Ácidos grasos omega 3 para prevenir o enlentecer la progresión de la degeneración macular senil
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010015.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 09 April 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Eyes and Vision Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
JL assessed studies for inclusion and exclusion, assessed risk of bias, extracted data, entered data and authored the first draft of the review
JE assessed studies for inclusion and exclusion, assessed risk of bias, extracted data, entered data reviewed and commented on the text of the review
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
-
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
JL: none known
JE: none known
Acknowledgements
We thank Catey Bunce, Andrew Law and William G Christen for their comments on this review. We are grateful to Iris Gordon from the Cochrane Eyes and Vision Group (CEVG) for preparing the electronic searches for this review and to Anupa Shah, Managing Editor for CEVG for her assistance throughout the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Apr 09 | Omega 3 fatty acids for preventing or slowing the progression of age‐related macular degeneration | Review | John G Lawrenson, Jennifer R Evans | |
| 2012 Nov 14 | Omega 3 fatty acids for preventing or slowing the progression of age‐related macular degeneration | Review | John G Lawrenson, Jennifer R Evans | |
| 2012 Aug 15 | Omega 3 fatty acid supplementation for preventing and slowing the progression of age‐related macular degeneration | Protocol | John G Lawrenson, Jennifer R Evans | |
| Adverse effects | Omega 3 N (%) | Placebo N (%) |
| AREDS 2 | ||
| Total number of participants | N = 1068 | N = 1012 |
| Participants with ≥ 1 adverse event · Cardiac disorders · Gastrointestinal disorders · Infections · Neoplasms · Nervous system disorders · Respiratory and chest disorders | 505 (47.3) 119 (11.1) 58 (5.4) 103 (9.6) 83 (7.8) 72 (6.7) 37 (3.5) | 479 (47.3) 96 (9.5) 76 (7.5) 90 (8.9) 80 (7.9) 66 (6.5) 44 (4.3) |
| NAT‐2 | ||
| Total number of participants | N = 134 | N = 129 |
| Total adverse events · Treatment emergent adverse events* · Ocular · Serious non‐ocular | 125 (83.3) 5 (4.7) 88 (58.4) 31 (23.1) | 115 (77.7) 2 (1.6) 74 (50.0) 30 (23.6) |
* As defined by the study authors (including gastrointestinal disorders, allergic dermatitis and breath odour)
Differences between protocol and review
None
Notes
None
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
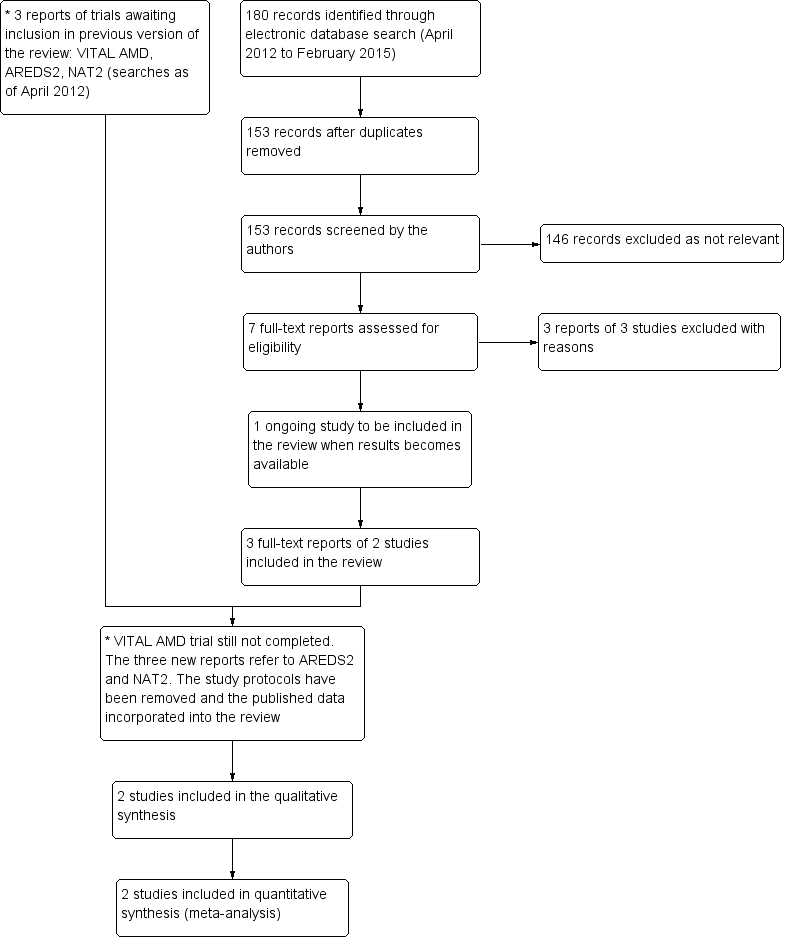
Results from searching for studies for inclusion in the review.
