Arteméter para el paludismo grave
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Trial design: Open label RCT | |
| Participants | Number of participants: 41 children enrolled Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether (Kunming Pharmaceuticals; China)
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Outpatient clinic in New Halfa, Eastern Sudan Funding: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each child was randomized". No further details provided. |
| Allocation concealment (selection bias) | Low risk | "Envelopes containing the assigned treatment were opened sequentially at the time when each patient was recruited to the study". |
| Blinding (performance bias and detection bias) | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: July to October 2007 | |
| Participants | Number of participants: 90 children enrolled Inclusion criteria: Children between six months and 12 years of age presenting with fever (> 37.5°C ) and P. falciparum infection with one or more general danger signs of severe or complicated malaria based on the WHO criteria for severe malaria Exclusion criteria: Serious concomitant illness, for example, sickle cell anaemia, HIV, tuberculosis and other chronic diseases, severe malnutrition, known hypersensitivity to one of the trial drugs. | |
| Interventions | 1. Intramuscular artemether (Paluther; May and Baker)
2.Intravenous or intramuscular quinine (Quinimax; Sanofi)
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Federal Medical Centre, Birnin Kudu, Jigawa State of Nigeria Transmission: Stable perennial transmission Funding: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were assigned to receive quinine if the last digit of their hospital identification number was odd and to receive artemether if the last digit of their |
| Allocation concealment (selection bias) | High risk | Trial authors did not describe any methods of allocation concealment, and this would not be possible using this randomization method. |
| Blinding (performance bias and detection bias) | Low risk | No blinding was described. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | No blinding is described, and blinding would not be feasible. |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up at day 14 were > 10% in both trial arms. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Double blind RCT Trial dates: Not stated | |
| Participants | Number of participants: 560 adults aged 15 to 79 years enrolled Inclusion criteria: Patients were included in the trial if they (or an accompanying relative) gave informed consent, had asexual forms of P. falciparum on a peripheral‐blood smear, were older than 14 years, were not in the first trimester of pregnancy, were not intravenous drug users, had received less than 3 g of quinine or two doses of artemisinin or a derivative in the previous 48 hours, and had one or more of the following: a score on the Glasgow Coma Scale of less than 11 (indicating cerebral malaria); anaemia (hematocrit, 20 percent), with a parasite count exceeding 100,000 parasites/mm3 on a peripheral‐blood smear; jaundice (serum bilirubin, 2.5 mg/dL (50 mmol per litre)), with a parasite count of more than 100,000 parasites/mm3 on a peripheral‐blood smear; renal impairment (urine output, 400 mL per 24 hours; and serum creatinine, 3 mg/dL (250 mmol/L)); hypoglycaemia (blood glucose, 40 mg/dL (2.2 mmol/L)); hyperparasitaemia (10% parasitaemia); and systolic blood pressure below 80 mmHg with cool extremities (indicating shock). Exclusion criteria: None stated | |
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical)
2.Intramuscular quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Special Research Ward, Centre for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: Not stated Funding: Wellcome Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Low risk | "The drugs for each patient were placed in a coded sealed envelope and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible." |
| Blinding (performance bias and detection bias) | Low risk | "To maintain blinding, a separate team of nurses, who were not otherwise involved with the care of the study patients, drew up and gave the injections. The drugs were kept in an opaque packet in a locked cabinet during the study". Both interventions were administered by intramuscular injection so blinding was feasible." |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: April 2000 to July 2001 | |
| Participants | Number: 46 children aged 6 months to 12 years enrolled Inclusion criteria: Asexual forms of P. falciparum from peripheral blood smear. One or more clinical manifestations of severe malaria present which included – cerebral malaria, severe anaemia (haemoglobin < 5 g/dL or hematocrit < 15%) metabolic abnormalities (hypoglycaemia: plasma glucose < 40 mg/dL or < 2.2 mmol/L), algid malaria (associated with peripheral circulatory failure or shock), black‐water fever, renal failure, spontaneous bleeding (thrombocytopenia, DIC), pulmonary edema and jaundice. Exclusion criteria: History of having received artemether /quinine within 24 hours preceding admission. Severe protein energy malnutrition or clinical/laboratory evidence of other significant illness not attributable to severe malaria. | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
Supportive therapy was given to all patients. | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None | |
| Notes | Location: Inpatient unit of Department of Pediatrics, and Parasitology laboratory, Department of Microbiology, Uttar Pradesh, India Transmission: Unknown Funding: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors provided no information on methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | Low risk | "The slides for malarial parasites were transported to the parasitology laboratory where the person examining the slides was unaware of the clinical status of the patient and also the treatment assignment group". |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: May to December 1991 | |
| Participants | Number of participants: 26 adults aged 15 to 45 years enrolled Inclusion criteria: Patients with severe falciparum malaria (WHO definition) with no history of antimalarials within 24 hours prior to admission, aged between 15 to 45 years and weighed 45 to 60kg Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether (Arthermin®)
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: Not stated Funding: Support from United Medical Ltd., Bangkok (provided artemether) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomized to receive either quinine or artemether". |
| Allocation concealment (selection bias) | Unclear risk | Trial authors provided no information on allocation concealment. |
| Blinding (performance bias and detection bias) | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | Trial authors provided no information on blinding, however it may not be feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates:1992 to 1994 | |
| Participants | Number of participants: 102 adults aged between 15 and 55 years enrolled Inclusion criteria: Male and female (non‐pregnant) patients with severe falciparum malaria (WHO definition) with no history of antimalarial treatment within 24 hours before admission aged 15 to 65 years and weighing 45 to 75kg Exclusion criteria: Patients with concurrent diseases were excluded | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Prapokklao Hospital, Chantaburi, Thailand Transmission: Not stated Funding: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization at WHO Office. |
| Allocation concealment (selection bias) | Low risk | "Each treatment was enclosed in a sealed envelope, which was opened only after the physician in charge had decided to recruit the patient into the study". |
| Blinding (performance bias and detection bias) | Low risk | Trial authors provided no information on blinding. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | Trial authors provided no information on blinding, however it may not be feasible due to different routes of administration for both interventions. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up about 5%. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: June 1993 to February 1994 and June 1994 to December 1994 | |
| Participants | Number of participants: 67 children aged three months to 15 years enrolled Inclusion criteria: Fever (core temperature ≥ 38 °C), positive blood smear for P. falciparum with ≥ 0.1% of parasitized erythrocytes, one major criterion or two minor criteria for severe malaria cases (WHO criteria) and parental consent Exclusion criteria: Patients with infection who had been treated within 24 hours with quinine or intramuscular injection was not eligible. | |
| Interventions | 1. Intramuscular artemether (Paluther)
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Gabriel Touré's Hospital, Mali Transmission: Unknown Funding: Rhône‐Poulenc Rorer Doma (France) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization by clinical monitor. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used to conceal allocation. |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. No blinding is described, and blinding would not be feasible. |
| Blinding (performance bias and detection bias) | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: Not stated | |
| Participants | Number: 160 children aged five months to 12 years enrolled Inclusion criteria: Children were admitted to the trial if they had P. falciparum asexual parasitaemia, were comatose and parental consent was obtained. Exclusion criteria: Children were excluded if there was evidence of a pre‐existing neurological deficit, head injury, or history of recent treatment with antimalarial drugs other than chloroquine. | |
| Interventions | 1. Intramuscular artemether (Paluther, Rhône‐Poulenc)
2. Intravenous quinine (Laboratoires Renaudin, Paris)
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Kenya Medical Research Institute (KEMRI) Coastal Research Unit, Kilifi district hospital, Kenya. Transmission: Unknown Funding:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally‐coded unique trial numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by the clinical monitor. |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | An open‐label trial. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) | Low risk | 40 patients (14 from artemether arm and 26 from quinine arm) excluded. Mostly for not meeting inclusion criteria. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: Not stated | |
| Participants | Number of participants: 37 children enrolled (age range not stated) Inclusion criteria: Children with unrousable coma, asexual forms of P. falciparum parasitaemia and no other identifiable cause of coma. Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None | |
| Notes | Location: University of Ilorin Teaching Hospital, Nigeria Transmission: Unknown Funding: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to either of the two treatment modalities". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Blinding (performance bias and detection bias) | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) | High risk | No information about blinding provided by trial authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label RCT Trial dates: Not stated | |
| Participants | Number of participants: 103 children aged 11 months to five years enrolled Inclusion criteria: Children aged six months to 5 years satisfying the WHO criteria for cerebral malaria, viz. unrousable coma lasting more than 30 minutes (with or without convulsions) with the presence of peripheral P. falciparum parasitaemia were included in the trial. Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine (Lemquine®)
Loading dose quinine was omitted in patients with a positive history of quinine or mefloquine ingestion in the preceding 24 hours before hospital presentation. | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Emergency Paediatric ward, University College Hospital, Ibadan, Nigeria Transmission: Unknown Funding: World Bank/UNDP/WHO special fund for Research and Training in Tropical Diseases (TDR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Unclear risk | Methods not described by trial authors. |
| Blinding (performance bias and detection bias) | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: Not stated | |
| Participants | Number: 32 children aged one to 12 years enrolled Inclusion criteria: Children aged one to 12 years, with fever (temperature > 37.5°C), presence of convulsion, vomiting, hypoglycaemia, anaemia and headache. Informed consent obtained from the parents and guardians. Assurance that patients will be resident within catchments of trial for follow‐up. Absence of concomitant illness such as bronchopneumonia, typhoid, meningitis, urinary tract infection. Exclusion criteria: History of blood transfusion in the last two months, presence of concomitant illness, history of previous allergy to quinine and artemether. | |
