Tratamiento con hierro en pacientes adultos anémicos sin nefropatías crónicas
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials
#1 MeSH descriptor Iron Compounds explode all trees
#2 MeSH descriptor Ferric Compounds explode all trees
#3 MeSH descriptor Ferrous Compounds explode all trees
#4 iron OR ferrous OR ferric
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Anemia explode all trees
#7 anemi* OR anaemi*
#8 (#6 OR #7)
#9 (#5 AND #8)
PubMed
("Iron Compounds"[Mesh] OR "Ferric Compounds"[Mesh] OR "Ferrous Compounds"[Mesh] OR iron OR ferrous OR ferric) AND ("Anemia"[Mesh] OR anemi* OR anaemi*) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
EMBASE (Ovid SP)
1 exp iron therapy/
2 (iron or ferrous or ferric).af.
3 1 or 2
4 exp anemia/
5 (anemi* OR anaemi*).af.
6 4 or 5
7 exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/
8 (random* or factorial* or crossover* or placebo*).af.
9 7 or 8
10 3 and 6 and 9
ISI Web of Science: Science Citation Index‐Expanded (SCI‐EXPANDED) and Conference Proceedings Citation Index‐Science (CPCI‐S)
# 1 TS=(iron OR ferrous OR ferric)
# 2 TS=(anemi* OR anaemi*)
# 3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*)
# 4 #3 AND #2 AND #1
CINAHL Plus (EBSCO)
S1 (MH "Clinical Trials+")
S2 PT Clinical trial
S3 TX clinic* n1 trial* or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) )
S4 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) )
S5 TX randomi* control* trial*
S6 (MH "Random Assignment")
S7 TX random* allocat*
S8 TX placebo*
S9 (MH "Placebos")
S10 (MH "Quantitative Studies")
S11 TX allocat* random*
S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S13 (MH "Iron")
S14 (iron OR ferrous OR ferric)
S15 S13 or S14
S16 (MH "Anemia+")
S17 (anemi* OR anaemi*)
S18 S16 or 17
S19 S15 and S18
S20 S12 and S20
Clinicaltrials.gov
Search terms: Randomized
Study type: Interventional Studies
Conditions: anemia OR anaemic
Interventions: iron OR ferrous OR ferric

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Twenty‐one studies are included in this review.
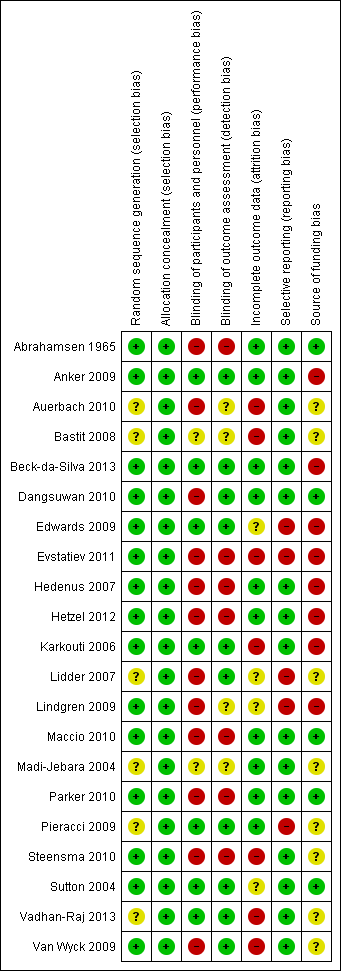
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Oral iron vs inactive control, Outcome 1 Mortality.

