Probiotics for vulvovaginal candidiasis in non‐pregnant women
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010496.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 23 November 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Sexually Transmitted Infections Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
HX and DF developed the review and were in charge of searching for studies, quality assessment, data extraction and data analysis.
FF was in charge of data analysis and review development, and offered clinical expertise.
DW participated in searching for studies and quality assessment.
HC participated in searching for studies and quality assessment.
LM participated in data extraction and data analysis.
XW participated in data extraction and data analysis.
Sources of support
Internal sources
-
West China Second University Hospital, West China Women's and Children's Hospital, Sichuan University, China.
External sources
-
No sources of support supplied
Declarations of interest
HX: none.
DF: none.
DW: none.
LM: none.
HC: none.
XW: none.
FF: none.
Acknowledgements
We would like to thank the Cochrane Sexually Transmitted Infections Group, the Cochrane Menstrual Disorders and Subfertility Group and the Cochrane Editorial Unit.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 23 | Probiotics for vulvovaginal candidiasis in non‐pregnant women | Review | Huan Yu Xie, Dan Feng, Dong Mei Wei, Ling Mei, Hui Chen, Xun Wang, Fang Fang | |
| 2013 Apr 30 | Probiotics for vulvovaginal candidiasis in non‐pregnant women | Protocol | Huan Yu Xie, Dan Feng, Dong Mei Wei, Hui Chen, Ling Mei, Xun Wang, Fang Fang | |
Differences between protocol and review
We redefined the "Clinical cure rate" in the primary outcome as "disappearance of symptoms and signs, and no evidence of fungal infection proved by microscopic examination or vaginal culture", split into 'short‐term clinical cure rate (zero to 14 days after treatment)' and 'long‐term clinical cure rate (one, three and six months after treatment).'
We redefined the types of interventions, deleted the type of "any probiotic used alone versus placebo or no intervention" and "used as adjuvants to conventional antifungal drugs (before, during or after antifungal treatment) versus placebo or no intervention".
We moved "rate of serious adverse events" from secondary outcomes to primary outcomes.
We redefined "the rate of non‐serious adverse events" in the secondary outcomes as mild symptoms include vomiting, diarrhea, abdominal pain, abnormal urination, pelvic cramps, dysmenorrhea, paresthesia, rhinorrhea, headache, dizziness, fever, chills, vaginal burning, stinging, itching and irritation.
We changed the "Sensitivity analysis" in the methods section as "We planned to perform a sensitivity analysis to explore whether the results of the review were robust, depending on study quality, for each outcome variable. We excluded studies with a high risk of bias, comparing findings within the remainder of the included studies with the original meta‐analysis."
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Intravaginal;
- Antifungal Agents [administration & dosage];
- Candidiasis, Vulvovaginal [prevention & control, *therapy];
- Clotrimazole [administration & dosage];
- Fluconazole [administration & dosage];
- Imidazoles [administration & dosage];
- Miconazole [administration & dosage];
- Probiotics [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Secondary Prevention;
Medical Subject Headings Check Words
Female; Humans;
PICOs

Study flow diagram. RCT: randomized controlled trial.
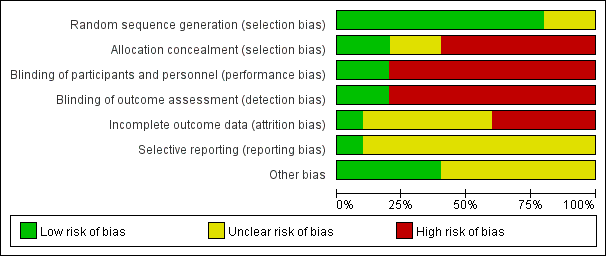
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.1 Clinical cure rate (short‐term).
