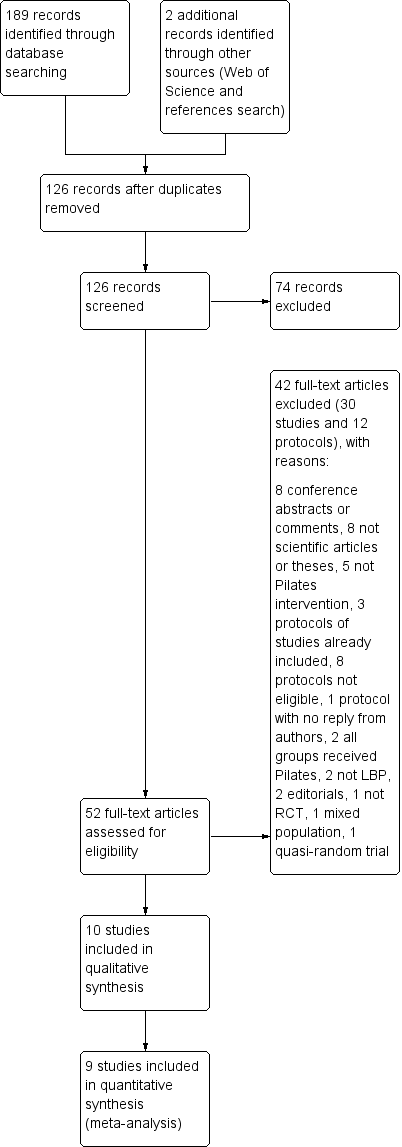
Pilates for low back pain
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010265.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 02 July 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Back and Neck Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Conception, design and drafting of the protocol: Leonardo OP Costa, Luciola da Cunha Menezes Costa and Cristina Maria Nunes Cabral.
Critical revision of the protocol for important intellectual content: Chris G Maher, Raymond Ostelo and Mark Hancock.
Final approval of the protocol: all authors.
Collection and assembly of data: Tiê P Yamato, Bruno T Saragiotto.
Analysis and interpretation of the data: Tiê P Yamato, Bruno T Saragiotto and Chris G Maher.
Drafting of the article: Tiê P Yamato, Bruno T Saragiotto and Chris G Maher.
Critical revision of the article for important intellectual content: Leonardo OP Costa, Luciola da Cunha Menezes Costa, Chris G Maher, Raymond Ostelo and Mark Hancock.
Final approval of the article: all authors.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
Tiê P Yamato has no conflict of interest.
Christopher G Maher has no conflict of interest.
Bruno T Saragiotto has no conflict of interest.
Mark J Hancock has no conflict of interest.
Raymond WJG Ostelo has no conflict of interest.
Cristina MN Cabral conducted two randomised controlled trials that use Pilates as an intervention for patients with chronic non‐specific low back pain and she is also author of one of the included trials (Miyamoto 2013).
Luciola da C Menezes Costa has no conflict of interest.
Leonardo OP Costa conducted two randomised controlled trials that use Pilates as an intervention for patients with chronic non‐specific low back pain and he is also author of one of the included trials (Miyamoto 2013).
Acknowledgements
Tiê Parma Yamato is supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. Chris Maher is supported by National Health and Medical Research Council of Australia. Bruno Tirotti Saragiotto is supported by CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jul 02 | Pilates for low back pain | Review | Tiê P Yamato, Christopher G Maher, Bruno T Saragiotto, Mark J Hancock, Raymond WJG Ostelo, Cristina MN Cabral, Luciola C Menezes Costa, Leonardo OP Costa | |
| 2012 Dec 12 | Pilates for low‐back pain | Protocol | Leonardo OP Costa, Mark Hancock, Christopher G. Maher, Raymond WJG Ostelo, Cristina MN Cabral, Luciola daC Menezes Costa | |
Differences between protocol and review
