Esteroides inhalados y riesgo de neumonía para la enfermedad pulmonar obstructiva crónica
Resumen
Antecedentes
Los corticosteroides inhalados (CEI) son fármacos antiinflamatorios que han probado tener efectos beneficiosos en personas en las que se observa el empeoramiento de los síntomas de la enfermedad pulmonar obstructiva crónica (EPOC) y que presentan exacerbaciones repetidas. Se usan comúnmente como inhaladores de combinación con agonistas beta2 de acción prolongada (ABAP) para reducir las tasas de exacerbaciones y la mortalidad por cualquier causa y para mejorar la función pulmonar y la calidad de vida. Las combinaciones más frecuentes de CEI y ABAP utilizadas en los inhaladores de combinación son fluticasona y salmeterol, budesonida y formoterol y una nueva formulación de fluticasona en combinación con vilanterol, que actualmente está disponible. Los CEI se han asociado con un aumento del riesgo de neumonía, aunque aún no se conoce la magnitud del riesgo y cómo se compara con diferentes CEI. Las revisiones recientes realizadas para considerar su seguridad no han comparado la seguridad relativa de estos dos fármacos cuando se utilizan solos o en combinación con ABAP.
Objetivos
Evaluar el riesgo de neumonía asociado con la administración de fluticasona y budesonida para la EPOC.
Métodos de búsqueda
Los ensayos se identificaron en el registro especializado de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group, CAGR), clinicaltrials.gov, listas de referencias de las revisiones sistemáticas existentes y en sitios web de fabricantes. Las búsquedas más recientes se realizaron en septiembre 2013.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) de grupos paralelos, de al menos 12 semanas de duración. Los estudios se incluyeron cuando comparaban los CEI budesonida o fluticasona versus placebo, o cualquier CEI en combinación con un ABAP versus el mismo ABAP como monoterapia para las personas con EPOC.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron de forma independiente las características de los estudios, los datos numéricos y la información del riesgo de sesgo para cada estudio incluido.
Se consideraron las comparaciones directas de CEI versus placebo por separado respecto de las comparaciones de CSI/ABAP versus ABAP para todos los desenlaces, y las mismas se combinaron con subgrupos cuando no se observó una heterogeneidad importante. Después de evaluar la transitividad, se realizó una comparación indirecta para comparar budesonida versus monoterapia con fluticasona, aunque no fue posible hacer lo mismo en el caso de los tratamientos combinados debido a las diferencias sistemáticas entre los grupos de datos de la combinación de budesonida y fluticasona.
De ser apropiado, se exploraron los efectos de la dosis del CEI, la duración del tratamiento con CEI y la gravedad inicial sobre el desenlace principal. Los hallazgos de todos los desenlaces se presentan en las tablas de “Resumen de los hallazgos” mediante el uso de GRADEPro.
Resultados principales
Se encontraron 43 estudios que cumplieron con los criterios de inclusión, y se proporcionó más evidencia sobre la fluticasona (26 estudios; n = 21.247) que sobre la budesonida (17 estudios; n = 10.150). La evidencia de los estudios sobre la budesonida era más inconsistente y menos precisa, y los estudios eran más cortos. Las poblaciones dentro de los estudios más a menudo incluyeron hombres con una media de edad de alrededor de 63, una media de paquetes‐años fumados mayor que 40 y un volumen espiratorio forzado en un segundo (VEF1) teórico medio menor que 50%.
El número alto o desigual de abandonos se consideró un riesgo de sesgo alto en casi un 40% de los ensayos, aunque las conclusiones para el desenlace principal no cambiaron al eliminar los ensayos en riesgo de sesgo alto en un análisis de sensibilidad.
La fluticasona aumentó los eventos adversos no mortales de neumonía (que requirieron ingreso hospitalario) (odds ratio [OR] 1,78, intervalo de confianza [IC] del 95%: 1,50 a 2,12; 18 más por cada 1000 tratados durante 18 meses; calidad alta), y ninguna evidencia sugirió que este desenlace se redujera al administrarla en combinación con salmeterol o vilanterol (diferencias de subgrupos: I2 = 0%, valor de p 0,51), o de que las diferencias en la dosis, la duración del ensayo o la gravedad inicial afectaran significativamente el cálculo. La budesonida también aumentó los eventos adversos graves no mortales de neumonía en comparación con placebo, aunque el efecto fue menos preciso y se basó en los ensayos más cortos (OR 1,62; IC del 95%: 1,00 a 2,62; seis más por cada 1000 tratados durante nueve meses; calidad moderada). Parte de la variación en los datos de la budesonida podría ser explicada por una diferencia significativa entre las dos dosis utilizadas comúnmente: la dosis de 640 mcg se asoció con un efecto más grande que la de 320 mcg en relación con el placebo (diferencias de subgrupos: I2 = 74%; valor de p = 0,05).
Una comparación indirecta de la budesonida versus monoterapia con fluticasona no reveló ninguna diferencia significativa en lo que se refiere a los eventos adversos graves (relacionados con la neumonía o por cualquier causa) o la mortalidad. El riesgo de cualquier evento de neumonía (es decir, casos menos graves tratados en la comunidad) fue mayor con fluticasona que con budesonida (OR 1,86; IC del 95%: 1,04 a 3,34). Esta fue la única diferencia significativa notificada de los dos fármacos. Sin embargo, este hallazgo debe interpretarse con cuidado debido a las posibles diferencias en la asignación del diagnóstico de neumonía, y debido a que ningún ensayo comparó directamente los dos fármacos.
No se observó ninguna diferencia significativa en las tasas de mortalidad global entre cualquiera de los esteroides inhalados y las intervenciones de control (ambas evidencia de calidad alta) , y las muertes relacionadas con la neumonía fueron muy poco frecuentes para permitir sacar conclusiones.
Conclusiones de los autores
La budesonida y la fluticasona, administradas solas o en combinación con un ABAP, se asocian con un aumento del riesgo de eventos adversos graves de neumonía, aunque ninguna afectó significativamente a la mortalidad en comparación con los controles. Los problemas de seguridad destacados en esta revisión deben compararse con los datos de cohortes recientes y la evidencia aleatorizada establecida sobre la eficacia con respecto a las exacerbaciones y la calidad de vida. La comparación de los dos fármacos no reveló ninguna diferencia estadísticamente significativa en la neumonía grave, la mortalidad o los eventos adversos graves. La fluticasona se asoció a un aumento del riesgo de cualquier tipo de neumonía comparada con budesonida (es decir menos casos graves tratados en la comunidad), aunque la variación en las definiciones utilizadas por los fabricantes respectivos es un factor de confusión potencial en su comparación.
La investigación primaria debe medir con exactitud los desenlaces de la neumonía y debe aclarar tanto la definición como el método de diagnóstico utilizado, especialmente para las nuevas formulaciones y combinaciones, sobre las que existe poca evidencia disponible actualmente en cuanto al riesgo de neumonía asociado. De igual manera, las revisiones sistemáticas y las cohortes deben considerar la fiabilidad de considerar la “neumonía” como un evento adverso o la causa de la muerte y deben determinar de qué forma lo anterior afecta a la aplicabilidad de los hallazgos.
PICOs
Resumen en términos sencillos
¿Los esteroides inhalados aumentan el riesgo de neumonía en los pacientes con enfermedad pulmonar obstructiva crónica (EPOC)?
¿Por qué es importante esta pregunta?
Los corticosteroides inhalados (CEI) son fármacos que pueden reducir la aparición exacerbaciones de la EPOC y mejorar la calidad de vida. En la EPOC, los CEI se usan comúnmente junto con agonistas 2 de acción prolongada (ABAP). Los inhaladores de combinación de CEI y ABAP más comunes incluyen fluticasona y salmeterol, y budesonida y formoterol, aunque el furoato de fluticasona también se usa una vez al día con un ABAP nuevo llamado vilanterol. Muchos estudios han mostrado efectos beneficiosos de los CEI, aunque los mismos también pueden aumentar el riesgo de neumonía. Además de esta inquietud, la neumonía puede ser difícil de diagnosticar, y la gravedad de la neumonía puede informarse de forma deficiente en los ensayos. Por lo tanto, aunque hay revisiones sobre los esteroides inhalados para la EPOC, se deseó realizar una revisión exclusivamente sobre la neumonía, de manera que la evidencia pudiera analizarse de forma más detallada.
El objetivo general de esta revisión es evaluar el riesgo de neumonía para las personas con EPOC que reciben fluticasona o budesonida.
¿Cómo se respondió la pregunta?
Se realizaron búsquedas de todos los estudios que comparaban budesonida o fluticasona versus un inhalador simulado (placebo), y de todos los estudios que comparaban su uso en combinación con un ABAP (es decir budesonida/formoterol, propinionato de fluticasona/salmeterol y furoato de fluticasona/vilanterol) versus la misma dosis de ABAP solo. Lo anterior permitió evaluar el riesgo del CEI utilizado solo o en combinación con ABAP.
¿Qué se encontró?
Se encontraron 43 estudios con más de 30.000 personas con EPOC. Más estudios utilizaron fluticasona (26 estudios; 21.247 personas) que budesonida (17 estudios; 10.150 personas). Una mayor proporción de personas en los estudios era hombre (alrededor del 70%) y la EPOC generalmente se clasificó como grave. La última búsqueda de estudios para incluir en la revisión se realizó en septiembre 2013.
Cada fármaco se comparó con controles y los resultados de los estudios que comparaban CEI versus placebo y una combinación de CEI/ABAP versus ABAP solo se evaluaron por separado. También se realizó una comparación indirecta de budesonida y fluticasona basada en los efectos en comparación con placebo, para explorar si un fármaco era más seguro que el otro.
La fluticasona aumentó las neumonías “graves” (que requieren el ingreso al hospital). Durante el plazo de 18 meses, 18 personas más de cada 1000 tratadas con fluticasona ingresaron en el hospital por neumonía.
La budesonida también aumentó las neumonías que se clasificaron como “graves”. Durante el plazo de nueve meses, se informaron seis ingresos más en el hospital por cada 1000 personas tratadas con budesonida. Una dosis inferior de budesonida (320 mcg) se asoció con menos neumonías graves que una dosis mayor (640 mcg).
No se informaron más muertes en general en los grupos de CEI en comparación con los controles, y las muertes relacionadas con la neumonía fueron muy poco frecuentes para sacar conclusiones.
Al comparar fluticasona y budesonida entre sí, la diferencia no era lo bastante clara como para decir si una es más segura (en cuanto a la neumonía, la necesidad de una estancia hospitalaria, los eventos adversos generales y la muerte). El riesgo de eventos de cualquier tipo de neumonía (es decir, casos menos graves que podrían ser tratados sin asistir al hospital) fue mayor con fluticasona que con budesonida.
La evidencia se consideró de calidad alta o moderada para la mayoría de los desenlaces. Cuando un desenlace se considera de alta calidad, es muy poco probable que la investigación adicional cambie la confianza en el cálculo del efecto, aunque las calificaciones moderadas reflejan algunas dudas en los hallazgos. Los desenlaces de los estudios de la budesonida en general fueron menos claros debido a que se basaron en menos personas, y los estudios fueron más cortos.
Conclusión
La budesonida y la fluticasona, administradas solas o en combinación con ABAP, pueden aumentar las neumonías graves que dan lugar a la hospitalización. No se ha demostrado que ninguna afecte a las posibilidades de morir en comparación con ninguna administración de CEI. La comparación de los dos fármacos no reveló ninguna diferencia en las neumonías graves o el riesgo de muerte. La fluticasona se asoció con un riesgo mayor de cualquier tipo de neumonía (es decir, casos que podrían ser tratados en la comunidad) que la budesonida, aunque las diferencias potenciales en la definición utilizada por los fabricantes del fármaco respectivo redujo la confianza en este hallazgo. Estas inquietudes deben equilibrarse con los efectos beneficiosos conocidos de los CEI (p.ej. menos exacerbaciones, mejoría en la función pulmonar y la calidad de vida).
Los investigadores deben seguir siendo conscientes de los riesgos asociados a los CEI y deben asegurarse de que la neumonía se diagnostique de forma adecuada en los estudios.
Authors' conclusions
Summary of findings
| Fluticasone for chronic obstructive pulmonary disease | |||||
| Patient or population: patients with chronic obstructive pulmonary disease Comparison: placebo or LABA monotherapy (dependent upon whether fluticasone was given with LABA in the intervention group) Setting: community | |||||
| Outcomes Follow‐ups presented as weighted means | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Fluticasone | ||||
| Non‐fatal, serious adverse pneumonia events (requiring hospital admission) | 25 per 1000 | 43 per 1000 | OR 1.78 | 19,504 | ⊕⊕⊕⊕ |
| Mortality, all‐cause | 58 per 1000 | 58 per 1000 | OR 0.99 | 20,861 | ⊕⊕⊕⊕ |
| Mortality, due to pneumonia | 2 per 1000 | 3 per 1000 | OR 1.23 | 19,532 | ⊕⊕⊕⊝ |
| Non‐fatal, serious adverse events (all) | 227 per 1000 | 237 per 1000 | OR 1.06 | 20,381 | ⊕⊕⊕⊕ |
| All pneumonia events | 72 per 1000 | 116 per 1000 | OR 1.68 | 15,377 | ⊕⊕⊕⊝ |
| Withdrawals | 343 per 1000 | 297 per 1000 | OR 0.81 | 21,243 | ⊕⊕⊕⊕ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Unless otherwise stated, subgroup differences between monotherapy studies (fluticasone versus placebo) and combination therapy studies (fluticasone/LABA versus LABA) were not significant. | |||||
| GRADE Working Group grades of evidence. | |||||
| 1Wide confidence intervals include significant benefit and harm, based on very few events (‐1 for imprecision). | |||||
| Budesonide for chronic obstructive pulmonary disease | |||||
| Patient or population: patients with chronic obstructive pulmonary disease Comparison: placebo or LABA monotherapy (dependent upon whether fluticasone was given with LABA in the intervention group) Setting: community | |||||
| Outcomes Follow‐ups presented as weighted means | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Budesonide | ||||
| Non‐fatal, serious adverse pneumonia events (requiring hospital admission) | 9 per 1000 | 15 per 1000 | OR 1.62 | 6472 | ⊕⊕⊕⊝ |
| Mortality, all‐cause | 17 per 1000 | 16 per 1000 | OR 0.90 | 10,009 | ⊕⊕⊕⊝ |
| Mortality, due to pneumonia | 0 per 1000 | 0 per 1000 | OR 4.46 | 1511 | ⊕⊝⊝⊝ |
| Non‐fatal, serious adverse events (all) | 145 per 1000 | 146 per 1000 | OR 1.01 | 10,009 | ⊕⊕⊕⊝ |
| All pneumonia events | 28 per 1000 | 31 per 1000 | OR 1.12 | 7011 | ⊕⊕⊕⊝ |
| Withdrawals | 280 per 1000 | 232 per 1000 | OR 0.78 | 10150 | ⊕⊕⊕⊕ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Unless otherwise stated, subgroup differences between monotherapy studies (budesonide versus placebo) and combination therapy studies (budesonide/LABA versus LABA) were not significant. | |||||
| GRADE Working Group grades of evidence. | |||||
| 1Confidence intervals are quite wide but are not considered serious enough to downgrade. | |||||
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterised by chronic and progressive breathlessness, cough, sputum production and airflow obstruction, which leads to restricted activity and poor quality of life (GOLD 2013). The World Health Organization (WHO 2012) has estimated that COPD is the fourth or fifth most common single cause of death worldwide, and that the treatment and management costs present a significant burden to public health. In the UK the annual cost of COPD to the National Health Service (NHS) is estimated to be GBP 1.3 million per 100,000 people (NICE 2011). Furthermore, because of its slow onset and under‐recognition of the disease by patients and healthcare professionals, COPD is heavily under diagnosed (GOLD 2013). COPD comprises a combination of bronchitis and emphysema and involves chronic inflammation and structural changes in the lung. Cigarette smoking is the most important risk factor, but air pollution and occupational dust and chemicals are also recognised risk factors. COPD is a progressive disease that leads to decreased lung function over time, even with the best available care. Currently no cure is known for COPD, although the condition is both preventable and treatable. As yet, apart from smoking cessation and non‐pharmacological treatments such as long‐term oxygen therapy in hypoxic patients and pulmonary rehabilitation, no intervention has been shown to reduce mortality (GOLD 2013;Puhan 2011). Management of the disease is multi‐faceted and includes interventions for smoking cessation (Van der Meer 2001), pharmacological treatments (GOLD 2013), education (Effing 2007) and pulmonary rehabilitation (Lacasse 2006; Puhan 2011). Pharmacological therapy is aimed at relieving symptoms, improving exercise tolerance and quality of life, improving lung function and preventing and treating exacerbations.
Description of the intervention
