Intensity of continuous renal replacement therapy for acute kidney injury
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group
Control group
Co‐interventions
| |
| Outcomes | Primary outcomes
Secondary outcomes
Tertiary endpoints
Economic analysis
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to one of the two treatment groups by means of a centralized, computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Central allocation process |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for mortality was low risk) |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Per cent followed: 99.55% |
| Selective reporting (reporting bias) | Low risk | The study reported mortality, kidney function recovery and adverse events |
| Other bias | Low risk | Funding sources were reported |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group 1
Treatment group 2
Control group
Co‐interventions
| |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to the treatment dosage using computer‐generated method |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were kept in numbered, sealed opaque envelopes that were opened at the time of enrolment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement (for kidney recovery was unclear risk but for mortality was low risk) |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome measurement is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data were reported |
| Selective reporting (reporting bias) | Low risk | The study reported mortality, kidney function recovery and adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group
Control group
Co‐interventions
| |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using computer‐generated methodology |
| Allocation concealment (selection bias) | Low risk | Central allocation process |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome measurement was unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow up data |
| Selective reporting (reporting bias) | Low risk | The study reported mortality, kidney function recovery and adverse events |
| Other bias | Low risk | Funding sources were reported |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group 1
Treatment group 2
Control group
Co‐interventions
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using computer‐generated methodology |
| Allocation concealment (selection bias) | Low risk | Central allocation process |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measurement unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow up |
| Selective reporting (reporting bias) | Low risk | Reported mortality, kidney function recovery and adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group
Control group
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using computer‐generated methodology in blocks of four and six patients |
| Allocation concealment (selection bias) | Unclear risk | Allocation was appropriate (sealed opaque envelopes). However, the table 1 showed a significant imbalance in the severity of illness observed between treatment arms |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measurement unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or losses to follow up |
| Selective reporting (reporting bias) | Low risk | Reported mortality, kidney function recovery and adverse events |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions |
Treatment group
Control group
Co‐interventions
| |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned using computer‐generated methodology (1:1 ratio between treatment dosages) |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were kept in numbered, sealed envelopes that were opened at the time of enrolment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measurement unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Per cent followed: 100% |
| Selective reporting (reporting bias) | Low risk | Reported mortality, kidney function recovery and adverse events |
| Other bias | Low risk | Funding sources were reported |
ACEi ‐ angiotensin‐converting enzyme inhibitors; AIDs ‐ acquired immune deficiency syndrome; AKI ‐ acute kidney injury; ATN ‐ acute tubular necrosis; BUN ‐ blood urea nitrogen; CrCl ‐ creatinine clearance; CRRT ‐ continuous renal replacement therapy; CVVH ‐ continuous venovenous haemofiltration; CVVHDF ‐ continuous venovenous haemodiafiltration; HD ‐ haemodialysis; ICU ‐ intensive care unit/s; IHD ‐ intermittent haemodialysis; M/F ‐ male/female; Qb ‐ extracorporeal blood flow; RCT ‐ randomised controlled trial; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine; SD ‐ standard deviation; SLED ‐ sustained low‐efficiency dialysis; SOFA ‐ Sequential Organ Failure Assessment
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Different intensity‐arms treatment; control arm was not within the pre‐specified range according to protocol: low volume HF (35 ml/kg/h) versus high volume HF (65 ml/kg/h); small sample size (< 20 participants) | |
| Not RCT | |
| Compared continuous dialysis therapy versus no haemofiltration | |
| Outcomes not relevant for this review; intensity of CRRT was not assessed | |
| 36% of patients in the control arm did not receive CRRT | |
| Different intensity arms treatment; control arm is not within the pre‐specified range according to protocol: standard volume HF (35 mL/kg/h) versus high volume HF (70 mL/kg/h) | |
| Different inclusion criteria; included patients with severe pancreatitis, but AKI was no obligatory condition for enrolment; AKI was observed in only 6 (16%) patients | |
| Different intensity arms treatment; less intensive arm is not within the pre‐specified range according to protocol: standard volume HF (40 mL/kg/h) versus high volume HF (80 mL/kg/h) | |
| Different intensity arms treatment; the control arm is not within the pre‐specified range according to protocol: high volume HF (50 mL/kg/h) versus extra high volume HF (85 mL/kg/h) | |
| Outcomes not relevant for this review; intensity of CRRT was not assessed | |
| Different intensity arms treatment; control dose arms are not within the pre‐specified range according to protocol: high volume HF (35 mL/kg/h) versus very high volume HF (> 55 mL/kg/h) | |
| Compared CRRT versus IHD | |
| Not RCT | |
| Different intensity arms treatment; the control arm is not within the pre‐specified range according to protocol: high volume HF (50 mL/kg/h) versus extra high volume HF (85 mL/Kg/h) |
CRRT ‐ continuous renal replacement therapy; HF ‐ haemofiltration; IHD ‐ intermittent haemodialysis; RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes |
|