| Interventions | 1. Intramuscular artemether (Rhône‐Poulence, Rorer France)
2. Intravenous quinine (Evans)
Treatment with quinine was for a total of seven days. | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review: None | |
| Notes | Location: Overcomers Specialist Clinic Ileshan and General Hospital Ikenne, Nigeria Transmission: Unknown Funding: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The children were randomly allocated into 2 treatment groups; treatment |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided by trial authors. |
| Blinding (performance bias and detection bias) | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by two different routes. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition about 6% and not likely to affect outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Authors have published three outcomes in three different publications from the same trial. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Double blind RCT Trial dates: May 1996 to June 2003 | |
| Participants | Number of participants: 370 adults aged between 15 and 77 years enrolled Inclusion criteria: Peripheral blood smears had asexual forms of P. falciparum and had at least one of the following severe complications: cerebral malaria (Glasgow Coma Score was less than 11), renal acute failure (oliguria and serum creatinine > 250 μmol/L), jaundice (total serum bilirubin > 50 μmol/L) with a parasite count of more than 100,000/μL or with serum creatinine > 250 μmol/L, hypoglycaemia (blood glucose < 2.2 mmol/L), anaemia (haematocrit < 20%) with a parasite count of more than 100,000/μL, hyperparasitaemia (parasite count > 500,000/μL), hyperlactataemia (plasma lactate > 4 mmol/L), metabolic acidosis (standard base excess > ‐ 5 mmol/L, base deficit < 10 mmol/L) and shock (systolic blood pressure < 80 mmHg with cool extremities). Exclusion criteria: Patients were not included if they were < 14 years, were pregnant in the first trimester, were known intravenous drug abusers, had received more than 3 g of quinine or two doses of any artemisinin derivatives in the previous 48 hours before admission, had a past history of allergy to any artemisinin derivatives, or if known to be HIV positive. | |
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical Company, Kunming, China)
2.Intramuscular artesunate (Guilin No 2 Pharmaceutical Factory, Guangxi,China)
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam Transmission: Not stated Funding: Wellcome Trust, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was generated from random number tables". |
| Allocation concealment (selection bias) | Low risk | "Labels with the name of drug for each patient were put in coded sealed opaque envelopes, and the envelopes were randomized in blocks of 20. Once a patient was enrolled in the study the envelope was opened". |
| Blinding (performance bias and detection bias) | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Blinding (performance bias and detection bias) | Low risk | "An independent team of nurses, not otherwise involved in the study or responsible for the care of these patients, open the envelope, randomized the patient and prepared the injection. Neither the treating physicians, study doctors and nurses, or patients knew which anti‐malarial drugs was administered". |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: RCT Trial dates: May 1995 to June 1996 | |
| Participants | Number: 77 children enrolled aged three months to 15 years Inclusion criteria: Children who satisfied the WHO criteria for diagnosis of cerebral malaria (such as unrousable coma for at least half an hour following convulsions, positive blood film for malaria, exclusion of other causes of encephalopathy and informed consent by parent or guardian). Exclusion criteria: Concomitant acute illness such as pneumonia, meningitis or acute renal failure and any contraindication to IM injection. | |
| Interventions | 1.Intramuscular artemether
2. Intravenous quinine
Treatment with quinine was for a total of seven days | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Khartoum Children's Emergency Hospital and Ahmed Gasim Specialist Hospital for Children, Sudan Transmission: Unknown Funding:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The cases were randomly allocated into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided by trial authors about allocation concealment. |
| Blinding (performance bias and detection bias) | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by 2 different routes. |
| Incomplete outcome data (attrition bias) | High risk | > 10% of participants in each arm dropped out before Day 28. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open‐label, RCT Trial dates: June 1992 to May 1995 | |
| Participants | Number: 33 adults aged above 12 years Inclusion criteria: Blood smear showed asexual forms of P. falciparum; in addition to fulfilling one or more of the WHO criteria for severe or complicated malaria Exclusion criteria: Patients under the age of 12 years, pregnant women, those who had received parenteral antimalarial treatment prior to admission and those with a co‐existent bacterial, viral, fungal or mixed malarial infection were excluded. | |
| Interventions | 1. Intramuscular artemether (Rhone‐Poulenc Rorer)
2. Intravenous Quinine (Medipharma)
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:None | |
| Notes | Location:Port Moresby General Hospital, Papua New Guinea Transmission: Seasonal/Sporadic Funding:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned to treatment with quinine or artemether. |
| Allocation concealment (selection bias) | Low risk | Envelopes containing the assigned treatment were opened sequentially when a patient was recruited. |
| Blinding (performance bias and detection bias) | Low risk | Unlikely to be biased whether blinding was done or not. |
| Blinding (performance bias and detection bias) | High risk | No information of blinding reported by authors. Blinding unlikely as artemether and quinine were given by two different routes. |