Pharmacological management for COPD is generally a stepwise process, commencing with therapy for symptoms, which is followed by introduction of additional therapeutic agents as needed to achieve control and to reduce the frequency and severity of exacerbations (GOLD 2013). Often the first step is to use a short‐acting bronchodilator for control of breathlessness when needed: a short‐acting beta2‐agonist (SABA) (e.g. salbutamol) or the short‐acting muscarinic antagonist (SAMA) ipratropium. For persistent or worsening breathlessness associated with lung function decline, long‐acting bronchodilators may be introduced (GOLD 2013). These comprise twice‐daily long‐acting beta2‐agonists (LABA), such as salmeterol or formoterol; once‐daily beta2‐agonists, such as indacaterol; and the long‐acting anticholinergic agent tiotropium. For patients with severe or very severe COPD (forced expiratory volume in one second (FEV1) < 50% predicted) and with repeated exacerbations, the Global initiative for chronic Obstructive Lung Disease (GOLD 2013) recommends the addition of inhaled corticosteroids (ICS) to bronchodilator treatment. ICS are anti‐inflammatory drugs that are licensed as combination inhalers for use with LABA. The most common ICS and LABA components in combination inhalers are fluticasone propionate and salmeterol, budesonide and formoterol and a new formulation of fluticasone furoate in combination with vilanterol, which is now available for once‐daily use. Patients with severe COPD may also be treated with the phosphodiesterase 4 (PDE4) inhibitor roflumilast, which may reduce the risk of exacerbations (GOLD).
How the intervention might work
ICS are anti‐inflammatory drugs. They reduce the rate of exacerbation and improve quality of life, but they have not been found to have an effect on overall mortality or on the long‐term decline in FEV1 (Agarwal 2010; GOLD 2013; Yang 2009). ICS and LABA combination inhalers reduce exacerbation rates and all‐cause mortality and improve lung function and quality of life (Nannini 2013). These effects are thought to be greater for combination inhalers than for the component preparations (GOLD 2013; Nannini 2013). ICS, alone or in combination with LABA, however, have been associated with increased risk of pneumonia (GOLD 2013; Singh 2009). Several mechanisms have been proposed by which ICS could increase the risk of pneumonia; these mechanisms are principally related to the immunosuppressive effects of ICS and include ICS reaching the lung in high concentrations. Particularly, inhibition of nuclear factor kappa B (NF‐κB) by ICS in COPD, one of the proposed mechanisms for their therapeutic effect, could lead to the suppression of normal host responses to bacterial infection (Singanayagam 2010).
Why it is important to do this review
Use of ICS for treatment of COPD may be beneficial, at least for some COPD patients. But the role of ICS therapy in patients with stable COPD is controversial, especially as an elevated risk of pneumonia has been found in studies of ICS use. Pneumonia in COPD is associated with high morbidity and mortality (Ernst 2007) and worsening quality of life and pulmonary function, so it is important to understand the strength and nature of the association between ICS use and this adverse event.
Several systematic reviews published in the last few years have looked at the risk of pneumonia with ICS use (Drummond 2008; Halpin 2011; Sin 2009; Singh 2009). Of these, only one compared different ICS versus each other and as combination inhaler therapy together with a LABA (Halpin 2011). Although ICS are usually administered in a combination inhaler in clinical practice, we are interested in the most comprehensive evidence on the risk of pneumonia with ICS. Differences in the molecular structures of ICS formulations are known to alter their relative potency ratios and durations of action (Johnson 1998; Rossios 2011), but potential differences between formulations in the magnitude of pneumonia risk remains unclear. It is also uncertain whether the association with pneumonia is altered by LABA in combination inhalers. We therefore included studies that examined ICS treatment both alone and in combination with a LABA. We focused on the risk of pneumonia with the two most frequently prescribed ICS—fluticasone and budesonide—compared with control, and on the difference in risk of pneumonia between these ICS. When there was a paucity of head‐to‐head trials directly comparing fluticasone and budesonide, we planned to complement the direct comparisons with an adjusted indirect comparison of budesonide and fluticasone using placebo as a common comparator (Figure 1). Indirect comparisons are considered valid if 'clinical and methodological homogeneity' is present between the budesonide and fluticasone studies (Cipriani 2013). The indirect comparison of budesonide and fluticasone when taken in a combination inhaler with different LABA assumes that the LABA salmeterol, formoterol and vilanterol do not have an important effect on the risk of pneumonia.
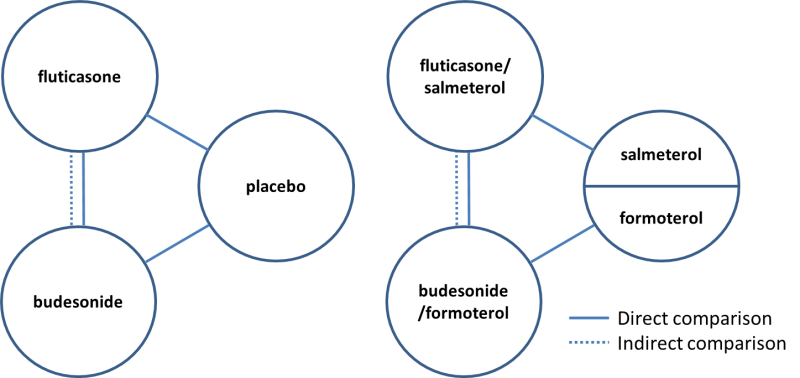
Direct and indirect comparisons of fluticasone and budesonide covered in the review.
Objectives
To assess the risk of pneumonia associated with the use of fluticasone and budesonide for COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a parallel‐group design of at least 12 weeks' duration. We did not exclude studies on the basis of blinding. Cross‐over trials were not included, as ICS can have long‐acting effects, and because the primary outcome is an adverse event.
Types of participants
We included RCTs that recruited participants with a diagnosis of COPD (e.g. based on criteria recommended by the American Thoracic Society and the European Respiratory Society) (ATS/ERS 2004).
-
Forced expiratory volume after one second (FEV1)/forced vital capacity (FVC) ratio < 0.7, which confirms the presence of persistent airflow limitation.
-
One or more of the following key indicators.
-
Progressive and/or persistent dyspnoea.
-
Chronic cough.
-
Chronic sputum production.
-
History of exposure to risk factors (tobacco smoke, smoke from home cooking and heating fuels, occupational dusts and chemicals).
-
Types of interventions
We included studies that performed any of the following comparisons.
-
Fluticasone versus placebo.
-
Budesonide versus placebo.
-
Fluticasone/salmeterol versus salmeterol.
-
Fluticasone/vilanterol versus vilanterol.
-
Budesonide/formoterol versus formoterol.
-
Fluticasone versus budesonide.
-
Fluticasone/salmeterol versus budesonide/formoterol.
-
Fluticasone/vilanterol versus budesonide/formoterol.
We allowed ICS/LABA combination treatment in a single inhaler and in separate inhalers. Participants were allowed to take other concomitant COPD medications as prescribed by their healthcare practitioner provided they were not part of the trial treatment under study. For example, we excluded studies that compared triple therapy of budesonide/formoterol combination inhaler plus tiotropium versus formoterol plus tiotropium.
Types of outcome measures
We were interested in events of pneumonia. Pneumonia is usually defined as an acute lower respiratory tract infection that generally includes symptoms and signs from the respiratory tract and noted in the general health of the patient, but the specific definition/diagnosis varies. We recorded the basis of diagnosis, specifically, radiological confirmation, and planned to conduct a subgroup analysis. One example of the definition of diagnostic criteria for pneumonia is found in BTS 2009.
-
Symptoms of an acute lower respiratory tract illness (cough and at least one other lower respiratory tract symptom).
-
New focal chest signs on examination.
-
At least one systemic feature (either a symptom complex of sweating, fevers, shivers, aches and pains and/or temperature of 38°C or higher).
-
No other explanation for the illness.
We primarily looked at pneumonia events leading to hospital admissions (i.e. serious adverse pneumonia events), which usually are better documented and diagnosed by imaging studies and laboratory investigations than pneumonia events of any severity, and are associated with substantial morbidity and mortality. One example of the definition of diagnostic criteria for pneumonia in hospital is found in BTS 2009.
-
Symptoms and signs consistent with an acute lower respiratory tract infection associated with new radiographic shadowing for which no other explanation is known (e.g. not pulmonary oedema or infarction).
-
The illness is the primary reason for hospital admission and is managed as pneumonia.
We used end of study as the time of analysis for all studies, which ranged from three to 36 months in duration.
Primary outcomes
-
Non‐fatal, serious adverse pneumonia events (requiring hospital admission).
We chose serious adverse pneumonia events as the primary outcome because of the increased burden these events have on the individual and on healthcare systems.
Secondary outcomes
-
Mortality: all‐cause and due to pneumonia.
-
Non‐fatal serious adverse events: all‐cause.
-
All pneumonia events.
-
Withdrawals.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified by systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and conference abstracts found through handsearching (see Appendix 1 for further details). We searched all records in the CAGR coded 'COPD' using the following terms.
((steroid* or corticosteroid*) and inhal*) or ICS or budesonide or fluticasone or pulmicort or flovent or flixotide or symbicort or viani or seretide or advair.
We also searched ClinicalTrials.gov using search terms provided in Appendix 2. We searched all databases with no restriction on date or language of publication up to September 2013.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched the manufacturers' websites (AstraZeneca and GlaxoSmithKline) for additional information on studies identified through the electronic searches.
Data collection and analysis
Selection of studies
Two review authors (AS and CK) independently screened the titles and abstracts of citations retrieved through literature searches and obtained those deemed to be potentially relevant. We assigned all references to a study identifier and assessed them against the inclusion criteria of this protocol. We resolved disagreements by consensus. Subsequent search updates were screened by AS and KMK.
Data extraction and management
Two review authors (KMK and AS or CK) independently extracted information from each included study (recording the data source) for the following characteristics.
-
Design (study design, total duration of study, number of study centres and locations).
-
Participants (number randomly assigned to each treatment, mean age, gender, baseline lung function, smoking history, inclusion criteria, exclusion criteria).
-
Interventions (run‐in, intervention and control treatment including concentration and formulation).
-
Outcomes (definitions of pneumonia events and data on the numbers of participants with one or more events with onset during the treatment period).
We resolved discrepancies in the data by discussion, or by consultation with a third party when necessary.
Assessment of risk of bias in included studies
We assessed the risk of bias according to recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for the following items.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
We graded each potential source of bias as high, low or unclear according to recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
Direct comparisons (fluticasone vs placebo; budesonide vs placebo; fluticasone/LABA vs LABA; budesonide/LABA vs LABA)
We analysed direct pair‐wise comparisons using Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CIs). When events were rare, we employed the Peto odds ratio. When count data were available as rate ratios, we transformed them into log rate ratios and analysed them using generic inverse variance (GIV). For the primary outcome, the number needed to treat for an additional harmful outcome was calculated from the pooled odds ratio and its confidence interval and was applied to appropriate levels of baseline risk.
Indirect comparisons (monotherapy: fluticasone vs budesonide; combination therapy: fluticasone/LABA vs budesonide/LABA)
We also conducted indirect comparisons of fluticasone and budesonide treatments using odds ratios with a 95% CI (Bucher 1997). When available, we planned to combine the indirect evidence with randomly assigned head‐to‐head comparisons of fluticasone and budesonide.
Assessing transitivity and similarity
To permit valid indirect comparisons of fluticasone and budesonide, the sets of trials for each drug must be similar in their distribution of effect modifiers (Cipriani 2013). Before conducting indirect comparisons, we constructed summary tables for monotherapy and combination therapy separately to compare the following characteristics between budesonide and fluticasone trials.
-
Inclusion and exclusion criteria (including allowed co‐medications).
-
Baseline characteristics (smoking history, % predicted FEV1, age, percentage male).
-
Intervention characteristics (dose distribution, inhaler device).
-
Methodology (risk of bias, study duration, sample size, funding).
-
Control group event rates.
Unit of analysis issues
The unit of analysis was the individual participant for dichotomous outcomes, but events were used to compare rates of exacerbation.
Dealing with missing data
When pneumonia data or key study characteristics were not reported in the primary publication, we searched clinical trial reports and contacted study authors and sponsors for additional information. We used intention‐to‐treat (ITT) analysis on outcomes of all randomly assigned participants when possible. We considered the impact of the unknown status of participants who withdrew from the trials as part of the sensitivity analysis.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by recording differences in study design, participant characteristics, study sponsorship and pneumonia definition between individual studies. We assessed the extent of statistical variation among study results by using the I2 measurement. We tested for inconsistency between direct and indirect data by calculating the log ratio of direct and indirect odds ratios (ROR) (Song 2008).
Assessment of reporting biases
We tried to minimise reporting bias from non‐publication of studies or selective outcome reporting by using a broad search strategy, checking references of included studies and relevant systematic reviews and contacting study authors for additional outcome data. We visually inspected funnel plots when 10 or more studies were included.
Data synthesis
We looked at direct comparisons of ICS versus placebo separately from comparisons of ICS/LABA versus LABA for all outcomes (Figure 1). When no important discrepancy was noted between the analyses with and without LABA, we combined the results. When a study comparing ICS/LABA versus LABA included arms for both a single inhaler (ICS/LABA) and separate inhalers (ICS + LABA), we split the control group (LABA) in half to avoid double‐counting.
The decision whether to perform indirect comparisons of studies was based on our assessment of their clinical and methodological differences.
If both direct and indirect comparison data were available, we planned to combine the estimates using a fixed‐effect model, but when statistical heterogeneity was evident (I2 > 30%), we used a random‐effects model to analyse the data and explore the heterogeneity (see below). When no important discrepancy was noted between direct and indirect estimates, we combined the resulting odds ratio and 95% CI from the indirect comparison with any direct pair‐wise data for the same comparison using inverse variance weighting (Glenny 2005).
We presented the findings of all outcomes in 'Summary of findings' tables using GRADEPro software and recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions.
Subgroup analysis and investigation of heterogeneity
When appropriate, we explored heterogeneity between studies by analysing data for the primary outcome by looking at the following subgroups.
-
ICS dose (separate subgroups for each of the following drugs and doses: fluticasone 500 and 1000 mcg; budesonide 320, 640 and 1280 mcg).
-
Duration of ICS therapy (≤ one year; > one year).
-
Diagnostic criteria of pneumonia.
-
Disease severity at baseline (FEV1 < 50% predicted; FEV1 ≥ 50% predicted).
Sensitivity analysis
We assessed the robustness of our analyses by performing sensitivity analyses, while systematically excluding studies from the overall analysis:
-
of high risk of bias; or
-
with high and or uneven withdrawal rates.
Results
Description of studies
Results of the search
Two thousand forty‐seven citations were identified by searching electronic databases. Twenty‐seven additional citations were found by searching reference lists, clinicaltrials.gov and drug company websites. Forty‐two duplicates were removed, and the remaining 2032 titles and abstracts were sifted. Two review authors excluded 1806 references that did not meet the inclusion criteria. Full texts were obtained and scrutinised for the final 226 references, and 195 (representing 43 studies) met all of the inclusion criteria. Figure 2 shows this information as a flow diagram and gives reasons for exclusion of the 31 references that were excluded after the full text was reviewed.

Study flow diagram.
Included studies
Forty‐three studies met all of the inclusion criteria and were included in the review: 26 using fluticasone and 17 using budesonide as the inhaled steroid. The fluticasone studies included more than twice as many people, with 21,247 people randomly assigned to the treatments of interest compared with 10,067 in the budesonide studies. Two of the included budesonide studies reported no data that could be used in the analyses and are not included in these numbers (Laptseva 2002; Senderovitz 1999).
No studies directly comparing fluticasone with budesonide met the inclusion criteria (either as monotherapy or in their combination preparations), so only indirect evidence was available for these comparisons.
Design and duration
All studies were randomised, double‐blind, parallel‐group trials of at least 12 weeks' duration. Most were funded by pharmaceutical companies, predominantly GlaxoSmithKline for the fluticasone studies and AstraZeneca for the budesonide studies. Duration ranged from three to 36 months for both drugs, but mean duration weighted by sample size was longer for the fluticasone studies (fluticasone, 18 months; budesonide, 14 months). A summary of each study and baseline characteristics can be found in Table 1 and Table 2.
| Study ID | Duration (m) | N Rand | Funder | ICS dose (mcg) | % Male | Mean age | Pack‐years | % pred FEV1 |
| Fluticasone versus placebo (n = 18) | ||||||||
| 3 | 41 | GSK | 1000 | 78 | 65 | 53 | 57 | |
| 36 | 740 | GSK | 1000 | 75 | 64 | 44 | 50 | |
| 12 | 763 | GSK | 1000 | 73 | 63 | 43 | 45 | |
| 36 | 3097 | GSK | 1000 | 76 | 65 | 49 | 44 | |
| 12 | 260 | Indep. | 1000 | 52 | 67 | 39 | 54 | |
| 6 | 640 | GSK | 500, 1000 | 69 | 64 | ‐ | ‐ | |
| 3 | 84 | GSK | 1000 | 77 | 64 | ‐ | ‐ | |
| 12 | 256 | GSK | 1000 | 82 | 65 | ‐ | ‐ | |
| 6 | 368 | GSK | 500 | 63 | 64 | 57 | 42 | |
| 3 | 36 | GSK | 1000 | 87 | 65 | 63 | 46 | |
| Kerwin 2013a,b | 6 | 413 | GSK | 100 | 66 | 62 | 46 | 42 |
| 30 | 55 | GSK | 1000 | 86 | 60 | 43 | 55 | |
| 6 | 349 | GSK | 1000 | 66 | 65 | 55 | 41 | |
| 6 | 612 | GSK | 100, 200 | 72 | 62 | 43 | 48 | |
| 6 | 281 | ‐ | 1000 | 74 | 63 | ‐ | 57 | |
| 36 | 190 | Indep. | 1000 | 71 | 59 | 28 | 64 | |
| 24 | 48 | GSK | 500 | 52 | 47 | 9 | 97 | |
| 6 | 23 | GSK | 1000 | 82 | 55 | 26 | 63 | |
| WM 22 m | 459 | ‐ | ‐ | 72 | 62 | 43 | 54 | |
| Fluticasone/LABA combination versus LABA monotherapy (n = 15) | ||||||||
| 12 | 797 | GSK | 500 | 54 | 65 | 57 | 34 | |
| 12 | 731 | GSK | 1000 | 73 | 63 | 43 | 45 | |
| 36 | 3088 | GSK | 1000 | 76 | 65 | 49 | 44 | |
| 12 | 12 | ‐ | 500 | 92 | ‐ | 42 | 50 | |
| 12 | 3255 | GSK | 50, 100, 200 | 57 | 64 | ‐ | 45 | |
| 12 | 782 | GSK | 500 | 55 | 65 | 56 | 33 | |
| 3 | 140 | GSK | 1000 | 66 | 62 | ‐ | ‐ | |
| 6 | 1050 | GSK | 500 | 78 | 64 | ‐ | ‐ | |
| 3 | 77 | GSK | 1000 | 77 | 64 | ‐ | ‐ | |
| 36 | 186 | GSK | 500 | 61 | 66 | ‐ | ‐ | |
| 6 | 355 | GSK | 500 | 63 | 64 | 57 | 42 | |
| 10 | 994 | GSK | 1000 | 76 | 64 | 37 | 40 | |
| Kerwin 2013a,b | 6 | 617 | GSK | 100 | 67 | 63 | 46 | 43 |
| 6 | 325 | GSK | 1000 | 66 | 65 | 55 | 41 | |
| 6 | 610 | GSK | 100, 200 | 72 | 62 | 43 | 48 | |
| WM 16 m | 867 | ‐ | ‐ | 69 | 64 | 53 | 42 | |
aMulti‐arm studies making both comparisons of interest (ICS vs placebo and ICS/LABA vs LABA).
bStudies using vilanterol as the LABA combination and monotherapy comparator, with fluticasone furoate.
Dose is given as the total received per day (i.e. 500 signifies 250 morning and evening).
WM = weighted mean.
| Study ID | Duration (m) | N Rand | Funder | ICS dose (mcg) | % Male | Mean age | Pack‐years | % pred FEV1 |
| Budesonide versus placebo (n = 13) | ||||||||
| 6 | 79 | AZ | 640 | 79 | 66 | 51 | 37 | |
| 12 | 513 | GSK | 640 | 76 | 64 | 35 | 36 | |
| 6 | 49 | NR | 640 | NR | NR | NR | NR | |
| 3 | 50 | NR | 640 | 75 | 53 | 27 | 62 | |
| 6 | 26 | NR | 640 | 69 | 65 | 45 | 59 | |
| 36 | 1277 | AZ | 640 | 73 | 52 | 39 | 77 | |
| 24 | 39 | AZ | 1280 | 100 | 55 | NR | 64 | |
| 6 | 26 | NR | 640 | 54 | 61 | NR | NR | |
| 36 | 254 | AZ | 640 | 58 | 64 | 56 | 52 | |
| 12 | 403 | AZ | 640 | 79 | 64 | 45 | 36 | |
| 6 | 575 | AZ | 640 | 68 | 63 | 41 | 40 | |
| 36 | 290 | AZ | 640 | 88 | 59 | NR | 87 | |
| 3 | 38 | ? | 1280 | 100 | 67 | 51 | 46 | |
| WM 23 m | 278 | ‐ | ‐ | 77 | 61 | 43 | 54 | |
| Budesonide/LABA combination versus LABA monotherapy (n = 7) | ||||||||
| 12 | 509 | GSK | 640 | 76 | 64 | 35 | 36 | |
| 11 | 481 | Chiesi | 640 | 81 | 64 | 39 | 42 | |
| 3 | 1293 | AZ | 640 | 89 | 65 | 44 | 41 | |
| 12 | 1483 | AZ | 320, 640 | 63 | 63 | NR | 39 | |
| 12 | 1219 | AZ | 320, 640 | 62 | 63 | 44 | 38 | |
| 12 | 409 | AZ | 640 | 79 | 64 | 45 | 36 | |
| 6 | 1129 | AZ | 320, 640 | 68 | 63 | 41 | 40 | |
| WM 9 m | 932 | ‐ | ‐ | 75 | 64 | 41 | 39 | |
aMulti‐arm studies making both comparisons of interest (ICS vs placebo and ICS/LABA vs LABA).
Dose is given as the total received per day (i.e. 640 signifies 320 morning and evening).
WM = weighted mean.
Participant inclusion and exclusion criteria
Full details of the inclusion and exclusion criteria for each trial can be found in Characteristics of included studies. Inclusion and exclusion criteria were largely similar across trials, with the exception of which medications participants were allowed to continue taking during the study period. In most studies, participants were required to be over the age of 40 and to have a smoking history of at least 10 pack‐years. In terms of lung function, most studies required values consistent with a GOLD diagnosis of COPD. Studies excluded participants if they had asthma or any other respiratory disorder. Other common exclusion criteria included recent lower respiratory tract infection, the need for long‐term or nocturnal oxygen therapy and recent use of antibiotics or oral corticosteroids (usually within four to six weeks of screening).
Baseline characteristics of participants
Baseline data are given for individual trial arms in Characteristics of included studies tables and are summarised across fluticasone and budesonide studies in Table 1 and Table 2, respectively. All of the trials recruited more men than women, with a mean of around 70% and 75% in the fluticasone and budesonide studies, respectively (range 52% to 100%). Mean age within the trials ranged from 47 to 67 years, and the overall mean was similar in the fluticasone and budesonide studies (˜63 years). Smoking history as measured by overall pack‐years (one pack‐year = one pack of 20 cigarettes per day for one year) was reported in three‐quarters of the studies, and the mean was higher in the fluticasone combination therapy studies (53 pack‐years) than in the other sets of trials; all were between 41 and 43 pack‐years overall. The range across all trials was 27 to 63 pack‐years. Percentage predicted FEV1, an indicator of disease severity, was reported in most trials. Overall means for fluticasone and budesonide were very similar (47% and 48%, respectively). One outlier in the fluticasone monotherapy studies recruited a much less severe population (those showing early signs and symptoms of COPD; van Grunsven 2003), with nine mean pack‐years and percentage predicted FEV1 of 97. Two budesonide monotherapy studies also recruited less severe populations, with percentage predicted FEV1 of 77 and 87 (Pauwels 1999; Vestbo 1999). None of these three studies reported the primary outcome.
Characteristics of the interventions
Of the 26 fluticasone studies, 18 compared fluticasone monotherapy versus placebo, and 15 compared fluticasone/LABA combination versus LABA monotherapy (12 using salmeterol and three using the new LABA, vilanterol). Seven trials used multi‐arm double‐dummy designs that performed both comparisons of interest (Calverley 2003 TRISTAN; Calverley 2007 TORCH; GSK SCO104925 2008; Hanania 2003; Mahler 2002), including two newly published trials using vilanterol and fluticasone furoate (Kerwin 2013; Martinez 2013). Fifteen fluticasone studies used fluticasone propionate at a total daily dose of 1000 mcg, and seven used 500 mcg. One further study, GSK FLTA3025 2005, included both doses. The three vilanterol studies used fluticasone furoate at total daily doses of 50, 100 and 200 mcg, and 25 mcg of vilanterol.
Of the 17 budesonide studies, 13 compared budesonide monotherapy versus placebo, and seven compared budesonide/formoterol combination versus formoterol monotherapy. Three studies had four or more arms and performed both comparisons of interest (Calverley 2003b; Szafranski 2003; Tashkin 2008 SHINE). Twelve studies used a total daily budesonide dose of 640 mcg, and two studies used a daily dose of 1280 mcg (Renkema 1996; Yildiz 2004). Three studies used more than one dose (Rennard 2009; Sharafkhaneh 2012), including one that had a total of six arms (Tashkin 2008 SHINE): three combination arms, budesonide 640 mcg, formoterol 18 mcg and placebo. Formoterol as monotherapy control or in combination with budesonide was given at a total daily dose of 18 mcg in all studies.
Table 3 presents the beclomethasone dipropionate (BDP) equivalent doses for the included treatments. As shown, higher‐dose budesonide (1280 mcg/d = 1280 BDP) is more similar to the lower dose of fluticasone (500 mcg/d = 1000 BDP). Fluticasone furoate doses of 100 mcg and 200 mcg daily are equivalent to fluticasone propionate 250 mcg twice daily (1000 BDP) and 500 mcg twice daily (2000 BDP) respectively, and the lowest dose of fluticasone furoate is equivalent to 500 BDP. Only the 100 mcg dose of fluticasone furoate is currently licensed for use in COPD
| Drug | Daily dose (mcg) | BDP equivalent (mcg) |
| Budesonide | 320 | 320 |
| 640 | 640 | |
| 1280 | 1280 | |
| Fluticasone | 500 (propionate) | 1000 |
| 1000 (propionate) | 2000 | |
| 50 (furoate) | 500 | |
| 100 (furoate) | 1000 | |
| 200 (furoate) | 2000 |
In most studies, participants were allowed short‐acting bronchodilators and treatment for acute exacerbations. Most studies also allowed people to continue on some long‐acting treatments that were not the treatments under study (usually theophylline, mucolytics, anticholinergics). Run‐in periods varied somewhat in length and nature. Most ranged from two to eight weeks; some required all bronchodilator treatment, ICS alone or ICS and LABA treatment to be tapered off; others used placebo and oral corticosteroids; and a subset did not describe the procedures used.
Transitivity and similarity
-
Inclusion and exclusion criteria: Inclusion and exclusion criteria, as described above, were considered comparable between the two sets of trials; although variation was noted in the allowed co‐medications between individual trials, this was not systematically different between the fluticasone and budesonide studies.
-
Baseline characteristics: Although variation between trials was seen, we did not consider that baseline characteristics systematically differed between budesonide monotherapy and fluticasone monotherapy trials, or between budesonide combination therapy and fluticasone combination therapy trials (see above and Table 1; Table 2).
-
Intervention characteristics: Budesonide and fluticasone studies most often used the respective commonly used twice‐daily dose, although the once‐daily fluticasone furoate studies introduced a potential source of heterogeneity. More important, the fluticasone studies generally used the Diskus or Accuhaler, and the budesonide studies used the Turbuhaler device; this may have confounded the common placebo comparator.
-
Methodology: We were concerned that funding for the fluticasone and budesonide trials was systematically different, but in light of similar inclusion criteria, baseline characteristics and study designs, we believed that funding alone was not a reason to believe that the transitivity assumption did not hold. However, although the monotherapy trials were of a comparable duration (weighted means of 22 and 23 months), the fluticasone combination therapy trials were a lot longer than the budesonide combination therapy trials (16 and nine months, respectively). Risk of bias was similar across all studies and did not differ systematically between those funded by the two main drug companies. Similarly, although fluticasone monotherapy trials had somewhat larger sample sizes than budesonide monotherapy trials, this finding was not deemed significant. The two sets of combination therapy trials had very similar mean sample sizes.
-
Control group event rates: Event rates for placebo monotherapy comparisons and for LABA combination therapy comparisons are presented in Table 4. Differences were noted between fluticasone and placebo for both monotherapy and combination therapy comparisons, with fluticasone studies consistently showing higher control group event rates. Inspection of control events showed that Calverley 2007 TORCH, which observed a large population over a longer time scale than most other studies (three years), was skewing the event rates. Although four other long‐term fluticasone monotherapy studies (two to three years) were identified, they had smaller populations, did not contribute to all of the outcomes and did not observe the same magnitude of event rates. With this study removed, control group events were much more similar between the two drugs (presented in brackets). Overall, considered in light of similar baselines and inclusion criteria, it is unlikely that the figures represent true differences in baseline event rates of the two populations.
| Monotherapy comparison—Placebo control events | Combination comparison—LABA control events | |||
| Fluticasone | Budesonide | Fluticasone | Budesonide | |
| Pneumonia‐related serious adverse events | 2.5%, 77/310 | 0.9%, 4/445 | 2.5%, 134/5420 | 0.9%, 19/2079 |
| 0.5% without TORCH | 0.7% without TORCH | |||
| All‐cause mortality | 7.6%, 282/3713 | 2.1%, 37/1763 | 5.1%, 254/5489 | 1.5%, 37/2534 |
| 2.4% without TORCH | 1.2% without TORCH | |||
| All‐cause serious adverse events | 25%, 882/3471 | 15%, 268/1763 | 21%, 1152/5489 | 14%, 356/2534 |
| 14% without TORCH | 14% without TORCH | |||
For the fluticasone control groups with and without the large 3‐year TORCH study.
In light of all of the information collected, we decided to calculate the indirect comparison of fluticasone and budesonide monotherapy via placebo because the only potential confound was the inhaler device used, and all other moderating factors were considered comparable. For the reasons outlined regarding control group event rates, we conducted a sensitivity analysis excluding Calverley 2007 TORCH.
For the combination therapy comparison, we considered the common LABA comparison to systematically differ between the two sets of combination studies; fluticasone studies used either salmeterol or vilanterol, which differed in their delivery and dosing schedules, and budesonide was always compared with formoterol. In addition, the fluticasone studies were much longer, and the same funding and device issues existed as for the monotherapy studies. As such, we did not perform an indirect comparison to compare fluticasone/LABA versus budesonide/LABA.
Outcomes and analysis structure
Unless otherwise stated, all of the analyses were conducted as proposed with fixed‐effect models using Mantel‐Haenszel methods. Several outcomes included some zero cells, but estimates were barely affected in sensitivity analyses using the Peto method. Therefore, Peto odds ratios were used only for 'mortality due to pneumonia', because events for this outcome were very rare. The quality of evidence for each outcome was rated using GRADEPro software, and this information is presented in summary of findings Table for the main comparison (fluticasone) and summary of findings Table 2 (budesonide).
Indirect comparisons performed to compare fluticasone with budesonide monotherapy are presented for the primary outcome and for three secondary outcomes (all‐cause mortality, all non‐fatal serious adverse events and all pneumonia events). Although we did not foresee the inclusion of fluticasone furoate when we conceived of the protocol, we decided to combine these data with the fluticasone propionate data on the basis of consistency observed in the monotherapy subgroup of each of the relevant direct analyses (Analysis 1.1; Analysis 1.2; Analysis 1.4).
For the primary outcome, 'non‐fatal serious adverse pneumonia events', data were organised into three sets of subgroups to explore the effects of three prespecified potential moderators. Subgroup analyses for daily dose, duration of ICS therapy (≤ one year and > one year) and baseline severity (< 50% FEV1 predicted and ≥ 50% FEV1 predicted) are presented separately for fluticasone and budesonide in comparisons three and four, respectively.
We also conducted a sensitivity analysis while removing studies judged to be at high risk of bias because of high or uneven levels of dropout. Of the 17 studies that fell into this category, eight reported the primary outcome and were removed from the analysis (fluticasone: Calverley 2003 TRISTAN; Ferguson 2008; GSK SCO40041 2008; Lapperre 2009; Mahler 2002; budesonide: Rennard 2009; Shaker 2009; Sharafkhaneh 2012).
Excluded studies
Thirty‐one references were excluded after full texts were consulted. Reasons for exclusion were 'wrong comparison' (n = 19), discontinuation of study (n = 6), 'asthma diagnosis' (n = 2) and 'treatment period less than 12 weeks (n = 2). One study is awaiting classification (only abstract is available), and recruitment for one study was ongoing in March 2014. Full details are listed in Characteristics of excluded studies.
Risk of bias in included studies
Studies generally were well conducted and were rated as low or unclear risk of bias for all four of the allocation and blinding parameters. However, in almost half of the studies, potential for bias was due to attrition and selective outcome reporting. Full details of our judgements for each study, as well as supporting information for each judgement, can be found in Characteristics of included studies. A summary of risk of bias across all studies is shown in Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Studies were rated for potential biases introduced by the method of sequence generation (e.g. computerised random number generator) and by the methods used to conceal the allocation sequence from those recruiting people into the studies.
Most studies were judged to be at low risk of bias for sequence generation (n = 39). Although not all of these studies adequately described sequence generation methods, all were funded by pharmaceutical companies that had previously confirmed their methods. The remaining four studies were rated 'unclear' because they did not describe their methods in detail and did not appear to be funded by a pharmaceutical company (Dal Negro 2003; Senderovitz 1999; Yildiz 2004).
Allocation concealment was not well reported, and only 17 studies were given a rating of low risk of bias because they adequately described the methods used. However, no studies were considered to be at high risk of bias, and the remaining 26 studies were given an 'unclear' rating.
Blinding
The risk of bias introduced by methods of blinding was rated separately for blinding of participants and personnel and for blinding of the people assessing outcomes.
Most studies stated that double‐blind procedures were used, and trial reports or registrations usually confirmed that this approach included both participants and investigators. For this reason, most studies were rated as low risk of bias (n = 39), and the remaining four were rated as 'unclear'. No studies were open‐label or used inadequate blinding procedures, so none were judged to be at high risk of bias.
Quite often, it was difficult to ascertain from the study reports who the outcome assessors were and for which outcomes the blinding applied. Only 10 studies gave enough information to allow a judgement of low risk of bias to be made, and the remaining 33 were rated as 'unclear'.
Incomplete outcome data
Around half of the studies were rated as low risk of bias because of incomplete outcome data (n = 21), either because the number of dropouts per group was low and even, or because the quantity and distribution of missing data were deemed acceptable given the method of imputation (e.g. intention‐to‐treat analysis using last observation carried forward). Sixteen studies were rated as high risk of bias, usually because dropout was very high in both groups, or because dropout was much higher in one group than in another. In the remaining six studies, authors considered that the information regarding attrition was not sufficient to permit judgement of whether dropout and methods of data imputation were likely to have affected the results.
Selective reporting
More than half of the studies were rated as low risk of bias for selective outcome reporting (n = 24), either because reported outcomes could be checked against the outcomes stated in a prospectively registered protocol, or because study authors provided additional data through personal communication. Five studies were rated as unclear, usually because no clear evidence on missing outcomes was available, but no trial registration could be found to confirm that all prespecified outcomes were properly reported. In all cases, attempts were made to contact trial authors for clarification; this is detailed in each study's risk of bias table in Characteristics of included studies. The remaining 14 studies were judged to be at high risk of bias, either because outcomes stated in the trial registration were missing or poorly reported in the published report, or because several key outcomes analysed in this review were not reported.
Other potential sources of bias
No additional sources of bias were identified.
Effects of interventions
See: Summary of findings for the main comparison Fluticasone for chronic obstructive pulmonary disease; Summary of findings 2 Budesonide for chronic obstructive pulmonary disease
In comparisons one and three, studies are pooled for fluticasone and budesonide, respectively, and are subgrouped in each case in terms of whether each randomly assigned group also received a long‐acting beta‐agonist. Comparisons two and four show additional subgroup analyses for the primary outcome of non‐fatal serious adverse events for fluticasone and budesonide, respectively.
Comparison five presents results for the indirect comparison of fluticasone and budesonide via the common placebo comparator (Figure 1). Comparison six refers to the equivalent comparison of fluticasone with budesonide given as combination therapy with a long‐acting beta‐agonist. As described above, no indirect comparison was undertaken for combination therapy because of violations of transitivity and similarity.
For illustration and visual comparison of the two drugs, effects of all fluticasone studies (comparison one) and all budesonide studies (comparison three) are presented together for each outcome in Figure 4. No statistical heterogeneity was noted between the pooled effect for budesonide and that of fluticasone for all outcomes except 'all pneumonia events'; for this reason the effects are not pooled for this outcome.
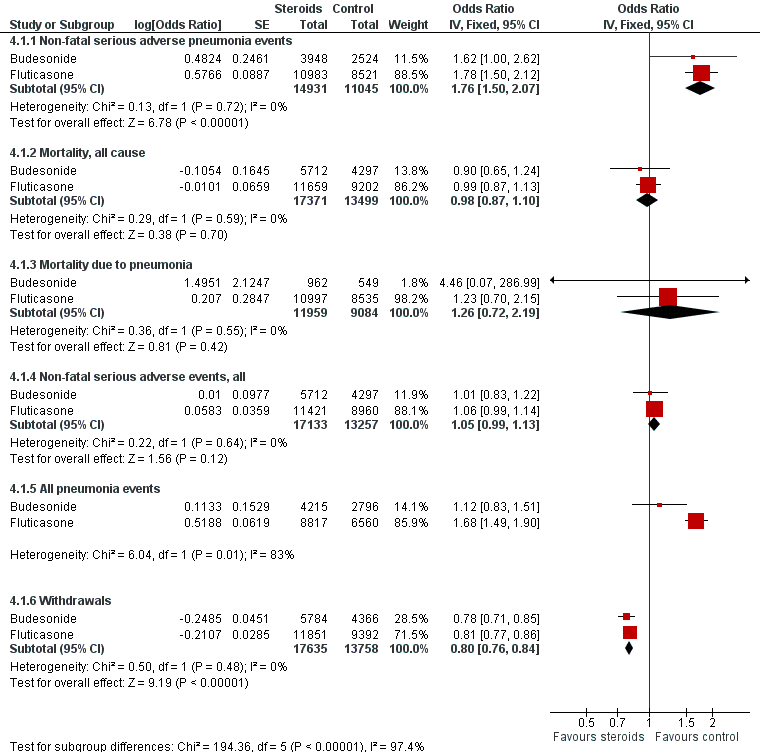
Summary of pooled effects of trials comparing ICS versus placebo and combination versus LABA.
Non‐fatal serious adverse pneumonia events were those requiring hospital admission. Data for all pneumonia events were not pooled because of heterogeneity.
Comparison one: fluticasone versus controls (all outcomes subgrouped to compare ICS vs placebo with ICS/LABA vs LABA)
1.1 Primary outcome: non‐fatal, serious adverse pneumonia events (requiring hospital admission)
Twenty‐four comparisons in 17 studies were analysed (n = 19,504), with seven studies contributing data to both the 'fluticasone versus placebo' and 'fluticasone/LABA versus LABA' subgroups. Fluticasone increased the incidence of non‐fatal serious adverse pneumonia events (OR 1.78, 95% CI 1.50 to 2.12; I2 = 0%, P value 0.65), with no significant heterogeneity noted between studies. No significant evidence indicated that the odds of having a serious adverse pneumonia event were differentially affected by fluticasone alone (against placebo) compared with fluticasone/LABA combination (against LABA alone) (I2 = 0%, P value 0.77). The outcome was rated of high quality.
1.2 Mortality, all‐cause
Twenty‐nine comparisons in 22 fluticasone studies were included in the analysis (n = 20,861), although seven comparisons did not contribute to the pooled estimate because no events were reported in either group. No evidence suggested a difference between fluticasone and controls (OR 0.99, 95% CI 0.87 to 1.13; I2 = 0%, P value 0.73), and a test for subgroup differences between 'fluticasone versus placebo' and 'fluticasone/LABA versus LABA' comparisons was not significant (I2 = 0%, P value 0.39). Evidence was rated of high quality.
1.3 Mortality, due to pneumonia
Data for pneumonia‐related deaths were available for 25 comparisons in 18 studies (n = 19,532). However, all but five comparisons observed no events, and Calverley 2007 TORCH accounted for 80% of the analysis weight across its two comparisons. No difference was detected between fluticasone and control overall (Peto OR 1.23, 95% CI 0.70 to 2.15; I2 = 0%, P value 0.95), and no observable subgroup differences were reported between the monotherapy and combination therapy subgroups (I2 = 0%, P value 0.44). Evidence was rated of moderate quality, being downgraded once for imprecision because so few events were reported.
1.4. Non‐fatal serious adverse events, all‐cause
Nineteen studies across 26 comparisons reported all‐cause serious adverse events (n = 20,381). The odds of a serious adverse event were higher with fluticasone than with control (OR 1.06, 95% CI 0.99 to 1.16; I2 = 0%, P value 0.66). The lower confidence just crossed the line of no effect, but no significant heterogeneity was noted between studies. No evidence suggested a difference between monotherapy and combination therapy subgroups (I2 = 0%, P value 0.58). Evidence was rated of high quality.
1.5. All pneumonia events
Fifteen studies making 11 comparisons reported the outcome (n = 15,377), and Calverley 2007 TORCH carried almost 80% of the weight across the analysis. The odds of any pneumonia event were significantly greater with fluticasone than with control (OR 1.68, 95% CI 1.49 to 1.90), with no important heterogeneity observed between studies (I2 = 0%, P value 0.76) or subgroups (I2 = 0%, P value 0.64). The outcome was underreported across the studies, so evidence was downgraded for publication bias, and was rated of moderate quality.
1.6. Withdrawals
Data from 26 studies (33 comparisons, n = 21,243) show that withdrawals were much less common on fluticasone than on control, with no significant heterogeneity noted (OR 0.81, 95% CI 0.77 to 0.86; I2 = 3%, P value 0.43). The effect was larger for fluticasone monotherapy, but the difference between subgroups was not statistically significant (I2 = 66%, P value 0.09). No reasons suggested the need to downgrade the evidence from high quality.
Comparison two: subgroup analyses—fluticasone versus controls
Dose: Combining all studies and organising by fluticasone dose did not reveal significant subgroup differences between doses (I2 = 0%, P value 0.90; Analysis 2.1). Pooled effects for the three furoate dose subgroups (50 mcg, 100 mcg and 200 mcg once a day) contained fewer data and therefore had much wider confidence intervals than the more widely used propionate preparation doses. Higher‐dose fluticasone propionate was the most widely studied and hence has the most precise estimate, but the pooled effect was not statistically different from the other dose subgroups.
Trial duration: No evidence shows significant differences between the trials with duration of one year or less and the three trials (four comparisons) that followed participants for three years (I2 = 0%, P value 0.61; Analysis 2.2). No significant heterogeneity between individual studies was noted within either subgroup (I2 = 0% in both cases).
Baseline percentage predicted FEV1 : Studies with a mean baseline percentage predicted FEV1 of less than 50% accounted for 99% of the analysis weight (Analysis 2.3), so no conclusions could be drawn regarding the moderating effect of baseline severity.
Comparison three: budesonide versus controls (all outcomes subgrouped to compare ICS vs placebo with ICS/LABA vs LABA)
3.1 Primary outcome: non‐fatal, serious adverse pneumonia events (requiring hospital admission)
Data for eight comparisons in seven studies were analysed (n = 6472), with Tashkin 2008 SHINE contributing data to both 'budesonide versus placebo' and 'budesonide/LABA versus LABA' subgroups. Budesonide increased non‐fatal serious adverse pneumonia events (OR 1.62, 95% CI 1.00 to 2.62), and, although a degree of variation was noted between study results, it was not significant (I2 = 28%, P value 0.21). Heterogeneity was evident between the monotherapy and combination subgroups, but the test for differences was not statistically significant (I2 = 55%, P value 0.14). The confidence intervals around the pooled estimate were quite wide but were not considered serious enough to warrant downgrading. However, because two‐thirds of the budesonide studies did not appear in the analysis, the outcome was downgraded once for publication bias and was rated of moderate quality.
3.2. Mortality, all‐cause
Budesonide did not significantly affect all‐cause mortality relative to control interventions (OR 0.90, 95% CI 0.65 to 1.24), based on 15 comparisons in 12 studies (n = 10,009). Heterogeneity was not significant across studies (I2 = 0%, P value 0.76), and no statistically significant difference was noted between the monotherapy and combination therapy subgroups (I2 = 0%, P value 0.75). Evidence was rated of moderate quality after being downgraded once for imprecision because the confidence intervals included significant benefit and harm.
3.3. Mortality, due to pneumonia
Only three budesonide studies reported the outcome (n = 1511), of which two studies observed no events in either group. No conclusions could be made from Sharafkhaneh 2012, which observed one event in the budesonide/LABA group. Evidence was rated of very low quality, being downgraded twice for imprecision and once for publication bias.
3.4. Non‐fatal serious adverse events, all‐cause
Fifteen comparisons in 12 studies were analysed (n = 10,009). Budesonide was not found to increase the odds of a serious adverse event (OR 1.01, 95% CI 0.83 to 1.22), although significant heterogeneity was noted between studies (I2 = 59%, P value 0.002), so a random‐effects analysis was used and the outcome was downgraded for inconsistency to moderate quality. No heterogeneity was observed between the subgroups (I2 = 0%, P value 0.68).
An outlier in the budesonide versus placebo subgroup was removed in a post hoc sensitivity analysis (Vestbo 1999), which changed the effect for the subgroup to favour the control (OR 1.27, 95% CI 1.04 to 1.55) with no within‐subgroup heterogeneity (previously I2 = 72%). The study recruited a less severe population, which might explain the difference in effect.
3.5. All pneumonia events
Not enough evidence was obtained to rule out a significant increase or a potential reduction in pneumonia events on budesonide compared with controls (OR 1.12, 95% CI 0.83 to 1.51; eight comparisons in six studies; n = 7011). A degree of unexplained heterogeneity was observed between studies (I2 = 13%, P value 0.33) and between treatment subgroups (I2 = 14%, P value 0.28), neither of which was significant. Although confidence intervals were quite wide, findings were not deemed serious enough to warrant downgrading of the evidence. However, because most of the budesonide studies did not appear in the analysis, the outcome was downgraded for publication bias and was rated of moderate quality.
3.6. Withdrawals
When 18 comparisons in 15 studies were combined (n = 10,150), withdrawals were seen to be less common in the budesonide groups than in the control groups (OR 0.78, 95% CI 0.71 to 0.85). No important heterogeneity was noted between individual studies (I2 = 12%, P value 0.31) or between monotherapy and combination therapy subgroups (I2 = 0%, P value 0.57). Evidence was rated of high quality.
Comparison four: subgroup analyses—budesonide versus controls
Dose: When all budesonide studies were subgrouped according to daily dose, the difference between 320 mcg and 640 mcg was significant (I2 = 74%, P value 0.05; Analysis 4.1); the higher dose increased non‐fatal serious adverse pneumonia events (OR 2.02, 95% CI 1.15 to 3.57), and no significant difference was observed for the lower dose (OR 0.68, 95% CI 0.27 to 1.71). The only study using the highest dose of 1280 mcg, Yildiz 2004, did not contribute data to the analysis because no events occurred in either group.
Trial duration: The difference between five studies lasting a year or less and Shaker 2009 (which followed participants for a minimum of two years) was not significant (I2 = 39%, P value 0.20; Analysis 4.2).
Baseline percentage predicted FEV1: When studies were subgrouped according to baseline severity, differences were not significant (I2 = 40%, P value 0.20; Analysis 4.3), and only one study reported a baseline mean FEV1 above 50% predicted (Shaker 2009).
Comparison five: sensitivity analysis—risk of bias
5.1 Non‐fatal serious adverse pneumonia events
5.1.1 Fluticasone versus controls
Four fluticasone studies representing six comparisons rated at high risk for attrition were removed from the primary outcome in a sensitivity analysis. The estimate gave a slightly larger effect of fluticasone on pneumonia than the main analysis (15 RCTs; n = 16,338; OR 1.82, 95% CI 1.52 to 2.19).
5.1.2 Budesonide versus controls
Three studies judged to be at high risk of bias due to high or uneven levels of attrition were removed from the primary outcome in a sensitivity analysis. The effect from the remaining four studies was larger but more imprecise because far fewer events were included in the analysis (5 RCTs; n = 3515; OR 3.28, 95% CI 1.22 to 8.81).
Comparison six: indirect comparison of fluticasone and budesonide monotherapy
We calculated the relative effects of fluticasone and budesonide for four outcomes by comparing their effects against placebo (see Figure 1). No studies directly comparing the two drugs met this review's inclusion criteria, but cohort data are summarised in Agreements and disagreements with other studies or reviews.
All four outcomes were downgraded for indirectness because no direct evidence was found and the estimate was obtained purely from indirect comparisons. None of the outcomes were downgraded for risk of bias or inconsistency. The indirect comparisons are presented in Figure 5 and are summarised below, and the sensitivity analysis removing Calverley 2007 TORCH is shown in Figure 6.

Indirect comparisons of fluticasone and budesonide monotherapy.
Non‐fatal serious adverse pneumonia events were defined as those requiring hospital admission.

Indirect comparisons of fluticasone and budesonide monotherapy—Sensitivity analysis removing (Calverley 2007 TORCH).
6.1 Non‐fatal serious adverse pneumonia events
The point estimate favoured fluticasone, but the difference was not significant and the confidence intervals were very wide (OR 0.53, 95% CI 0.16 to 1.71). In addition to being downgraded for indirectness, the outcome was downgraded twice for imprecision and was rated as very low quality.
6.2 Mortality, all‐cause
The point estimate favoured budesonide, but the difference was not significant and the confidence intervals were wide (OR 1.24, 95% CI 0.74 to 2.07). Evidence was also downgraded once for imprecision and was rated of low quality.
6.3 All non‐fatal serious adverse events
The difference between fluticasone and budesonide was not significant, and the confidence intervals were much tighter than for the other two indirect comparisons (OR 0.96, 95% CI 0.77 to 1.20). The evidence was not downgraded for any other reason and was rated of moderate quality.
6.4 All pneumonia events
A significant difference was noted between fluticasone and budesonide, although the confidence interval was quite wide (OR 1.86, 95% CI 1.04 to 3.34). The evidence was not downgraded for any other reason and was rated of moderate quality. When Calverley 2007 TORCH was removed from the sensitivity analysis, the difference was larger in magnitude but was much less precise and was not statistically significant (OR 2.30, 95% CI 0.79 to 6.67).
Comparison seven: indirect comparison of fluticasone/LABA and budesonide/LABA combination therapy
For the reasons described in 'Transitivity and similarity', we chose not to calculate an indirect comparison for combination therapy. Recent studies directly comparing fluticasone/salmeterol with budesonide/formoterol were not comparable with the design or time scales used in the rest of this review (Blais 2010; Janson 2013 [PATHOS]; Partridge 2009; Roberts 2011), but their findings are discussed in Agreements and disagreements with other studies or reviews.
Discussion
Summary of main results
We found a total of 43 studies that met the inclusion criteria for this review, with more evidence for fluticasone (26 studies, n = 21,247) than budesonide (17 studies, n = 10,150). Evidence from the budesonide studies was more inconsistent and less precise, and the mean duration of trials was shorter.
Fluticasone increased non‐fatal serious adverse pneumonia events (i.e. those requiring a hospital admission) (OR 1.78, 95% CI 1.50 to 2.12; high‐quality evidence), and no significant evidence suggested that this was different when fluticasone was delivered in combination with salmeterol or vilanterol (subgroup differences, I2 = 0%, P value 0.51). We did not find that different doses, trial duration or baseline severity significantly affected this estimate. Budesonide also increased non‐fatal serious adverse pneumonia events, but the effect was less precise (OR 1.62, 95% CI 1.00 to 2.62; moderate‐quality evidence). Some of the variation in the budesonide data could be explained by a significant difference between the two commonly used doses: 640 mcg showed a larger increase than 320 mcg (subgroup differences, I2 = 74%, P value 0.05). An indirect comparison of budesonide and fluticasone using placebo as a common comparator showed no significant differences with respect to serious adverse events (pneumonia‐related or all‐cause) or mortality.
No difference in overall mortality rates was noted between either of the inhaled steroids and the control interventions (both high‐quality evidence), but no conclusions could be drawn regarding deaths that were pneumonia‐related, as so few events were reported. Evidence of total pneumonia events on fluticasone was dominated by the three‐year Calverley 2007 TORCH study, and far fewer budesonide studies reported the outcome.
Indirect comparisons of budesonide and fluticasone monotherapy showed no significant differences with respect to serious adverse events (pneumonia‐related or all‐cause) or mortality. The risk of any pneumonia event (i.e. less serious cases treated in the community) was higher with fluticasone than with budesonide (OR 1.86, 95% CI 1.04 to 3.34); this was the only significant difference found between the two drugs. However, this finding should be interpreted with caution because of possible differences in assignment of a diagnosis of pneumonia, and because no trials directly compared the two drugs.
Fewer people in the inhaled steroid groups withdrew from study medication; this was true for both monotherapy versus placebo and combination therapy versus LABA trials. It is possible that this was a result of lack of efficacy in the control groups, but it was not formally evaluated. High or uneven dropout was considered to show a high risk of bias in almost 40% of the trials, but conclusions for the primary outcome did not change when the trials at high risk of bias were removed in a sensitivity analysis.
Overall completeness and applicability of evidence
Efforts were made to contact all trial authors to obtain additional data when outcomes did not appear in the available reports. Although this resulted in some additional data being provided—both numerical and related to study conduct—most studies do not appear in every analysis; only around a third of the studies contribute data to the primary outcome.
We chose to look at fluticasone and budesonide as the two most widely used inhaled steroids that are also commonly prescribed in combination with the long‐acting beta2‐agonists salmeterol and formoterol, respectively, and we did not include trials studying other available inhaled steroids (e.g. mometasone, triamcinolone, ciclesonide, beclomethasone, flunisolide). As such, the study results can be applied only to the inhaled steroids in question, not to the class in general.
The plan to indirectly compare budesonide/formoterol and fluticasone/salmeterol combination therapies was more complicated than it was for monotherapy, and by choosing not to conduct the indirect comparison, we were unable to draw conclusions about the relative safety of budesonide and fluticasone when used in combination with a LABA. Even without the addition of the fluticasone furoate/vilanterol studies, we considered the LABA comparator to differ systematically between fluticasone and budesonide combination therapy studies, hence violating the transitivity assumption. Variation in the LABA used within the fluticasone trials further reduced our confidence in the indirect comparison.
Inhaled corticosteroids are not equal in strength, and their doses are normally expressed as equivalents to budesonide dipropionate (BDP) doses (Table 3). By pooling data for all doses of fluticasone for comparison with all doses of budesonide, the indirect comparison could not account for the dose‐related effects that we found with budesonide, or for differences in dose equivalence between the two sets of data.
Quality of the evidence
We wrote to all study authors when numerical outcome data or details related to risk of bias could not be obtained from available reports. Of all the studies, nine reported all information and did not need to be contacted; we failed to find contact details for a further six. Of the remaining 28 studies, 13 study authors did not reply, six provided additional data or confirmed that all measured outcomes were reported in the original reports, four forwarded the request to the drug company that held the data, four were requests sent directly to GlaxoSmithKline and one could not provide data. No drug company data were provided by the time the review was published, and the application for data is ongoing and may be incorporated in future updates. In addition, nearly half of the studies were conducted 10 or more years ago; this made it difficult for review authors to locate contact details and for study authors to locate the data requested.
The definition of pneumonia and the method of diagnosis were routinely missing from trial reports, and this information could not be obtained to conduct a subgroup analysis on this basis. We were unsure whether pneumonia was radiographically confirmed in most cases, and the trials were not designed for the purpose of measuring the incidence of pneumonia. In addition, the extent to which pneumonia events are misclassified as acute exacerbations of COPD (and vice versa) is somewhat unclear (Marzoratti 2013). We could not obtain information to judge whether ascertainment of pneumonia was systematically different between funding drug companies or between healthcare systems in which studies were conducted; this is a potential source of bias.
Despite a relatively low rate of response from trial authors, all outcomes based on direct evidence were rated as high or moderate quality with GRADE. All trials were generally of good methodological quality, having been conducted by large pharmaceutical companies. In addition, more than 40 studies were found that contributed data to at least one analysis. Some imprecision was noted for rare events such as mortality due to pneumonia; we were unable to draw conclusions in these cases, especially for budesonide, for which less evidence was available. For this reason, we were generally less confident in the budesonide outcomes.
We identified two potential confounds, which we did not consider to be sufficient to downgrade the quality of the evidence. First, most of the budesonide studies were funded by AstraZeneca, and most of the fluticasone studies by GlaxoSmithKline. However, systematic differences in study conduct were not identified in the assessments of bias. Second, budesonide monotherapy and combination therapy are delivered via a Turbuhaler, and fluticasone is given via a Diskus or Accuhaler device. We assumed that participants in the trials were given adequate training for the device used, and that this did not systematically affect the amount of drug delivered or the likelihood of compliance between the two drugs. For this reason, and because in each double‐blind trial the placebo device matched that of the active drug, we did not consider different drug devices a reason not to conduct the indirect comparison of budesonide versus fluticasone.
When the protocol for this review was written, we did not anticipate the inclusion of a new preparation of fluticasone furoate in combination with the long‐acting beta2‐agonist vilanterol. Including these studies may have introduced heterogeneity into the direct fluticasone versus placebo comparison, and into the fluticasone/LABA versus LABA comparison, although a recent randomised trial comparing fluticasone/vilanterol with fluticasone/salmeterol found no significant differences in their efficacy or safety profiles (Agusti 2013). Inclusion of these studies may also have reduced the reliability of the indirect comparisons by increasing variation within the fluticasone monotherapy node and the fluticasone/salmeterol node (by including fluticasone/vilanterol studies in a combined fluticasone/LABA node), but the absence of statistical heterogeneity in the analyses did not suggest that this was the case. The expected publication of further RCTs of fluticasone furoate and other new preparations will increase the likelihood of detecting any differences which may exist between their safety profiles and more established corticosteroids. This may warrant dealing with different preparations separately in future updates of this review, which was not possible with the current evidence base.
Our decision to conduct the monotherapy indirect comparison was based on statistical consistency within the two sets of monotherapy trials (budesonide vs placebo and fluticasone vs placebo) and on a comprehensive assessment of transitivity across these two sets of trials. As such, we considered the indirect comparison to be valid, but the quality of the evidence remains limited by the observational nature of indirect comparisons, and by the lack of head‐to‐head trials comparing fluticasone and budesonide.
Potential biases in the review process
We made every effort to adhere to Cochrane methods during the review process. As stated in the protocol, all numerical data were extracted by two review authors, and discrepancies were resolved through discussion. The same was true for the risk of bias ratings. Neither of the review authors have conflicting interests.
It is unlikely that the review is biased as a result of published studies missed during study selection. The electronic searches were relatively broad, and review authors searched additional resources to locate any studies that might have been missed (e.g. trial registration websites, drug company databases, reference lists of included studies). In addition, review authors attempted to contact all study authors when data or details of study methodology were not reported in the published reports.
Agreements and disagreements with other studies or reviews
Two Cochrane reviews that are related to this safety review have recently been updated: Yang 2012, looking at the safety and efficacy of any inhaled corticosteroid versus placebo, and Nannini 2012, focusing on ICS/LABA combination therapy versus LABA alone. Both reviews are consistent with the findings and conclusions of this review; no effect was observed for mortality, and increased rates of pneumonia seen with inhaled steroids were not significantly different between types of inhaled steroids. Nannini 2012 called for more evidence regarding differential safety of different doses of inhaled steroids, which this review has helped to clarify. Both author teams correctly point out in their reviews that evidence for harms associated with these medications needs to be assessed in conjunction with good evidence of the clinical benefit of inhaled steroids (notably, fewer exacerbations and improved quality of life). In contrast to other previous meta‐analyses (Ruiz 2011; Sin 2009), this review found evidence for an association between budesonide and serious adverse pneumonia events, and indirect comparison with fluticasone yielded little evidence for differential safety between the two drugs. It is problematic to incorporate evidence for dose‐related safety with efficacy data to assess whether a particular dose of a drug is preferable to another; evidence from Calverley 2007 TORCH suggests that the trade‐off between reducing exacerbations and increasing pneumonia changes over time for fluticasone, but it is not yet clear whether this is true for different doses and products (Cates 2013).
Evidence from cohorts corroborates the data from randomised trials, showing increased rates of pneumonia in those treated with inhaled steroids and more clearly demonstrating a dose‐related effect (Ernst 2007; Janson 2013 [PATHOS]; Suissa 2013; Yawn 2013). Evidence from one of the cohorts, which observed more than 20,000 serious pneumonias, revealed increased risk with fluticasone (RR 2.01, 95% CI 1.93 to 2.10) compared with budesonide (RR 1.17, 95% CI 1.09 to 1.26) (Suissa 2013); this conclusion could not be drawn from the randomised trial evidence. Although we believe that it was not viable to make an indirect comparison of budesonide combination therapy versus fluticasone combination therapy, cohort data suggest that the fluticasone combination is associated with higher rates of pneumonia, deaths related to pneumonia and admissions to hospital than budesonide/formoterol, but the duration of admissions for pneumonia and overall mortality were similar (Janson 2013 [PATHOS]). We found no indirect comparisons of budesonide/formoterol and fluticasone/salmeterol using a LABA control group, but indirect evidence based on randomised trials using placebo as the common comparator supported the cohort findings (Halpin 2011).

Direct and indirect comparisons of fluticasone and budesonide covered in the review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Summary of pooled effects of trials comparing ICS versus placebo and combination versus LABA.
Non‐fatal serious adverse pneumonia events were those requiring hospital admission. Data for all pneumonia events were not pooled because of heterogeneity.

Indirect comparisons of fluticasone and budesonide monotherapy.
Non‐fatal serious adverse pneumonia events were defined as those requiring hospital admission.

Indirect comparisons of fluticasone and budesonide monotherapy—Sensitivity analysis removing (Calverley 2007 TORCH).

Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 1 Non‐fatal, serious adverse pneumonia events.
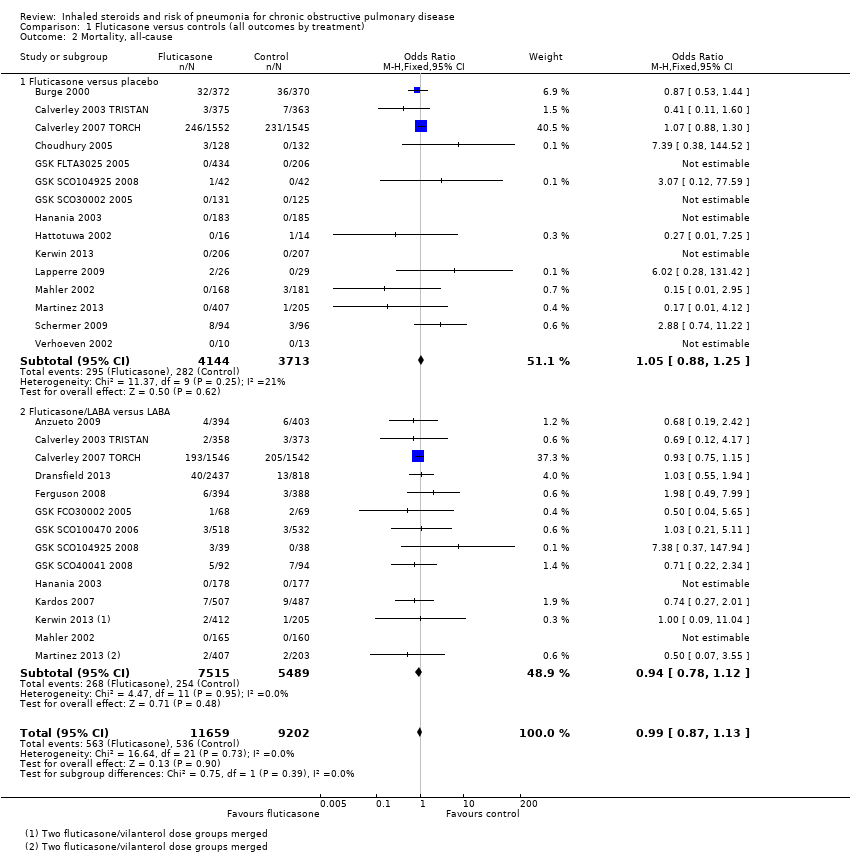
Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 2 Mortality, all‐cause.

Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 3 Mortality, due to pneumonia.
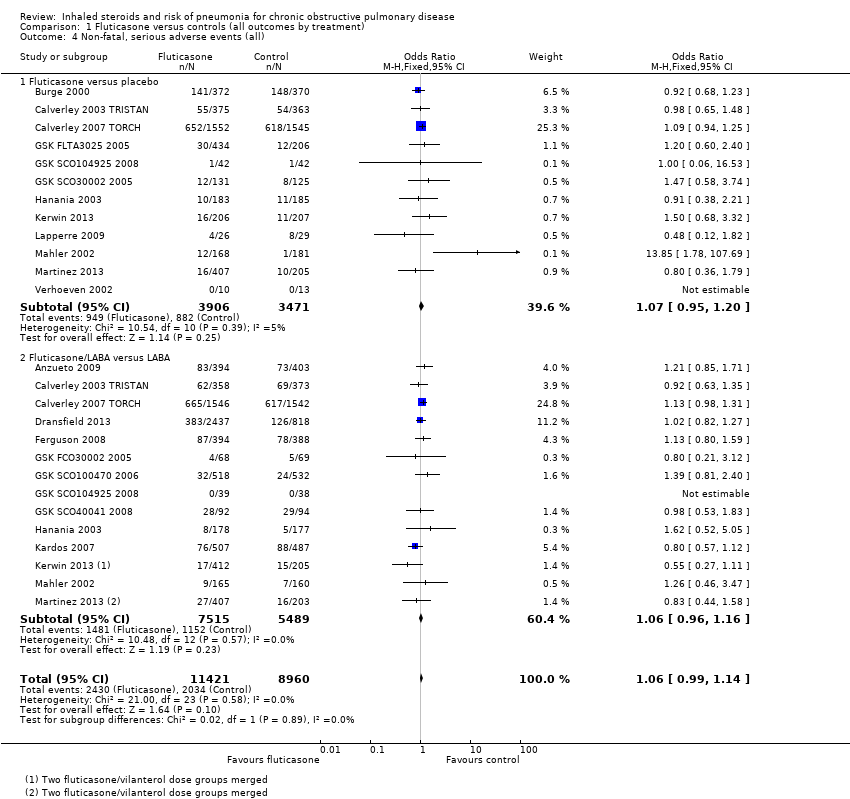
Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 4 Non‐fatal, serious adverse events (all).

Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 5 All pneumonia events.
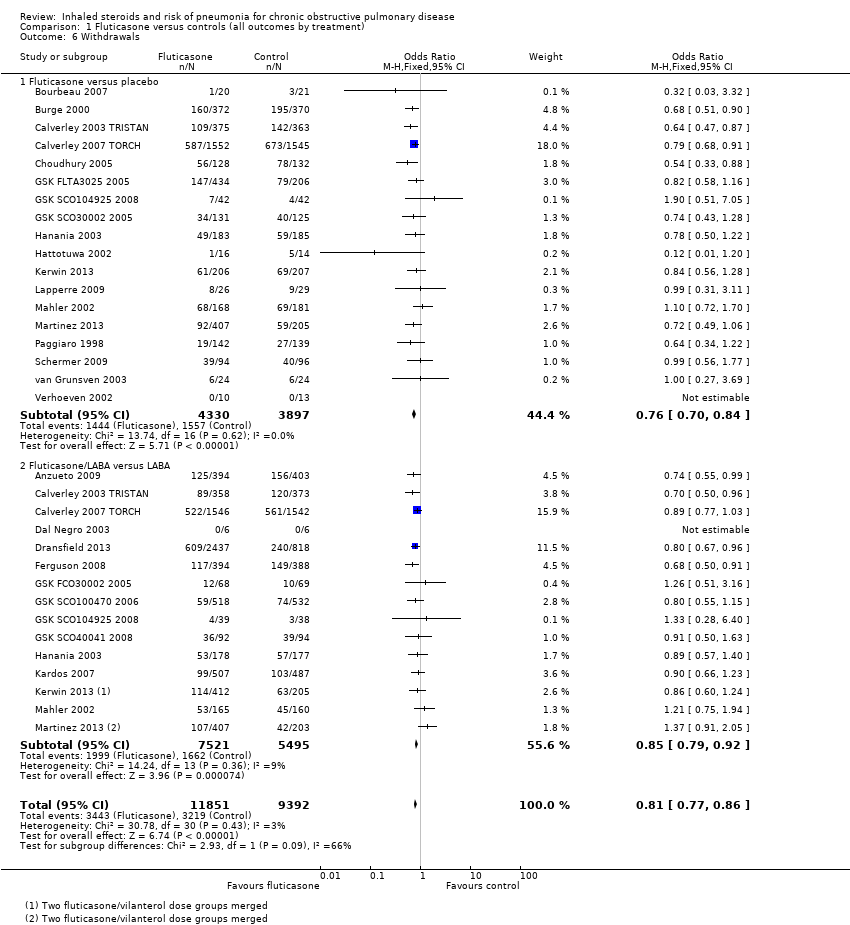
Comparison 1 Fluticasone versus controls (all outcomes by treatment), Outcome 6 Withdrawals.
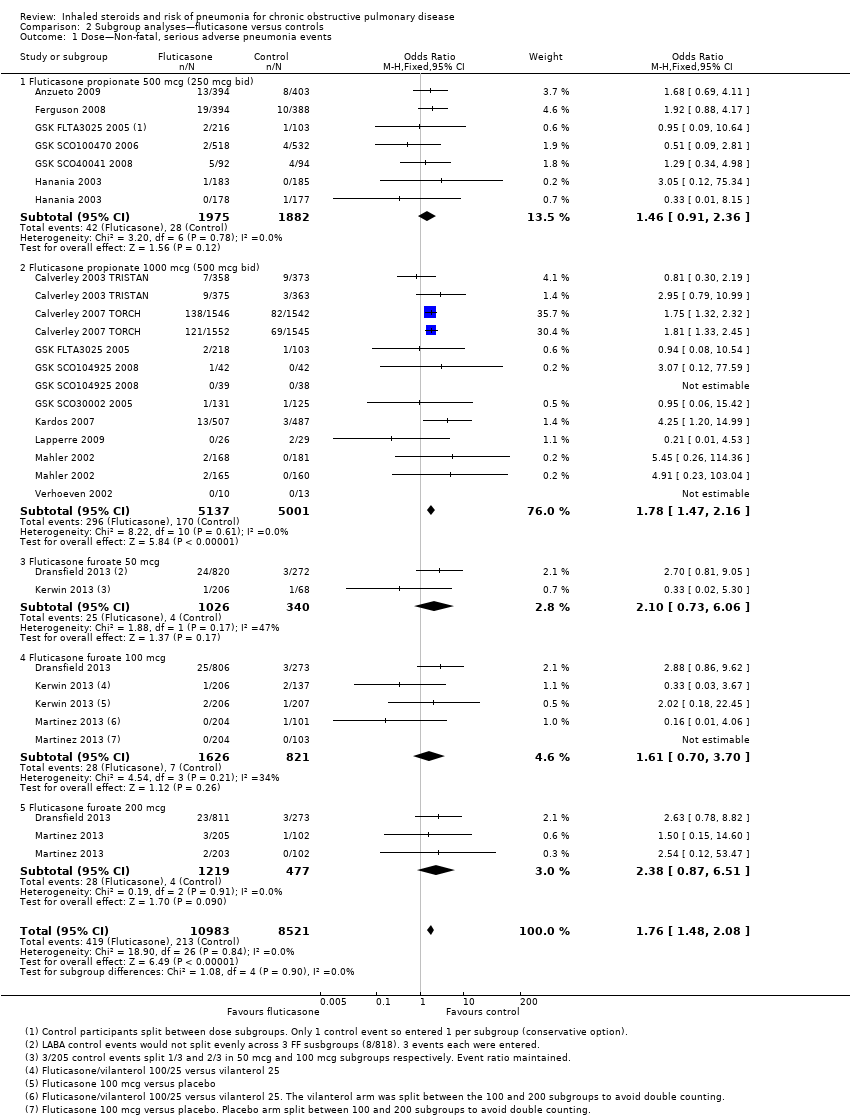
Comparison 2 Subgroup analyses—fluticasone versus controls, Outcome 1 Dose—Non‐fatal, serious adverse pneumonia events.
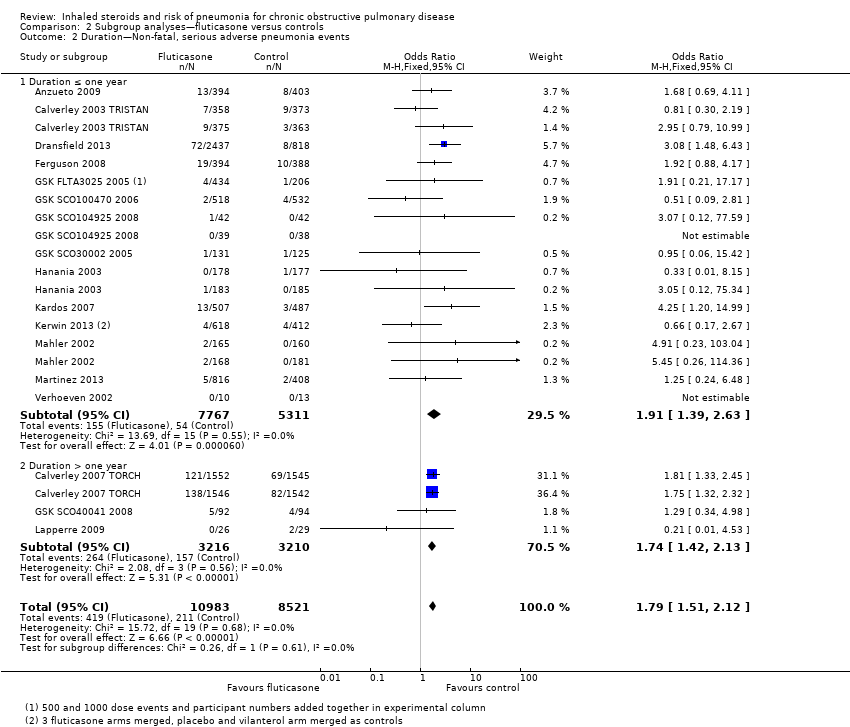
Comparison 2 Subgroup analyses—fluticasone versus controls, Outcome 2 Duration—Non‐fatal, serious adverse pneumonia events.
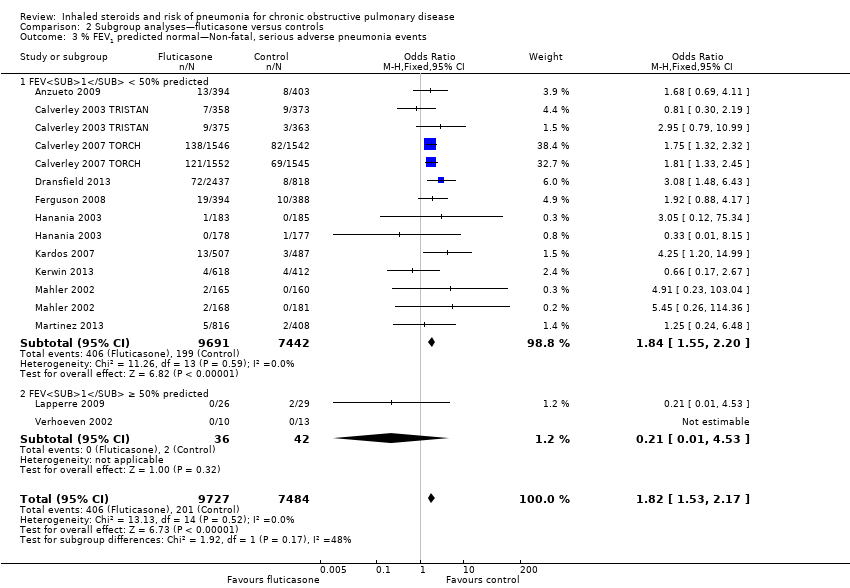
Comparison 2 Subgroup analyses—fluticasone versus controls, Outcome 3 % FEV1 predicted normal—Non‐fatal, serious adverse pneumonia events.
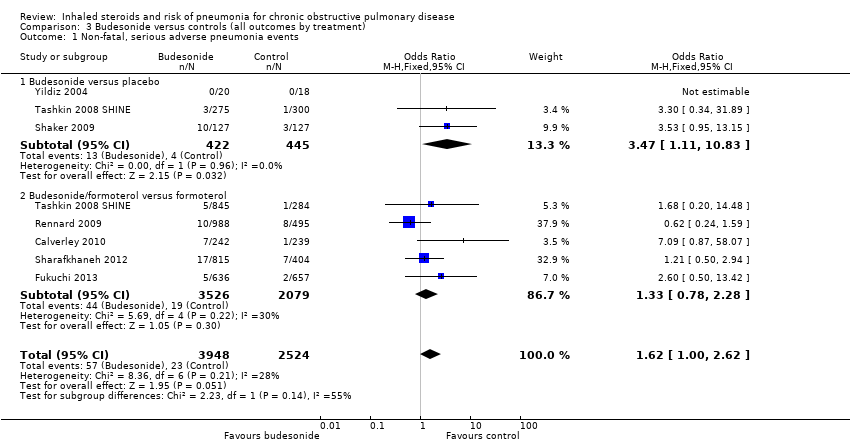
Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 1 Non‐fatal, serious adverse pneumonia events.

Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 2 Mortality, all‐cause.

Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 3 Mortality, due to pneumonia.

Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 4 Non‐fatal, serious adverse events (all).

Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 5 All pneumonia events.
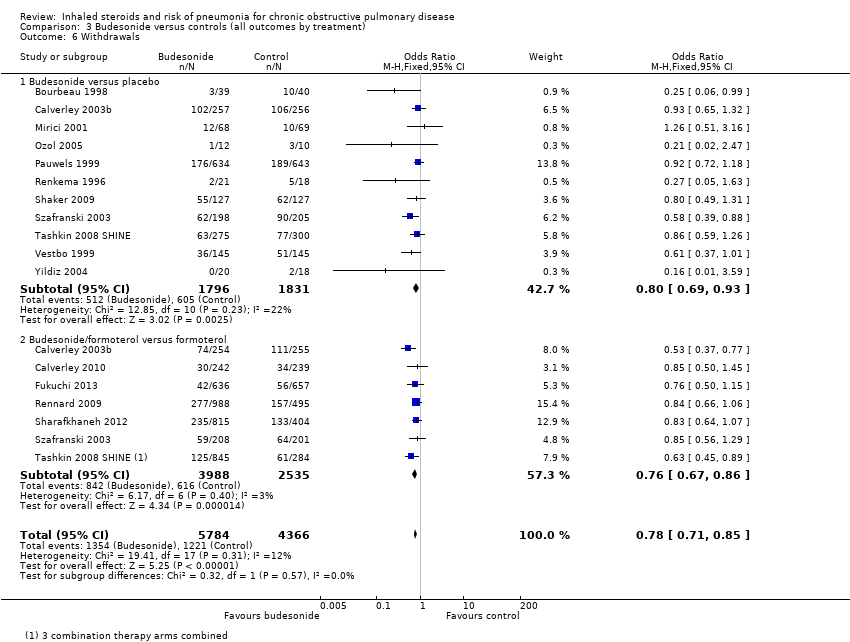
Comparison 3 Budesonide versus controls (all outcomes by treatment), Outcome 6 Withdrawals.
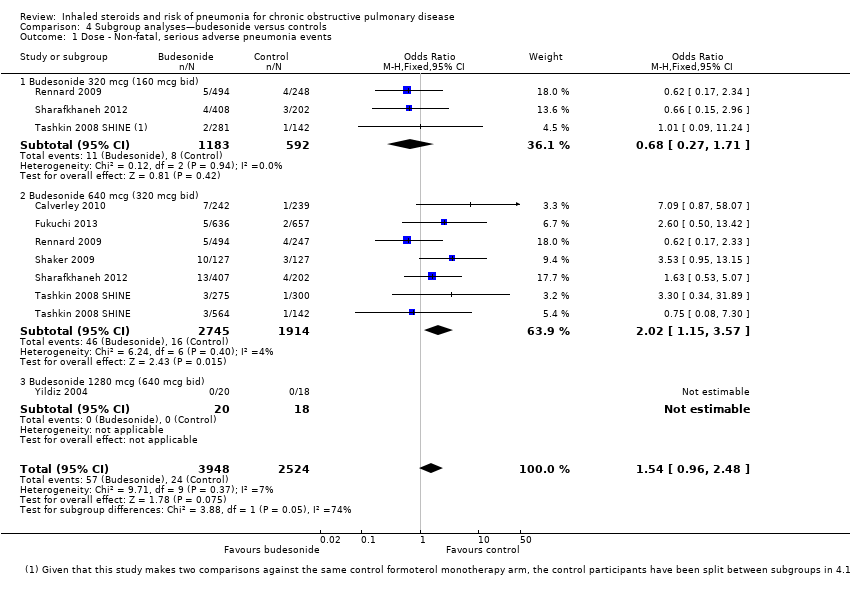
Comparison 4 Subgroup analyses—budesonide versus controls, Outcome 1 Dose ‐ Non‐fatal, serious adverse pneumonia events.

Comparison 4 Subgroup analyses—budesonide versus controls, Outcome 2 Duration ‐ Non‐fatal, serious adverse pneumonia events.

Comparison 4 Subgroup analyses—budesonide versus controls, Outcome 3 % FEV1 predicted normal ‐ Non‐fatal, serious adverse pneumonia events.
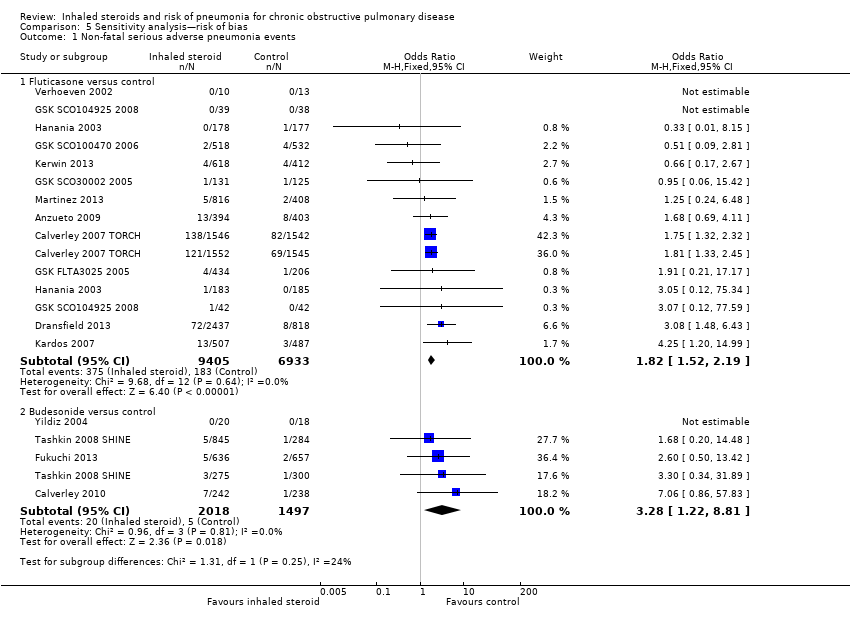
Comparison 5 Sensitivity analysis—risk of bias, Outcome 1 Non‐fatal serious adverse pneumonia events.
| Fluticasone for chronic obstructive pulmonary disease | |||||
| Patient or population: patients with chronic obstructive pulmonary disease Comparison: placebo or LABA monotherapy (dependent upon whether fluticasone was given with LABA in the intervention group) Setting: community | |||||
| Outcomes Follow‐ups presented as weighted means | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Fluticasone | ||||
| Non‐fatal, serious adverse pneumonia events (requiring hospital admission) | 25 per 1000 | 43 per 1000 | OR 1.78 | 19,504 | ⊕⊕⊕⊕ |
| Mortality, all‐cause | 58 per 1000 | 58 per 1000 | OR 0.99 | 20,861 | ⊕⊕⊕⊕ |
| Mortality, due to pneumonia | 2 per 1000 | 3 per 1000 | OR 1.23 | 19,532 | ⊕⊕⊕⊝ |
| Non‐fatal, serious adverse events (all) | 227 per 1000 | 237 per 1000 | OR 1.06 | 20,381 | ⊕⊕⊕⊕ |
| All pneumonia events | 72 per 1000 | 116 per 1000 | OR 1.68 | 15,377 | ⊕⊕⊕⊝ |
| Withdrawals | 343 per 1000 | 297 per 1000 | OR 0.81 | 21,243 | ⊕⊕⊕⊕ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Unless otherwise stated, subgroup differences between monotherapy studies (fluticasone versus placebo) and combination therapy studies (fluticasone/LABA versus LABA) were not significant. | |||||
| GRADE Working Group grades of evidence. | |||||
| 1Wide confidence intervals include significant benefit and harm, based on very few events (‐1 for imprecision). | |||||
| Budesonide for chronic obstructive pulmonary disease | |||||
| Patient or population: patients with chronic obstructive pulmonary disease Comparison: placebo or LABA monotherapy (dependent upon whether fluticasone was given with LABA in the intervention group) Setting: community | |||||
| Outcomes Follow‐ups presented as weighted means | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Budesonide | ||||
| Non‐fatal, serious adverse pneumonia events (requiring hospital admission) | 9 per 1000 | 15 per 1000 | OR 1.62 | 6472 | ⊕⊕⊕⊝ |
| Mortality, all‐cause | 17 per 1000 | 16 per 1000 | OR 0.90 | 10,009 | ⊕⊕⊕⊝ |
| Mortality, due to pneumonia | 0 per 1000 | 0 per 1000 | OR 4.46 | 1511 | ⊕⊝⊝⊝ |
| Non‐fatal, serious adverse events (all) | 145 per 1000 | 146 per 1000 | OR 1.01 | 10,009 | ⊕⊕⊕⊝ |
| All pneumonia events | 28 per 1000 | 31 per 1000 | OR 1.12 | 7011 | ⊕⊕⊕⊝ |
| Withdrawals | 280 per 1000 | 232 per 1000 | OR 0.78 | 10150 | ⊕⊕⊕⊕ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Unless otherwise stated, subgroup differences between monotherapy studies (budesonide versus placebo) and combination therapy studies (budesonide/LABA versus LABA) were not significant. | |||||
| GRADE Working Group grades of evidence. | |||||
| 1Confidence intervals are quite wide but are not considered serious enough to downgrade. | |||||
| Study ID | Duration (m) | N Rand | Funder | ICS dose (mcg) | % Male | Mean age | Pack‐years | % pred FEV1 |
| Fluticasone versus placebo (n = 18) | ||||||||
| 3 | 41 | GSK | 1000 | 78 | 65 | 53 | 57 | |
| 36 | 740 | GSK | 1000 | 75 | 64 | 44 | 50 | |
| 12 | 763 | GSK | 1000 | 73 | 63 | 43 | 45 | |
| 36 | 3097 | GSK | 1000 | 76 | 65 | 49 | 44 | |
| 12 | 260 | Indep. | 1000 | 52 | 67 | 39 | 54 | |
| 6 | 640 | GSK | 500, 1000 | 69 | 64 | ‐ | ‐ | |
| 3 | 84 | GSK | 1000 | 77 | 64 | ‐ | ‐ | |
| 12 | 256 | GSK | 1000 | 82 | 65 | ‐ | ‐ | |
| 6 | 368 | GSK | 500 | 63 | 64 | 57 | 42 | |
| 3 | 36 | GSK | 1000 | 87 | 65 | 63 | 46 | |
| Kerwin 2013a,b | 6 | 413 | GSK | 100 | 66 | 62 | 46 | 42 |
| 30 | 55 | GSK | 1000 | 86 | 60 | 43 | 55 | |
| 6 | 349 | GSK | 1000 | 66 | 65 | 55 | 41 | |
| 6 | 612 | GSK | 100, 200 | 72 | 62 | 43 | 48 | |
| 6 | 281 | ‐ | 1000 | 74 | 63 | ‐ | 57 | |
| 36 | 190 | Indep. | 1000 | 71 | 59 | 28 | 64 | |
| 24 | 48 | GSK | 500 | 52 | 47 | 9 | 97 | |
| 6 | 23 | GSK | 1000 | 82 | 55 | 26 | 63 | |
| WM 22 m | 459 | ‐ | ‐ | 72 | 62 | 43 | 54 | |
| Fluticasone/LABA combination versus LABA monotherapy (n = 15) | ||||||||
| 12 | 797 | GSK | 500 | 54 | 65 | 57 | 34 | |
| 12 | 731 | GSK | 1000 | 73 | 63 | 43 | 45 | |
| 36 | 3088 | GSK | 1000 | 76 | 65 | 49 | 44 | |
| 12 | 12 | ‐ | 500 | 92 | ‐ | 42 | 50 | |
| 12 | 3255 | GSK | 50, 100, 200 | 57 | 64 | ‐ | 45 | |
| 12 | 782 | GSK | 500 | 55 | 65 | 56 | 33 | |
| 3 | 140 | GSK | 1000 | 66 | 62 | ‐ | ‐ | |
| 6 | 1050 | GSK | 500 | 78 | 64 | ‐ | ‐ | |
| 3 | 77 | GSK | 1000 | 77 | 64 | ‐ | ‐ | |
| 36 | 186 | GSK | 500 | 61 | 66 | ‐ | ‐ | |
| 6 | 355 | GSK | 500 | 63 | 64 | 57 | 42 | |
| 10 | 994 | GSK | 1000 | 76 | 64 | 37 | 40 | |
| Kerwin 2013a,b | 6 | 617 | GSK | 100 | 67 | 63 | 46 | 43 |
| 6 | 325 | GSK | 1000 | 66 | 65 | 55 | 41 | |
| 6 | 610 | GSK | 100, 200 | 72 | 62 | 43 | 48 | |
| WM 16 m | 867 | ‐ | ‐ | 69 | 64 | 53 | 42 | |
| aMulti‐arm studies making both comparisons of interest (ICS vs placebo and ICS/LABA vs LABA). bStudies using vilanterol as the LABA combination and monotherapy comparator, with fluticasone furoate. Dose is given as the total received per day (i.e. 500 signifies 250 morning and evening). WM = weighted mean. | ||||||||
| Study ID | Duration (m) | N Rand | Funder | ICS dose (mcg) | % Male | Mean age | Pack‐years | % pred FEV1 |
| Budesonide versus placebo (n = 13) | ||||||||
| 6 | 79 | AZ | 640 | 79 | 66 | 51 | 37 | |
| 12 | 513 | GSK | 640 | 76 | 64 | 35 | 36 | |
| 6 | 49 | NR | 640 | NR | NR | NR | NR | |
| 3 | 50 | NR | 640 | 75 | 53 | 27 | 62 | |
| 6 | 26 | NR | 640 | 69 | 65 | 45 | 59 | |
| 36 | 1277 | AZ | 640 | 73 | 52 | 39 | 77 | |
| 24 | 39 | AZ | 1280 | 100 | 55 | NR | 64 | |
| 6 | 26 | NR | 640 | 54 | 61 | NR | NR | |
| 36 | 254 | AZ | 640 | 58 | 64 | 56 | 52 | |
| 12 | 403 | AZ | 640 | 79 | 64 | 45 | 36 | |
| 6 | 575 | AZ | 640 | 68 | 63 | 41 | 40 | |
| 36 | 290 | AZ | 640 | 88 | 59 | NR | 87 | |
| 3 | 38 | ? | 1280 | 100 | 67 | 51 | 46 | |
| WM 23 m | 278 | ‐ | ‐ | 77 | 61 | 43 | 54 | |
| Budesonide/LABA combination versus LABA monotherapy (n = 7) | ||||||||
| 12 | 509 | GSK | 640 | 76 | 64 | 35 | 36 | |
| 11 | 481 | Chiesi | 640 | 81 | 64 | 39 | 42 | |
| 3 | 1293 | AZ | 640 | 89 | 65 | 44 | 41 | |
| 12 | 1483 | AZ | 320, 640 | 63 | 63 | NR | 39 | |
| 12 | 1219 | AZ | 320, 640 | 62 | 63 | 44 | 38 | |
| 12 | 409 | AZ | 640 | 79 | 64 | 45 | 36 | |
| 6 | 1129 | AZ | 320, 640 | 68 | 63 | 41 | 40 | |
| WM 9 m | 932 | ‐ | ‐ | 75 | 64 | 41 | 39 | |
| aMulti‐arm studies making both comparisons of interest (ICS vs placebo and ICS/LABA vs LABA). Dose is given as the total received per day (i.e. 640 signifies 320 morning and evening). WM = weighted mean. | ||||||||
| Drug | Daily dose (mcg) | BDP equivalent (mcg) |
| Budesonide | 320 | 320 |
| 640 | 640 | |
| 1280 | 1280 | |
| Fluticasone | 500 (propionate) | 1000 |
| 1000 (propionate) | 2000 | |
| 50 (furoate) | 500 | |
| 100 (furoate) | 1000 | |
| 200 (furoate) | 2000 |
| Monotherapy comparison—Placebo control events | Combination comparison—LABA control events | |||
| Fluticasone | Budesonide | Fluticasone | Budesonide | |
| Pneumonia‐related serious adverse events | 2.5%, 77/310 | 0.9%, 4/445 | 2.5%, 134/5420 | 0.9%, 19/2079 |
| 0.5% without TORCH | 0.7% without TORCH | |||
| All‐cause mortality | 7.6%, 282/3713 | 2.1%, 37/1763 | 5.1%, 254/5489 | 1.5%, 37/2534 |
| 2.4% without TORCH | 1.2% without TORCH | |||
| All‐cause serious adverse events | 25%, 882/3471 | 15%, 268/1763 | 21%, 1152/5489 | 14%, 356/2534 |
| 14% without TORCH | 14% without TORCH | |||
| For the fluticasone control groups with and without the large 3‐year TORCH study. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐fatal, serious adverse pneumonia events Show forest plot | 17 | 19504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.50, 2.12] |
| 1.1 Fluticasone versus placebo | 11 | 6635 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.39, 2.44] |
| 1.2 Fluticasone/LABA versus LABA | 13 | 12869 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.41, 2.17] |
| 2 Mortality, all‐cause Show forest plot | 22 | 20861 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.13] |
| 2.1 Fluticasone versus placebo | 15 | 7857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 2.2 Fluticasone/LABA versus LABA | 14 | 13004 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.12] |
| 3 Mortality, due to pneumonia Show forest plot | 18 | 19532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.70, 2.15] |
| 3.1 Fluticasone versus placebo | 12 | 6665 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.52, 2.77] |
| 3.2 Fluticasone/LABA versus LABA | 13 | 12867 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.59, 2.65] |
| 4 Non‐fatal, serious adverse events (all) Show forest plot | 19 | 20381 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| 4.1 Fluticasone versus placebo | 12 | 7377 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.20] |
| 4.2 Fluticasone/LABA versus LABA | 14 | 13004 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.16] |
| 5 All pneumonia events Show forest plot | 11 | 15377 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.49, 1.90] |
| 5.1 Fluticasone versus placebo | 6 | 4971 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.33, 1.97] |
| 5.2 Fluticasone/LABA versus LABA | 9 | 10406 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.47, 2.01] |
| 6 Withdrawals Show forest plot | 26 | 21243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.77, 0.86] |
| 6.1 Fluticasone versus placebo | 18 | 8227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.70, 0.84] |
| 6.2 Fluticasone/LABA versus LABA | 15 | 13016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.79, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dose—Non‐fatal, serious adverse pneumonia events Show forest plot | 17 | 19504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.48, 2.08] |
| 1.1 Fluticasone propionate 500 mcg (250 mcg bid) | 6 | 3857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.91, 2.36] |
| 1.2 Fluticasone propionate 1000 mcg (500 mcg bid) | 9 | 10138 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.47, 2.16] |
| 1.3 Fluticasone furoate 50 mcg | 2 | 1366 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.73, 6.06] |
| 1.4 Fluticasone furoate 100 mcg | 3 | 2447 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.70, 3.70] |
| 1.5 Fluticasone furoate 200 mcg | 2 | 1696 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.87, 6.51] |
| 2 Duration—Non‐fatal, serious adverse pneumonia events Show forest plot | 17 | 19504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.51, 2.12] |
| 2.1 Duration ≤ one year | 14 | 13078 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.39, 2.63] |
| 2.2 Duration > one year | 3 | 6426 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.42, 2.13] |
| 3 % FEV1 predicted normal—Non‐fatal, serious adverse pneumonia events Show forest plot | 12 | 17211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.53, 2.17] |
| 3.1 FEV1 < 50% predicted | 10 | 17133 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.55, 2.20] |
| 3.2 FEV1 ≥ 50% predicted | 2 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐fatal, serious adverse pneumonia events Show forest plot | 7 | 6472 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.00, 2.62] |
| 1.1 Budesonide versus placebo | 3 | 867 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.11, 10.83] |
| 1.2 Budesonide/formoterol versus formoterol | 5 | 5605 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.78, 2.28] |
| 2 Mortality, all‐cause Show forest plot | 12 | 10009 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.24] |
| 2.1 Budesonide versus placebo | 8 | 3487 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| 2.2 Budesonide/formoterol versus formoterol | 7 | 6522 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.61, 1.46] |
| 3 Mortality, due to pneumonia Show forest plot | 3 | 1511 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.46 [0.07, 286.99] |
| 3.1 Budesonide versus placebo | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Budesonide/formoterol versus formoterol | 1 | 1219 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.46 [0.07, 286.99] |
| 4 Non‐fatal, serious adverse events (all) Show forest plot | 12 | 10009 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.83, 1.22] |
| 4.1 Budesonide versus placebo | 8 | 3487 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.50] |
| 4.2 Budesonide/formoterol versus formoterol | 7 | 6522 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.11] |
| 5 All pneumonia events Show forest plot | 6 | 7011 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.83, 1.51] |
| 5.1 Budesonide versus placebo | 3 | 1378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| 5.2 Budesonide/formoterol versus formoterol | 5 | 5633 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.87, 1.77] |
| 6 Withdrawals Show forest plot | 15 | 10150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.85] |
| 6.1 Budesonide versus placebo | 11 | 3627 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.93] |
| 6.2 Budesonide/formoterol versus formoterol | 7 | 6523 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dose ‐ Non‐fatal, serious adverse pneumonia events Show forest plot | 7 | 6472 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.96, 2.48] |
| 1.1 Budesonide 320 mcg (160 mcg bid) | 3 | 1775 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.27, 1.71] |
| 1.2 Budesonide 640 mcg (320 mcg bid) | 6 | 4659 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.15, 3.57] |
| 1.3 Budesonide 1280 mcg (640 mcg bid) | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Duration ‐ Non‐fatal, serious adverse pneumonia events Show forest plot | 7 | 6471 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.00, 2.62] |
| 2.1 Duration ≤ one year | 6 | 6217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.83, 2.37] |
| 2.2 Duration > one year | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.95, 13.15] |
| 3 % FEV1 predicted normal ‐ Non‐fatal, serious adverse pneumonia events Show forest plot | 7 | 6471 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.99, 2.59] |
| 3.1 FEV1 < 50% predicted | 6 | 6217 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.82, 2.34] |
| 3.2 FEV1 ≥ 50% predicted | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.95, 13.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐fatal serious adverse pneumonia events Show forest plot | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fluticasone versus control | 12 | 16338 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.52, 2.19] |
| 1.2 Budesonide versus control | 4 | 3515 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.28 [1.22, 8.81] |

