Drenaje abdominal para la prevención del absceso intraperitoneal después de una apendicectomía abierta por una apendicitis complicada
Appendices
Appendix 1. Search strategy for CENTRAL (2017, Issue 6)
#1 MeSH descriptor: [Appendectomy] explode all trees
#2 MeSH descriptor: [Appendicitis] explode all trees
#3 appendectom* or appendic*:ti,ab,kw
#4 (#1 or #2 or #3)
#5 MeSH descriptor: [Drainage] explode all trees
#6 MeSH descriptor: [Suction] explode all trees
#7 MeSH descriptor: [Negative‐Pressure Wound Therapy] explode all trees
#8 ((negative pressure or negative‐pressure) near/3 (dressing* or therap*)):ti,ab,kw
#9 ((vacuum‐assisted or vacuum assisted) near/3 closure*):ti,ab,kw
#10 (drain* or suction*):ti,ab,kw
#11 (#5 or #6 or #7 or #8 or #9 or #10)
#12 (#4 and #11)
Appendix 2. Search strategy for MEDLINE (Ovid) (1946 to 30 June 2017)
1. exp Appendectomy/
2. exp Appendicitis/
3. (appendectom* or appendic*).mp.
4. 1 or 2 or 3
5. exp Drainage/
6. exp Negative‐Pressure Wound Therapy/
7. exp Suction/
8. ((negative pressure or negative‐pressure) adj3 (dressing* or therap*)).mp.
9. ((vacuum‐assisted or vacuum assisted) adj closure*).mp.
10. (drain* or suction*).mp.
11. 5 or 6 or 7 or 8 or 9 or 10
12. 4 and 11
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. clinical trials as topic.sh.
18. randomly.ab.
19. trial.ti.
20. 13 or 14 or 15 or 16 or 17 or 18 or 19
21. exp animals/ not humans.sh.
22. 20 not 21
23. 12 and 22
Appendix 3. Search strategy for Embase (Ovid) (1974 to 30 June 2017)
1. exp appendectomy/
2. exp acute appendicitis/
3. exp appendicitis/
4. (appendectom* or appendic*).mp.
5. 1 or 2 or 3 or 4
6. exp drain/
7. exp abscess drainage/
8. exp abdominal drainage/
9. exp wound drainage/
10. exp surgical drainage/
11. exp vacuum assisted closure/
12. exp suction/
13. ((negative pressure or negative‐pressure) adj3 (dressing* or therap*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
14. ((vacuum‐assisted or vacuum assisted) adj closure*).mp.
15. (drain* or suction*).mp.
16. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 5 and 16
18. CROSSOVER PROCEDURE.sh.
19. DOUBLE‐BLIND PROCEDURE.sh.
20. SINGLE‐BLIND PROCEDURE.sh.
21. (crossover* or cross over*).ti,ab.
22. placebo*.ti,ab.
23. (doubl* adj blind*).ti,ab.
24. allocat*.ti,ab.
25. trial.ti.
26. RANDOMIZED CONTROLLED TRIAL.sh.
27. random*.ti,ab.
28. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
30. 28 not 29
31. 17 and 30
Appendix 4. Search strategy for Science Citation Index Expanded (1900 to 30 June 2017)
#1 Topic=(appendectom* OR appendic*)
#2 Topic=(drain* OR suction* OR negative pressure wound therap* OR negative‐pressure wound therap* OR vacuum‐assisted closure OR vacuum assisted closure*)
#3 Topic=(random* OR control* OR RCT* OR placebo OR trial* OR group*)
#4 (#1 AND #2 AND #3)
Appendix 5. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
| Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of 'Low risk' of bias. | The investigators described a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of 'High risk' of bias. | The investigators described a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, e.g.:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, e.g.:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk.' |
| Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of 'Low risk' of bias. | Participants and investigators enrolling participants could not have foreseen assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of 'High risk' of bias. | Participants or investigators enrolling participants could possibly have foreseen assignments and thus introduced selection bias, such as allocation based on:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information to permit judgement of 'Low risk' or 'High risk.' This is usually the case if the method of concealment was not described or not described in sufficient detail to allow a definite judgement; e.g. if the use of assignment envelopes was described, but it remained unclear whether envelopes were sequentially numbered, opaque and sealed. |
| Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
| Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
| Incomplete outcome data Attrition bias due to amount, nature, or handling of incomplete outcome data. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
| Selective reporting Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of 'Low risk' of bias. | Any of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information to permit judgement of 'Low risk' or 'High risk.' It is likely that the majority of studies will fall into this category. |
| Other bias Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of 'Low risk' of bias. | Study appeared to be free of other sources of bias. |
| Criteria for the judgement of 'High risk' of bias. | There was ≥ 1 important risk of bias; e.g. the study:
|
| Criteria for the judgement of 'Unclear risk' of bias. | There may be a risk of bias, but there was either:
|

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Trial sequential analysis of drain use versus no drain use for intra‐peritoneal abscess. Analysis was performed with an event rate of 10.7% (Pc) in the control group, a risk ratio reduction of 20%, alpha 5%, beta 20%, and observed diversity 63%. The cumulative Z‐curve did not cross the trial sequential boundaries (inward sloping etched lines). The results showed that the observed diversity‐adjusted required information size was 2,570 participants, corresponding to 20.3% of the total sample size in the included trials. Accordingly, the meta‐analysis did not support or refute an intervention effect as data were too few.
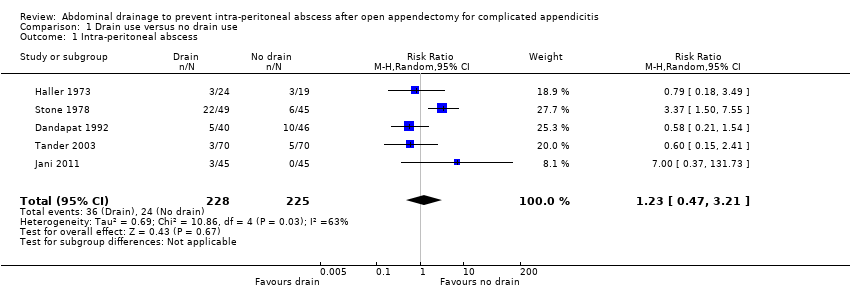
Comparison 1 Drain use versus no drain use, Outcome 1 Intra‐peritoneal abscess.

Comparison 1 Drain use versus no drain use, Outcome 2 Wound infection.

Comparison 1 Drain use versus no drain use, Outcome 3 Morbidity.

Comparison 1 Drain use versus no drain use, Outcome 4 Mortality.
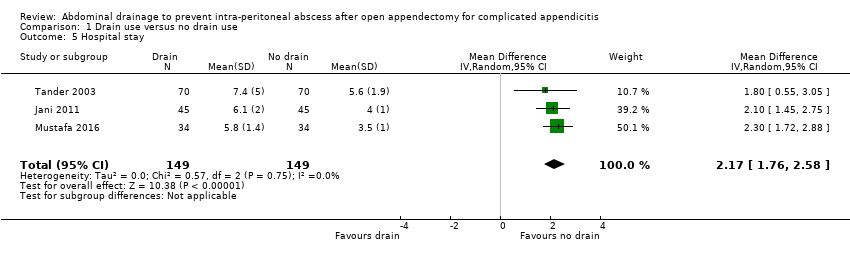
Comparison 1 Drain use versus no drain use, Outcome 5 Hospital stay.

Comparison 2 Drain use versus no drain use (sensitivity analyses by excluding quasi‐randomised trials), Outcome 1 Intra‐peritoneal abscess.
| Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | ||||||
| Patient or population: people undergoing emergency open appendectomy for complicated appendicitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with no drain use | Risk with drain use | |||||
| Intra‐peritoneal abscess Follow‐up: 14 days | 107 per 1000 | 131 per 1000 | RR 1.23 | 453 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 254 per 1000 | 511 per 1000 | RR 2.01 | 478 | ⊕⊝⊝⊝ | |
| Morbidity Follow‐up: 30 days | 67 per 1000 | 445 per 1000 | RR 6.67 | 90 | ⊕⊝⊝⊝ | |
| Mortality Follow‐up: 30 days month | 6 per 1000 | 27 per 1000 | Peto OR 4.88 | 363 | ⊕⊕⊕⊝ | |
| Hospital stay (days) | The mean hospital stay in the control groups was 4.60 days | The mean hospital stay in the intervention groups was | MD 2.17 days higher | 298 | ⊕⊝⊝⊝ | |
| Hospital cost | Not reported | |||||
| Pain | Not reported | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk is the mean comparison group proportion in the studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded two levels for very serious risk of bias. b Downgraded one level for severe inconsistency (substantial heterogeneity as indicated by the I2 statistic). c Downgraded one level for serious imprecision. For abscess, morbidity and infection, the confidence interval includes appreciable benefit and harm, and the sample size is small. For mortality, there are few events (8 deaths in total) d Downgraded one level for serious imprecision (small sample size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.47, 3.21] |
| 2 Wound infection Show forest plot | 5 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.88, 4.56] |
| 3 Morbidity Show forest plot | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 6.67 [2.13, 20.87] |
| 4 Mortality Show forest plot | 4 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.88 [1.18, 20.09] |
| 5 Hospital stay Show forest plot | 3 | 298 | Mean Difference (IV, Random, 95% CI) | 2.17 [1.76, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.02] |