There were some differences between the protocol and review in three subsections of Data collection and analysis:
-
In Selection of studies, the screening for potentially eligible studies was conducted by two pairs of review authors instead of two review authors as stated in the protocol.
-
In Measures of treatment effect, we had pre‐specified in our protocol that for different scales we were going to quantify effect using the standardised mean difference (SMD) and the mean difference (MD) for studies using the same scale. However, we decided to quantify the effects of treatments using the MD for all continuous outcomes. If different scales were used we converted the scales to a 0 to 100 point scale.
-
We did not perform a sensitivity analysis by excluding trials where the definition of the intervention was not clear because all definitions of Pilates exercise were consistent with our criteria.
-
We did not perform a subgroup analysis for duration of symptoms as all trials included chronic patients.
-
In Assessment of heterogeneity, we included an acceptable range of the I2 value (< 50%) to combine the results in a meta‐analysis when no clear heterogeneity was identified by visual inspection. We used the 50% cut‐off as I2 values above this value may represent substantial heterogeneity.
-
The approach to GRADE has been clarified further since the protocol with more detail about downgrading.
-
We found three potentially eligible studies in trial registries reported as completed at least two years ago, for which no publicly available report was found. Additionally, we were unable to contact the authors for these trials. Thus, we considered that there was a possibility of publication bias in this review and downgraded all studies regarding publication bias for the analysis of quality of evidence (GRADE). This condition was not mentioned previously in the protocol.
-
Two studies measured quality of life, but the data from the physical and mental components were not available in the text and the authors did not provide this information on request (Natour 2014; Wajswelner 2012), so we were unable to analyse this primary outcome cited in the protocol.
-
We did not find any studies that reported return to work, so we were unable to analyse this secondary outcome cited in the protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
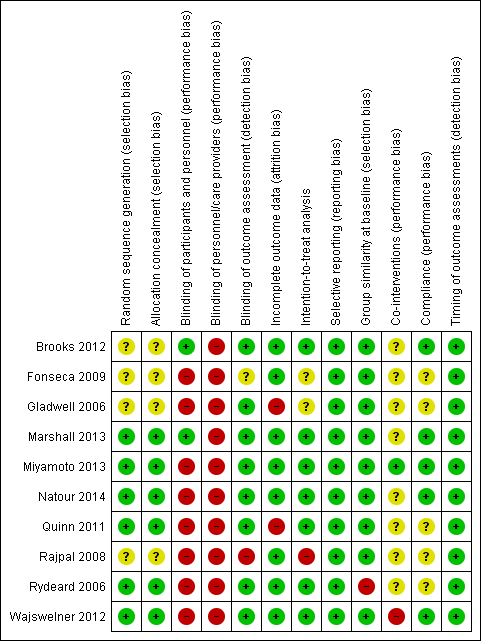
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Pilates versus minimal intervention, outcome: 1.1 Pain.