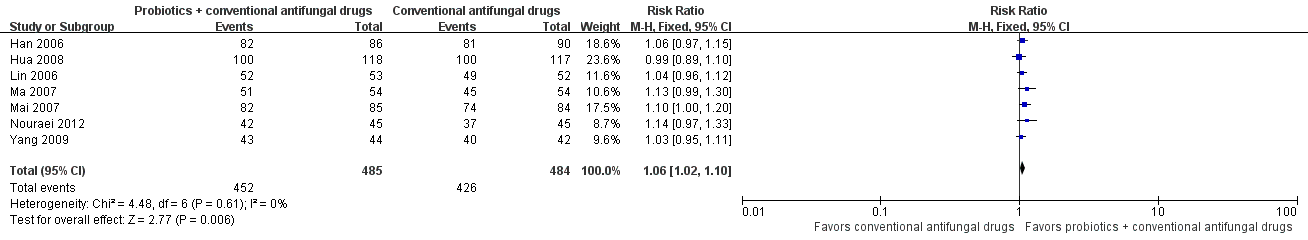
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.6 Mycological cure rate (short‐term).

Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.10 Mycological cure rate (long‐term/1 month after treatment).
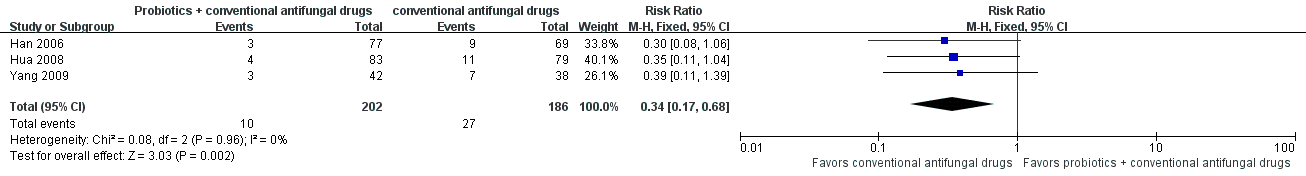
Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.13 Relapse rate.

Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.14 Non‐serious adverse events.

Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.3 Clinical cure rate (short‐term): subgroup analysis.

Forest plot of comparison: 2 Probiotics + conventional antifungal drugs versus conventional antifungal drugs, outcome: 1.8 Mycological cure rate (short‐term): subgroup analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 1 Clinical cure rate (short‐term).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 2 Clinical cure rate (short‐term): sensitivity analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 3 Clinical cure rate (short‐term): subgroup analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 4 Clinical cure rate (long‐term/1 month after treatment).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 5 Clinical cure rate (long‐term/3 months after treatment).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 6 Mycological cure rate (short‐term).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 7 Mycological cure rate (short‐term): sensitivity analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 8 Mycological cure rate (short‐term): subgroup analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 9 Mycological non‐cure (short‐term/Candida albicans versus non‐albicans).
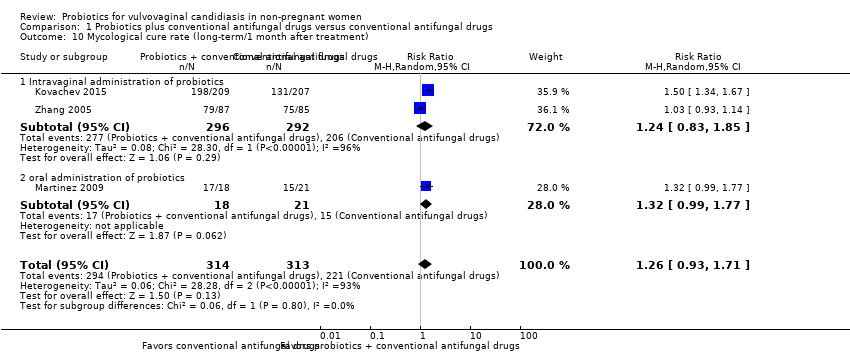
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 10 Mycological cure rate (long‐term/1 month after treatment).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 11 Mycological cure rate (long‐term/1 month after treatment): sensitivity analysis.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 12 Mycological cure rate (long‐term/3 months after treatment).

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 13 Relapse rate.
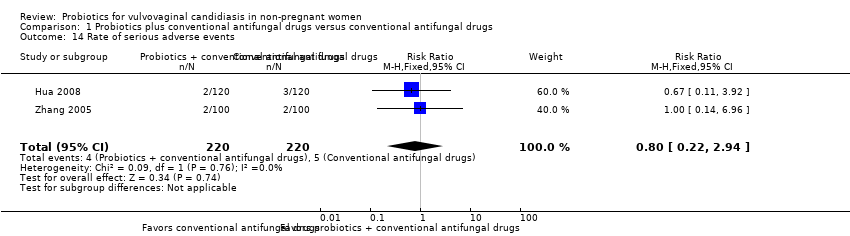
Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 14 Rate of serious adverse events.