AKI ‐ acute kidney injury; CKD ‐ chronic kidney disease; CRRT ‐ continuous renal replacement therapy; CVVH ‐ continuous venovenous haemofiltration; GFR ‐ glomerular filtration rate; ICU ‐ intensive care unit/s; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 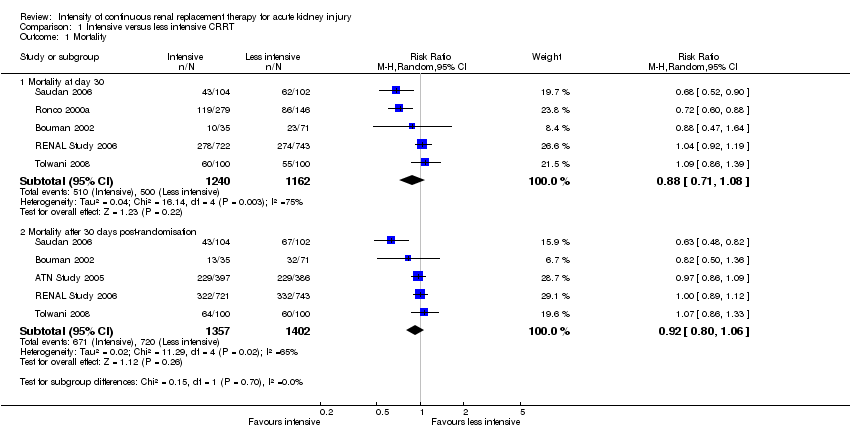 Comparison 1 Intensive versus less intensive CRRT, Outcome 1 Mortality. | ||||
| 1.1 Mortality at day 30 | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.08] |
| 1.2 Mortality after 30 days post‐randomisation | 5 | 2759 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 2 Mortality in prespecified groups Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Intensive versus less intensive CRRT, Outcome 2 Mortality in prespecified groups. | ||||
| 2.1 Patients with sepsis | 5 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 2.2 Patients without sepsis | 4 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 2.3 Patients with SOFA cardiovascular score < 3 | 1 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 2.4 Patients with SOFA cardiovascular ≥ 3 | 1 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.5 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.88] |
| 2.6 Patients with AKI unrelated to surgical causes | 3 | 1871 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 3 Recovery of kidney function Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Intensive versus less intensive CRRT, Outcome 3 Recovery of kidney function. | ||||
| 3.1 Free of RRT after discontinuing CRRT | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.37] |
| 3.2 Free of RRT after discontinuing CRRT at day 30 | 5 | 1416 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 3.3 Free of RRT after discontinuing CRRT at day 90 | 3 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.01] |
| 4 Kidney function recovery in prespecified subgroup Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Intensive versus less intensive CRRT, Outcome 4 Kidney function recovery in prespecified subgroup. | ||||
| 4.1 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.53] |
| 4.2 Patients with AKI related to non‐surgical causes | 3 | 1870 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.71] |
| 5 Length of stay Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 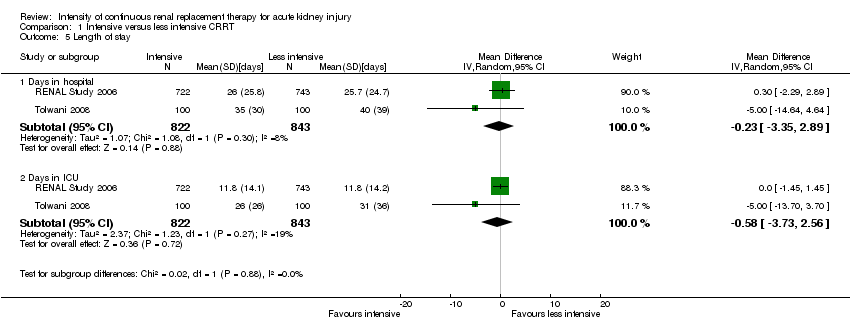 Comparison 1 Intensive versus less intensive CRRT, Outcome 5 Length of stay. | ||||
| 5.1 Days in hospital | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐3.35, 2.89] |
| 5.2 Days in ICU | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐3.73, 2.56] |
| 6 Metabolic control Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Intensive versus less intensive CRRT, Outcome 6 Metabolic control. | ||||
| 6.1 Normalised metabolic acidosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 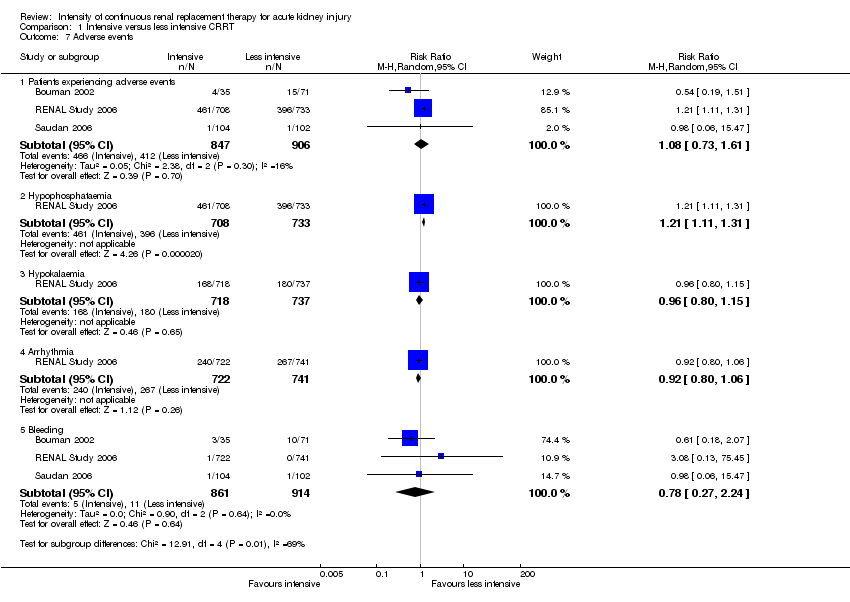 Comparison 1 Intensive versus less intensive CRRT, Outcome 7 Adverse events. | ||||
| 7.1 Patients experiencing adverse events | 3 | 1753 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.61] |
| 7.2 Hypophosphataemia | 1 | 1441 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.31] |
| 7.3 Hypokalaemia | 1 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 7.4 Arrhythmia | 1 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 7.5 Bleeding | 3 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.24] |
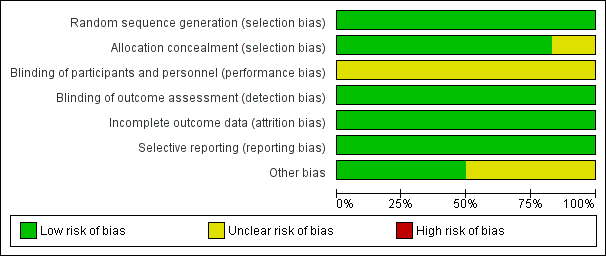
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 High Intensity versus less intensive CRRT, outcome: 1.1 Mortality