Forest plot of comparison: 2 Pilates versus other exercises, outcome: 2.2 Disability.

Comparison 1 Pilates versus minimal intervention, Outcome 1 Pain.

Comparison 1 Pilates versus minimal intervention, Outcome 2 Disability.

Comparison 1 Pilates versus minimal intervention, Outcome 3 Function.

Comparison 1 Pilates versus minimal intervention, Outcome 4 Global impression of recovery.

Comparison 2 Pilates versus other exercises, Outcome 1 Pain.

Comparison 2 Pilates versus other exercises, Outcome 2 Disability.

Comparison 2 Pilates versus other exercises, Outcome 3 Function.
| Pilates compared with minimal intervention for low back pain | ||||||
| Patient or population: patients with low back pain Settings: primary or tertiary care Intervention: Pilates Comparison: minimal intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Minimal intervention | Pilates | |||||
| Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: short‐term (less than 3 months from randomisation) | The mean pain at short‐term follow‐up ranged across control groups from 33.9 to 52 points | The mean pain at short‐term follow‐up in the intervention groups was (18.9 to 9.2 lower) | Mean difference ‐14.05 (‐18.91 to ‐9.19) | 265 participants | ⊕⊕⊝⊝ | This is a moderate effect that is clinically relevant in this patient group |
| Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean pain at intermediate‐term follow‐up ranged across control groups from 53 to 58.3 points | The mean pain at intermediate‐term follow‐up in the intervention group was 10.5 lower (18.5 to 2.6 lower) | Mean difference ‐10.54 (‐18.46 to ‐2.62) | 146 participants (2 studies) | ⊕⊕⊕⊝ | This is a moderate effect that is clinically relevant in this patient group |
| Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: short‐term (less than 3 months from randomisation) | The mean disability at short‐term follow‐up ranged across control groups from 13.3 to 44.1 points | The mean disability at short‐term follow‐up in the intervention groups was 7.95 lower (13.2 to 2.7 lower) | Mean difference ‐7.95 (‐13.23 to ‐2.67) | 248 participants (5 studies) | ⊕⊕⊝⊝ | This is a small effect that may be clinically relevant in this patient group |
| Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean disability at intermediate‐term follow‐up ranged across control groups from 27.9 to 44.4 points | The mean disability at intermediate‐term follow‐up in the intervention groups was (18.4 to 3.9 lower) | Mean difference ‐11.17 (‐18.41 to ‐3.92) | 146 participants (2 study) | ⊕⊕⊕⊝ | This is a moderate effect that is clinically relevant in this patient group |
| Function Patient Specific Functional Scale: used in a 11‐point scale from 0 to 10 (greater functional ability) Follow‐up: short‐term (less than 3 months from randomisation) | The mean function at short‐term follow‐up in the control group was 6.4 points | The mean function at short‐term follow‐up in the intervention group was 1.1 higher (0.2 to 2.0 higher) | Mean difference 1.10 (0.23 to 1.97) | 86 participants (1 study) | ⊕⊕⊝⊝ | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
| Function Patient Specific Functional Scale: used in a 11‐point scale from 0 to 10 (greater functional ability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean function at intermediate‐term follow‐up in the control group was 6.1 points | The mean function at intermediate‐term follow‐up in the intervention group was 0.8 higher (0.0 lower to 1.6 higher) | Mean difference 0.80 (‐0.00 to 1.60) | 86 participants (1 study) | ⊕⊕⊝⊝ | The difference is not statistically or clinically significant (results from 1 single study) |
| Global impression of recovery Global Perceived Effect Scale: scale from ‐5 to +5 (greater recovery) Follow‐up: short‐term (less than 3 months from randomisation) | The mean global impression of recovery at short‐term follow‐up in the control group was 1.7 points | The mean global impression of recovery at short‐term follow‐up in the intervention group was 1.5 higher (0.7 to 2.3 higher) | Mean difference 1.50 (0.70 to 2.30) | 86 participants (1 study) | ⊕⊕⊝⊝ | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
| Global impression of recovery Global Perceived Effect Scale: scale from ‐5 to +5 (greater recovery) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean global impression of recovery at intermediate‐term follow‐up in the control group was 1.7 points | The mean global impression of recovery at intermediate‐term follow‐up in the intervention group was 0.7 higher (0.1 lower to 1.5 higher) | Mean difference 0.70 (‐0.11 to 1.51) | 86 participants (1 study) | ⊕⊕⊝⊝ | The difference is not statistically or clinically significant (results from 1 single study) |
| Adverse events | See comment | See comment | Not estimable | See comment | Only 1 included trial assessed adverse events and none were reported | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to imprecision (fewer than 400 participants, total). 2 Downgraded one level due to risk of bias (> 25% of the participants were from studies with a high risk of bias). 3Downgraded one level due to clear inconsistency of results. 4Downgraded one level due to inconsistency (I² > 50%). | ||||||
| Pilates compared with other exercises for low back pain | ||||||
| Patient or population: participants with low back pain Settings: primary and tertiary care Intervention: Pilates Comparison: other exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other exercises | Pilates | |||||
| Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: short‐term (less than 3 months from randomisation) | Not estimated | Not estimated | Not estimated | 181 participants (3 studies) | ⊕⊕⊝⊝ | Pooled results not estimated due to high heterogeneity |
| Pain NRS: scale from 0 to 100 (worse pain) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | Not estimated | Not estimated | Not estimated | 151 participants (2 studies) | ⊕⊕⊝⊝ | Pooled results not estimated due to high heterogeneity |
| Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: short‐term (less than 3 months from randomisation) | The mean disability at short‐term follow‐up ranged across control groups from 17.1 to 20 points | The mean disability at short‐term follow‐up in the intervention groups was (6.8 lower to 0.2 higher) | Mean difference ‐3.29 (‐6.82 to 0.24) | 149 participants (2 studies) | ⊕⊕⊕⊝ | The difference is not statistically or clinically significant |
| Disability Multiple scales: scale from 0 to 100 (worse disability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean disability at intermediate‐term follow‐up ranged across control groups from 13 to 18.1 points | The mean disability at intermediate‐term follow‐up in the intervention groups was (5.0 lower to 3.2 higher) | Mean difference ‐0.91 (‐5.02 to 3.20) | 151 participants (2 studies) | ⊕⊕⊕⊝ | The difference is not statistically or clinically significant |
| Function Patient Specific Functional Scale: scale from 0 to 30 (greater functional ability) Follow‐up: short‐term (less than 3 months from randomisation) | The mean function at short‐term follow‐up in the control group was 18.9 points | The mean function at short‐term follow‐up in the intervention group was 0.1 lower (2.4 lower to 2.6 higher) | Mean difference 0.10 (‐2.44 to 2.64) | 87 participants (1 study) | ⊕⊕⊝⊝ | The difference is not statistically or clinically significant (results from 1 single study) |
| Function Patient Specific Functional Scale: scale from 0 to 30 (greater functional ability) Follow‐up: intermediate‐term (more than 3 months and less than 12 months) | The mean function at intermediate‐term follow‐up in the control group was 22.8 points | The mean function at intermediate‐term follow‐up in the intervention group was 3.6 lower (7 to 0.2 lower) | Mean difference ‐3.60 (‐7.00 to ‐0.20) | 87 participants (1 study) | ⊕⊕⊝⊝ | This is a small effect that may be clinically relevant in this patient group (results from 1 single study) |
| Adverse events | See comment | See comment | Not estimable | See comment | 1 trial assessed adverse events and reported minor events | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to imprecision (fewer than 400 participants, total). 2Downgraded one level due to inconsistency (I² > 50%). 3Downgraded one level due to clear inconsistency of results. | ||||||
| Studies/criteria | Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice? | Are the interventions and treatment settings described well enough so that you can provide the same for your patients? | Were all clinically relevant outcomes measured and reported? | Is the size of the effect clinically important?* | Are the likely treatment benefits worth the potential harms? |
| Yes | Yes | Yes | No | Yes | |
| Yes | Yes | No | No | Yes | |
| Yes | Yes | Yes | No | Yes | |
| Yes | Yes | Yes | No | Yes | |
| Yes | Yes | Yes | Yes1 | Yes | |
| Yes | Yes | Yes | Yes1 | Yes | |
| Yes | Yes | Yes | Yes2 | Yes | |
| No | Yes | No | No | Yes | |
| Yes | Yes | Yes | No | Yes | |
| Yes | Yes | Yes | No | Yes | |
| *Clinical importance: consider 30% on VAS/NRS for pain intensity as clinically significant, and 2 to 3 points (or 8% to 12%) on the Roland‐Morris Disability Questionnaire for disability. 1Disability (short and intermediate‐term). 2Disability (short‐term). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (< 3/12 months from randomisation) | 6 | 265 | Mean Difference (IV, Random, 95% CI) | ‐14.05 [‐18.91, ‐9.19] |
| 1.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐10.54 [‐18.46, ‐2.62] |
| 2 Disability Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3/12 months from randomisation) | 5 | 248 | Mean Difference (IV, Random, 95% CI) | ‐7.95 [‐13.23, ‐2.67] |
| 2.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐11.17 [‐18.41, ‐3.92] |
| 3 Function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intermediate‐term (more than 3/12 months, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Global impression of recovery Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Short‐term (< 3/12 months from randomisation) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Disability Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term (< 3/12 months from randomisation) | 2 | 149 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐6.82, 0.24] |
| 2.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐5.02, 3.20] |
| 3 Function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Short‐term (< 3/12 months from randomisation) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Intermediate‐term (more than 3/12, less than 12/12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |