Intervenciones farmacológicas para la prevención de las fracturas por insuficiencia y necrosis avascular asociada con la radioterapia pelviana en adultos
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Fractures, Bone] explode all trees
#2 fracture*.mp.
#3 bone* near/5 (break or broke*)
#4 MeSH descriptor: [Femur Head Necrosis] this term only
#5 femur head necrosis
#6 avascular necrosis
#7 (bone* near/5 (fragility or fragile))
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Radiotherapy] explode all trees
#10 radiotherap* or brachytherap* or IMRT
#11 radiat* or irradiat*
#12 Any MeSH descriptor with qualifier(s): [Radiotherapy ‐ RT]
#13 #9 or #10 or #11 or #12
#14 Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT]
#15 MeSH descriptor: [Calcium, Dietary] explode all trees
#16 MeSH descriptor: [Vitamin D] explode all trees
#17 MeSH descriptor: [Diphosphonates] explode all trees
#18 MeSH descriptor: [Selective Estrogen Receptor Modulators] explode all trees
#19 MeSH descriptor: [Hormone Replacement Therapy] explode all trees
#20 MeSH descriptor: [Contraceptives, Oral] explode all trees
#21 MeSH descriptor: [Testosterone] explode all trees
#22 (calcium or vitamin D or bisphosphonate* or alendronate or alendronic acid or etidronate or ibandronate or risedronate or zoledronate or pamidronate or (selective adj (oestrogen or estrogen) adj receptor modulator*) or raloxifene or hormone replacement or ((oestrogen or estrogen) adj replacement therapy) or oral contraceptive pill or testosterone or sustanon or strontium ranelate or teriparatide or denosumab or calcitonin)
#23 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
#24 #8 and #13 and #23
Appendix 2. MEDLINE search strategy
1 exp Fractures, Bone/
2 fracture*.mp.
3 (bone* adj5 (break or broke*)).mp.
4 Femur Head Necrosis/
5 femur head necrosis.mp.
6 avascular necrosis.mp.
7 (bone* adj5 (fragility or fragile)).mp.
8 1 or 2 or 3 or 4 or 5 or 6 or 7
9 exp Radiotherapy/
10 (radiotherap* or brachytherapy or IMRT).mp.
11 (radiat* or irradiat*).mp.
12 radiotherapy.fs.
13 9 or 10 or 11 or 12
14 drug therapy.fs.
15 Calcium, Dietary/
16 exp Vitamin D/
17 exp Diphosphonates/
18 exp Selective Estrogen Receptor Modulators/
19 exp Hormone Replacement Therapy/
20 exp Contraceptives, Oral/
21 exp Testosterone/
22 (calcium or vitamin D or bisphosphonate* or alendronate or alendronic acid or etidronate or ibandronate or risedronate or zoledronate or pamidronate or (selective adj (oestrogen or estrogen) adj receptor modulator*) or raloxifene or hormone replacement or ((oestrogen or estrogen) adj replacement therapy) or oral contraceptive pill or testosterone or sustanon or strontium ranelate or teriparatide or denosumab or calcitonin).mp.
23 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 8 and 13 and 23
key:
mp = Title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
Appendix 3. Embase Ovid search strategy
1 exp fracture/
2 fracture*.mp.
3 (bone* adj5 (break or broke*)).mp.
4 femur head necrosis/
5 femur head necrosis.mp.
6 avascular necrosis.mp.
7 (bone* adj5 (fragility or fragile)).mp.
8 1 or 2 or 3 or 4 or 5 or 6 or 7
9 exp radiotherapy/
10 (radiotherap* or brachytherapy or IMRT).mp.
11 (radiat* or irradiat*).mp.
12 rt.fs.
13 9 or 10 or 11 or 12
14 dt.fs.
15 calcium intake/
16 exp vitamin D/
17 exp bisphosphonic acid derivative/
18 selective estrogen receptor modulator/
19 exp hormone substitution/
20 exp oral contraceptive agent/
21 androgen therapy/
22 (calcium or vitamin D or bisphosphonate* or alendronate or alendronic acid or etidronate or ibandronate or risedronate or zoledronate or pamidronate or (selective adj (oestrogen or estrogen) adj receptor modulator*) or raloxifene or hormone replacement or ((oestrogen or estrogen) adj replacement therapy) or oral contraceptive pill or testosterone or sustanon or strontium ranelate or teriparatide or denosumab or calcitonin).mp.
23 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 8 and 13 and 23
25 (exp animal/ or nonhuman/ or exp animal experiment/) not human/
26 24 not 25
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
fs=floating subheading
Appendix 4. Risk of bias
Random sequence generation
-
Low risk of bias (e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers).
-
High risk of bias (e.g. participants assigned to treatments on basis of date of birth or clinic ID number or surname, or no attempt made to randomly assign participants).
-
Unclear risk of bias (e.g. not reported, information not available).
Allocation concealment
-
Low risk of bias (e.g. allocation sequence could not be foretold).
-
High risk of bias (e.g. allocation sequence could be foretold by participants, investigators or treatment providers).
-
Unclear risk of bias (e.g. not reported).
Blinding of participants and personnel
-
Low risk of bias if participants and personnel were adequately blinded.
-
High risk of bias if participants were not blinded to the intervention that they received.
-
Unclear risk of bias if this was not reported or was unclear.
Blinding of outcomes assessors
-
Low risk of bias if outcomes assessors were adequately blinded.
-
High risk of bias if outcomes assessors were not blinded to the interventions that participants received.
-
Unclear risk of bias if this was not reported or was unclear.
Incomplete outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome as:
-
low risk of bias if less than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment groups;
-
high risk of bias if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment groups; and
-
unclear risk of bias if loss to follow‐up was not reported.
Selective reporting of outcomes
-
Low risk of bias (e.g. review reported all outcomes specified in the protocol).
-
High risk of bias (e.g. suspected that outcomes were selectively reported).
-
Unclear risk of bias (e.g. unclear whether outcomes were selectively reported).
Other bias
-
Low risk of bias: no suspicion of any other source of bias and trial appeared to be methodologically sound.
-
High risk of bias: suspected that the trial was prone to an additional bias.
-
Unclear risk of bias: uncertain whether an additional bias may have been present.
Non‐randomised studies
We assessed the risk of bias in non‐randomised controlled trials in accordance with four additional criteria concerning cohort selection and comparability of treatment groups.
Relevant details of criteria for assignment of participants to treatments
-
Low risk of bias (e.g. yes).
-
High risk of bias (e.g. no).
-
Unclear risk of bias.
Representative group of participants who received the experimental intervention
-
Low risk of bias if representative of adults undergoing pelvic radiotherapy.
-
High risk of bias if groups of participants were selected.
-
Unclear risk of bias if selection of group was not described.
Representative group of participants who received the comparison intervention
-
Low risk of bias if drawn from the same population as the experimental cohort.
-
High risk of bias if drawn from a different source.
-
Unclear risk of bias if selection of group was not described.
No differences between the two groups or differences controlled for, in particular with reference to age, gender, type/dose of radiotherapy, use of chemotherapy and performance status
-
Low risk of bias if at least two of these characteristics were reported.
-
High risk of bias if the 2 groups differed and differences were not controlled for.
-
Unclear risk of bias if fewer than two of these characteristics were reported even if no other differences were noted between the groups and other characteristics were controlled for.
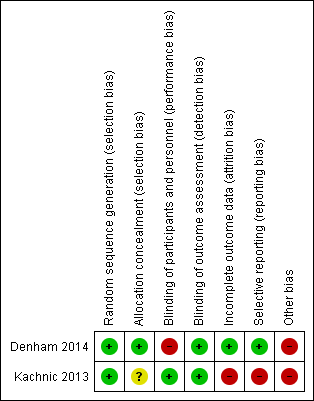
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Pharmacological intervention compared with no intervention for prevention of radiation‐related insufficiency fractures and avascular necrosis | |||
| Patient or population: Adults undergoing pelvic radiotherapy Settings: Intervention prior or during radiotherapy; hospital Intervention: Zoledronic acid Comparison: No intervention | |||
| Outcomes | No of participants | Quality of the evidence | Comments |
| The 2 included studies did not fully describe the radiotherapy techniques used. The dose, delivery of treatment (conformal, intensity modulated, brachytherapy) and, therefore, volume of pelvis irradiated could influence the pattern of bone fractures, changes in BMD and risk of avascular necrosis. Studies were heterogeneous in other aspects so meta‐analyses were not performed and results were reported as a narrative. | |||
| Insufficiency fractures and avascular necrosis | Kachnic 2013 (96 eligible participants) Out of the 91 men (95% of eligible participants) who consented to QoL data collection, participant compliance for completing the FACT‐G was 96% at baseline, 82% at 12 months, 71% at 24 months and 58% at 36 months. Denham 2014 (905 participants had thoracolumbar x‐rays available for assessment out of a possible 1167) (222 participants for BMD sub study). | ⊕⊝⊝⊝ |
|
| BMD and fracture risk | ⊕⊝⊝⊝ |
| |
| Bone turnover markers | ⊕⊝⊝⊝ |
| |
| QoL | ⊕⊝⊝⊝ |
| |
| Mortality | ⊕⊝⊝⊝ |
| |
| Adverse events | ⊕⊝⊝⊝ |
| |
| BMD: bone mineral density; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; QoL: quality of life; RCT: randomised controlled trial. | |||
| GRADE Working Group grades of evidence | |||
| 1Downgraded as both studies had a low reported number of fractures but the details of the fracture (location, nature) were not described and hence there was uncertainty regarding these being insufficiency fractures from radiotherapy. 2Quality of evidence downgraded due to heterogeneity, poor reporting of results and limitations in study methodology (risk of bias). 3Downgraded as QoL not reported in the large trial (Denham 2014). 4Downgraded as neither trial reported other potentially important outcomes such as survival, bone symptoms and unplanned hospital stay. | |||