Comparison 1 Intensive versus less intensive CRRT, Outcome 1 Mortality.

Comparison 1 Intensive versus less intensive CRRT, Outcome 2 Mortality in prespecified groups.

Comparison 1 Intensive versus less intensive CRRT, Outcome 3 Recovery of kidney function.

Comparison 1 Intensive versus less intensive CRRT, Outcome 4 Kidney function recovery in prespecified subgroup.

Comparison 1 Intensive versus less intensive CRRT, Outcome 5 Length of stay.

Comparison 1 Intensive versus less intensive CRRT, Outcome 6 Metabolic control.

Comparison 1 Intensive versus less intensive CRRT, Outcome 7 Adverse events.
| Intensive versus less intensive CRRT for AKI | ||||||
| Patient or population: patients with AKI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Less intensive CRRT | Intensive CRRT | |||||
| Mortality at day 30 | Study population | RR 0.88 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 430 per 1000 | 420 per 1000 | |||||
| Moderate | ||||||
| Mortality after 30 days post‐randomisation | Study population | RR 0.92 | 2759 (5) | ⊕⊕⊝⊝ | ||
| 514 per 1000 | 483 per 1000 | |||||
| Moderate | ||||||
| 593 per 1000 | 557 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 1.12 | 2402 (5) | ⊕⊕⊝⊝ | ||
| 483 per 1000 | 541 per 1000 | |||||
| Moderate | ||||||
| 390 per 1000 | 437 per 1000 | |||||
| Patients free of RRT after discontinuing CRRT | Study population | RR 0.98 | 988 (3) | ⊕⊕⊕⊝ | ||
| 923 per 1000 | 904 per 1000 | |||||
| Moderate | ||||||
| 800 per 1000 | 784 per 1000 | |||||
| Adverse events: hypophosphataemia | Study population | RR 1.21 | 1441 (1) | ⊕⊕⊕⊕ | ||
| 540 per 1000 | 654 per 1000 | |||||
| Moderate | ||||||
| 540 per 1000 | 653 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects ³ Indirectness: critically ill patients with AKI in CRRT have high short‐term mortality risk; mortality is a competing end point for kidney recovery at day 90 | ||||||
| Intensive versus less intensive CRRT for AKI: subgroups | ||||||
| Patient or population: patients with AKI who need CRRT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard dose | High dose | |||||
| Mortality: patients with sepsis | Study population | RR 0.94 | 966 (5) | ⊕⊕⊝⊝ | ||
| 524 per 1000 | 492 per 1000 | |||||
| Moderate | ||||||
| 618 per 1000 | 581 per 1000 | |||||
| Mortality: patients without sepsis | Study population | RR 0.89 | 1216 (4) | ⊕⊕⊝⊝ | ||
| 465 per 1000 | 414 per 1000 | |||||
| Moderate | ||||||
| 564 per 1000 | 502 per 1000 | |||||
| Mortality: patients with AKI related to cardiac or general surgery | Study population | RR 0.73 | 531 (2) | ⊕⊕⊕⊕ | ||
| 505 per 1000 | 368 per 1000 | |||||
| Moderate | ||||||
| 459 per 1000 | 335 per 1000 | |||||
| Mortality: patients with AKI not related to surgery | Study population | RR 0.94 | 1871 (3) | ⊕⊕⊝⊝ | ||
| 414 per 1000 | 389 per 1000 | |||||
| Moderate | ||||||
| 550 per 1000 | 517 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ inconsistency: due to substantial heterogeneity (I2 values ranged from 73% to 78%) ² imprecision: due to wide CI which crossed the threshold for clinically meaningful effects | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mortality at day 30 | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.08] |
| 1.2 Mortality after 30 days post‐randomisation | 5 | 2759 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 2 Mortality in prespecified groups Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Patients with sepsis | 5 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 2.2 Patients without sepsis | 4 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 2.3 Patients with SOFA cardiovascular score < 3 | 1 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.18] |
| 2.4 Patients with SOFA cardiovascular ≥ 3 | 1 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 2.5 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.88] |
| 2.6 Patients with AKI unrelated to surgical causes | 3 | 1871 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 3 Recovery of kidney function Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Free of RRT after discontinuing CRRT | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.37] |
| 3.2 Free of RRT after discontinuing CRRT at day 30 | 5 | 1416 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 3.3 Free of RRT after discontinuing CRRT at day 90 | 3 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.01] |
| 4 Kidney function recovery in prespecified subgroup Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Patients with AKI related to surgical causes | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.53] |
| 4.2 Patients with AKI related to non‐surgical causes | 3 | 1870 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.73, 1.71] |
| 5 Length of stay Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Days in hospital | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐3.35, 2.89] |
| 5.2 Days in ICU | 2 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐3.73, 2.56] |
| 6 Metabolic control Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Normalised metabolic acidosis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Patients experiencing adverse events | 3 | 1753 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.61] |
| 7.2 Hypophosphataemia | 1 | 1441 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.31] |
| 7.3 Hypokalaemia | 1 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 7.4 Arrhythmia | 1 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 7.5 Bleeding | 3 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.24] |