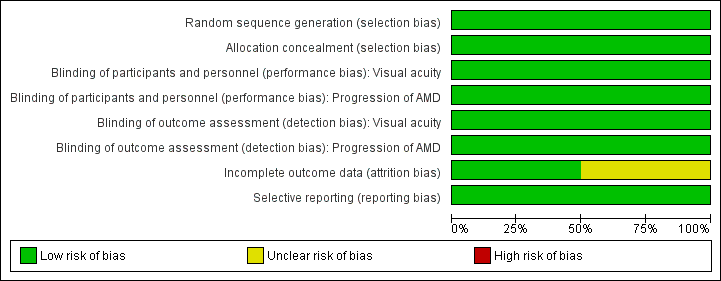
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison (Analysis 1.1): 1 Omega 3 fatty acids versus control, outcome: 1.10 Progression of AMD.
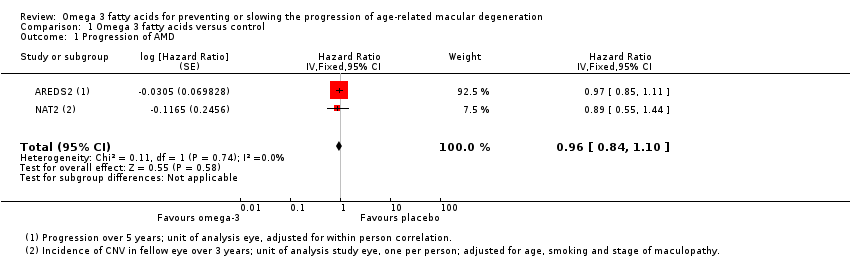
Comparison 1 Omega 3 fatty acids versus control, Outcome 1 Progression of AMD.
| Omega 3 fatty acids compared to placebo for slowing the progression of age‐related macular degeneration | ||||||
| Patient or population: people with AMD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No omega 3 fatty acids (placebo) | Omega 3 fatty acids | |||||
| Loss of 3 or more lines of VA at 24 months | 100 per 1000 | 114 per 1000 | RR 1.14, 95% CI 0.53 to 2.45 | 236 | ⊕⊕⊕⊝ | |
| Loss of 3 or more lines of VA at 36 months | 150 per 1000 | 187 per 1000 | RR 1.25, 95% CI 0.69 to 2.26) | 230 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 24 months | 100 per 1000 | 106 per 1000 | RR 1.06, 95% CI 0.47 to 2.40 | 224 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 36 months | 150 per 1000 | 168 per 1000 | RR 1.12, 95% CI 0.53 to 2.38 | 195 | ⊕⊕⊕⊝ | |
| Progression of AMD over 5 years | 300 per 1000 | 290 per 1000 | HR 0.96 | 2343 | ⊕⊕⊕⊕ | |
| Adverse effects | 500 per 1000 | 505 per 1000 | RR 1.01, 95% CI 0.94 to 1.09 | 2343 | ⊕⊕⊕⊕ | AREDS2 reported participants with one or more serious adverse events (AE). NAT‐2 reported total AE including treatment emergent and serious non‐ocular events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision | ||||||
| Adverse effects | Omega 3 N (%) | Placebo N (%) |
| AREDS 2 | ||
| Total number of participants | N = 1068 | N = 1012 |
| Participants with ≥ 1 adverse event · Cardiac disorders · Gastrointestinal disorders · Infections · Neoplasms · Nervous system disorders · Respiratory and chest disorders | 505 (47.3) 119 (11.1) 58 (5.4) 103 (9.6) 83 (7.8) 72 (6.7) 37 (3.5) | 479 (47.3) 96 (9.5) 76 (7.5) 90 (8.9) 80 (7.9) 66 (6.5) 44 (4.3) |
| NAT‐2 | ||
| Total number of participants | N = 134 | N = 129 |
| Total adverse events · Treatment emergent adverse events* · Ocular · Serious non‐ocular | 125 (83.3) 5 (4.7) 88 (58.4) 31 (23.1) | 115 (77.7) 2 (1.6) 74 (50.0) 30 (23.6) |
| * As defined by the study authors (including gastrointestinal disorders, allergic dermatitis and breath odour) | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of AMD Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.84, 1.10] | |