| Incomplete outcome data (attrition bias) | High risk | Differential proportion of withdrawals from both arms (25% versus 10%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label RCT Trial dates: January 1992 to June 1994 | |
| Participants | Number: 183 children enrolled (age range not stated) Inclusion criteria: Children with asexual forms of P. falciparum detected in a peripheral blood smear, and a Blantyre Coma Score ≤ 2, and if no other cause of fever or altered consciousness could be discovered. Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Paediatric ward at the Queen Elizabeth Central Hospital, Malawi Transmission: Unknown Funding: UNDP/World Bank/WHO special programme for research and training in tropical diseases (TDR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. |
| Allocation concealment (selection bias) | Low risk | "Randomized treatment assignments were prepared in blocks of ten by the sponsoring agency.Following initial stabilization, diagnosis and examination, a sealed envelope containing the treatment group was opened, and the patient |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) | High risk | Differential proportion of withdrawals from both arms (13% versus 8%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open label trial Trial dates: 1992 to 1994 | |
| Participants | Number: 576 children aged one to nine years enrolled Inclusion criteria: Unconscious children one to nine years of age with a Blantyre coma score of 2 or less, asexual forms of P. falciparum were identified on a thick blood film, and a parent or guardian gave informed consent. Exclusion criteria: Patients with diseases other than malaria at the time of admission and those who recovered consciousness immediately after correction of hypoglycaemia or within one hour if they were convulsing on admission. Patients treated with quinine before admission. | |
| Interventions | 1. Intramuscular artemether (Paluther, Rhone‐Poulenc)
2. Intravenous quinine (Rotexmedica, Germany)
An oral dose of approximately 1.25 mg/kg pyrimethamine and 25 mg/kg sulfadoxine was given to both arms to reduce recrudescence (in the 2nd and 3rd years of the trial). | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Royal Victoria Hospital and Sibanor Health Centre,Banjul Gambia Transmission:Unknown Funding:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details of sequence generation |
| Allocation concealment (selection bias) | Low risk | "The treatment code for each child was stored in a sealed envelope that was opened after the admission procedure was completed and parental consent had been obtained". |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | Low risk | "Each blood film was examined by two independent observers who were unaware of the treatment code". |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were recorded. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: March 1992 to September 1994 | |
| Participants | Number: 124 adults aged between 16 and 66 years Inclusion criteria: 15 years of age or older, with clinical symptoms and signs of malaria and the presence of asexual forms of P. falciparum in their peripheral blood. In addition to having at least one of the following signs: (i) unrousable coma (Glasgow coma score < ll); (ii) hypoglycaemia (blood glucose < 2.2 mmol/L (40 mg %); (iii) acute renal failure (plasma creatinine > 265.2 µmol/L (3 mg %) with or without oliguria); (iv) jaundice (total bilirubin > 51.3 mol/L (3 mg %)) with parasitaemia > 100,000/µL or with plasma creatinine >1.5 mg %; (v) anaemia (haematocrit < 20%) Exclusion criteria: Patients were excluded from the trial if prior treatment with more than 3 g of quinine or two doses of artemisinin or a derivative had been recorded by the peripheral health care worker. Pregnant patients in the | |
| Interventions | 1.Intramuscular artemether (Kunming Pharmaceutical, Yunnan, China)
2.Intramuscular artesunate (Guilin No. 2 Pharmaceutical Factory, Guangxi, China)
3. Intravenous artesunate (Guilin No. 2 Pharmaceutical Factory, Guangxi, China)
All patients received 750 mg mefloquine (Lariam®, Roche) as a single dose after regaining consciousness or at day 4. | |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: Tan Phu regional Hospital, Vietnam Transmission: Endemic Funding: Roche Asian Research Foundation, Hong Kong | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial authors do not provide details about random sequence generation. |
| Allocation concealment (selection bias) | Low risk | "When a patient fulfilled the enrolment criteria, a sealed envelope containing the code for the treatment regimen was opened to allocate him/her to one of the following 4 treatment groups". |
| Blinding (performance bias and detection bias) | Low risk | Described as open‐label. However, lack of blinding is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | An open‐label trial. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial design: Open RCT Trial dates: Not stated | |
| Participants | Number: 54 children aged one to five years enrolled Inclusion criteria: Patients were admitted if they satisfied the strict WHO definition of cerebral malaria. Exclusion criteria: None stated | |
| Interventions | 1. Intramuscular artemether
2. Intravenous quinine
| |
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
| |
| Notes | Location: University College Hospital, Ibadan, Nigeria Transmission:Unknown Funding: World Bank/UNDP/WHO special fund for Research and Training in Tropical Diseases (TDR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers used. |
| Allocation concealment (selection bias) | Low risk | "Each child was then assigned a random number from a list prepared by an independent collaborator and thus allocated at random to receive either intramuscular artemether or intravenous quinine". |
| Blinding (performance bias and detection bias) | Low risk | An open‐label trial is unlikely to bias an objective outcome like death. |
| Blinding (performance bias and detection bias) | High risk | "This was a randomized, open, controlled study in which no attempt was made to ‘blind’ the investigators, as the test drug and the control drug were given by 2 different routes". |
| Incomplete outcome data (attrition bias) | Low risk | Only one patient excluded from fever clearance time outcome assessment because of urinary tract infection. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No other bias identified. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Intervention was rectal artemisinin. | |
