| Aspiration versus no aspiration for treating radial head fractures |
| Patient or population: patients being treated for radial head fractures; subsequent management was non‐surgical Settings: emergency or outpatients (within a day or so post injury) Intervention: aspiration (early intervention in first one to three days to remove bloody fluid (hematoma) from the elbow joint capsule) Comparison: no aspiration |
| Impaired function Unable to carry heavy weights or > 10° loss in extension (discomfort when carrying objects) At 12 months | Study population | RR 1.43 (0.57 to 3.58) | 108
(2 studies) | ⊕⊝⊝⊝
very low2 | Neither trial used validated clinical scores to report on patient function. All participants were treated conservatively. |
| 177 per 1000 | 254 per 1000
(101 to 634) |
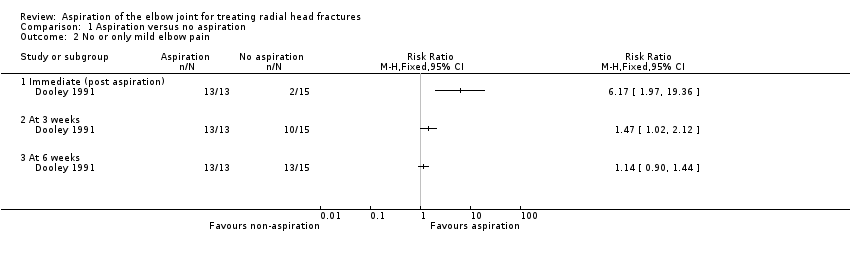
| No or only mild elbow pain Immediate (post aspiration or at an equivalent time) | Study population | RR 6.17 (1.97 to 19.36) | 28
(1 study) | ⊕⊝⊝⊝
very low2 | Improvement post aspiration was also reported in the second trial, with 18/38 (47%) with 'excellent' pain relief; 17 (45%) with 'fair' pain relief; and 3 (8%) with no relief. There were no data for the control group in this trial. |
| 134 per 1000 | 827 per 1000
(264 to 1000) |
| No or only mild elbow pain At 3 weeks | Study population | RR 1.47
(1.02 to 2.12) | 28
(1 study) | ⊕⊝⊝⊝
very low2 | |
| 667 per 1000 | 980 per 1000
(654 to 1000) |
| No or only mild elbow pain At 6 weeks | Study population | RR 1.14
(0.90 to 1.44) | 28
(1 study) | ⊕⊝⊝⊝
very low2 | |
| 867 per 1000 | 989 per 1000 (781 to 1000) |
| Adverse effects of procedure or Aspiration failure | See comment | See comment | Not estimable | 108 (2 studies) | See comment | Neither study provided information on adverse outcomes (e.g. infection, nerve injuries) from the procedure. One study reported aspiration failure in 3 (7.8%) of 38 participants allocated aspiration. |
| 'Full' range of motion At 6 weeks | Study population | RR 2.31 (0.9 to 5.92) | 28
(1 study) | ⊕⊝⊝⊝
very low2 | Defined as participants with full extension. |
| 267 per 1000 | 616 per 1000
(241 to 1000) |
| 'Full' range of motion At 12 months | Study population | RR 0.92
(0.78 to 1.08) | 108 (2 studies) | ⊕⊝⊝⊝
very low2 | In one study, defined as participants with full extension; in the other study, defined as participants with extension loss less than 10°. |
| 878 per 1000 | 808 per 1000
(685 to 948) |
| Adverse effects (of fracture and management) At 12 months | See comment | See comment | Not estimable | 80 (1 study) | See comment | One study (80 participants) reported that no participants had myositis ossificans, joint instability, or late displacement of the fracture. The other study (28 participants) did not specifically report this outcome. |
| *The basis for the assumed risk is provided in footnote 1. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio. |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. |