Aspiration of the elbow joint for treating radial head fractures
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009949.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 22 November 2014see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Bone, Joint and Muscle Trauma Group
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Thanit Foocharoen drafted the protocol and is the guarantor. Chingching Foocharoen, Thavatchai Tiamklang and Malinee Laopaiboon revised and approved the protocol.
Thanit Foocharoen and Chingching Foocharoen selected studies for the review, extracted data and assessed study risk of bias. Thavatchai Tiamklang resolved any disagreements. Thavatchai Tiamklang also entered data into Review Manager software; this was checked by the other three review authors. Thanit Foocharoen drafted the full review and assessed the quality of the evidence; this was checked by Malinee Laopaiboon.
Sources of support
Internal sources
-
Thai Cochrane Network, Khon Kaen University, Thailand.
External sources
-
No sources of support supplied
Declarations of interest
Thanit Foocharoen: none known
Chingching Foocharoen: none known
Malinee Laopaiboon: none known
Thavatchai Tiamklang: none known
Acknowledgements
We would like to thank Mario Lenza and Helen Handoll for valuable comments on the protocol and review and Paula Harding for her helpful feedback on the review. Thanks, too, to Joanne Elliott for her assistance with the development of the search strategies and Lindsey Elstub and Laura MacDonald for help during editorial processing. We would also like to acknowledge Diane Horsley for her support.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Nov 22 | Aspiration of the elbow joint for treating radial head fractures | Review | Thanit Foocharoen, Chingching Foocharoen, Malinee Laopaiboon, Thavatchai Tiamklang | |
| 2012 Jul 11 | Aspiration for treating radial head fractures | Protocol | Thanit Foocharoen, Chingching Foocharoen, Malinee Laopaiboon, Thavatchai Tiamklang | |
Differences between protocol and review
1. Type of outcome measures
-
We clarified that the 'Adverse events' in Secondary outcomes covered those more generally relating to the fracture or subsequent treatment.
-
Timing of outcome measurement: We reported pain outcomes at intermediate times of three and six weeks, as this allowed for an assessment of whether the initial pain relief from aspiration persisted in the short term.
2. Measure of treatment effect
-
We could not report mean differences with 95% confidence intervals due to lack of standard deviations.
3. Unit of analysis issues. As anticipated, the unit of analysis in both included trials was the individual participant.
4. Subgroup analysis. There were insufficient data to perform subgroup analysis.
5. The title has been changed to 'Aspiration of the elbow joint for treating radial head fractures'.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Aspiration versus no aspiration, Outcome 1 Impaired function at 12 months.
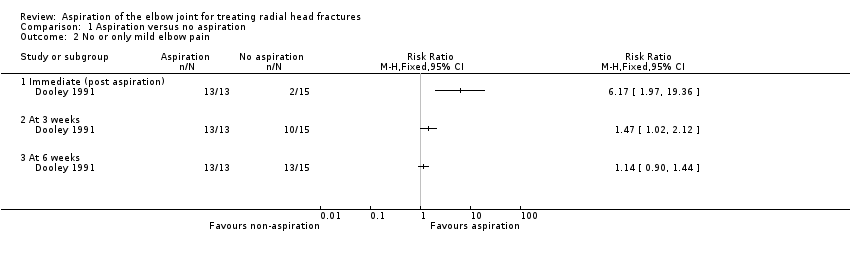
Comparison 1 Aspiration versus no aspiration, Outcome 2 No or only mild elbow pain.

Comparison 1 Aspiration versus no aspiration, Outcome 3 'Full' range of motion.
| Aspiration versus no aspiration for treating radial head fractures | ||||||
| Patient or population: patients being treated for radial head fractures; subsequent management was non‐surgical Settings: emergency or outpatients (within a day or so post injury) Intervention: aspiration (early intervention in first one to three days to remove bloody fluid (hematoma) from the elbow joint capsule) Comparison: no aspiration | ||||||
| Outcomes | Illustrative comparative risks*1 (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Correspondingrisk | |||||
| No aspiration | Aspiration | |||||
| Impaired function Unable to carry heavy weights or > 10° loss in extension (discomfort when carrying objects) At 12 months | Study population | RR 1.43 (0.57 to 3.58) | 108 | ⊕⊝⊝⊝ | Neither trial used validated clinical scores to report on patient function. All participants were treated conservatively. | |
| 177 per 1000 | 254 per 1000 | |||||
| No or only mild elbow pain Immediate (post aspiration or at an equivalent time) | Study population | RR 6.17 (1.97 to 19.36) | 28 | ⊕⊝⊝⊝ | Improvement post aspiration was also reported in the second trial, with 18/38 (47%) with 'excellent' pain relief; 17 (45%) with 'fair' pain relief; and 3 (8%) with no relief. There were no data for the control group in this trial. | |
| 134 per 1000 | 827 per 1000 | |||||
| No or only mild elbow pain At 3 weeks | Study population | RR 1.47 | 28 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 980 per 1000 | |||||
| No or only mild elbow pain At 6 weeks | Study population | RR 1.14 | 28 | ⊕⊝⊝⊝ | ||
| 867 per 1000 | 989 per 1000 (781 to 1000) | |||||
| Adverse effects of procedure or Aspiration failure | See comment | See comment | Not estimable | 108 (2 studies) | See comment | Neither study provided information on adverse outcomes (e.g. infection, nerve injuries) from the procedure. One study reported aspiration failure in 3 (7.8%) of 38 participants allocated aspiration. |
| 'Full' range of motion At 6 weeks | Study population | RR 2.31 (0.9 to 5.92) | 28 | ⊕⊝⊝⊝ | Defined as participants with full extension. | |
| 267 per 1000 | 616 per 1000 | |||||
| 'Full' range of motion At 12 months | Study population | RR 0.92 | 108 (2 studies) | ⊕⊝⊝⊝ | In one study, defined as participants with full extension; in the other study, defined as participants with extension loss less than 10°. | |
| 878 per 1000 | 808 per 1000 | |||||
| Adverse effects (of fracture and management) At 12 months | See comment | See comment | Not estimable | 80 (1 study) | See comment | One study (80 participants) reported that no participants had myositis ossificans, joint instability, or late displacement of the fracture. The other study (28 participants) did not specifically report this outcome. |
| *The basis for the assumed risk is provided in footnote 1. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The assumed control risk is either that of the control group of the individual trials, where data from only one trial are available, or the pooled control group, where data from both trials are available. 2. The evidence was downgraded two levels due to major limitations in study design and implementation (both studies were at very high risk of bias) and one level for imprecision (reflecting wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Impaired function at 12 months Show forest plot | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.57, 3.58] |
| 1.1 Unable to carry heavy weights | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.06, 5.66] |
| 1.2 > 10° loss in extension (discomfort when carrying objects) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.63, 4.94] |
| 2 No or only mild elbow pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Immediate (post aspiration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 'Full' range of motion Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 weeks | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.90, 5.92] |
| 3.2 At 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 3.3 At one year | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.08] |