Comparison 1 Oral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion.
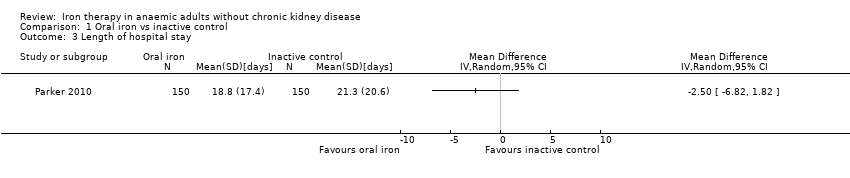
Comparison 1 Oral iron vs inactive control, Outcome 3 Length of hospital stay.
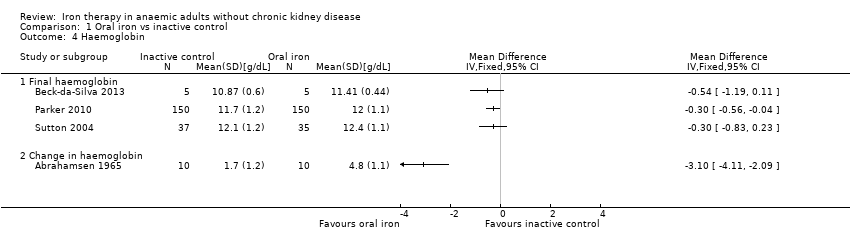
Comparison 1 Oral iron vs inactive control, Outcome 4 Haemoglobin.
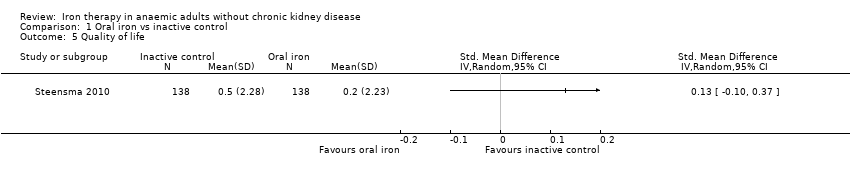
Comparison 1 Oral iron vs inactive control, Outcome 5 Quality of life.

Comparison 1 Oral iron vs inactive control, Outcome 6 Serious adverse events.

Comparison 2 Parenteral iron vs inactive control, Outcome 1 Mortality.

Comparison 2 Parenteral iron vs inactive control, Outcome 2 Proportion requiring blood transfusion.

Comparison 2 Parenteral iron vs inactive control, Outcome 3 Haemoglobin.
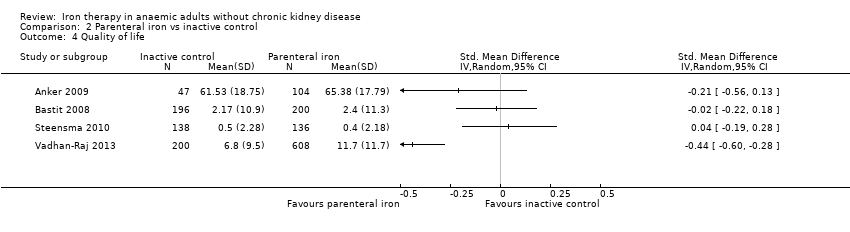
Comparison 2 Parenteral iron vs inactive control, Outcome 4 Quality of life.

Comparison 2 Parenteral iron vs inactive control, Outcome 5 Serious adverse events.
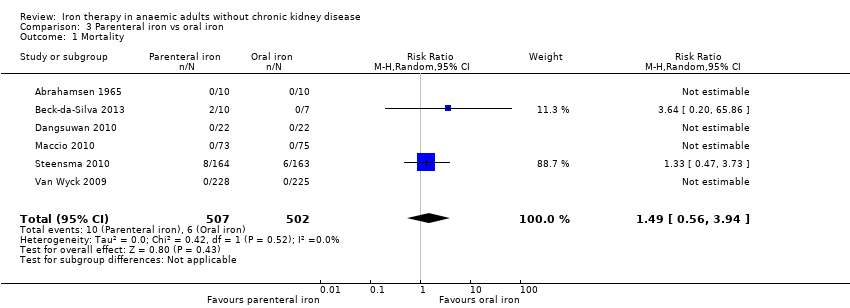
Comparison 3 Parenteral iron vs oral iron, Outcome 1 Mortality.
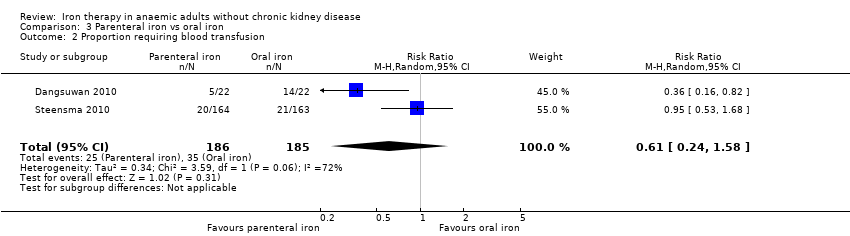
Comparison 3 Parenteral iron vs oral iron, Outcome 2 Proportion requiring blood transfusion.

Comparison 3 Parenteral iron vs oral iron, Outcome 3 Mean blood transfused.

Comparison 3 Parenteral iron vs oral iron, Outcome 4 Haemoglobin.

Comparison 3 Parenteral iron vs oral iron, Outcome 5 Quality of life.

Comparison 3 Parenteral iron vs oral iron, Outcome 6 Serious adverse events.
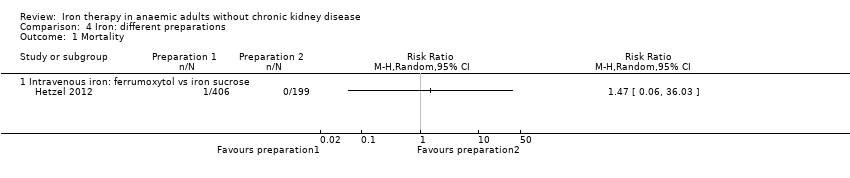
Comparison 4 Iron: different preparations, Outcome 1 Mortality.
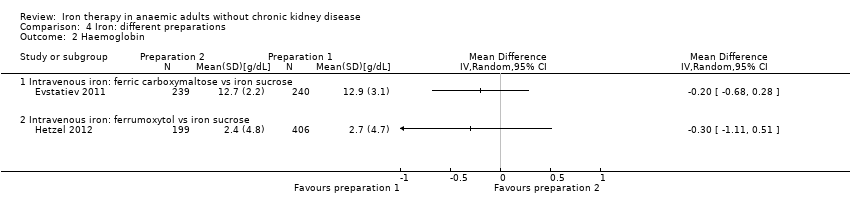
Comparison 4 Iron: different preparations, Outcome 2 Haemoglobin.

Comparison 4 Iron: different preparations, Outcome 3 Serious adverse events.
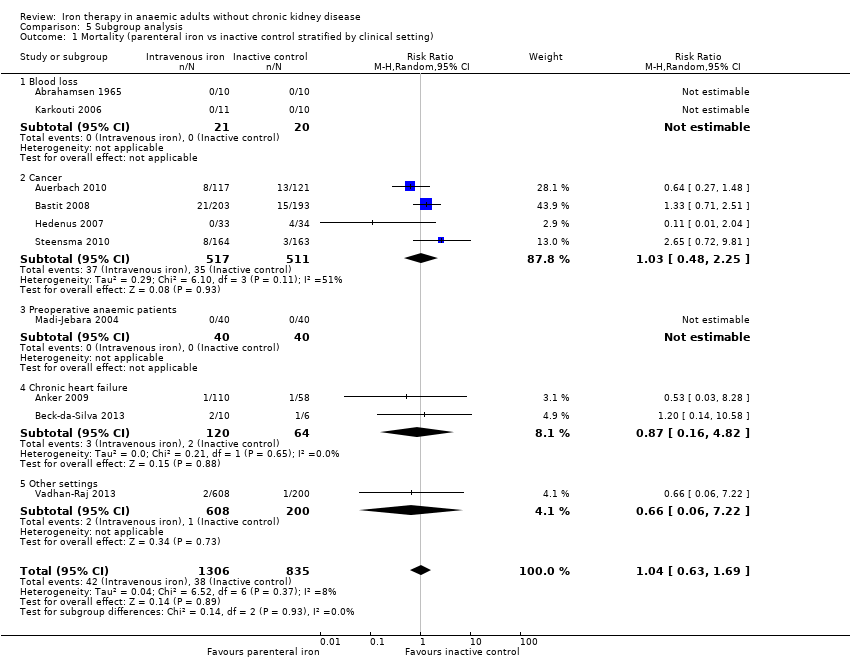
Comparison 5 Subgroup analysis, Outcome 1 Mortality (parenteral iron vs inactive control stratified by clinical setting).