Comparison 1 Probiotics plus conventional antifungal drugs versus conventional antifungal drugs, Outcome 15 Rate of non‐serious adverse events.
| Probiotics used as adjuvants to conventional antifungal drugs compared with conventional antifungal drugs for the treatment of vulvovaginal candidiasis in non‐pregnant women | |||||||
| Patient or population: non‐pregnant women with vulvovaginal candidiasis Settings: outpatient clinics in Brazil, Bulgaria, Iran and China Intervention: probiotics used as adjuvants to conventional antifungal drugs Comparison: conventional antifungal drugs | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Conventional antifungal drugs | Probiotics used as adjuvants to conventional antifungal drugs | ||||||
| Clinical cure rate (short‐term) follow‐up: 5‐10 days | Medium risk population | RR 1.14 (1.05 to 1.24) | 695 | ⊕⊕⊝⊝ | ‐ | ||
| 721 per 1000 | 822 per 1000 | ||||||
| Clinical cure rate (long‐term) | 1 month after treatment follow‐up: 1 month | Medium risk population | RR 1.07 (0.86 to 1.33) | 172 | ⊕⊝⊝⊝ | ‐ | |
| 635 per 1000 | 679 per 1000 | ||||||
| 3 months after treatment follow‐up: 3 months | Medium risk population | RR 1.30 (1.00 to 1.70) | 172 | ⊕⊝⊝⊝ | ‐ | ||
| 494 per 1000 | 642 per 1000 | ||||||
| Mycological cure rate (short‐term) follow‐up: 5‐10 days | Medium risk population | RR 1.06 (1.02 to 1.10) | 969 | ⊕⊕⊝⊝ | See 3 in footnotes. | ||
| 880 per 1000 | 933 per 1000 | ||||||
| Mycological cure rate (long‐term) | 1 month after treatment follow‐up: 28‐30 days | Medium risk population | RR 1.26 (0.93 to 1.71) | 627 | ⊕⊝⊝⊝ | See 5 in footnotes. | |
| 706 per 1000 | 890 per 1000 | ||||||
| 3 months after treatment follow‐up: 3 months | Medium risk population | RR 1.16 (1.00 to 1.35) | 172 | ⊕⊝⊝⊝ | ‐ | ||
| 741 per 1000 | 860 per 1000 | ||||||
| Relapse rate follow‐up: 30‐37 days after treatment | Medium risk population | RR 0.34 (0.17 to 0.68) | 388 | ⊕⊝⊝⊝ | ‐ | ||
| 145 per 1000 | 49 per 1000 | ||||||
| Rate of serious adverse events follow‐up: 5‐90 days after treatment | Medium risk population | RR 0.80 (0.22 to 2.94) | 440 | ⊕⊕⊝⊝ | ‐ | ||
| 23 per 1000 | 18 per 1000 | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded two levels due to very serious risk of bias: the included studies had a high or unclear risk of bias in at least one key domain i.e. random sequence generation, allocation concealment or blinding. 2Downgraded one level due to serious imprecision: the 95% CI were wide and included null effects. 3A sensitivity analysis that excluded studies with high risk of bias and included only one study showed that the rate of short‐term mycological cure was changed to no significantly difference between two arms. For the heterogeneity and difference in results, we think the reasons may be associated with the small sample of included study, high risk of bias of studies that were excluded for sensitivity analysis. 4Downgraded one level due to inconsistency: there was a substantial heterogeneity between studies. 5There was heterogeneity that may be attributable to differences in route of the administration of probiotics, we undertook a subgroup analysis, the results showed no statistically significant difference between two arms in either subgroup. 6Downgraded one level due to serious imprecision: small sample size and few events. | |||||||
| Species | Frequency | Response to azoles |
| Candida albicans | 80‐90% | Sensitive |
| Candida glabrata | 5‐10% | Resistant |
| Candida krusei | < 1% | Tends to be resistant |
| Candida lusitaniae | < 1% | Tends to be resistant |
| Candida parapsilosis | < 1% | Tends to be resistant |
| Candida pseudotropicalis | < 1% | Tends to be resistant |
| Candida tropicalis | < 1% | Tends to be resistant |
| Source: Bieber 2006. | ||
| Non‐prescription intravaginal agents | Prescription intravaginal agents |