| Not a RCT. | |
| Drug‐resistant malaria not severe malaria. | |
| Uncomplicated malaria. | |
| Comparison not parenteral treatment. | |
| Not a RCT. | |
| Comparison not parenteral treatment. | |
| Not a RCT. | |
| Not a RCT. | |
| No desired review outcomes. | |
| Patients not randomly selected (non‐probability consecutive sampling used). | |
| Design is a case‐control prospective study. | |
| Not a RCT. | |
| Participants in the artemether arm also received single dose of mefloquine. | |
| Participants not randomized. |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Multicentre trial |
| Participants | Information not available |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
| Methods | RCT |
| Participants | 105 adults enrolled |
| Interventions | Information not available |
| Outcomes | Information not available |
| Notes | Information not available |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
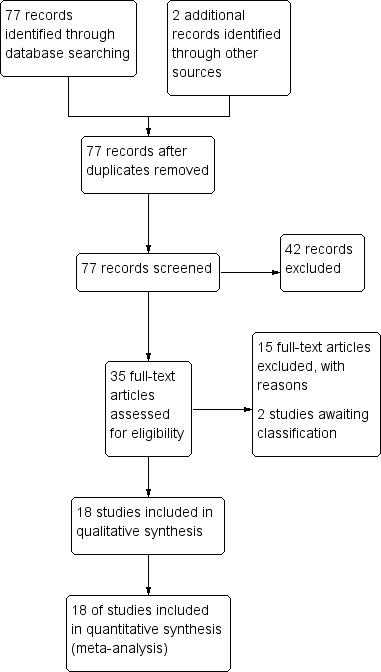
| 1 Death Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 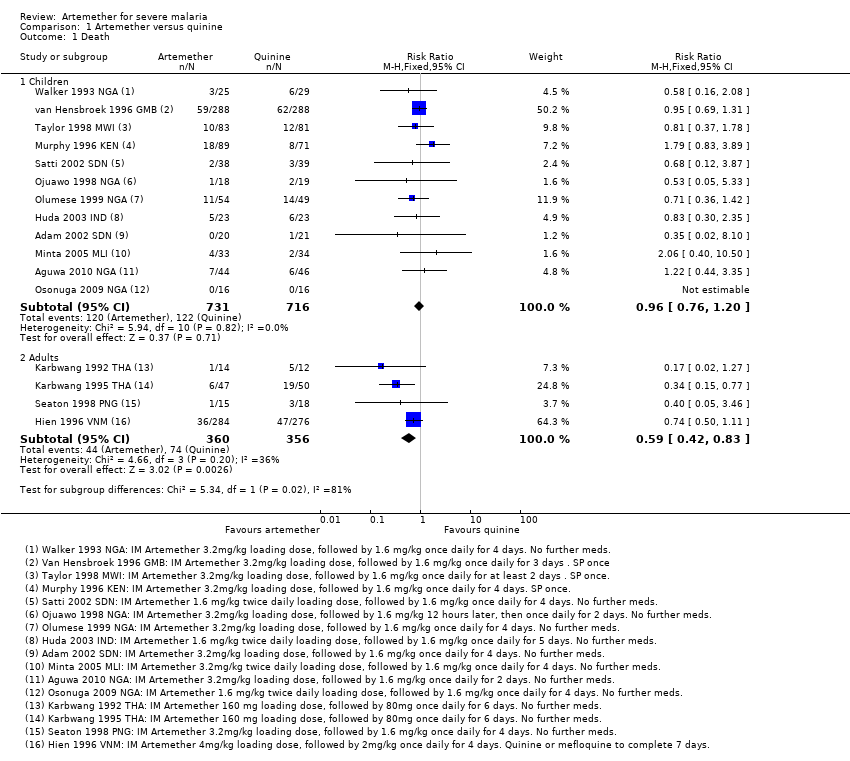 Comparison 1 Artemether versus quinine, Outcome 1 Death. | ||||
| 1.1 Children | 12 | 1447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.20] |
| 1.2 Adults | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Death: Time since admission to hospital Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Artemether versus quinine, Outcome 2 Death: Time since admission to hospital. | ||||
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Artemether versus quinine, Outcome 3 Coma resolution time (hours). | ||||
| 3.1 Children | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐5.45 [‐7.90, ‐1.00] |
| 4 Neurological sequelae at discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 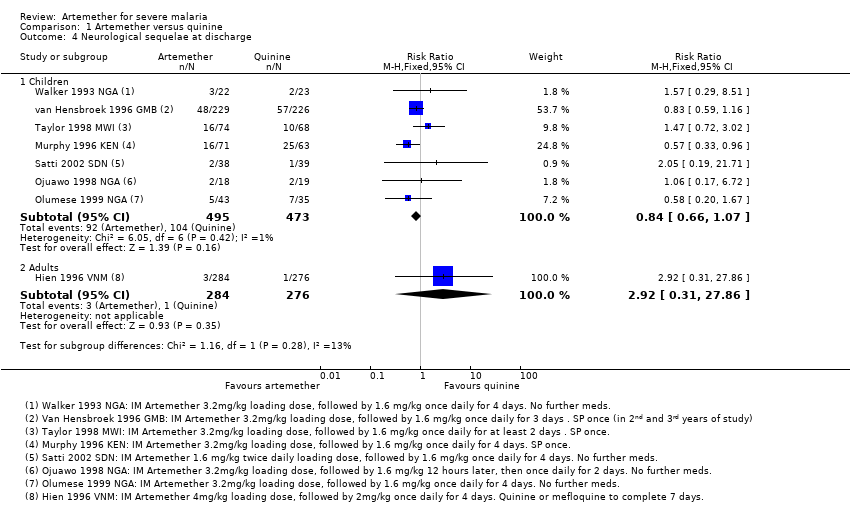 Comparison 1 Artemether versus quinine, Outcome 4 Neurological sequelae at discharge. | ||||
| 4.1 Children | 7 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up Show forest plot | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| Analysis 1.5 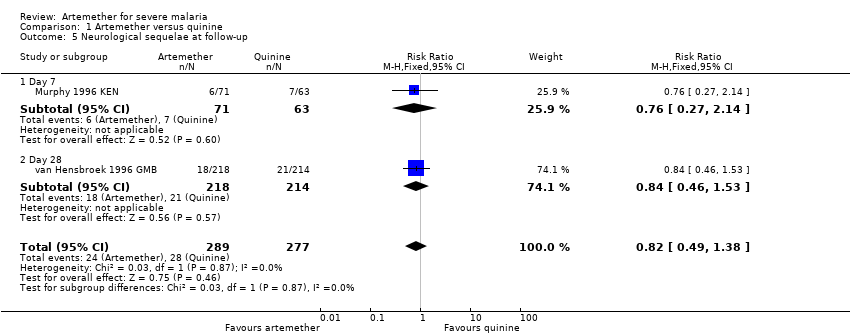 Comparison 1 Artemether versus quinine, Outcome 5 Neurological sequelae at follow‐up. | ||||
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time Show forest plot | 8 | 446 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐11.20, ‐6.45] |
| Analysis 1.6 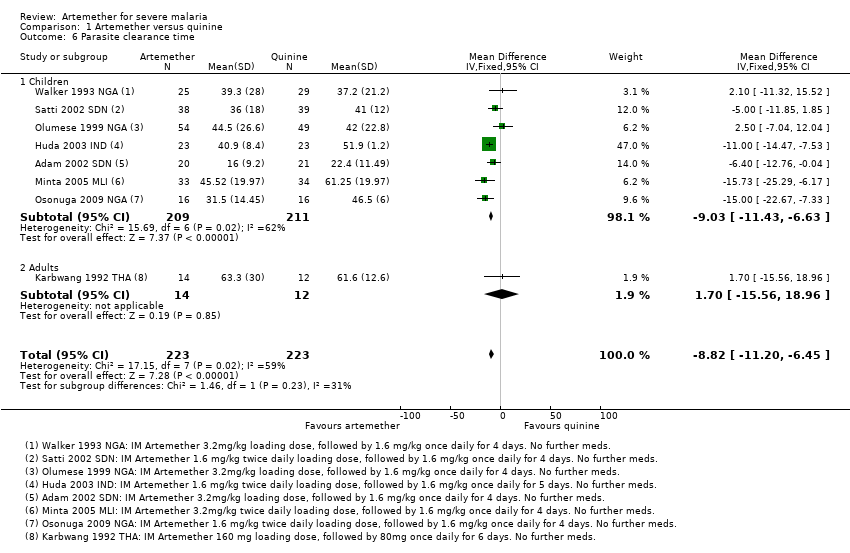 Comparison 1 Artemether versus quinine, Outcome 6 Parasite clearance time. | ||||
| 6.1 Children | 7 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐11.43, ‐6.63] |
| 6.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐15.56, 18.96] |
| 7 Proportion with parasite clearance Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| Analysis 1.7 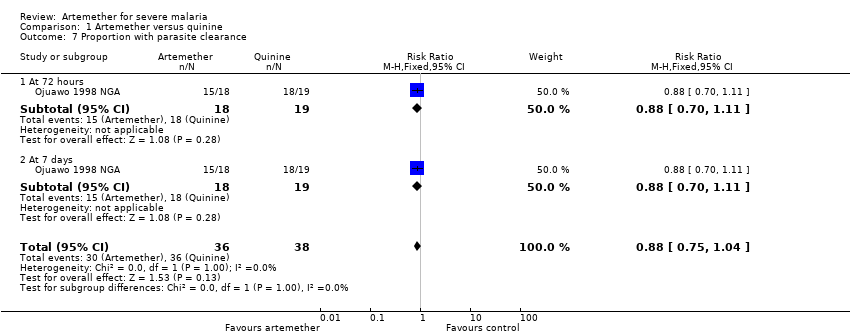 Comparison 1 Artemether versus quinine, Outcome 7 Proportion with parasite clearance. | ||||
| 7.1 At 72 hours | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 7.2 At 7 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 8 Fever clearance time (hours) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Artemether versus quinine, Outcome 8 Fever clearance time (hours). | ||||
| 8.1 Children | 8 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.55, ‐0.92] |
| 8.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐29.70 [‐54.14, ‐5.26] |
| 9 Need for blood transfusion Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Artemether versus quinine, Outcome 9 Need for blood transfusion. | ||||
| 9.1 Children | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.62, 2.59] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 10 Episodes of hypoglycaemia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Artemether versus quinine, Outcome 10 Episodes of hypoglycaemia. | ||||
| 10.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 10.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 11 Adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Artemether versus quinine, Outcome 11 Adverse events. | ||||
| 11.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 11.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 11.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 11.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 11.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 11.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 11.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 11.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 11.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 11.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 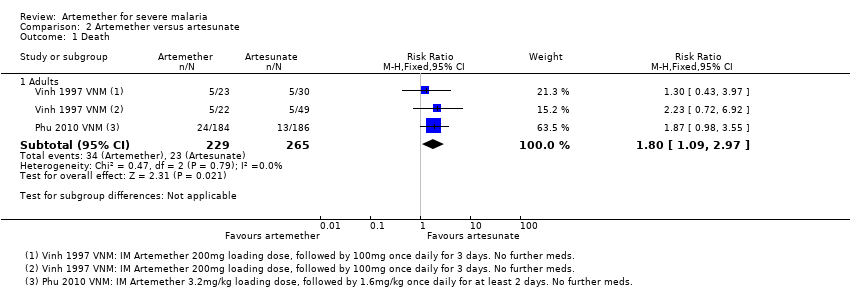 Comparison 2 Artemether versus artesunate, Outcome 1 Death. | ||||
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 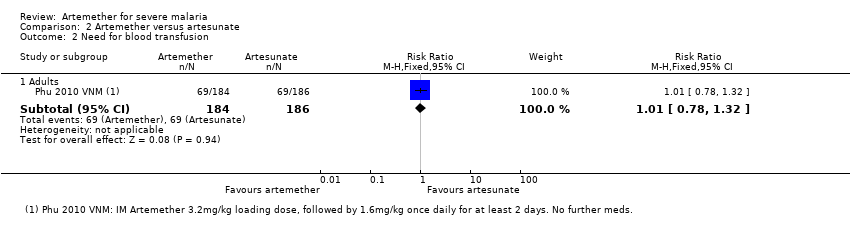 Comparison 2 Artemether versus artesunate, Outcome 2 Need for blood transfusion. | ||||
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 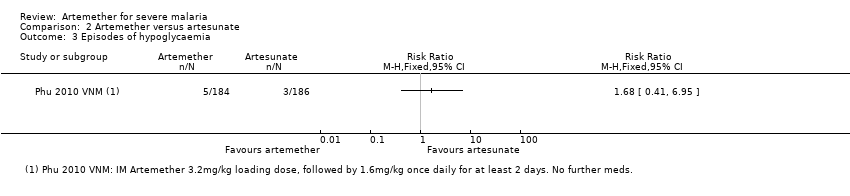 Comparison 2 Artemether versus artesunate, Outcome 3 Episodes of hypoglycaemia. | ||||
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Artemether versus artesunate, Outcome 4 Adverse events. | ||||
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Comparison 1 Artemether versus quinine, Outcome 1 Death.

Comparison 1 Artemether versus quinine, Outcome 2 Death: Time since admission to hospital.

Comparison 1 Artemether versus quinine, Outcome 3 Coma resolution time (hours).

Comparison 1 Artemether versus quinine, Outcome 4 Neurological sequelae at discharge.

Comparison 1 Artemether versus quinine, Outcome 5 Neurological sequelae at follow‐up.

Comparison 1 Artemether versus quinine, Outcome 6 Parasite clearance time.

Comparison 1 Artemether versus quinine, Outcome 7 Proportion with parasite clearance.

Comparison 1 Artemether versus quinine, Outcome 8 Fever clearance time (hours).

Comparison 1 Artemether versus quinine, Outcome 9 Need for blood transfusion.

Comparison 1 Artemether versus quinine, Outcome 10 Episodes of hypoglycaemia.

Comparison 1 Artemether versus quinine, Outcome 11 Adverse events.

Comparison 2 Artemether versus artesunate, Outcome 1 Death.

Comparison 2 Artemether versus artesunate, Outcome 2 Need for blood transfusion.

Comparison 2 Artemether versus artesunate, Outcome 3 Episodes of hypoglycaemia.

Comparison 2 Artemether versus artesunate, Outcome 4 Adverse events.
| Artemether compared with quinine for treating children with severe malaria | |||||
| Patient or population: Children with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 170 per 1000 | 164 per 1000 | RR 0.96 | 1447 | ⊕⊕⊕⊝ |
| Coma resolution time | The mean coma resolution time ranged across control groups from | The mean coma resolution time in the intervention groups was | ‐ | 358 | ⊕⊕⊝⊝ |
| Neurological sequelae at discharge | 220 per 1000 | 185 per 1000 | RR 0.84 | 968 | ⊕⊕⊝⊝ |
| Parasite clearance time | The mean parasite clearance time ranged across control groups from | The mean parasite clearance time in the intervention groups was | ‐ | 420 | ⊕⊕⊕⊝ |
| Fever clearance time | The mean fever clearance time ranged across control groups from | The mean fever clearance time in the intervention groups was | ‐ | 457 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials were variable in their risk of bias, but exclusion of the trials at high or unclear risk of selection bias did not change this result. | |||||
| Artemether compared with quinine for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 208 per 1000 | 123 per 1000 | RR 0.59 | 716 | ⊕⊕⊕⊝ |
| Coma resolution time | ‐ | ‐ | Not pooled. Little difference. | 657 | ⊕⊕⊝⊝ |
| Neurological sequelae at discharge | 4 per 1000 | 12 per 1000 (1 to 111) | RR 2.92 | 560 | ⊕⊕⊝⊝ |
| Parasite clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 | ⊕⊕⊕⊝ |
| Fever clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials are generally well conducted and at low risk of bias. 7 No serious risk of bias: This single trial was at low risk of bias. 8 Downgraded by 1 for serious imprecision: Neurological sequelae in adults were uncommon. This trial is underpowered to detect or exclude clinically important differences. | |||||
| Artemether compared with artesunate for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Artesunate | Artemether | ||||
| Death | 87 per 1000 | 156 per 1000 | RR 1.80 | 494 | ⊕⊕⊕⊝ |
| Coma resolution time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊕⊝ |
| Neurological sequelae at discharge | ‐ | ‐ | ‐ | 0 | ‐ |
| Parasite clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊕⊝ |
| Fever clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials were generally well conducted and at low risk of bias. | |||||
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | Embase2 | LILACS2 | ISI Web of Science |
| 1 | malaria | Malaria ti, ab, MeSH | Malaria ti, ab, MeSH | Malaria ti, ab, Emtree | malaria | malaria |
| 2 | artemether | Artemether ti, ab | Artemether ti, ab | Artemether ti, ab, Emtree | artemether | artemether |
| 3 | Artemisinin* | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* | Artemisinin* |
| 4 | intramuscular | Intramuscular ti, ab | Intramuscular ti, ab | Intramuscular ti, ab | intramuscular | intramuscular |
| 5 | parenteral | Injections, Intramuscular [MeSH] | Injections, Intramuscular [MeSH] | Intramuscular drug administration [Emtree] | parenteral | parenteral |
| 6 | 2 or 3 | Parenteral ti, ab | Parenteral ti, ab | Parenteral drug administration [Emtree] | 2 or 3 | 2 or 3 |
| 7 | 4 or 5 | 2 or 3 | 2 or 3 | 2 or 3 | 4 or 5 | 4 or 5 |
| 8 | 1 and 5 and 7 | 4 or 5 or 6 | 4 or 5 or 6 | 4 or 5 or 6 | 1 and 5 and 7 | 1 and 5 and 7 |
| 9 | ‐ | 1 and 7 and 8 | 1 and 7 and 8 | 1 and 7 and 8 | ‐ | Randomised clinical trial* |
| 10 | ‐ | ‐ | ‐ | ‐ | ‐ | 8 and 9 |
| 1Cochrane Infectious Diseases Group Specialized Register. | ||||||
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 2002 | 'Children' | 20 mg/kg IV | 10 mg/kg IV every eight hours for 72 hours | Oral quinine for 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| 2007 | 6 months to 12 yrs | 20 mg/kg IV or IM | 10 mg/kg IV/IM every eight hours | None | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days | None | |
| 2001 | < 14 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 5 days | None | |
| 2004 | 3 months to 15 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine 10 mg/kg every eight hours | 3.2mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1996 | 5 months to 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | SP once | |
| 1998 | Mean age about 4 yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM 12 hrs later, then once daily for 2 days | None | |
| 1999 | 11 months to 5 yrs | 20mg/kg IV | 10mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| 2009 | 1 to 12yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1996 | 3 months to 15yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1994 | Mean age of 3 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours for at least 2 doses | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days at least | SP once | |
| 1994 | 1 to 9yrs | 20 mg/kg IV | 10 mg/kg IV every twelve hours | Quinine to complete 5 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 3 days | SP once1 | |
| 1993 | 1 to 5yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| IM = intramuscular; IV = intravenous; SP = sulphadoxine‐pyrimethamine. 1Only in the second and third years of the study. | ||||||||
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 1996 | 15 to 79 yrs | 20 mg/kg IM | 10 mg/kg IM every eight hours | Quinine or mefloquine to complete 7 days | 4 mg/kg IM | 2 mg/kg IM once daily for 4 days | Quinine or mefloquine to complete 7 days | |
| 1991 | 15 to 45 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None | |
| 1994 | 15 to 55 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None | |
| 1995 | > 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| IM = intramuscular; IV = intravenous. | ||||||||
| Trial ID | Year of study | Age limits | Artemether dosing schedule | Artesunate dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 2003 | 15 to 77 yrs | 3.2 mg/kg IM | 1.6 mg/kg IM daily | None | 2.4 mg/kg IM | 1.2 mg/kg IM once daily | 2 mg/kg of artesunate to complete 7 days | |
| 1994 | 15 to 66 yrs | 200 mg IM | 100 mg IM once daily for 3 days | Mefloquine once | 120 mg IM or IV | 60 mg IM or IV once daily for 3 days | Mefloquine once | |
| IM = intramuscular; IV = intravenous. | ||||||||
| Trial ID | Coma resolution time | Fever clearance time | Parasite clearance time | Hypoglycaemia |
| Mean value (h) reported and defined as a Blantyre coma score of 5 recorded for at least 24 hours | Mean value (h) reported and defined as the time after which the temperature remained normal (axillary temperature < 37.5°C) | Mean value (h) reported and defined as the time passed from admission and start of treatment until two consecutive negative smears. Blood films repeated every 8 hours. | Number of episodes (n/N) reported but not defined | |
| Proportions with coma resolution on D3 reported but not defined | Proportions with fever clearance on D3 and D14 reported and defined as body temperature ≤ 37.5°C after commencement of treatment | Proportions with parasite clearance on D3 and D14. Parasite clearance was taken as adequate clinical and parasitological response (ACPR) | Not reported | |
| Median value (h) reported and defined as the time to reach a score of 15 on the Glasgow Coma Scale | Median value (h) reported but not defined. | Median value (h) reported and defined as the time to Assessed every 4 hours for the first 24 hours and every 6 hours until three consecutive negative blood smears | Number of episodes (n/N) reported but not defined | |
| Glasgow coma scale was used in grading the level of consciousness of the patients every eight hours | Mean value (h) reported and defined as time to clearance of fever | Mean value (h) reported but not defined | Not reported | |
| Unclear if values reported are means or medians (h) | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Mean value (h) reported and defined as the time for the parasite count to fall below the level of microscopic detection (thick film) | Not reported | |
| Median value (h) reported and defined as the time taken for the patients to recover completely from unconciousness | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Median value (h) reported and defined as the time taken for parasite count to fall below the level of microscopic detection (thick film) | Not reported | |
| Mean value (h) reported and defined as the time to normalization of consciousness | Mean value (h) reported but not defined | Mean value (h) reported and defined as time till negative parasitaemia result | Not reported | |
| Median value (h) reported but not described | Median value (h) reported but not described | Median value (h) reported but not described. Every four hours until clearance | Not reported | |
| Mean value (h) reported and defined as the interval between onset of therapy and the attainment of full consciousness | Mean value (h) reported and defined as the interval between the onset of therapy and the time the body temperature is ≤ 37°C and remained so | Defined as two successive thick blood films done at 12 hours interval are negative for asexual forms of plasmodium species | Not reported | |
| Mean value (h) reported and defined as time to regain full consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so for at least 48 hours | Mean value (h) reported and defined as the time from start of drug administration to the first of two consecutive negative thick smears remaining negative until day 7 | Not reported | |
| Mean value (h) reported and defined as time to attainment of a Blantyre score of 5 for at least 24 hours from initiation of treatment | Mean value (h) reported but not defined | Mean value (h) reported but not defined. Thick and thin film done on D0 and repeated on Days 3, 7 and 14 | Not reported | |
| Median value (h) reported and defined as time to Glasgow coma score of 15. | Median value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so | Median value (h) reported and defined as the time to clear all parasites | Number of episodes (n/N) reported but not defined | |
| Mean value (h) reported and defined as time to regaining consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C | Mean value (h) reported and defined as time to clear parasites measured every six hours till clearance | Not reported | |
| Median value (h) reported but not defined | Median value (h) reported and defined as a temperature <37.5 °C on two successive readings | Median value (h) reported and defined as as the time at which the blood films were negative for P. falciparum for at least eight hours | Number of episodes (n/N) reported but not defined | |
| Median value (h) reported and defined as time required for a child to achieve a Blantyre Coma Score of 5 | Median value (h) reported and defined as the time at which the rectal or axillary temperature dropped below 37.5°C and remained < 37.5°C for 24 consecutive hours | Median value (h) reported and defined as the time at which the first of two negative (0 parasites/200 WBC) thick blood films was prepared. Every four hours till clearance | Not reported | |
| Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time needed for the rectal temperature to fall below | Median value (h) reported and defined as time needed for all parasites to clear relative to parasite density at admission and assessed every 12 hours till clearance | Number of episodes (n/N) reported and defined as a blood glucose level below 40 mg/dL (2.2 mmol/L) | |
| Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time for axillary temperature to fall to, and remain for ≥ 24 hours at 37.5°C or lower | Median value (h) reported and defined as time to clear parasites | Not reported | |
| Mean value (h) reported but not defined | Mean value (h) reported | Mean value (h) reported and defined as the time for parasitaemia to be cleared and to remain so up to Day 7. Assessed every six hours during period of coma and then every 12 hours. | Not reported | |
| WBC = white blood cell. | ||||
| Outcome | Type of test | Proportion in control group3 | Proportion in Intervention group | Estimated RR | Total sample size1,2 |
| Death | Superiority | 0.17 | 0.136 | 0.80 | 3514 |
| Equivalence | 0.17 | 0.14 to 0.204 | ‐ | 6592 | |
| Neurological sequelae | Superiority | 0.25 | 0.20 | 0.80 | 2184 |
| Equivalence | 0.25 | 0.22 to 0.284 | ‐ | 8760 | |
| 1 These calculation were performed using a power calculator available at: http://www.sealedenvelope.com/power/ | |||||
| Outcome | Type of test | Mean in control group3 | Mean in Intervention group4 | SD of outcome | Total sample size1,2 |
| Coma resolution time | Superiority | 25 | 19 | 20 | 350 |
| Equivalence | 25 | 19 to 31 | 20 | 382 | |
| Parasite clearance time | Superiority | 42 | 36 | 20 | 350 |
| Equivalence | 42 | 36 to 48 | 20 | 382 | |
| Fever clearance time | Superiority | 48 | 42 | 20 | 350 |
| Equivalence | 48 | 36 to 54 | 20 | 382 | |
| 1 These calculations were performed using a power calculator available at: http://www.sealedenvelope.com/power/ | |||||
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) | 160 | 12 | 13 | Not significantly different | |
| Median (IQR) | 576 | 26 | 20 | P = 0.046 | ||
| Median (IQR) | 164 | 18 | 20 | Not significantly different | ||
| Coma recovery (%) on Day 3 | 90 | 15.9% | 21.4% | RR = 0.763 (95% CI 0.065 to 9.015) | ||
| Mean (SD) | 32 | 4.5 (13.05) | 9 (24.59) | P = 0.523 | ||
| Mean (SD) | 67 | 30.57 (29.02) | 25.15 (31.62) | P = 0.53 | ||
| Time to hospital discharge | % spending less than one week in hospital | 90 | 61.76% | 71.74% | P = 0.829 | |
| Fever clearance (h) | Median (IQR) | 160 | 32 | 32 | Not significantly different | |
| 576 | 30 | 33 | P = 0.8 | |||
| 164 | 31 | 45 | "Significant" | |||
| Fever clearance (%) on Day 3 | 90 | 90.0% | 87.7% | P = 0.753 | ||
| Parasite clearance (h) | Median (IQR) | 160 | 39.5 | 48 (37 to 56) | P < 0.001 | |
| 576 | 48 | 60 | P < 0.001 | |||
| 164 | 32 | 40 | 'significant' | |||
| Parasite clearance (%) on Day 3 | 90 | 99.0% n = 44 | 96.8% n = 46 | P = 0.422 | ||
| Needing blood transfusion | ‐ | ‐ | ‐ | ‐ | "The two groups were similar in terms of the need for blood transfusions,and the incidence of secondary bacterial infections (data not shown)." | |
| IQR = interquartile range. | ||||||
| Study ID | Sample size | Clinical symptoms monitoring | Biochemistry | Haematological | Electrocardiogram | Additional comments on adverse events |
| 41 | Not reported | Not reported | Not reported | Not reported | "Neurological deficits were not observed in any patient during the follow‐up period" | |
| 90 | Not reported | Not reported | Not reported | Not reported | None | |
| 560 | Clinical assessment every 4 hours for the first 24 hours and 6 hourly afterwards | Blood glucose, lactate and cytokine levels measured 4, 8, 12 and 24 hours after admission | Full blood count on admission | Pre‐treatment and 12 hours after initiation of treatment on Day 0, 4 hours after last dose and at discharge | None | |
| 46 | Lumbar puncture Chest x‐ray on day 0 | Blood Glucose, Renal Function Test, Liver Function Test and Serum Electrolyte on Days 0 and 3 | Full Blood Count on Days 0 and 3 | Day 0 | "No serious side effects of either of the drugs were observed in our study...... Closer and more frequent monitoring and larger sample size would have probably revealed more subtle adverse drug effects." | |
| 26 | Clinical evaluation daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 | Biochemistry on Days 0, 2, 4 and 7 | Full Blood Count on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | "The side effects in the quinine group were dizziness and vertigo. No side effects were detected with artemether". | |
| 102 | Clinical evaluation on admission and twice daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 | Biochemistry on Days 0, 2, 4 and 7 | FBC on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | QTc prolongation and tinnitus were the major adverse events in Quinine arm. Mild transient pain at injection site for approximately 15 mins after artemether treatment. | |
| 67 | Clinical examination daily on Days 1 to7, and 14 | Blood glucose on Days 1, 2, 3, 5, 7 and 14 Urea and Serum Electrolyte, transaminases, phosphatases on Days 1, 3, 5, 7 and 14 | FBC on Days 1, 3, 5, 7 and 14 | Once daily on Days 1, 3, 5, 7 and 14 | None | |
| 160 | Clinical assessment on admission, then at six, then 12 hour intervals till discharge | Blood glucose, urea, electrolytes, blood gases and when clinically indicated | FBC on Day 0 and when clinically indicated Blood cultures on Day 0 | On admission and at 6, 24, 30, 48 and 54 hours | None | |
| 37 | Clinical assessment on Day 0 | Urea and electrolyte Blood sugar and liver function test on Day 0 | FBC on Day 0 | None | None | |
| 103 | Clinical assessments on Days 0, 3, 7, 14, 28 | Blood glucose, Urea and creatinine, electrolytes on Days 0, 3, 7, 14, 28 | WBC count on Days 0, 3, 7, 14, 28 | None | "No adverse reactions to the two drugs were recorded during the study". | |
| 32 | Clinical examination on Days 0 to 7 and 14 | None | None | None | None | |
| 370 | Clinical examination on admission Chest x‐ray on admission Lumbar puncture | Blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine transaminase, plasma lactate | Full blood count on admission | None | None | |
| 77 | Clinical evaluation on admission and every six hours on Days 0 to 4, and then once daily on Days 14, 21 and 28 | Blood glucose, serum creatinine, serum aspartate, aminotransferase on Day 0 | WBC, Haemoglobin on Days 0 and 3 | None | None | |
| 33 | Chest X‐ray on admission | Renal and Liver function tests on admission, Days 3 and 7 | Full Blood Count on Days 0, 3 and 7 | None | None | |
| 183 | CSF collected on admission | Blood glucose, Blood pH, on D0 (every four hours for the first 24 hours | Haematocrit every eight hours Full Blood Count, urea and electrolytes on Days 0, 3, 7 and 28 | On admission, 6, 48,54 and 96 hours | "Of the initial 127 patients on whom serial electrocardiographic tracings were made, more patients in the quinine group | |
| 576 | Clinical examination on Day 0 Lumbar puncture on admission | Blood glucose on admission, after 4hours and 12 hours | PCV, Haemoglobin, Blood culture on Day 0 | None | None | |
| 124 | Clinical examination on admission | Blood glucose, serum creatinine, serum bilirubin on admission | Full blood count on admission | None | None | |
| 54 | Clinical examination twice daily Spinal taps | Urea and Electrolyte, on days 3, 7, 14, 28 | PCV on days 3, 7, 14, 28 | On admission, at 4 and 6 hours | None |
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) | 560 | 66 (30 to 132) | 48 (20 to 84) | P = 0.003 | |
| Median (Range) | 97 | 48 (6 to 144) | 48 (6 to 144) | Not significantly different | ||
| Fever clearance (h) | Median (IQR) | 560 | 127 (60 to 216) | 90 (54 to 144) | < 0.001 | |
| Median (Range) | 33 | 32 (20 to 112) | 48 (28 to 88) | P = 0.034 | ||
| Median (Range) | 97 | 79 (16 to 147) | 84 (36 to 144) | Not significantly different | ||
| Parasite clearance (h) | Median (IQR) | 560 | 72 (54 to 102) | 90 (66 to 108) | < 0.001 | |
| Median (range) | 33 | 48(4 to 72) | 52 (12 to112) | P = 0.381 | ||
| Median (Range) | 97 | 54 (30 to 164) | 78 (18 to 168) | P = 0.007 | ||
| IQR = interquartile range. | ||||||
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Artesunate IM | Artesunate IV | Comparative results reported in article |
| Coma resolution time (h) | Median (range) | 370 | 72(2 to 2232) n = 184 | 60(4 to 2136) n = 186 | ‐ | P = 0.11 | |
| Median (95% CI) | 124 | 47 (31 to 63) | 30 (18 to 42) | 24 (4 to 44) | ‐ | ||
| Fever clearance (h) | Median (range) | 370 | 108 (0 to 888) n = 184 | 108 (0 to 888) n = 186 | ‐ | P = 0.27 | |
| Median (95% CI) | 124 | 48 (38 to 58) | 36 (30 to 42) | 30 (18 to 42) | ‐ | ||
| Parasite clearance (h) | Median (range) | 370 | 72 (2 to 204) | 72 (7 to 330) | ‐ | P = 0.97 | |
| Median (95% CI) | 124 | 30 (26 to 34) | 24 (15 to 33) | 24 (15 to 33) | Not statistically significant | ||
| IM = intramuscular; IV = intravenous. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 12 | 1447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.20] |
| 1.2 Adults | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Death: Time since admission to hospital Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Children | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐5.45 [‐7.90, ‐1.00] |
| 4 Neurological sequelae at discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 7 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up Show forest plot | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time Show forest plot | 8 | 446 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐11.20, ‐6.45] |
| 6.1 Children | 7 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐11.43, ‐6.63] |
| 6.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐15.56, 18.96] |
| 7 Proportion with parasite clearance Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 7.1 At 72 hours | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 7.2 At 7 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 8 Fever clearance time (hours) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 8 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.55, ‐0.92] |
| 8.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐29.70 [‐54.14, ‐5.26] |
| 9 Need for blood transfusion Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.62, 2.59] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 10 Episodes of hypoglycaemia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 10.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 11 Adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 11.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 11.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 11.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 11.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 11.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 11.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 11.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 11.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 11.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |