en diciembre de 2017 y octubre de 2019.
Objetivos
Evaluar la eficacia y la seguridad de los agonistas de los PPAR‐γ en la prevención secundaria del accidente cerebrovascular y los eventos vasculares relacionados en las personas con accidente cerebrovascular o accidente isquémico transitorio (AIT).
Métodos de búsqueda
Se realizaron búsquedas en el Registro de ensayos del Grupo Cochrane de Accidentes cerebrovasculares (1 de enero de 2022), en el Registro Cochrane central de ensayos controlados (CENTRAL; 2021, número 12), en MEDLINE (1949 hasta el 1 de enero de 2022), en Embase (1980 hasta el 1 de enero de 2022), en CINAHL (1982 hasta el 1 de enero de 2022), en AMED (1985 hasta el 1 de enero de 2022) y en 11 bases de datos chinas (1 de enero de 2022). En un esfuerzo por identificar ensayos adicionales publicados, no publicados y en curso, se realizaron búsquedas en registros de ensayos en curso, listas de referencias y actas de congresos relevantes y se estableció contacto con autores y compañías farmacéuticas. No se impuso restricción de idioma.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) que evaluaron los agonistas de los PPAR‐γ versus placebo para la prevención secundaria del accidente cerebrovascular y los eventos vasculares relacionados en personas con accidente cerebrovascular o AIT, con los desenlaces de accidente cerebrovascular recurrente, eventos vasculares y eventos adversos.
Obtención y análisis de los datos
Dos autores de la revisión examinaron de forma independiente los títulos y los resúmenes de las entradas identificadas, seleccionaron los estudios para inclusión, extrajeron los datos elegibles, cotejaron la exactitud de los datos y evaluaron la calidad metodológica y el riesgo de sesgo. La calidad general de la evidencia para cada desenlace se evaluó con él método GRADE.
Resultados principales
Se identificaron cinco ECA con 5039 participantes; dos estudios tuvieron bajo riesgo de sesgo en todos los dominios. Cuatro estudios evaluaron el fármaco pioglitazona y un estudio evaluó la rosiglitazona. Los participantes en diferentes estudios fueron heterogéneos.
Accidente cerebrovascular recurrente
Tres estudios evaluaron el número de participantes con accidente cerebrovascular recurrente (4979 participantes, un solo estudio aportó 3876 de ellos). Es probable que los agonistas de los receptores activados por proliferadores peroxisómicos gamma reduzcan la recurrencia del accidente cerebrovascular en comparación con placebo (razón de riesgos [RR] 0,66; intervalo de confianza [IC] del 95%: 0,44 a 0,99; evidencia de certeza moderada).
Eventos adversos
La evidencia de que los eventos adversos se produjeron con mayor frecuencia en los participantes tratados con agonistas de los PPAR‐γ en comparación con placebo fue incierta debido al amplio intervalos de confianza y a los altos niveles de heterogeneidad estadística: diferencia de riesgos 10%; IC del 95%: ‐8% a 28%; evidencia de certeza baja).
Hubo datos disponibles sobre desenlaces compuestos adicionales que reflejaron eventos vasculares graves (muerte por todas las causas y otros eventos vasculares graves; mortalidad por todas las causas, infarto de miocardio no mortal o accidente cerebrovascular no mortal) a partir de un estudio con 984 personas. Este estudio proporcionó evidencia de certeza baja de que los agonistas de los PPAR‐γ provocaron menos eventos (los datos no fueron metanalizados).
Eventos vasculares
Los agonistas de los receptores activados por proliferadores peroxisómicos gamma administrados durante una duración media de 34,5 meses en un único ensayo con 984 participantes podrían reducir los eventos vasculares graves expresados como un desenlace compuesto de los eventos totales de muerte cardiovascular, infarto de miocardio no mortal o accidente cerebrovascular no mortal (RR 0,73; IC del 95%: 0,54 a 0,99; evidencia de certeza baja).
