Intervenciones para el tratamiento de la gangrena gaseosa
Appendices
Appendix 1. Glossary
Antitoxin: antibody produced in response to a toxin of bacterial, animal, or plant origin (the origin in this text refers to the clostridial bacteria causing gas gangrene) with the ability to neutralise the effects of the toxin.
Barotrauma: physical damage to body tissues caused by a difference in pressure between an air space inside or beside the body and the surrounding fluid. Damage occurs in the tissues around the body's air spaces because gases are compressible and the tissues are not. During increases in external pressure, the internal air space provides the surrounding tissues with little support to resist the higher pressure. During decreases in external pressure, the higher pressure of gas trapped inside air spaces within the body causes damage to the surrounding tissues.
Central venous pressure: the venous pressure as measured at the right atrium, one of the two blood collection chambers of the heart. The pressure can be measured by means of a catheter (tube) introduced through the median cubital vein, a superficial vein of the upper limb, to the superior vena cava, one of the veins that carry blood to the heart's right atriums.
Clostridial bacteraemia: the presence of clostridial bacteria in the blood. Since the blood is normally a sterile environment, the detection of bacteria in the blood (most commonly with blood cultures) is always abnormal.
Crepitus: a sound or feeling that resembles the crackling noise heard when rubbing hair between the fingers or throwing salt on an open fire. In gas gangrene, crepitus is caused by rubbing of bone fragments and air in superficial tissues.
Debridement: surgical removal of foreign material and dead tissue from a wound in order to promote healing and prevent infection.
Detumescence: reduction of swelling or the subsidence of anything swollen.
Exotoxins: potent toxins secreted by micro‐organisms and released into their surroundings. These can cause damage to people by destroying cells or disrupting normal cellular metabolism. Exotoxins are proteins and so can be destroyed by heat, or detoxified by treatment with formaldehyde. Bacteria of the genus Clostridium are one of the most frequent producers of exotoxins.
Haematogenous spread: the term given to the dissemination of Clostridium through the blood stream, usually to an extremity or abdominal wall, causing necrotising infection.
Haemorrhagic bulla: a fluid‐containing, elevated lesion of the skin, usually more than 5 mm in diameter and characterised by bleeding beneath the skin, with a very clear boundary.
Hydrogen peroxide: an unstable compound of hydrogen and oxygen that is easily broken down into water and oxygen. A 3% solution is used as a mild antiseptic for the skin and mucous membranes.
Hypersensitivity: a state in which the body reacts with an exaggerated immune response to something it perceives to be a foreign substance.
Malodour: gangrene is accompanied by a distinctive odour that is offensively unpleasant.
Myositis: inflammation of a muscle, especially a skeletal muscle which is used to move bones, characterised by pain, tenderness, and sometimes spasm in the affected area.
Necrosis: the death of cells or tissues from severe injury or disease, especially in a localised area of the body. Causes of necrosis include inadequate blood supply (as in infarcted tissue), bacterial infection, traumatic injury, and hyperthermia (being too hot).
Packed cell volume: the ratio of the volume occupied by packed red blood cells to the volume of the whole blood.
Potassium permanganate: a dark purple crystalline compound used as a disinfectant in medicine.
Pulmonary capillary wedge pressure: an indirect measure of the pressure in the leftatrium, one of the two blood collection chambers of the heart. The pressure can be obtained by wedging a catheter (tube) into a small pulmonary artery (artery which carries blood from the heart to the lungs) tightly enough to block flow from behind and thus to sample the pressure beyond.
Serosanguinous exudate: the liquid that drains from open wounds in the human body. Exudate is made up of the serum around inflamed and damaged tissue. One type of exudate, called serosanguinous exudate, appears pink due to a small number of blood cells mixing with the fluid that is draining out.
Appendix 2. Search strategies
Ovid MEDLINE
1 exp Gas Gangrene/
2 (gas* adj gangrene).tw.
3 (clostridi* adj myonecrosis).tw.
4 ((nonclostridi* or non‐clostridi*) adj myonecrosis).tw.
5 or/1‐4
6 randomized controlled trial.pt.
7 controlled clinical trial.pt.
8 randomized.ab.
9 placebo.ab.
10 clinical trials as topic.sh.
11 randomly.ab.
12 trial.ti.
13 or/6‐12
14 (animals not (humans and animals)).sh.
15 13 not 14
16 5 and 15
Ovid EMBASE
1 exp gas gangrene/
2 (gas* adj gangrene).tw.
3 (clostridi* adj myonecrosis).tw.
4 ((nonclostridi* or non‐clostridi*) adj myonecrosis).tw.
5 or/1‐4
6 Randomized controlled trials/
7 Single‐Blind Method/
8 Double‐Blind Method/
9 Crossover Procedure/
10 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab.
11 (doubl$ adj blind$).ti,ab.
12 (singl$ adj blind$).ti,ab.
13 or/6‐12
14 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
15 human/ or human cell/
16 and/14‐15
17 14 not 16
18 13 not 17
19 5 and 18
EBSCO CINAHL
S18 S5 AND S17
S17 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16
S16 MH "Quantitative Studies"
S15 TI placebo* or AB placebo*
S14 MH "Placebos"
S13 TI random* allocat* or AB random* allocat*
S12 MH "Random Assignment"
S11 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S10 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S9 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S8 TI clinic* N1 trial* or AB clinic* N1 trial*
S7 PT Clinical trial
S6 MH "Clinical Trials+"
S5 S1 OR S2 OR S3 OR S4
S4 TI ( (nonclostridi* or non‐clostridi*) N1 myonecrosis ) OR AB ( (nonclostridi* or non‐clostridi*) N1 myonecrosis )
S3 AB clostridi* N1 myonecrosis OR TI clostridi* N1 myonecrosis
S2 AB gas* N1 gangrene OR TI gas* N1 gangrene
S1 (MH "Gas Gangrene")
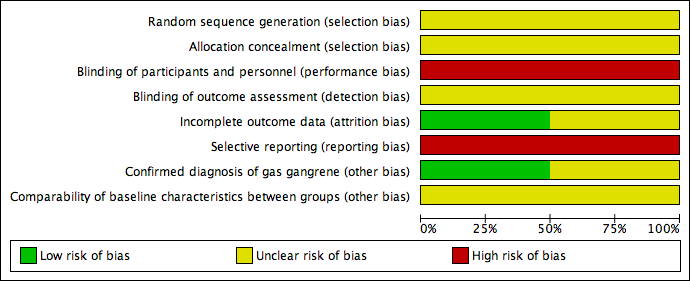
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
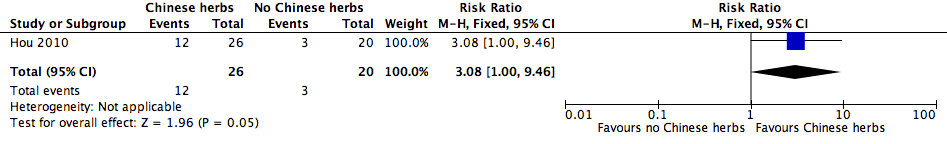
Forest plot of comparison: 1 Additional Chinese herbs versus no additional Chinese herbs, outcome: 1.1 Cure rate.