| Butoconazole 2% cream 5 g intravaginally for 3 days OR Clotrimazole 1% cream 5 g intravaginally for 7‐14 days OR Clotrimazole 2% cream 5 g intravaginally for 3 days OR Miconazole 2% cream 5 g intravaginally for 7 days OR Miconazole 4% cream 5 g intravaginally for 3 days OR Miconazole 100 mg vaginal suppository, 1 suppository for 7 days OR Miconazole 200 mg vaginal suppository, 1 suppository for 3 days OR Miconazole 1200 mg vaginal suppository, 1 suppository for 1 day OR Tioconazole 6.5% ointment 5 g intravaginally in a single application | Butoconazole 2% cream (single dose bioadhesive product), 5 g intravaginally for 1 day OR Nystatin 100,000 unit vaginal tablet, 1 tablet for 14 days OR Terconazole 0.4% cream 5 g intravaginally for 7 days OR Terconazole 0.8% cream 5 g intravaginally for 3 days OR Terconazole 80 mg vaginal suppository, 1 suppository for 3 days |
| CDC: Centers for Disease Control and Prevention. Source: CDC 2015. | |
| Recurrent vulvovaginal candidiasis | |
| Initial regimen: 7‐14 days of any topical azole drug OR Fluconazole 100 mg, 150 mg or 200 mg orally once daily every 3rd day for a total of 3 doses (days 1, 4 and 7) | Maintenance regimen: Fluconazole 100 mg, 150 mg or 200 mg orally once weekly for 6 months |
| Severe vulvovaginal candidiasis | |
| Intravaginally once daily for 7 to 14 days of any topical azole drug OR Fluconazole 150 mg orally once daily in 2 doses (second dose 72 hours after initial dose) | |
| Non‐albicans vulvovaginal candidiasis | |
| Non‐fluconazole azole (oral or topical) 7‐14 days OR Boric acid gelatin capsule Intravaginally once daily for 14 days | |
| Abnormal host | |
| More prolonged (i.e. 7‐14 days) conventional antifungal drugs is necessary | |
| CDC: Centers for Disease Control and Prevention. Source: CDC 2015. | |
| Lactobacillus species: Lactobacillus acidophilus Lactobacillus bulgaricus Lactobacillus casei Lactobacillus crispatus Lactobacillus delbrueckii Lactobacillus fermentum Lactobacillus gasseri Lactobacillus johnsonii Lactobacillus lactis Lactobacillus plantarum Lactobacillus reuteri Lactobacillus rhamnosus GG | Streptococcus species: Streptococcus thermophilus
|
| Yeast: Saccharomyces boulardii
| |
| Other species: Bacillus cereus Enterococcus faecalisa Enterococcus faeciuma Escherichia coli Nissle
| |
| Bifidobacterium species: Bifidobacterium adolescentis Bifidobacterium animalis Bifidobacterium bifidum Bifidobacterium breve Bifidobacterium infantis Bifidobacterium lactis Bifidobacterium longum | |
| Sources: Senok 2005; Doron 2006; Santosa 2006. | |
| Included study | Intervention groups | Intervention doses and duration | Intervention administration route |
| Clotrimazole + probiotic group | 1 tablet of clotrimazole 500 mg on day 1 and day 4 + 1 capsule of Lactobacillus delbrueckii Subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal | |
| Clotrimazole group | 1 tablet of clotrimazole 500 mg on day 1 and day 4. | Vaginal | |
| Miconazole + probiotic group | 1 suppository of miconazole nitrate 400 mg, QD from day 1 to day 6, and then 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 7 to day 16. | Vaginal | |
| Miconazole group | 1 vaginal suppository of Miconazole nitrate (400 mg), QD from day 1 to day 6 | Vaginal | |
| Azole + vaginal probiotic group | Fluconazole 150 mg + 1 globule of fenticonazole 600 mg on the same day; however, 10 applications of probiotics (Lactobacillus acidophilus,Lactobacillus rhamnosus,Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus) were also administered beginning the 5th day after azole treatment. | Fluconazole: oral. Fenticonazole: vaginal Probiotics: vaginal | |
| Azole group | Fluconazole 150 mg + 1 globule of fenticonazole 600 mg on the same day. | Fluconazole: oral Fenticonazole: vaginal | |
| Clotrimazole + probiotic group | 1 suppository of clotrimazole 150 mg, QD from day 1 to day 7, then, 1 capsule of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. | Vaginal | |
| Clotrimazole group | 1 suppository of clotrimazole 150 mg, QD from day 1 to day 7. | Vaginal | |
| Miconazole + probiotic group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 14, and on day 8, 1 capsule of Streptococcus faecalis (each capsule contained 6 × 107 colony forming units), QD from day 8 to day 14. | Vaginal | |
| Miconazole group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 14. | Vaginal | |
| Clotrimazole + probiotic group | 1 suppository of clotrimazole 150 mg, QN + 1 capsule of Lactobacillus delbrueckii Subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal | |
| Clotrimazole group | 1 suppository of clotrimazole 150 mg, QN from day 1 to day 10. | Vaginal | |
| Fluconazole + probiotic group | 1 dose of fluconazole 150 mg + 2 capsules of Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 (each capsule contained 1 × 109 viable cells of both strains) for 28 days | Oral | |
| Fluconazole + placebo group | 1 dose of fluconazole 150 mg + 2 oral capsules of placebo once daily (every morning) for 28 days. | Oral | |
| Fluconazole + oral protexin group | 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 protexin capsules per day (after meals in the morning and evening) for 3 days. | Oral | |
| Fluconazole + placebo group | 1 dose of fluconazole 300 mg (2 × 150 mg) + 2 placebo capsules daily (after meals in the morning and evening) for 3 days. | Oral | |
| Clotrimazole + probiotic group | 1 tablet of clotrimazole 500 mg on day 1 and day 4 + 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 1 to day 10. | Vaginal | |
| Clotrimazole group | 1 tablet of clotrimazole 500 mg on day 1 and day 4. | Vaginal | |
| Miconazole + probiotic group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 7, and then, 1 capsule of Lactobacillus delbrueckii subsp. Lactis DM8909 (each capsule contained 0.25 × 106 colony forming units), QD from day 8 to day 17. | Vaginal | |
| Miconazole group | 1 suppository of miconazole nitrate 200 mg, QD from day 1 to day 7. | Vaginal | |
| QD: every day; QN: every night. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure rate (short‐term) Show forest plot | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.05, 1.24] |
| 2 Clinical cure rate (short‐term): sensitivity analysis Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.84] |
| 3 Clinical cure rate (short‐term): subgroup analysis Show forest plot | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.05, 1.24] |
| 3.1 Intravaginal administration and single species of probiotics | 4 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.02, 1.21] |
| 3.2 Oral administration and multiple species of probiotics | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.07, 1.84] |
| 4 Clinical cure rate (long‐term/1 month after treatment) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 5 Clinical cure rate (long‐term/3 months after treatment) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.00, 1.70] |
| 6 Mycological cure rate (short‐term) Show forest plot | 7 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.02, 1.10] |
| 7 Mycological cure rate (short‐term): sensitivity analysis Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
| 8 Mycological cure rate (short‐term): subgroup analysis Show forest plot | 7 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.02, 1.10] |
| 8.1 Intravaginal administration and single species of probiotics | 6 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.10] |
| 8.2 Oral administration and multiple species of probiotics | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.33] |
| 9 Mycological non‐cure (short‐term/Candida albicans versus non‐albicans) Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.16, 1.30] |
| 9.1 Candida albicans | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.52] |
| 9.2 Non‐albicans | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.53] |
| 10 Mycological cure rate (long‐term/1 month after treatment) Show forest plot | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.71] |
| 10.1 Intravaginal administration of probiotics | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.85] |
| 10.2 oral administration of probiotics | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.99, 1.77] |
| 11 Mycological cure rate (long‐term/1 month after treatment): sensitivity analysis Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.99, 1.77] |
| 12 Mycological cure rate (long‐term/3 months after treatment) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.00, 1.35] |
| 13 Relapse rate Show forest plot | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.68] |
| 14 Rate of serious adverse events Show forest plot | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.94] |
| 15 Rate of non‐serious adverse events Show forest plot | 7 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.48, 1.70] |