Otros desenlaces
Un estudio con 20 pacientes midió la sensibilidad a la insulina y un estudio con 40 personas midió la actividad de la ubiquitina‐proteasoma en las placas carotídeas. La confianza en las mejorías observadas con los agonistas de los PPAR‐γ se vio limitada por el tamaño muestral pequeño y el riesgo de sesgo. Ninguno de los estudios informó sobre el número de participantes con discapacidad debido a eventos vasculares ni sobre la mejoría en la calidad de vida.
Conclusiones de los autores
Los agonistas de los receptores activados por proliferadores peroxisómicos gamma probablemente reduzcan el accidente cerebrovascular recurrente y los eventos totales de muerte cardiovascular, infarto de miocardio no mortal o accidente cerebrovascular no mortal y podrían mejorar la sensibilidad a la insulina y la estabilización de las placas carotídeas. Sus efectos sobre los eventos adversos no están claros. Las conclusiones se deben interpretar con cautela, teniendo en cuenta el escaso número de estudios incluidos y la calidad de los estudios. Se necesitan más ECA doble ciego bien diseñados con muestras grandes para evaluar la eficacia y la seguridad de los agonistas de los PPAR‐γ en la prevención secundaria del accidente cerebrovascular y los eventos vasculares relacionados en personas con accidente cerebrovascular o AIT.
PICOs
Resumen en términos sencillos
Medicamentos para la diabetes para prevenir el ictus y otras enfermedades vasculares en personas que han sufrido un ictus o un accidente isquémico transitorio anterior
Pregunta
Se deseaba evaluar la efectividad y la seguridad de los nuevos medicamentos para la diabetes (agonistas de los receptores activados por proliferadores peroxisómicos gamma [PPAR‐γ]) para prevenir el ictus y la enfermedad vascular relacionada en personas que ya han sufrido un ictus o un accidente isquémico transitorio.
Antecedentes
Los agonistas de los receptores activados por proliferadores peroxisómicos gamma son medicamentos que mejoran la forma en que la insulina funciona en el cuerpo humano. Son muy utilizados en el tratamiento de la diabetes tipo adulto (diabetes tipo 2). Además, también pueden proteger contra la presencia de exceso de grasas en la sangre y contra la enfermedad de las paredes de las arterias, que son factores de riesgo para el ictus.
Características de los estudios
Se identificaron cinco estudios hasta el 1 de enero de 2022, con un total de 5039 participantes. Cuatro estudios evaluaron el fármaco pioglitazona y un estudio evaluó la rosiglitazona. Cuatro estudios incluyeron a participantes sin antecedentes de diabetes y un estudio solo incluyó a participantes con diabetes.
Resultados clave
En comparación con los comprimidos de placebo, los agonistas de los PPAR‐γ redujeron los ictus recurrentes y otras enfermedades vasculares, mejoraron la respuesta del cuerpo a la insulina y estabilizaron los depósitos de grasa en las paredes arteriales. Los medicamentos también parecieron ser bien tolerados, pero la evidencia al respecto no fue concluyente.
Calidad de la evidencia
Las conclusiones se deben interpretar con cautela, teniendo en cuenta el escaso número de estudios incluidos y la calidad limitada de algunos de los estudios. Se necesitan más ensayos controlados aleatorizados grandes y bien diseñados.
Authors' conclusions
Summary of findings
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke
Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ |
|
| Reported adverse events
Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝
|
|
| Serious vascular events | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: 126 of 498 participants (25%).
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: 98 of 498 participants (20%). | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: 98 of 486 participants (20%).
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: 76 of 486 participants (16%). | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: RR 0.80, 95% CI 0.63 to 1.01.
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: RR 0.79, 95% CI 0.61 to 1.04.
Composite of reduced fatal or non‐fatal stroke: RR 0.54, 95% CI 0.35 to 0.85.