Forest plot of comparison: 2 Additional topical HBOT versus additional systemic HBOT, outcome: 2.1 Cure rate.

Comparison 1 Additional Chinese herbs versus no additional Chinese herbs, Outcome 1 Cure rate.

Comparison 2 Additional topical HBOT versus additional systemic HBOT, Outcome 1 Cure rate.
| Additional Chinese herbs compared with no additional Chinese herbs for treating gas gangrene | ||||||
| Patient or population: patients with gas gangrene | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no Chinese herbs | Risk with Chinese herbs | |||||
| Cure rate | Study population | RR 3.08 | 46 | ⊕⊝⊝⊝ | ||
| 150 per 1000 | 462 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to limitations in design; high risk of performance and reporting bias, and unclear risk of bias in other bias sources. 2 Downgraded two levels due to imprecision; only one trial with small sample size and very wide confidence interval that included the possibility of an effect in either direction (crosses line of no effect). | ||||||
| Additional topical HBOT compared with additional systemic HBOT for treating gas gangrene | ||||||
| Patient or population: patients with gas gangrene | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic HBOT | Risk with Topical HBOT | |||||
| Cure rate | Study population | RR 1.10 | 44 | ⊕⊝⊝⊝ | ||
| 130 per 1000 | 143 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to limitations in design; high risk of performance and reporting bias, and unclear risk of bias in selection and detection bias. 2 Downgraded two levels for imprecision; only one trial with small sample size and very wide confidence interval that included the possibility of an effect in either direction (crosses line of no effect). | ||||||
| Search strategies |
| China Biological Medicine Database (CBM‐disc) |
| #1 "气性坏疽"[常用字段:智能] |
| China National Knowledge Infrastructure (CNKI) |
| (主题="气性坏疽") OR ((主题="梭菌"+"梭状") AND (主题="肌坏死"+"肌炎")) |
| Chinese scientific periodical database of VIP INFORMATION (VIP) |
| ((题名或关键词=肌坏死 或 文摘=肌坏死 或 题名或关键词=肌炎 或 文摘=肌炎 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊) 与 (题名或关键词=梭状 或 文摘=梭状 或 题名或关键词=梭菌 或 文摘=梭菌 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊)) 或者 (题名或关键词=气性坏疽 或 文摘=气性坏疽 与 专业=经济管理+图书情报+教育科学+自然科学+农业科学+医药卫生+工程技术+社会科学 与 范围=全部期刊) |
| Science Citation Index |
| Gas gangrene or clostridi* myonecrosis |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
| "Gas Gangrene"(By topics) |
| Current Controlled Trials (www.controlled‐trials.com) |
| "gas gangrene" or "myonecrosis" |
| WHO International Clinical Trials Registry Platform (www.who.int/trialsearch) |
| gas gangrene or clostridi* myonecrosis or non‐clostridi* myonecrosis or nonclostridi* myonecrosis |
| Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) |
| "gas gangrene" or "clostridial myonecrosis" or "non‐clostridial myonecrosis" or "nonclostridial myonecrosis" |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure rate Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.00, 9.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure rate Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.84] |