Comparison 5 Subgroup analysis, Outcome 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use).
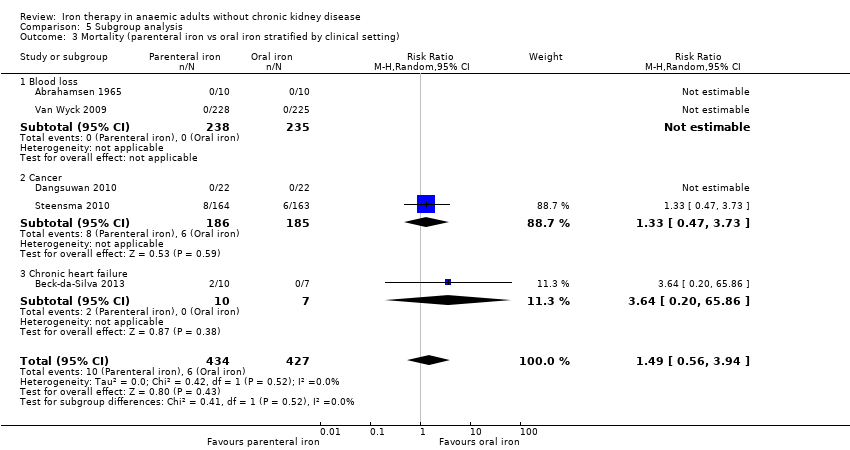
Comparison 5 Subgroup analysis, Outcome 3 Mortality (parenteral iron vs oral iron stratified by clinical setting).
| Oral iron vs inactive control for anaemic patients | |||||
| Patient or population: patients with anaemia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Inactive control | Oral iron | ||||
| Mortality | 100 per 1000 | 105 per 1000 (68 to 161) | RR 1.05 (0.68 to 1.61) | 659 | ⊕⊝⊝⊝ |
| Proportion requiring blood transfusion | 279 per 1000 | 206 per 1000 | RR 0.74 | 546 | ⊕⊝⊝⊝ |
| Length of hospital stay | Mean hospital stay in control groups was | Mean hospital stay in intervention groups was | 300 | ⊕⊝⊝⊝ | |
| Haemoglobin | Mean haemoglobin in control groups ranged between 11.4 g/dL and 12.4 g/dL | Point estimate of haemoglobin in intervention groups in the individual studies was 0.30 to 3.10 higher | 402 | ⊕⊝⊝⊝ | |
| Quality of life | ‐ | Mean quality of life in intervention groups was | 276 | ⊕⊝⊝⊝ | |
| Serious adverse events | 205 per 1000 | 197 per 1000 | RR 0.96 (0.76 to 1.22) | 731 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is the average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | |||||
| Parenteral iron vs inactive control for anaemic patients | |||||
| Patient or population: patients with anaemia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Inactive control | Parenteral iron | ||||
| Mortality | 46 per 1000 | 47 per 1000 | RR 1.04 (0.63 to 1.69) | 2141 | ⊕⊝⊝⊝ |
| Proportion requiring blood transfusion | 182 per 1000 | 153 per 1000 | RR 0.84 (0.66 to 1.06) | 1315 | ⊕⊝⊝⊝ |
| Haemoglobin | Mean haemoglobin in control groups ranged between 11.2 g/dL and 13.0 g/dL | The point estimate of haemoglobin in intervention groups in the individual studies was from 0.30 to 3.00 higher | 1371 | ⊕⊝⊝⊝ | |
| Quality of life | ‐ | The point estimate of haemoglobin in intervention groups in the individual studies was between 0.04 standard deviations lower and 0.44 standard deviations higher | 1629 | ⊕⊝⊝⊝ | |
| Serious adverse events | 184 per 1000 | 184 per 1000 | RR 1 (0.74 to 1.34) | 1802 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk is the average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | |||||
| Parenteral iron vs oral iron for anaemic patients | ||||||
| Patient or population: patients with anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Parenteral iron | |||||
| Mortality | 12 per 1000 | 18 per 1000 | RR 1.49 (0.56 to 3.94) | 1009 | ⊕⊝⊝⊝ | |
| Proportion requiring blood transfusion | 189 per 1000 | 115 per 1000 | RR 0.61 (0.24 to 1.58) | 371 | ⊕⊝⊝⊝ | |
| Mean blood transfused | Mean mean blood transfused in control groups was | Mean blood transfused in intervention groups was | 44 | ⊕⊝⊝⊝ | ||
| Haemoglobin | Mean haemoglobin in control groups was between | Mean haemoglobin in intervention groups was | 769 | ⊕⊕⊝⊝ | ||
| Quality of life | ‐ | Mean quality of life in intervention groups was | 771 | ⊕⊝⊝⊝ | SMD 0.09 (‐0.04 to 0.22) | |
| Serious adverse events | 131 per 1000 | 162 per 1000 | RR 1.23 (0.99 to 1.54) | 1100 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk is average risk among controls. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aTrial(s) reporting this outcome was/were at high risk of bias. | ||||||
| Group characteristics | Haemoglobin | Haematocrit | Haematocrit |
| Children (6 months to 59 months) | 110 | 6.83 | 0.33 |
| Children (5 to 11 years) | 115 | 7.13 | 0.34 |
| Children (12 to 14 years) | 120 | 7.45 | 0.36 |
| Non‐pregnant women (over 15 years of age) | 120 | 7.45 | 0.36 |
| Pregnant women | 110 | 6.83 | 0.33 |
| Men (over 15 years of age) | 130 | 8.07 | 0.39 |
| aHaematocrit is the volume of packed red blood cells expressed in terms of fraction or percentages in a whole blood specimen (NCBI‐Hematocrit). | |||
|
Study name | Participant characteristics | Clinical setting | Co‐interventions | Routes and methods of administration of parenteral iron |
Comparison | |||||||||||
| Sample size (after postrandomisation dropouts) | Mean age (years) | Females | Postrandomisation dropouts | Blood loss conditions | Cancer | Preoperative | Chronic heart failure | Autoimmune | Miscellaneous | Added erythropoietin | Added oral iron | Parenteral iron—intravenous | Parenteral iron—intramuscular | Total dose infusion | ||
| 30 | Not stated | Not stated | 0 (0%) | Yes | No | No | No | No | No | No | Not applicable | No | Yes | No | Parenteral iron vs oral iron vs inactive control | |
| 158 | Not stated | Not stated | 0 (0%) | No | No | No | Yes | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 238 | 63 | 158 (66.4%) | 5 (2.1%) | No | Yes | No | No | No | No | Yes | Oral iron was allowed as part of standard treatment | Yes | No | No | Parenteral iron vs inactive control | |
| 396 | 61 | 240 (60.6%) | 2 (0.5%) | No | Yes | No | No | No | No | Yes | Oral iron was allowed as part of standard treatment | Yes | No | No | Parenteral iron vs inactive control | |
| 23 | 66 | 7 | Not stated | No | No | No | Yes | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron vs inactive control | |
| 44 | 51 | 44 (100%) | 0 (0%) | No | Yes | No | No | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 18 | Not stated | Not stated | Not stated | No | No | Yes | No | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 483 | 39 | 284 (58.8%) | 2 (0.4%) | No | No | No | No | Yes | No | No | No | Yes | No | Yes | Different preparations | |
| 67 | 76 | 42 (62.7%) | 0 (0%) | No | Yes | No | No | No | No | Yes | No | Yes | No | No | Parenteral iron vs inactive control | |
| 605 | Not stated | Not stated | Not stated | No | No | No | No | No | Yes | No | No | Yes | No | No | Different preparations | |
| 21 | 62 | 5 (23.8%) | 5 (19.2%) | Yes | No | No | No | No | No | No | Yes | Yes | No | No | Parenteral iron vs inactive control | |
| 20 | Not stated | 9 (45%) | Not stated | No | No | Yes | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 91 | 42 | 63 (69.2%) | Not stated | No | No | No | No | Yes | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 148 | 68 | 59 (39.9%) | 0 (0%) | No | Yes | No | No | No | No | Yes | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| 80 | Not stated | Not stated | 0 (0%) | No | No | Yes | No | No | No | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 300 | 82 | 245 (81.7%) | 0 (0%) | Yes | No | No | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 200 | 57 | 103 (51.5%) | 0 (0%) | No | No | No | No | No | Yes | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 490 | 64 | 320 (65.3%) | 12 (2.4%) | No | Yes | No | No | No | No | Yes | Not applicable | Yes | No | No | Parenteral iron vs oral iron vs inactive control | |
| 72 | 70 | 30 (41.7%) | Not stated | Yes | No | No | No | No | No | No | Not applicable | Not applicable | Not applicable | Not applicable | Oral iron vs inactive control | |
| 808 | 45 | 720 | 4 (0.5%) | No | No | No | No | No | Yes | No | No | Yes | No | No | Parenteral iron vs inactive control | |
| 453 | 39 | 453 (100%) | 24 (5%) | Yes | No | No | No | No | No | No | Not applicable | Yes | No | No | Parenteral iron vs oral iron | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.68, 1.61] |
| 2 Proportion requiring blood transfusion Show forest plot | 3 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 3 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Haemoglobin Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Final haemoglobin | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Change in haemoglobin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Serious adverse events Show forest plot | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 2 Proportion requiring blood transfusion Show forest plot | 8 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.06] |
| 3 Haemoglobin Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Final haemoglobin | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change in haemoglobin | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Serious adverse events Show forest plot | 7 | 1802 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| 2 Proportion requiring blood transfusion Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 3 Mean blood transfused Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Haemoglobin Show forest plot | 6 | 769 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.73, ‐0.27] |
| 4.1 Final haemoglobin | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.74, ‐0.21] |
| 4.2 Change in haemoglobin | 3 | 621 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.29, 0.46] |
| 5 Quality of life Show forest plot | 3 | 771 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.13] |
| 6 Serious adverse events Show forest plot | 7 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.99, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haemoglobin Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serious adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Intravenous iron: ferric carboxymaltose vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intravenous iron: ferrumoxytol vs iron sucrose | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (parenteral iron vs inactive control stratified by clinical setting) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 1.1 Blood loss | 2 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Cancer | 4 | 1028 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.25] |
| 1.3 Preoperative anaemic patients | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Chronic heart failure | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.16, 4.82] |
| 1.5 Other settings | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.06, 7.22] |
| 2 Mortality (parenteral iron vs inactive control stratified by erythropoietin use) Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.69] |
| 2.1 Supplementary erythropoietin | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.91] |
| 2.2 No supplementary erythropoietin | 7 | 1440 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.58, 3.90] |
| 3 Mortality (parenteral iron vs oral iron stratified by clinical setting) Show forest plot | 5 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.94] |
| 3.1 Blood loss | 2 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cancer | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.73] |
| 3.3 Chronic heart failure | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 3.64 [0.20, 65.86] |