Composite of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke: RR 0.73, 95% CI 0.54 to 0.99. | 984 | ⊕⊕⊝⊝
| Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — |
|
| Disability due to vascular events | Not reported | Not reported | — | — | — |
|
| Improvement in quality of life | Not reported | Not reported | — | — | — |
|
| Insulin sensitivity
Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; PPAR‐γ: peroxisome proliferator‐activated receptor gamma | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. | ||||||
Background
Description of the condition
Stroke is one of the most common neurological diseases and one of the most common causes of mortality worldwide (WHO 2010). On average 80% of strokes are caused by ischaemia, and recurrent strokes account for around 30% of all events (Goldstein 2006; Simon 2009). Significantly higher mortality was found in recurrent stroke compared with first‐ever stroke (Jørgensen 1997). Secondary prevention therefore plays an important role in reducing stroke recurrence and other related vascular events. Diabetes is an important risk factor for ischaemic stroke. It has been estimated that around one in eight or nine strokes in people with a history of stroke or transient ischaemic attack (TIA) could be attributed to diabetes (Emerging Risk Factors Collaboration 2010). Management of blood glucose can therefore be regarded as one possible target for stroke prevention.
Description of the intervention
Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists are insulin‐sensitising drugs used for the treatment of hyperglycaemia with insulin resistance. To date, PPAR‐γ agonists such as rosiglitazone and pioglitazone have been widely recommended in the treatment of people with type 2 diabetes (Mooradian 2002). In certain cases PPAR‐γ agonists can be used in combination with insulin or other hypoglycaemic agents. In view of their effect on lowering glucose, PPAR‐γ agonists are believed to be beneficial for stroke prevention. Common adverse events associated with PPAR‐γ agonists include oedema, anaemia, liver dysfunction, and cardiac failure (Fogg 2009). Moreover, an increased risk of mortality and vascular events was found with rosiglitazone compared with pioglitazone in people with diabetes older than 65 years of age (Graham 2010).
How the intervention might work
The mechanisms of hyperglycaemia and stroke have been widely discussed. Blood flow and vascular reactivity can be affected by hyperglycaemia due to the abnormal metabolism of endothelium‐derived nitric oxide (Melikian 2009). Hyperglycaemia is also associated with reduced penumbral salvage in the large‐vessel thromboembolic stroke (Els 2002). Peroxisome proliferator‐activated receptor gamma agonists can therefore prevent these pathological processes by controlling blood glucose. In addition to lowering glucose, PPAR‐γ agonists may protect against hyperlipidaemia and arteriosclerosis, which are complications of diabetes and risk factors for stroke (Collino 2010; Dasu 2009). Moreover, PPAR‐γ agonists have been shown to reduce inflammation, which may prevent vascular events to some extent (Nakamura 2007).
Why it is important to do this review
Evidence from trials indicates the benefits of aspirin, clopidogrel, ticlopidine, triflusal, and the combination of aspirin and dipyridamole in the secondary prevention of stroke and other vascular events (Costa 2005; De Schryver 2007; Sudlow 2009). Anticoagulants appear to be less effective, at least where the cause is not cardioembolic (Sandercock 2009). Vitamin K antagonists are not more efficacious than antiplatelet therapy (De Schryver 2012). Peroxisome proliferator‐activated receptor gamma agonists have been tested in clinical trials focusing on the prevention of stroke and other vascular events, particularly for people with diabetes. We thus aimed to evaluate the efficacy and safety of PPAR‐γ agonists for preventing recurrent stroke and other vascular events in people with stroke or TIA. To our knowledge, no other systematic review or meta‐analysis on this topic exists in the literature.
Objectives
To assess the efficacy and safety of PPAR‐γ agonists in the secondary prevention of stroke and related vascular events for people with stroke or transient ischaemic attack (TIA).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and excluded quasi‐randomised or confounded studies.
Types of participants
We included studies of people over the age of 18 years with a history of stroke or TIA. We used the definition of TIA as provided in the original publications. We excluded studies of people with diabetes who lacked a clear history of stroke or TIA.
Types of interventions
We included trials comparing PPAR‐γ agonists (including pioglitazone, rosiglitazone, glitazone, troglitazone, netoglitazone, rivoglitazone, ciglitazone, balaglitazone, darglitazone, edaglitazone, englitazone, and lobeglitazone) with placebo, regardless of the length of treatment period and dosage of treatment. We included other concomitant therapies providing they were administered to both the intervention and control groups.
Types of outcome measures
We measured all outcomes at the end of follow‐up.
Primary outcomes
-
The number of participants with recurrent stroke, as defined in the original publications.
-
The number of participants who experienced any adverse events, such as oedema, anaemia, or cardiac failure.
Secondary outcomes
-
The number of participants with serious vascular events, such as myocardial infarction, stroke, or vascular death.
-
The number of deaths due to vascular events.
-
The number of participants with disability due to vascular events.
-
Improvement in quality of life.
-
Insulin sensitivity.
-
Ubiquitin‐proteasome activity in carotid plaques.
Search methods for identification of studies
We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (1 January 2022) and the following electronic bibliographic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 12) in the Cochrane Library (searched 1 January 2022) (Appendix 1);
-
MEDLINE (Ovid) (1949 to 1 January 2022) (Appendix 2);
-
Embase (Ovid) (1980 to 1 January 2022) (Appendix 3);
-
CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature; 1982 to 1 January 2022) (Appendix 4);
-
AMED (Ovid) (Allied and Complementary Medicine Database; 1985 to 1 January 2022) (Appendix 5).
We developed all the search strategies with the help of the Cochrane Stroke Group Information Specialist.
We also searched the following ongoing trials registers on 1 January 2022:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/) (Appendix 6);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (Appendix 7).
Searching other resources
We searched the following resources with the Chinese equivalents of keywords (Appendix 8):
-
Chinese Clinical Trials Registry (searched on 1 January 2022);
-
CBM‐disc (China Biological Medicine Databases) (1979 to 1 January 2022);
-
CNKI (China National Knowledge Infrastructure) (1979 to 1 January 2022);
-
Chinese MD and DD Dissertations in CNKI (searched on 1 January 2022);
-
CACP (Chinese Academic Conference Papers Database) (1998 to 1 January 2022);
-
CDDB (Chinese Dissertations Database) (1977 to 1 January 2022);
-
Chinese Evidence‐Based Medicine Database (searched on 1 January 2022);
-
CMAC (China Medical Academic Conferences) (1994 to 1 January 2022);
-
CMCC (Chinese Medical Current Contents) (1994 to 1 January 2022);
-
Chinese Science and Technique Journals Database (VIP) (1989 to 31 January 2022);
-
Wanfang Data (www.wanfangdata.com/) (1984 to 1 January 2022).
We also:
-
used Science Citation Index Cited Reference Search for forward tracking of important articles;
-
searched reference lists of relevant reviews and retrieved articles;
-
searched relevant conference proceedings, including the 7th European Stroke Organisation Conference (2021) and the 4th to 13th World Stroke Congress (from 2000 to 2021);
-
contacted authors where necessary for missing information;
-
contacted the manufacturers (Takeda Pharmaceutical Company and GlaxoSmithKline Pharmaceuticals) for updated information.
Data collection and analysis
Selection of studies
Two review authors (JL, LW) independently screened titles and abstracts of the references identified by the search and excluded obviously irrelevant reports. We retrieved the full‐text articles for the remaining references, and the same two review authors independently screened the full‐text articles and identified studies for inclusion, and also identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted an external third party. We collated multiple reports of the same study so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Figure 1).
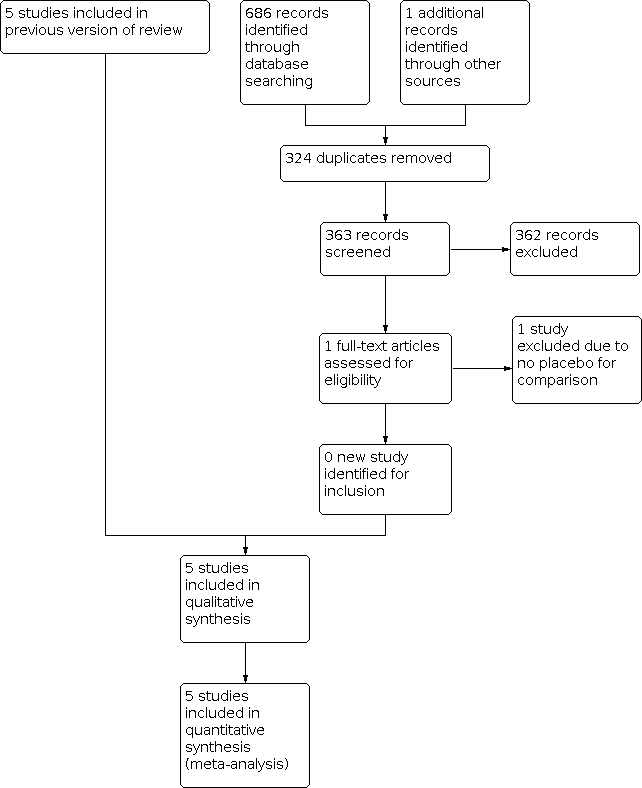
Study flow diagram
Data extraction and management
Two review authors (JL, LW) independently extracted data from the published reports onto standardised forms, and cross‐checked the data for accuracy. We used checklists to independently record details including methods of generating the randomisation schedule, method of concealment of allocation, blinding of assessors, intention‐to‐treat analysis, adverse events and dropouts for all reasons, important imbalance in prognostic factors, participants (socio‐demographic and related clinical information), interventions (medications and non‐pharmacological interventions), and outcomes. We resolved disagreements with an external third party.
Assessment of risk of bias in included studies
Two review authors (JL, LW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving an external third party. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded the risk of bias for each domain as high, low, or unclear and provided information from the study report together with a justification for our judgement in the risk of bias tables.
Measures of treatment effect
We expected the randomised controlled trials to measure dichotomous data. We expressed dichotomised data as risk ratios (RRs) with their 95% confidence intervals (CI). If a trial (or group within a trial) reported no adverse events or dropouts, we calculated risk differences (RDs) instead of RRs with 95% CI. We entered and analysed data using Review Manager 5 (RevMan 2020).
Unit of analysis issues
We dealt with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Dealing with missing data
We planned to contact the authors of the studies for further details if any data were missing or to establish the characteristics of unpublished trials. According to the intention‐to‐treat principle, all randomised participants should be included. We considered different scenarios (best‐case and worst‐case) to account for missing data.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis, considering I² values over 50% as suggestive of substantial heterogeneity.
Assessment of reporting biases
We planned to use funnel plots to examine potential publication bias if there was a sufficient number of trials (Egger 1997).
Data synthesis
Where we considered studies to be sufficiently similar, we conducted a meta‐analysis by pooling the appropriate data using Review Manager 5 (RevMan 2020). We expressed dichotomised data as RRs with their 95% CI. If a trial (or group within a trial) reported no adverse events or dropouts, we calculated RDs instead of RRs with 95% CI. We calculated the overall effects using a random‐effects model regardless of the level of heterogeneity. We provided a descriptive summary of the results when substantial heterogeneity between the studies prevented us from combining outcome data.
Subgroup analysis and investigation of heterogeneity
We intended to undertake subgroup analyses according to the age and ethnicity of participants, TIA definition, different PPAR‐γ agonists, and dosage and duration of treatment. We intended to use the Chi² test to examine the significance of differences between subgroups.
Sensitivity analysis
We analysed sensitivity by assessing the robustness of results in fixed‐effect versus random‐effects models. We planned to assess the impact of studies at high risk of bias. We also examined potential sources of methodological heterogeneity.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013), in relation to the following criteria.
-
Study design
-
Risk of bias
-
Inconsistency
-
Indirectness
-
Imprecision
-
Other considerations (including publication bias)
We used GRADEpro GDT software to create a summary of findings table for the comparison evaluated in the review (GRADEpro GDT). We included the following outcomes: recurrence of stroke, reported adverse events, serious vascular events, deaths due to vascular events, disability due to vascular events, improvement in quality of life, and insulin sensitivity, and used the table to guide our conclusions.
Results
Description of studies
Results of the search
The previous version of this review included five studies. On rerunning the searches in 1 January 2022, we identified 363 papers after deduplicating the results (Figure 1). We acquired and screened the full text of one article, which did not meet the inclusion criteria. We therefore included a total of five studies in this update. Agreement between the review authors on exclusion was 100%.
Included studies
In accordance with the inclusion criteria, we included five studies with 5039 participants. IRIS investigated the efficacy of pioglitazone in participants with insulin resistance and ischaemic stroke or TIA no less than 14 days and no more than six months before randomisation. J‐SPIRIT tested the effect of pioglitazone on the reduction of recurrent stroke in participants with abnormal glucose metabolisms and insulin resistance after ischaemic stroke. Kernan 2003 evaluated the effect of pioglitazone in improving insulin sensitivity among non‐diabetic patients with a recent TIA or non‐disabling ischaemic stroke. Marfella 2006 investigated the effect of rosiglitazone in participants with symptomatic carotid stenosis by testing ubiquitin‐proteasome activity in carotid plaques. PROactive focused on the efficacy and safety of pioglitazone on the reduction of stroke recurrence and related vascular events in participants with type 2 diabetes. The details of the included studies are provided in the Characteristics of included studies table.
Excluded studies
We excluded 15 studies after full‐text evaluation (CIMT Trial; Erdmann 2015; Forst 2008; Hedblad 2007; ISRCTN54951661; Kiran 2020; Koshiyama 2001; Lincoff 2014; Meisner 2006; NCT00879970; Schrieks 2018; Sidhu 2004; Tanaka 2015; TART; TRIPOD). The reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Information regarding risk of bias is provided in Figure 2 and Figure 3. The data for J‐SPIRIT were only released in abstracts, therefore insufficient information was available for us to judge the risk of bias.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Two studies clearly stated the methods of randomisation and allocation concealment (IRIS; Kernan 2003). Information for the remaining studies was insufficient to make a judgement of high or low risk of bias, therefore we judged selection bias to be at unclear risk.
Blinding
Information regarding blinding was insufficient in two studies (J‐SPIRIT; PROactive). In Kernan 2003 and IRIS, information about performance bias and detection bias was provided, while only information about detection bias was reported in Marfella 2006. We therefore assessed this to be at unclear risk of bias.
Incomplete outcome data
J‐SPIRIT did not report on completeness of outcome data. No dropouts were reported in Kernan 2003 or Marfella 2006. In IRIS, a total of 227 participants (5.9%) withdrew consent and 99 (2.6%) were lost to follow‐up; we assessed this study to be at low risk of bias. In PROactive, 882 of 984 participants (90%) completed the final visit: 439/486 (90%) in the pioglitazone group and 443/498 (89%) in the placebo group. We assessed this as a high risk of attrition bias.
Selective reporting
With the exception of J‐SPIRIT, all prespecified outcomes were reported in the included trials. We therefore assessed this as low risk of reporting bias.
Other potential sources of bias
The role of financial support was clearly stated in Kernan 2003, so we judged this study to be at low risk of other bias. We judged the remaining four studies to have an unclear risk of other bias (IRIS; J‐SPIRIT; Marfella 2006; PROactive).
Effects of interventions
summary of findings Table 1 presents key outcomes for this review.
Primary outcome measures
Number of participants with recurrent stroke
Three studies with 4979 participants reported the number of recurrent strokes at the end of the study: 156/2483 (6%) participants in the PPAR‐γ agonist group and 212/2496 (8%) participants in the placebo group (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.44 to 0.99; moderate‐certainty evidence) (Analysis 1.1) (IRIS; J‐SPIRIT; PROactive). When we conducted a sensitivity analysis with a fixed‐effect model, the results did not vary substantially (RR 0.74, 95% CI 0.61 to 0.90).
Number of participants who experienced any adverse events
Three studies reported the number of participants with any adverse events (Kernan 2003; Marfella 2006; PROactive). Adverse events were experienced by 247/516 (48%) and 260/528 (49%) participants in the PPAR‐γ agonists and placebo groups, respectively (risk difference (RD) 10%, 95% CI ‐8% to 28%; low‐certainty evidence), with strong heterogeneity (test for heterogeneity I² = 86%) (Analysis 1.2). When we conducted a sensitivity analysis with a fixed‐effect model, the result was: RD ‐1%, 95% CI ‐7% to 5%. The RDs of the random‐effects and fixed‐effect models were essentially the same. In Kernan 2003, certain adverse events such as nausea, oedema, muscle aches, sore throat, and dizziness were more common in the pioglitazone group. In Marfella 2006, no clinical events were reported in either group during the study. The proportion of participants who experienced adverse events was the highest in PROactive, with definitions of adverse events including: resulting in death, life‐threatening, needing or prolonging in‐patient admission, resulting in persistent or significant disability, or needing intervention to prevent any of the above. As a result, heart failure requiring hospitalisation was reported in 31 (6.4%) participants in the pioglitazone group versus 20 (4.0%) in the placebo group (P = 0.09). Fatal heart failure was reported in 6 (1.2%) participants in the pioglitazone group versus 4 (0.8%) participants in the placebo group (P = 0.50). IRIS only reported the number of each adverse event. In general, participants in the pioglitazone group had more weight gain, oedema, shortness of breath, and bone fractures than did participants in the placebo group.
Secondary outcome measures
Number of participants with serious vascular events
Only one study reported the number of participants with serious vascular events (PROactive). For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98/486 (20%) and 126/498 (25%) participants in the PPAR‐γ agonists and placebo groups, respectively (RR 0.80, 95% CI 0.63 to 1.01; low‐certainty evidence). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76/486 (16%) and 98/498 (20%) participants in the PPAR‐γ agonists and placebo groups, respectively (RR 0.79, 95% CI 0.61 to 1.04; low‐certainty evidence). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85; low‐certainty evidence) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99; low‐certainty evidence).
Number of deaths due to vascular events
None of the studies reported the number of deaths due to vascular events. However, PROactive reported all‐cause mortality: 46/486 (9%) and 49/498 (10%) deaths in the PPAR‐γ agonists and placebo groups, respectively (RR 0.96, 95% CI 0.66 to 1.41).
Number of participants with disability due to vascular events
None of the studies reported the number of participants with disability due to vascular events.
Improvement in quality of life
None of the studies reported improvement in quality of life.
Insulin sensitivity
In Kernan 2003, insulin sensitivity was measured with the composite insulin sensitivity index. The change in the composite index was 1.2 ± 0.6 (mean ± standard deviation) in the pioglitazone group and ‐0.1 ± 0.6 in the placebo group (P = 0.0003). The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L in the pioglitazone group, but increased from 0.41 to 0.45 mg/L in the placebo group (P = 0.06).
Ubiquitin‐proteasome activity in carotid plaques
Marfella 2006 examined the ubiquitin‐proteasome activity in carotid plaques. Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01); less ubiquitin (322 ± 79 ng/mg in the rosiglitazone group and 468.7 ± 89 ng/mg in the placebo group, P < 0.01), proteasome 20S (46.8 ± 10 pmol/mg in the rosiglitazone group and 79.8 ± 25 pmol/mg in the placebo group, P < 0.01), and nuclear factor kappa B (NFkB) (P < 0.01); less nitrotyrosine (2.2 ± 0.21 nmol/pg in the rosiglitazone group and 3.5 ± 0.42 nmol/pg in the placebo group, P < 0.01) and superoxide anion production (3.57 ± 1.1 pmol/L in the rosiglitazone group and 6.26 ± 1.4 pmol/L in the placebo group, P < 0.01); and more collagen content (P < 0.01), suggesting greater plaque stabilisation.
Discussion
Summary of main results
Five studies with 5039 participants met the inclusion criteria. Four of these studies evaluated the effect of pioglitazone versus placebo (IRIS; J‐SPIRIT; Kernan 2003; PROactive); the fifth focused on rosiglitazone versus placebo (Marfella 2006). Three studies evaluated the number of participants with recurrent stroke (IRIS; J‐SPIRIT; PROactive), where PPAR‐γ agonists reduced the recurrence of stroke compared with placebo (RR 0.66, 95% CI 0.44 to 0.99; moderate‐certainty evidence). Moreover, PPAR‐γ agonists reduced total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke and improved insulin sensitivity and stabilisation of carotid plaques (Kernan 2003; Marfella 2006; PROactive). Regarding safety, evidence was inconclusive of any difference in reported adverse events in the PPAR‐γ agonists group versus the placebo group (RD 10%, 95% CI ‐8% to 28%; low‐certainty evidence) in three studies (Kernan 2003; Marfella 2006; PROactive).
Overall completeness and applicability of evidence
All participants had a past history of stroke or TIA; however, none of the randomised controlled trials precisely described stroke type or diagnosis criteria. Kernan 2003 evaluated non‐diabetic participants, IRIS and J‐SPIRIT participants with an abnormal glucose metabolism and insulin resistance, PROactive participants with type 2 diabetes, and Marfella 2006 participants with carotid plaques. The clinical heterogeneity of the participants in the included studies could potentially induce heterogeneity in the results of the meta‐analysis. Four studies evaluated pioglitazone versus placebo (IRIS; J‐SPIRIT; Kernan 2003; PROactive), and one study evaluated rosiglitazone versus placebo (Marfella 2006). Regarding outcomes, three studies recorded recurrent stroke (IRIS; J‐SPIRIT; PROactive). The other two studies investigated insulin sensitivity and ubiquitin‐proteasome activity in carotid plaques, respectively (Kernan 2003; Marfella 2006). Due to insufficient data, we did not undertake any subgroup analyses.
Quality of the evidence
We judged IRIS and Kernan 2003 to be of high certainty with a low risk of bias for all domains. J‐SPIRIT was only published as abstracts, therefore information was insufficient to judge. We assessed Marfella 2006 and PROactive to be at unclear risk of bias for the method of randomisation, allocation concealment, and blinding, and PROactive to be at high risk of bias for incomplete outcome data. In summary, the methodological limitations of the included studies should be considered when interpreting the results. In addition, the sample sizes in Kernan 2003 and Marfella 2006 were relatively small.
Potential biases in the review process
We performed the search strategy as per protocol and identified five completed studies. However, we cannot be certain that we have not missed other unpublished studies. Our contact with relevant authors and manufacturers yielded no additional information. In preparing this review, we independently screened trials for inclusion, extracted data, and assessed the risk of bias of included trials to minimise potential biases. We used the RD as a way of addressing studies with zero events. The event rates of the number of participants with adverse events reported, based on the aggregate event rates for the two groups, were 48% versus 49%, a difference of 1%. With the random‐effects model, the outlying estimate from Kernan 2003 effectively pushed the RD out towards 10%. This might well explain why the difference in the aggregate data of 1% did not translate to a RD of 10% (the fixed‐effect result was much closer to the aggregate events). We found no other potential biases.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review of this intervention in the field of secondary prevention of stroke and related vascular events for people with stroke or TIA.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1: Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1: Recurrence of stroke

Comparison 1: Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2: Reported adverse events
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke
Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ |
|
| Reported adverse events
Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝
|
|
| Serious vascular events | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: 126 of 498 participants (25%).
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: 98 of 498 participants (20%). | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: 98 of 486 participants (20%).
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: 76 of 486 participants (16%). | Composite of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation: RR 0.80, 95% CI 0.63 to 1.01.
Composite of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke: RR 0.79, 95% CI 0.61 to 1.04.
Composite of reduced fatal or non‐fatal stroke: RR 0.54, 95% CI 0.35 to 0.85.
Composite of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke: RR 0.73, 95% CI 0.54 to 0.99. | 984 | ⊕⊕⊝⊝
| Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — |
|
| Disability due to vascular events | Not reported | Not reported | — | — | — |
|
| Improvement in quality of life | Not reported | Not reported | — | — | — |
|
| Insulin sensitivity
Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; PPAR‐γ: peroxisome proliferator‐activated receptor gamma | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| 1.2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |

